Abstract
We examined whether an additive treatment with an angiotensin receptor blocker, olmesartan, reduces the mortality and morbidity in hypertensive patients with chronic heart failure (CHF) treated with angiotensin-converting enzyme (ACE) inhibitors, β-blockers, or both. In this prospective, randomized, open-label, blinded endpoint study, a total of 1147 hypertensive patients with symptomatic CHF (mean age 66 years, 75% male) were randomized to the addition of olmesartan (n = 578) to baseline therapy vs. control (n = 569). The primary endpoint was a composite of all-cause death, non-fatal acute myocardial infarction, non-fatal stroke, and hospitalization for worsening heart failure. During a median follow-up of 4.4 years, the primary endpoint occurred in 192 patients (33.2%) in the olmesartan group and in 166 patients (29.2%) in the control group [hazard ratio (HR) 1.18; 95% confidence interval (CI), 0.96–1.46, P = 0.112], while renal dysfunction developed more frequently in the olmesartan group (16.8 vs. 10.7%, HR 1.64; 95% CI 1.19–2.26, P = 0.003). Subgroup analysis revealed that addition of olmesartan to combination of ACE inhibitors and β-blockers was associated with increased incidence of the primary endpoint (38.1 vs. 28.2%, HR 1.47; 95% CI 1.11–1.95, P = 0.006), all-cause death (19.4 vs. 13.5%, HR 1.50; 95% CI 1.01–2.23, P = 0.046), and renal dysfunction (21.1 vs. 12.5%, HR 1.85; 95% CI 1.24–2.76, P = 0.003). Additive use of olmesartan did not improve clinical outcomes but worsened renal function in hypertensive CHF patients treated with evidence-based medications. Particularly, the triple combination therapy with olmesartan, ACE inhibitors and β-blockers was associated with increased adverse cardiac events. This study is registered at clinicaltrials.gov-NCT00417222.
Keywords: Heart failure, Hypertension, Angiotensin II receptor blocker, Olmesartan
See page 899 for the editorial comment on this article (doi:10.1093/eurheartj/ehv033)
Introduction
In patients with heart failure (HF) with reduced ejection fraction (EF) (HFrEF), inhibition of renin–angiotensin system (RAS) by angiotensin-converting enzyme (ACE) inhibitors is generally recommended to improve mortality and morbidity in the clinical guidelines.1,2 Angiotensin receptor blockers (ARBs) are considered as reasonable alternatives of ACE inhibitors since they improve outcomes in patients with HFrEF and intolerance of ACE inhibitors.3 However, it is still controversial whether the additive use of ARBs to evidence-based medications is beneficial in patients with HFrEF, particularly in the subset of those who have a history of hypertension.4–6 Furthermore, unlike in HFrEF patients, beneficial impacts of RAS inhibitors (ACE inhibitors or ARBs) or β-blockers have not been shown in the randomized clinical trials in patients with HF with preserved EF (HFpEF),7–10 and thus neither RAS inhibitors nor β-blockers are recommended for the treatment of HFpEF in the clinical guidelines.1,2 In case of hypertensive patients with HFpEF, however, RAS inhibitors and/or β-blockers could be used as anti-hypertensive medication,1,2 but the supplemental benefit of ARBs in combination with anti-hypertensive medications has not been examined.
These lines of evidence suggest a need to elucidate whether additive use of ARB is beneficial or not in the general practice of hypertensive chronic heart failure (CHF) patients treated with ACE inhibitors and/or β-blockers. In the supplemental benefit of ARB in hypertensive patients with stable heart failure using olmesartan (SUPPORT) trial, we thus aimed to examine whether a supplemental use of olmesartan provides beneficial impacts in patients with a broad spectrum of HF, who are treated with conventional therapies, namely with ACE inhibitors and/or β-blockers.11
Methods
Study design
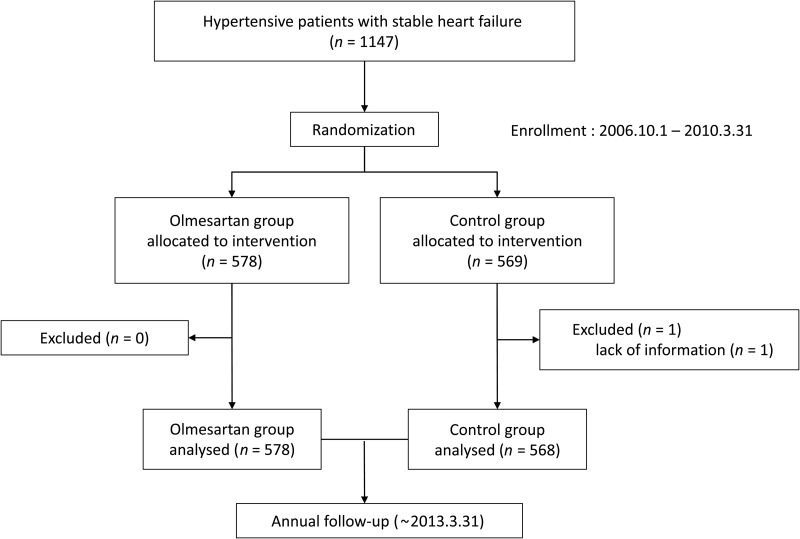
The SUPPORT trial was a prospective, randomized, open-label blinded endpoint study,11 which was conducted according to the ethical principles described in Declaration of Helsinki. The study protocol was approved by the institutional ethics committees of the 17 participating institutions in the Tohoku District of Japan (Appendix). The primary objective of the study was to examine whether an additive treatment with an ARB, olmesartan, reduces the mortality and morbidity of hypertensive patients with stable CHF (NCT00417222). The inclusion criteria were designed to enroll hypertensive patients aged 20–79 with symptomatic CHF but in stable condition and were treated with ACE inhibitors and/or β-blockers, while the exclusion criteria were designed to exclude patients with substantive confounding medical conditions or an inability to meaningfully participate in the SUPPORT trial (Table 1). Finally, a total of 1147 symptomatic CHF patients with a history of hypertension who met the inclusion and exclusion criteria and gave written informed consent for the trial were assigned to either the olmesartan or the control group according to a 1 : 1 ratio of olmesartan to control, through stratification by participating institute, sex and age between October 2006 and March 2010. The patients were followed up until the study ended on 31 March 2013 (Figure 1). If contact could not be made at the end of the study, data of these patients were censored at the date when they were known to be alive last. Olmesartan was initiated at a dose of 5–10 mg/day, and then up titrated to 40 mg/day, if tolerable, in the olmesartan group, while no ARB use was allowed in the control group. The diagnosis of HF was made based on the criteria of the Framingham study12 by an attending physician at each hospital. All physicians were encouraged to control blood pressure of the patients in each group according to the recommendations in the JNC7.13 The primary endpoint was the composite of all-cause death, non-fatal acute myocardial infarction, non-fatal stroke, and worsening HF requiring hospitalization, while secondary endpoints consisted of the modes of death, hospitalization for cardiovascular reasons, surrogate markers for HF and development of cardiovascular disease, atrial fibrillation, diabetes, and renal dysfunction (see Supplementary material online, Table S1).
Table 1.
Inclusion and exclusion criteria
|
Other patients deemed unsuitable as subjects of the study by physician in charge.
Figure 1.
Trial profile.
Interim analysis and data monitoring
Meetings of the Data Safety Monitoring Board, which is independent of the study committees, were held twice a year throughout the trial period to monitor the study safety until the end of the trial. Interim analyses were conducted at the end of and 2 years after the end of the registration period, namely March 2010 and September 2011, to evaluate the primary endpoint and the safety for the continuation of the trial.
Statistical analysis
All analyses were performed according to a predefined statistical analysis plan. Y.S., S.M., and H.S. had a full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of data analysis. The primary and secondary endpoints were analysed based on the time to the first occurrence, according to the intention-to-treat principle, including all patients of lost to follow-up censored at the day of the last contact. Survival curves were estimated using the Kaplan–Meier procedure and compared with a two-sided log-rank test. Based on the results of the CHART-1 Study,14,15 we assumed that the annual incidence of the primary composite endpoint would be ∼12% in the SUPPORT trial and thus 480 patients would be required on the condition of 3-year follow-up for each arm to provide 80% power to detect 30% risk reduction by olmesartan, using a two-sided significance level of 0.05 by the log-rank test.11 Considering that ∼15% of cases would be lost during follow-up or unsuitable for analysis, we estimated that >565 patients in each group (control and olmesartan) would be needed to complete the trial.
The P-value of the interim analysis was 0.005 while the P-value of the final analysis was 0.048. The effects of olmesartan were examined using Cox proportional hazards models. Subgroup analyses were performed according to baseline medication and other baseline characteristics and clinical parameters. Continuous variables are presented as means ± standard deviations except brain natriuretic peptide (BNP). Brain natriuretic peptide levels are presented as medians and interquartile ranges. Categorical variables were presented as numerals and percentages. Group comparisons were made with the Mann–Whitney test for continuous variables, and the χ2-test without continuity correction for categorical variables. All statistical analyses were performed using IBM SPSS Statistics 21.0 (IBM, Somers, NY, USA) and R 3.0.2 (R Foundation for Statistical Computing, Vienna. http://www.R-project.org/). A two-sided probability values of <0.05 and P-values for interaction <0.1 were considered to be statistically significant.
Results
Patient characteristics
Baseline characteristics were comparable between the olmesartan and the control groups (Table 2). The mean age was 66 and 75% of the patients were male. Functional class of HF was in NYHA Class II in 93% and Class III in 7%, and underlying heart disease included ischaemic heart disease in 48% and dilated cardiomyopathy in 21%. Mean systolic/diastolic blood pressure was 128/74 mmHg, and mean left ventricular ejection fraction (LVEF) was 54%. At the time of randomization, ACE inhibitors and β-blockers were prescribed in 81 and 72% of the patients, respectively.
Table 2.
Baseline characteristics
| Control (n = 569) | Olmesartan (n = 578) | P-value | |
|---|---|---|---|
| Age (years) | 65.5 ± 10.1 | 65.8 ± 10.4 | 0.445 |
| Males (%) | 427 (75.2%) | 429 (74.2%) | 0.71 |
| Body weight (kg) | 64.1 ± 12.9 | 63.2 ± 12.7 | 0.297 |
| Height (cm) | 161.0 ± 9.1 | 160.8 ± 9.6 | 0.655 |
| Body mass index (kg/m2) | 24.6 ± 4.1 | 24.2 ± 4.1 | 0.185 |
| NYHA functional class | 0.564 | ||
| II | 530 (93.5%) | 535 (92.6%) | |
| III | 37 (6.5%) | 43 (7.4%) | |
| Baseline cardiovascular disease | |||
| Ischaemic heart disease | 262 (46.1%) | 283 (49%) | 0.337 |
| Dilated cardiomyopathy | 132 (23.2%) | 110 (19%) | 0.081 |
| Diabetes mellitus | 292 (51.4%) | 283 (49%) | 0.408 |
| Haemodynamics and LV function | |||
| Systolic blood pressure (mmHg) | 127.1 ± 18.0 | 128.7 ± 18.2 | 0.081 |
| Diastolic blood pressure (mmHg) | 73.9 ± 11.7 | 74.8 ± 12.2 | 0.311 |
| Heart rate (bpm) | 71.5 ± 14.6 | 71.2 ± 13.8 | 0.808 |
| LVDd (mm) | 54.0 ± 8.7 | 53.3 ± 9.0 | 0.113 |
| LVEF (%) | 53.7 ± 14.5 | 54.5 ± 14.9 | 0.277 |
| ≤40% | 106 (18.8%) | 110 (19.2%) | 0.874 |
| >40 and <50% | 112 (19.9%) | 101 (17.6%) | 0.328 |
| ≥50% | 346 (61.3%) | 363 (63.2%) | 0.510 |
| Laboratory findings | |||
| Haemoglobin (g/dL) | 13.7 ± 1.9 | 13.8 ± 1.7 | 0.279 |
| Blood urea nitrogen (mg/dL) | 18.0 ± 6.9 | 18.3 ± 7.5 | 0.556 |
| Creatinine (mg/dL) | 0.95 ± 0.36 | 0.94 ± 0.33 | 0.956 |
| Albumin (mg/dL) | 4.2 ± 0.4 | 4.2 ± 0.4 | 0.28 |
| LDL-C (mg/dL) | 107.3 ± 30.0 | 108.2 ± 30.8 | 0.775 |
| eGFR (mL/min/1.73 m2) | 70.4 ± 24.4 | 70.0 ± 22.6 | 0.887 |
| BNP (pg/mL) | 78.2 (37.8, 173.0) | 84.2 (36.7, 188.8) | 0.63 |
| Baseline medication | |||
| β-Blocker | 416 (73.2%) | 405 (70.1%) | 0.234 |
| ACEI | 460 (81.0%) | 469 (81.1%) | 0.946 |
| Diuretics | 322 (56.7%) | 328 (56.7%) | 0.984 |
| Thiazides | 22 (3.9%) | 19 (3.3%) | 0.593 |
| Loop diuretics | 296 (52.1%) | 292 (50.5%) | 0.589 |
| Spironolactone | 153 (26.9%) | 152 (26.3%) | 0.807 |
| Calcium-channel blocker | 212 (37.3%) | 222 (38.4%) | 0.705 |
| Statin | 274 (48.2%) | 287 (49.7%) | 0.632 |
NYHA, New York Heart Association; LVDd, left ventricular diastolic dimension; LVEF, left ventricular ejection fraction; LDL-C, low-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; BNP, brain natriuretic peptide.
Drug adherence and blood pressure
At the time of randomization, systolic/diastolic blood pressure was 128.7/74.8 ± 18.2/12.2 and 127.1/73.8 ± 18.1/11.7 mmHg in the olmesartan and the control group, respectively (P = 0.081/0.311). Changes in systolic/diastolic blood pressure in both groups are shown in Figure 2. There was no significant difference in systolic/diastolic blood pressure at any point between the two groups.
Figure 2.
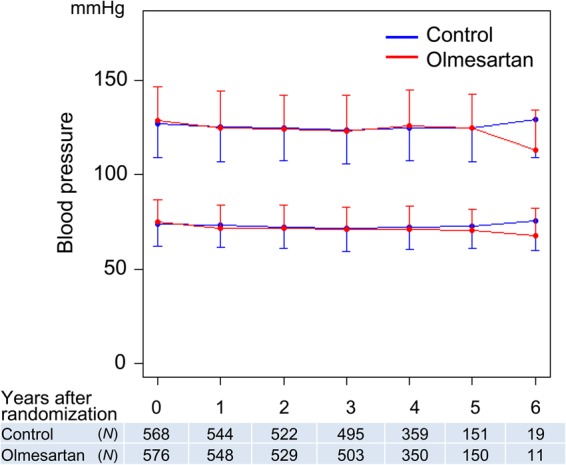
Time course in blood pressure. Blood pressure values are presented as mean ± standard deviations. There were no statistical differences in systolic or diastolic blood pressure at each point between the olmesartan and the control groups.
In the olmesartan group, olmesartan was not prescribed in 18 (3.1%), 56 (9.4%), 70 (12.1%), 65 (11.2%), 48 (10.7%), 19 (9.7%), and 1 (4.5%) patients at 0, 1, 2, 3, 4, 5, and 6 years after randomization, respectively. In the control group, the cumulative incidence of any ARB use was none (0%), 2 (0.3%), 4 (0.7%), 8 (1.4%), 8 (1.4%), 8 (1.4%), and 8 (1.4%) at 0, 1, 2, 3, 4, 5, and 6 years after randomization, respectively. In the olmesartan group, mean dose of olmesartan (mg/day) at 0, 1, 2, 3, 4, 5, and 6 years after randomization was 9.5, 13.3, 15.4, 16.1, 17.4, 17.9, and 16.5, respectively.
When the primary endpoint occurred or the SUPPORT trial ended, 58 patients (10.0%) were not taking olmesartan in the olmesartan group, whereas four patients (0.7%) were taking ARB in the control group. Subgroup analysis revealed that in the olmesartan group treated with ACE inhibitors alone, β-blockers alone, and both of them at the time of randomization, discontinuation of the ARB was noted in 13 (8.0%), 15 (14.2%), and 30 patients (10.0%), respectively, due to the occurrence of the primary endpoint or the end of the study.
Primary endpoint
During a median follow-up of 4.4 years, the primary endpoint occurred in 192 patients (33.2%) in the olmesartan group and in 166 patients (29.2%) in the control group [hazard ratio (HR) 1.18; 95% confidence interval (CI), 0.96–1.46, P = 0.112] (Figure 3 and Table 3). Subgroup analysis according to the baseline medication revealed that the incidence of the primary endpoint was more frequent in the olmesartan group than that in the control group, when combined with both ACE inhibitors and β-blockers [38.1% (114/299) vs. 28.2% (88/312), HR 1.47; 95% CI 1.12–1.95, P = 0.006], whereas there was no difference in the primary endpoint when combined with β-blockers alone or ACE inhibitors alone (Figure 4). Similarly, when combined with both ACE inhibitors and β-blockers, olmesartan was associated with increased incidence of all-cause death [19.4% (58/299) vs. 13.5% (42/312), HR 1.50; 95% CI 1.01–2.23, P = 0.046], a component of the primary endpoint, whereas olmesartan was associated with decreased mortality when combined with β-blockers alone [9.4% (10/106) vs. 22.1% (23/104), HR 0.41; 95% CI 0.19–0.85, P = 0.017], but not with ACE inhibitors alone (see Supplementary material online, Figure S1 and Table S2). Subgroup analysis revealed that additive use of olmesartan was associated with an increase in the primary endpoint in the subgroups of systolic blood pressure (SBP) of <130 mmHg, estimated glomerular filtration rate (eGFR) of <60 mL/min/1.73 m2, BNP level of >100 pg/mL, and high-sensitive C-reactive protein level of >1 mg/L (see Supplementary material online, Table S3), which was primarily due to the triple combination therapy (see Supplementary material online, Figure S2). There were no interactions of the impacts of olmesartan with age, gender, body mass index, diabetes, diastolic blood pressure, left ventricular hypertrophy, LVEF, or use of spironolactone (see Supplementary material online, Table S3).
Figure 3.
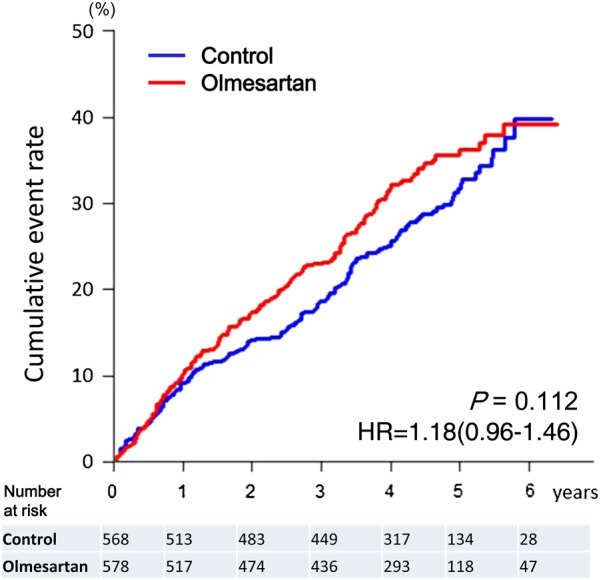
Kaplan–Meier curves for the primary endpoint for overall patients.
Table 3.
Incidence of the primary outcome measures, all-cause death, and secondary outcome measures in the overall population
| Control (n = 568) | Olmesartan (n = 578) | Hazard ratio | 95% CI |
P-value | ||
|---|---|---|---|---|---|---|
| Events, n (%) | Events, n (%) | Lower | Upper | |||
| Primary endpoint | 166 (29.2%) | 192 (33.2%) | 1.18 | 0.96 | 1.46 | 0.112 |
| All-cause death | 85 (15.0%) | 98 (17.0%) | 1.15 | 0.86 | 1.54 | 0.338 |
| Non-fatal acute myocardial infarction | 8 (1.4%) | 12 (2.1%) | 1.479 | 0.604 | 3.617 | 0.391 |
| Non-fatal stroke | 26 (4.6%) | 34 (5.9%) | 1.313 | 0.788 | 2.188 | 0.296 |
| Worsening HF requiring hospitalization | 99 (17.4%) | 113 (19.6%) | 1.148 | 0.877 | 1.504 | 0.316 |
| Secondary endpoints | ||||||
| Cardiovascular death | 38 (6.7%) | 48 (8.3%) | 1.26 | 0.82 | 1.93 | 0.290 |
| Death due to HF | 18 (3.2%) | 10 (1.7%) | 0.56 | 0.26 | 1.20 | 0.137 |
| Sudden death | 8 (1.4%) | 18 (3.1%) | 2.24 | 0.97 | 5.15 | 0.058 |
| Acute myocardial infarction | 12 (2.1%) | 13 (2.2%) | 1.07 | 0.49 | 2.35 | 0.866 |
| Stroke | 26 (4.6%) | 34 (5.9%) | 1.31 | 0.79 | 2.19 | 0.296 |
| Hospitalization for cardiovascular reasons | 179 (31.5%) | 199 (34.4%) | 1.13 | 0.92 | 1.38 | 0.230 |
| Fatal arrhythmia or appropriate ICD discharge | 29 (5.1%) | 30 (5.2%) | 1.02 | 0.61 | 1.69 | 0.947 |
| New-onset diabetes | 60 (10.6%) | 70 (12.1%) | 1.17 | 0.83 | 1.65 | 0.376 |
| Development of renal dysfunction | 61 (10.7%) | 97 (16.8%) | 1.64 | 1.19 | 2.26 | 0.003 |
| New-onset atrial fibrillation | 31 (5.5%) | 21 (3.6%) | 0.67 | 0.38 | 1.16 | 0.149 |
| Need to modify HF treatments | 131 (23.1%) | 142 (24.6%) | 1.08 | 0.85 | 1.37 | 0.534 |
| Decrease in LVEF | 111 (19.5%) | 122 (21.1%) | 1.11 | 0.86 | 1.44 | 0.419 |
| Increase in BNP levels | 198 (34.9%) | 217 (37.5%) | 1.12 | 0.92 | 1.35 | 0.259 |
ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; BNP, brain natriuretic peptide.
Figure 4.
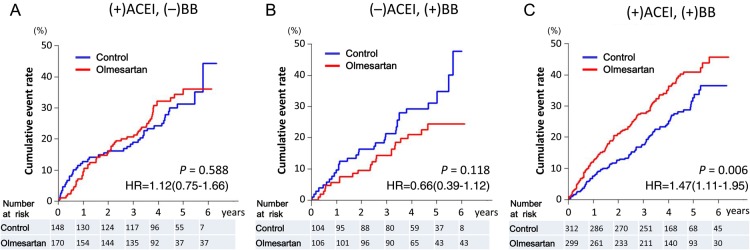
Kaplan–Meier curves for the primary endpoint for subgroups according to the baseline medication. (A) Patients treated with angiotensin-converting enzyme inhibitors but not with β-blockers. (B) Patients treated with β-blockers but not with angiotensin-converting enzyme inhibitors. (C) Patients treated with both angiotensin-converting enzyme inhibitors and β-blockers. ACEI, angiotensin-converting enzyme inhibitors; BB, β-blockers.
Secondary endpoints
There were no differences in secondary or other endpoints between the olmesartan and the control groups except for development of renal dysfunction [16.8% (97/578) vs. 10.7% (61/568), HR 1.64; 95% CI 1.19–2.26, P = 0.003] (Table 3). Subgroup analysis revealed that additive use of olmesartan, when combined with both ACE inhibitors and β-blockers, was significantly associated with increased incidence of renal dysfunction [21.1% (63/299) vs. 12.5% (39/312), HR 1.85; 95% CI 1.24–2.76, P = 0.003] (see Supplementary material online, Table S2). In contrast, when combined with ACE inhibitors alone, use of olmesartan was not associated with renal dysfunction and was rather associated with decreased incidence of atrial fibrillation [2.4% (4/170) vs. 8.8% (13/148), HR 0.26; 95% CI 0.09–0.80, P = 0.019] (see Supplementary material online, Table S2).
Adverse events
By the end of the study, 16.6% (96/578) of the patients in the olmesartan group discontinued olmesartan due to adverse events and other reasons (see Supplementary material online, Table S4). Besides hypotension (7.0%), renal dysfunction (1.2%), and worsening HF (0.7%) were the main adverse events (see Supplementary material online, Table S4).
Discussion
In the SUPPORT trial, we examined whether an additive treatment with an ARB, olmesartan, reduces the mortality and morbidity in CHF patients with a history of hypertension and treated with ACE inhibitors and β-blockers. The results revealed that additive use of olmesartan to ACE inhibitors and/or β-blockers did not improve clinical outcomes but worsened renal function in hypertensive CHF patients. Furthermore, subgroup analysis suggested that the triple combination therapy with olmesartan, ACE inhibitors and β-blockers was associated with increased adverse cardiac events and renal dysfunction.
Angiotensin receptor blockers for the management of hypertensive chronic heart failure patients treated with angiotensin-converting enzyme inhibitors and/or β-blockers
Angiotensin receptor blockers have been shown to provide various cardioprotective effects for patients with hypertension and other cardiovascular diseases,16–19 and are now widely used for the management of CHF worldwide.20–23 However, there have been no reports to evaluate the additive impact of ARBs in CHF patients with hypertension treated with evidence-based medications. The SUPPORT trial was the first to examine the efficacy of additive use of an ARB to ACE inhibitors and/or β-blockers in hypertensive patients with CHF. The results demonstrated that additive use of olmesartan did not decrease the primary endpoint, a composite of all-cause death, non-fatal acute myocardial infarction, non-fatal stroke, and hospitalization for worsening HF, but increased renal dysfunction in hypertensive CHF patients. Importantly, the triple combination of olmesartan, ACE inhibitors, and β-blockers was associated with an increase in the primary endpoint, particularly in patients with SBP of <130 mmHg, eGFR of <60 mL/min/1.73 m2, serum BNP levels of ≥100 pg/mL, and high-sensitive CRP level of ≥1.0 mg/L. The triple combination of olmesartan, ACE inhibitors, and β-blockers was also associated with increased incidence of all-cause death and renal dysfunction, whereas the dual combination of olmesartan and ACE inhibitors or β-blockers was not associated with any increase in the primary or secondary endpoints. In addition, it was noteworthy that dual combination of olmesartan and ACE inhibitors was associated with a decrease in new-onset atrial fibrillation and that of olmesartan and β-blockers was associated with decreased mortality without development of renal dysfunction. Thus, it is suggested that the triple combination is harmful but the dual combination therapy, particularly that of olmesartan and β-blockers, could be beneficial in hypertensive patients with CHF. However, dual combination therapy of olmesartan and ACE inhibitors warrants a caution as recent studies suggested that dual blockade of RAS failed to reduce mortality but was associated with an excessive risk of adverse effects, including renal dysfunction, hyperkalaemia, and hypotension, particularly in those without HF.15–18
Triple combination of olmesartan, angiotensin-converting enzyme inhibitors, and β-blockers for chronic heart failure
Although it has been shown that combination of ACE inhibitors and ARBs without β-blockers was useful in patients with symptomatic CHF, there has been a controversy for the effectiveness of the dual RAS blockade with ACE inhibitors and ARBs in patients with HFrEF treated with β-blockers.4–6 In the valsartan heart failure (Val-HeFT) trial,4 valsartan significantly reduced the combined endpoint of mortality and morbidity in patients with symptomatic CHF; however, the post hoc analysis revealed an increase in adverse effect on mortality and morbidity in the subgroup receiving valsartan, an ACE inhibitor and a β-blocker.4 In contrast, the CHARM-added trial demonstrated that addition of an ARB candesartan to ACE inhibitors was beneficial in patients with symptomatic CHF regardless of β-blocker use.5 Our findings in the SUPPORT trial are consistent with the results of the Val-HeFT study as the triple combination therapy appeared harmful in hypertensive patients with CHF. Although the precise mechanism of the discrepancy for the effectiveness of the triple combination therapy between the Val-HeFT and the CHARM-added studies is unclear, it could be explained by the differences in patient backgrounds; the majority of the patients were in NYHA Class II (62%) in the Val-HeFT study but were in NYHA Class III (73%) in the CHARM-added study. In the present SUPPORT trial, the majority of the patients (93%) were in NYHA Class II. Thus, although the routine use of triple combination of ARBs, ACE inhibitors, and β-blockers should be avoided in hypertensive patients with mildly symptomatic CHF, it remains to be examined whether the triple combination therapy could be beneficial for patients with severe CHF.
Olmesartan for heart failure with preserved ejection fraction
Heart failure with preserved ejection fraction is now recognized as a new entity of HF,24,25 and represents more than half of HF patients in Japan19,26,27 and Western countries.28–30 Although previous RCTs have failed to show the beneficial effects of RAS inhibitors to improve mortality and morbidity in patients with HFpEF,7–9 a recent report from the Swedish Heart Failure Registry suggested that RAS inhibitors might be beneficial for the disorder.20 Furthermore, a post hoc analysis of irbesartan in patients with heart failure and preserved ejection fraction study demonstrated that the use of irbesartan was associated with improved outcomes in HFpEF patients with NT-proBNP levels of ≤339 pg/mL.31 These lines of evidence suggested an benefit of ARBs in patients with HFpEF. In the SUPPORT trial, we enrolled a considerable number of HFpEF patients and examined the clinical impact of olmesartan in a subgroup of patients with preserved EF (≥50%). However, the results remained unchanged even in the subgroups of HFpEF patients.
Relatively low dose olmesartan in the SUPPORT trial
In the ROADMAP trial, olmesartan at a dose of 40 mg once daily was associated with a delayed onset of microalbuminuria in patients with type 2 diabetes, whereas the higher rate of fatal cardiovascular events with olmesartan among patients with pre-existing coronary artery disease was of concern.32 In the present SUPPORT trial, the mean dose of olmesartan was 9.5 mg/day at the time of randomization and was then increased annually up to 13.3, 15.4, 16.1, 17.4, 17.9, and 16.5 mg/day at 1–6 years, respectively. Since the doses of olmesartan were relatively low compared with the previous studies examining the efficacy of olmesartan,32,33 there might be a critique that the dose of olmesartan was not enough to achieve its full clinical impacts in the present study. In the Japanese population, however, it was reported that a mean dose of 13.0 mg per day successfully provided adequate reductions in both systolic and diastolic blood pressures along with a significant increase in plasma renin activity as well as a decrease in angiotensin II levels.34 Thus, it is anticipated that an adequate blockade of angiotensin type I receptor was obtained in the olmesartan group in the SUPPORT trial. Anyway, since the adverse effects of the triple combination of olmesartan, ACE inhibitors, and β-blockers were noted with this relatively low doses of olmesartan, the present findings should be of clinical importance.
Study limitations
Several limitations should be mentioned for the present study. First, since the SUPPORT trial was conducted in an open-labelled fashion, the interpretation warrants caution. Second, it should be noted that the patients enrolled in the SUPPORT trial had been relatively well-controlled and mildly symptomatic before randomization compared with the previous HF studies; both the mean systolic and diastolic blood pressures (128/74 mmHg) had been already controlled below the target levels recommended by the JNC713 and other clinical guidelines,1,2 and majority of the patients (93%) had been controlled in NYHA Class II. As a result, the patients were characterized by relatively low BNP value (median 80 pg/mL) and modest prescription rate of diuretics (57%). These characteristics were comparable with those in the ALDO-DHF trial that mainly enrolled HFpEF patients in NYHA Class II,35 but were different from other HFpEF studies that enrolled more symptomatic patients7–9 and from previous HFrEF studies.3–5 Furthermore, since the sample size of the present population of patients with HFrEF was small, further studies with a large sample size are needed to examine whether or not the ARB olmesartan is effective for the treatment of those patients. Thus, cautions are warranted when interpreting the present results into other populations including more severely hypertensive or more symptomatic CHF patients. Third, the possible influence of the Great East Japan Earthquake in 2011 in our Tohoku area should be considered, which occurred after the randomization and during the follow-up period of the SUPPORT trial. However, since the results were unaltered even after exclusion of the results from the hospitals located in the area with severe damage, the influence of the disaster may be minimal.
Conclusions
The SUPPORT trial demonstrated that additive use of olmesartan did not improve clinical outcomes but worsened renal function in hypertensive CHF patients treated with evidence-based medications. Particularly, the triple combination therapy with olmesartan, ACE inhibitors, and β-blockers was associated with increased adverse cardiac events.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This study was supported in part by the grants-in-aid from the Ministry of Health, Labour, and Welfare and those from the Ministry of Education, Culture, Sports, Science, and Technology, Japan. Funding to pay the Open Access publication charges for this article was provided by the author.
Conflict of interest: The Department of Evidence-based Cardiovascular Medicine, Tohoku University Graduate School of Medicine is supported in part by the unrestricted research grants from Daiichi Sankyo Co., Ltd. (Tokyo, Japan), Bayer Yakuhin, Ltd. (Osaka, Japan), Kyowa Hakko Kirin Co., Ltd. (Tokyo, Japan), Kowa Pharmaceutical Co., Ltd. (Tokyo, Japan), Novartis Pharma K.K. (Tokyo, Japan), Dainippon Sumitomo Pharma, Co., Ltd. (Osaka, Japan), and Nippon Boehringer Ingelheim Co., Ltd. (Tokyo, Japan). H.S. has received lecture fees from Bayer Yakuhin, Ltd. (Osaka, Japan), Daiichi Sankyo Co., Ltd. (Tokyo, Japan), and Novartis Pharma K.K. (Tokyo, Japan).
Acknowledgements
We thank Toshimitsu Hamasaki and all the members of the Tohoku Heart Failure Society and staffs of the Departments of Cardiovascular Medicine and Evidence-based Cardiovascular Medicine, Tohoku University Graduate School of Medicine, for their valuable contributions.
Appendix
The SUPPORT trial investigators
Executive committee
H. Shimokawa (principal investigator), M. Fukuchi, T. Goto, T. Hiramoto, K. Inoue, A. Kato, T. Komaru, M. Ohe, N. Sekiguchi, N. Shiba, T. Shinozaki, M. Sugi, K. Tamaki.
Steering committee
T. Hiramoto, K. Inoue, A. Kato, M. Ogata, S. Sato, M. Sugi.
Endpoint evaluation committee
N. Ishide, S. Ibayashi, Y. Maruyama.
Ethics committee
I. Ohno, K. Tamaki.
Data safety monitoring board
H. Ogawa, M. Kitakaze.
Statistical analysis board
I. Tsuji, T. Watanabe, K. Sugiyama.
Collaborating hospitals and active investigators by prefecture
Aomori prefecture
S. Oyama (Towada City Hospital).
Iwate prefecture
E. Nozaki, A. Nakamura, T. Takahashi, H. Endo, S. Fukui, S. Nakajima (Iwate Prefectural Central Hospital). M. Nakagawa, T. Nozaki, T. Yagi (Iwate Prefectural Isawa Hospital).
Akita prefecture
S. Horiguchi, E. Fushimi, Y. Sugai, S. Takeda, K. Fukahori, K. Aizawa (Hiraka General Hospital).
Yamagata prefecture
M. Ohe, T. Tashima, K. Sakurai, T. Kobayashi (Kojirakawa Shiseido Hospital). T. Goto, M. Matsui, Y. Tamada, T. Yahagi, A. Fukui, K. Takahashi, K. Takahashi, Y. Kikuchi (Yamagata Prefectural Central Hospital).
Miyagi prefecture
K. Akai (Ishinomaki City Hospital). H. Kanno, J. Kaneko (Katta General Hospital). S. Suzuki, K. Takahashi (KKR Tohoku Kosai Hospital). K. Akai (Kurihara Central Hospital). D. Katayose (Miyagi Rifu Ekisaikai Hospital). S. Onodera, T. Hiramoto, S. Komatsu, M. Chida, K. Iwabuchi, M. Takeuchi, H. Yahagi, N. Takahashi (Osaki Citizen Hospital). K. Otsuka, Y. Koseki, M. Morita (Saito Hospital). T. Shinozaki, T. Ishizuka, N. Onoue, N. Yamaguchi, H. Fujita (Sendai Medical Center). A. Katoh, S. Namiuchi, T. Sugie, K. Saji, T. Takii, (Sendai Open Hospital). A. Sugimura, J. Ohashi (Sendai Red Cross Hospital). M. Fukuchi, M. Ogata, T. Tanikawa, O. Kitamukai (Sendai Tokushukai Hospital). Y. Matsumoto (Shizugawa Public Hospital). K. Inoue, J. Koyama, T. Tomioka, H. Shioiri, Y. Ito (South Miyagi Medical Center). H. Kato, C. Takahashi, A. Kawana (Tohoku Rosai Hospital). Y. Sakata, K. Ito, M. Nakayama, K. Fukuda, J. Takahashi, S. Miyata, K. Sugimura, K. Sato, Y. Matsumoto, M. Nakano, T. Shiroto, R. Tsuburaya, K. Nochioka, H. Yamamoto, T. Aoki, K. Hao, M. Miura, M. Kondo, S. Tatebe, S. Yamamoto, H. Suzuki, K. Nishimiya, N. Yaoita (Tohoku University Hospital).
Fukushima prefecture
M. Sugi, Y. Yamamoto, S. Toda, Y. Minatoya, Y. Takagi, Y. Hasebe, T. Nihei, K. Hanawa (Iwaki Kyouritsu Hospital). K. Fukuda (Watanabe Hospital).
Head office and coordinating center
Y. Sakata, J. Takahashi, S. Miyata, K. Nochioka, M. Miura, S. Tadaki, R. Ushigome, T. Yamauchi, K. Sato, K. Tsuji, T. Onose, R. Abe, C. Saga, J. Suenaga, Y. Yamada, J. Kimura, H. Ogino, I. Oikawa, S. Watanabe, M. Saga, M. Washio, K. Nagasawa, S. Nagasawa, S. Kotaka, W. Komatsu, R. Hashimoto, Y. Ikeno, T. Suzuki, H. Hamada (Tohoku University Hospital)
References
- 1.WRITING COMMITTEE MEMBERS Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;128:e240–e327. [DOI] [PubMed] [Google Scholar]
- 2.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A. ESC Committee for Practice Guidelines. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787–1847. Erratum in Eur Heart J 2013;34:158. [DOI] [PubMed] [Google Scholar]
- 3.Granger CB, McMurray JJ, Yusuf S, Held P, Michelson EL, Olofsson B, Ostergren J, Pfeffer MA, Swedberg K, CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet 2003;362:772–776. [DOI] [PubMed] [Google Scholar]
- 4.Cohn JN, Tognoni G. Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001;345:1667–1675. [DOI] [PubMed] [Google Scholar]
- 5.McMurray JJ, Ostergren J, Swedberg K, Granger CB, Held P, Michelson EL, Olofsson B, Yusuf S, Pfeffer MA, CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet 2003;362:767–771. [DOI] [PubMed] [Google Scholar]
- 6.Dimopoulos K, Salukhe TV, Coats AJ, Mayet J, Piepoli M, Francis DP. Meta-analyses of mortality and morbidity effects of an angiotensin receptor blocker in patients with chronic heart failure already receiving an ACE inhibitor (alone or with a β-blocker). Int J Cardiol 2004;93:105–111. [DOI] [PubMed] [Google Scholar]
- 7.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A, I-PRESERVE Investigators. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008;359:2456–2467. [DOI] [PubMed] [Google Scholar]
- 8.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J, PEP-CHF Investigators. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–2345. [DOI] [PubMed] [Google Scholar]
- 9.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM preserved trial. Lancet 2003;362:777–781. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto K, Origasa H, Hori M, J-DHF Investigators. Effects of carvedilol on heart failure with preserved ejection fraction: the Japanese Diastolic Heart Failure Study (J-DHF). Eur J Heart Fail 2013;15:110–118. [DOI] [PubMed] [Google Scholar]
- 11.Sakata Y, Nochioka K, Miura M, Takada T, Tadaki S, Miyata S, Shiba N, Shimokawa H. Supplemental benefit of an angiotensin receptor blocker in hypertensive patients with stable heart failure using olmesartan (SUPPORT) trial-Rationale and design. J Cardiol 2013;62:31–36. [DOI] [PubMed] [Google Scholar]
- 12.McKee PA, Castelli WP, McNamara PM, Kannel WB. Natural history of congestive heart failure: the Framingham Study. N Engl J Med. 1971;285:1441–1446. [DOI] [PubMed] [Google Scholar]
- 13.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ, National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 14.Shiba N, Nochioka K, Kohno H, Matsuki M, Takahashi J, Tada T, Kagaya Y, Shimokawa H. Emerging problems of heart failure practice in Japanese women: lessons from the CHART study. Circ J. 2008;72:2009–2014. [DOI] [PubMed] [Google Scholar]
- 15.Nochioka K, Shiba N, Kohno H, Miura M, Shimokawa H. Both high and low body mass indexes are prognostic risks in Japanese patients with chronic heart failure: implications from the CHART study. J Card Fail 2010;16:880–887. [DOI] [PubMed] [Google Scholar]
- 16.Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, Leehey DJ, McCullough PA, O'Connor T, Palevsky PM, Reilly RF, Seliger SL, Warren SR, Watnick S, Peduzzi P, Guarino P, VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013;369:1892–1903. [DOI] [PubMed] [Google Scholar]
- 17.ONTARGET Investigators Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008;358:1547–1559. [DOI] [PubMed] [Google Scholar]
- 18.Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S, Dickstein K, Keltai M, Metsärinne K, Oto A, Parkhomenko A, Piegas LS, Svendsen TL, Teo KK, Yusuf S. ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008;372:547–553. [DOI] [PubMed] [Google Scholar]
- 19.Makani H, Bangalore S, Desouza KA, Shah A, Messerli FH. Efficacy and safety of dual blockade of the renin-angiotensin system: meta-analysis of randomised trials. BMJ 2013;346:f360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lund LH, Benson L, Dahlström U, Edner M. Association between use of renin-angiotensin system antagonists and mortality in patients with heart failure and preserved ejection fraction. JAMA 2012;308:2108–2117. [DOI] [PubMed] [Google Scholar]
- 21.Shiba N, Nochioka K, Miura M, Kohno H, Shimokawa H. CHART-2 Investigators. Trend of westernization of etiology and clinical characteristics of heart failure patients in Japan -First report from the CHART-2 Study. Circ J 2011;75:823–833. [DOI] [PubMed] [Google Scholar]
- 22.Miura M, Sakata Y, Miyata S, Nochioka K, Takada T, Tadaki S, Takahashi J, Shiba N, Shimokawa H. CHART-2 Investigators. Usefulness of combined risk stratification with heart rate and systolic blood pressure in the management of chronic heart failure. A report from the CHART-2 study-. Circ J 2013;77:2954–2962. [DOI] [PubMed] [Google Scholar]
- 23.Miura Y, Fukumoto Y, Miura T, Shimada K, Asakura M, Kadokami T, Ando S, Miyata S, Sakata Y, Daida H, Matsuzaki M, Yasuda S, Kitakaze M, Shimokawa H. Impact of physical activity on cardiovascular events in patients with chronic heart failure. A multi-center prospective cohort study-. Circ J 2013;77:2963–2972. [DOI] [PubMed] [Google Scholar]
- 24.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med 2006;355:260–269. [DOI] [PubMed] [Google Scholar]
- 25.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355:251–259. [DOI] [PubMed] [Google Scholar]
- 26.Sakata Y, Shimokawa H. Epidemiology of heart failure in Asia. Circ J 2013;77:2209–2217. [DOI] [PubMed] [Google Scholar]
- 27.Miura M, Shiba N, Nochioka K, Takada T, Takahashi J, Kohno H, Shimokawa H. CHART-2 Investigators. Urinary albumin excretion in heart failure with preserved ejection fraction: an interim analysis of the CHART 2 study. Eur J Heart Fail 2012;14:367–376. [DOI] [PubMed] [Google Scholar]
- 28.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol 2011;8:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC. ADHERE Scientific Advisory Committee and Investigators. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol 2006;47:76–84. [DOI] [PubMed] [Google Scholar]
- 30.Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB. OPTIMIZE-HF Investigators and Hospitals. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF registry. J Am Coll Cardiol 2007;50:768–777. [DOI] [PubMed] [Google Scholar]
- 31.Anand IS, Rector TS, Cleland JG, Kuskowski M, McKelvie RS, Persson H, McMurray JJ, Zile MR, Komajda M, Massie BM, Carson PE. Prognostic value of baseline plasma amino-terminal pro-brain natriuretic peptide and its interactions with irbesartan treatment effects in patients with heart failure and preserved ejection fraction: findings from the I-PRESERVE trial. Circ Heart Fail 2011;4:569–577. [DOI] [PubMed] [Google Scholar]
- 32.Haller H, Ito S, Izzo JL, Jr, Januszewicz A, Katayama S, Menne J, Mimran A, Rabelink TJ, Ritz E, Ruilope LM, Rump LC, Viberti G. ROADMAP Trial Investigators. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med 2011;364:907–917. [DOI] [PubMed] [Google Scholar]
- 33.Imai E, Chan JC, Ito S, Yamasaki T, Kobayashi F, Haneda M, Makino H, ORIENT study investigators. Effects of olmesartan on renal and cardiovascular outcomes in type 2 diabetes with overt nephropathy: a multicentre, randomised, placebo-controlled study. Diabetologia 2011;54:2978–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ichikawa S, Takayama Y. Long-term effects of olmesartan, an Ang II receptor antagonist, on blood pressure and the renin-angiotensin-aldosterone system in hypertensive patients. Hypertens Res 2001;24:641–646. [DOI] [PubMed] [Google Scholar]
- 35.Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W, Duvinage A, Stahrenberg R, Durstewitz K, Löffler M, Düngen HD, Tschöpe C, Herrmann-Lingen C, Halle M, Hasenfuss G, Gelbrich G, Pieske B. Aldo-DHF Investigators. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA 2013;309:781–791. [DOI] [PubMed] [Google Scholar]