Abstract
Bacterial zoonoses comprise a group of diseases in humans or animals acquired by direct contact with or by oral consumption of contaminated animal materials, or via arthropod vectors. Among neglected infections, bacterial zoonoses are among the most neglected given emerging data on incidence and prevalence as causes of acute febrile illness, even in areas where recognized neglected tropical diseases occur frequently. While many other bacterial infections could also be considered in this neglected category, five distinct infections stand out because they are globally distributed, are acute febrile diseases, have high rates of morbidity and case fatality, and are reported as commonly as malaria, typhoid or dengue virus infections in carefully designed studies in which a broad spectrum diagnoses are actively sought. Thus, this review will focus attention on leptospirosis, relapsing fever borreliosis, and rickettsioses, including scrub typhus, murine typhus and spotted fever group rickettsiosis. Of greatest interest is the lack of distinguishing clinical features among these infections when in humans, which confounds diagnosis where laboratory confirmation is lacking, and in regions where clinical diagnosis is often attributed to one of several perceived more common threats. As diseases such as malaria come under improved control, the real impact of these common and under-recognized infections will become evident, as will the requirement for the strategies and allocation of resources for their control.
Keywords: Leptospira, Relapsing fever Borrelia, Orientia, Rickettsia, acute febrile illness, zoonosis, vector-borne bacteria, ticks, fleas, chiggers
Introduction
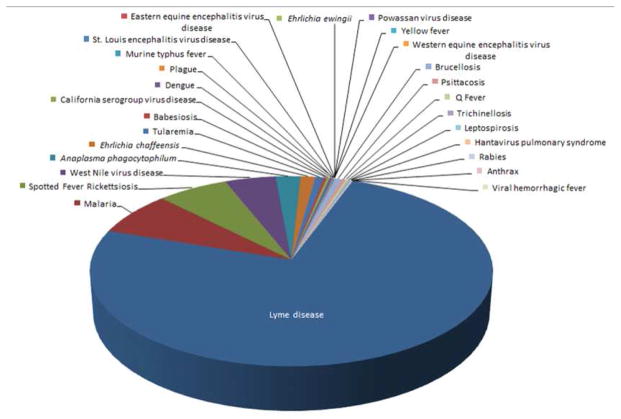
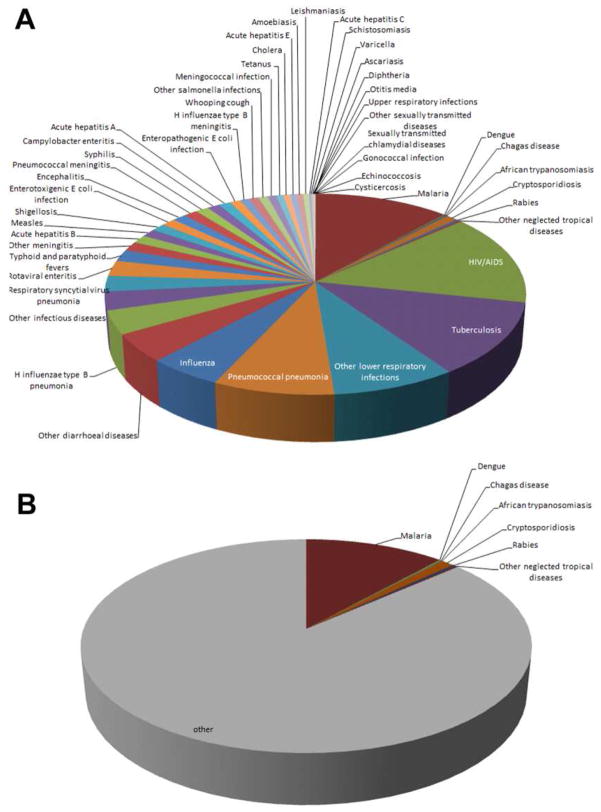
Zoonoses comprise an array of infections, many known throughout man’s history, and others that were only recently recognized to cause human or animal disease. The phrase “neglected” infectious disease recently emerged based on the perception that neglected infections are largely tropical or affect large poverty-ridden populations, are chronic, and are perceived to take a steep toll on society and development [1]. However, the concept of neglected infections is neither new nor fixed, and depends on the interpretation context. Figure 1 shows zoonoses in the US as reported in Morbidity Mortality Weekly Reports spanning 1981 to 2012 [2] and lacks any priority neglected zoonosis as cited by the World Health Organization (WHO) or the U.S. Centers for Disease Control and Prevention (CDC) [3, 4]. Depending on the source, bacterial infections defined as “neglected” by the Bill & Melinda Gates Foundation (BMGF) include only trachoma and buruli ulcer [5], while WHO and CDC add yaws and leprosy, and the list expands to include infantile diarrhea (enterotoxigenic Escherichia coli) in the European Union [3, 4, 6]. The BMGF-sponsored Global Burden of Diseases, Injuries, and Risk Factors Study 2010 list of deaths from globally-important infectious diseases includes only a small proportion that is zoonotic (Figure 2) [7]. The biased data presentation occurs largely because neglected diseases cannot be compared if not accurately diagnosed and reported, or assessed based on disability-adjusted life years (DALYs) if that factor is unable to be calculated given the absence of meaningful incidence or prevalence data. Rather the list reflects existing data shaped by infections for which diagnosis or reporting is easy, or based on evidence estimates which could be biased given the lack of such data [8, 9]. Much speculation has been made in recent years about priorities for allocation of resources as malaria comes under increasing control [10]. A growing number of investigations to discern the etiology of acute febrile illness in developed and under-resourced regions illustrate major gaps in our knowledge, and these gaps identify a greater expanse of neglected infectious diseases, many of which are bacterial zoonoses [11–16]. Such investigations shed light on prevalence estimates and the likely high human toll of inattention to other infections, many bacterial, and the topic of this article.
Figure 1.
Zoonoses reported to the CDC and published in Morbidity Mortality Weekly Reports between 1981 and 2012. Not all diseases were reportable over this interval, and some case definitions changed. No neglected infectious disease as cited by the CDC or WHO appears on this list. Aside from malaria that is most often imported, the most commonly reported zoonoses in the US over this interval were Lyme disease, spotted fever group rickettsioses, West Nile virus infection, Anaplasma phagocytophilum infection, and Ehrlichia chaffeensis infection.
Figure 2.
The proportional contribution of various infectious disease fatalities as reported in the Global Burden of Diseases, Injuries, and Risk Factors Study 2010 [7]. (A) Pie chart demonstrating all infectious diseases examined by the study. B. Zoonoses occupy a minority of the reported deaths and disease burden, and include no bacterial zoonoses.
Bacterial zoonoses occur with transmission via one of several mechanisms: 1) direct contact with animals or infected materials; 2) animal bites and scratches; 3) bites or mechanical transmission by arthropod vectors; and 4) consumption of contaminated foods (Table 1). The bacteria that cause the infections can sometimes be acquired by more than one transmission mechanism, complicating control measures. Most do not appear on lists of neglected infections, in part because of serious problems with definitive etiologic diagnosis and reporting: most acute febrile illnesses in Sub-Saharan Africa are diagnosed and reported on clinical grounds as malaria, and in Southeast Asia, as typhoid fever or dengue virus infection. When investigated objectively using pathogen-specific diagnostics, infections such as leptospirosis, rickettsioses, and melioidosis are diagnosed as frequently as dengue, typhoid or malaria [11–13, 16, 17]. Owing to the biases toward the “neglected diseases” and the paucity of objective data accumulated by major public health agencies, this article will focus on only selected bacterial zoonoses for which their recognition as important or relevant is not generally acknowledged, and for which diagnosis, treatment and research priorities are minimal globally. Despite the exclusion of certain bacterial zoonoses, the approach is intended to highlight what is known and not known as examples of why improvements in the study of these diseases are needed.
Table 1.
Bacterial Zoonoses by transmission mechanism and causative agent(s)
| Bacterial Zoonoses transmitted by direct contact with animals or infected animal materials | |
|
| |
| Anthrax | Bacillus anthracis |
| Brucellosis | Brucella spp. |
| Cat Scratch Disease | Bartonella spp. |
| Erysipelothrix infections | Erysipelothrix rhusiopathiae |
| Glanders and Melioidosis | Burkholderia mallei and B. pseudomallei |
| Leptospirosis | Leptospira interrogans spp. |
| Mycobacterioses | Mycobacteria spp. |
| Q fever | Coxiella burnetii |
|
| |
| Bacterial Zoonoses transmitted principally by animal bites or scratches | |
|
| |
| Pasteurellosis | Pasteurella multocida and other spp. |
| Capnocytophaga infections | Capnocytophaga canimorsus |
| Cat scratch disease | Bartonella henselae |
| Rat-Bite fever | Spirillum minus and Streptobacillus moniliformis |
|
| |
| Vector-borne Bacterial Zoonoses | |
|
| |
| Lyme borreliosis | Borrelia burgdorferi sensu lato (incl. B. garinii, B. afzelii) |
| Tick- and louse-borne relapsing fever borreliosis | Borrelia recurrentis, B. turicatae, B. hermsii, others |
| Plague | Yersinia pestis |
| Tularemia | Francisella tularensis |
| Rickettsioses | Spotted fever and typhus group Rickettsia species |
| Ehrlichiosis and Anaplasmosis | Ehrlichia chaffeensis, Anaplasma phagocytophilum |
| Scrub typhus | Orientia tsutsugamushi |
|
| |
| Foodborne Bacterial Zoonoses and Intoxications | |
|
| |
| Salmonellosis | Salmonella enteritidis |
| Campylobacteriosis | Campylobacter spp. |
| Listeriosis | Listeria monocytogenes |
| E. coli O157:H7 infections | Escherichia coli STEC |
| Yersinia enterocolitica infections | Yersinia enterocolitica |
| Clostridium perfringens gastroenteritis | Clostridium perfringens |
| Botulism | Clostridium botulinum |
| Staphylococcal food poisoning | Staphylococcus aureus |
Leptospirosis
Leptospirosis is a zoonosis caused by pathogenic spirochetes of the genus Leptospira [18–20]. Leptospires are spiral shaped, motile aerobic spirochetes distinguished morphologically from other spirochetes by characteristic hooked ends [19]. Members of the Leptospira genus were previously grouped according to antigenic determinants, with pathogenic leptospires belonging to the species Leptospira interrogans and nonpathogenic leptospires grouped under the species Leptospira biflexa. A new classification system proposes grouping of leptospires by DNA relatedness into 20 species: 9 pathogenic, 5 of intermediate or unclear pathogenicity, and 6 non-pathogenic saprophytes [21, 22].
Animals serve as natural hosts and can be asymptomatic [19]. Leptospires are excreted in their urine, contaminating water and soil where they can remain viable for days to months. Rodents are the most significant reservoirs for transmission and when infected can shed the leptospires throughout their lifetime. Humans are accidental hosts, acquiring infection after exposure of mucous membranes and abraded skin to animal urine, contaminated water or soil, or infected animal tissue. After entering the bloodstream, the spirochetes multiply in organs, most commonly the CNS, kidneys, and liver. They are cleared from the blood and most tissues by the immune response but can persist and multiply in the tubules of the kidneys.
The distribution of leptospirosis is worldwide but it occurs with the greatest frequency in tropical and subtropical environments [18, 20]. It is estimated more than 10 million cases occur each year around the world and it is a significant cause of morbidity and mortality [21, 23]. Leptospirosis is most common in developing urban and rural areas with inadequate sewage disposal and water treatment, yet outbreaks in temperate regions, including the U.S. and Europe are well documented [21, 24]. The burden of disease is highest in the Caribbean, Central and South America, Southeast Asia, Oceania, India and South Asia, and Eastern Europe. The incidence in the Seychelles in the Indian Ocean is as high as 432 per 1,000,000 population and globalization continues to occur through international travel to these and other similar areas where leptospirosis is endemic [25]. Although well-validated global data on leptospirosis are lacking, the estimated global annual incidence of leptospirosis in temperate regions is 0.1–1 cases per 100,000 population while the incidence in tropical climates is >10 cases per 100,000 population [26]. These are likely underestimates because of misdiagnosis and under-reporting, particularly in regions where other diseases with similar non-specific presentations such as dengue and malaria are prevalent. For example, a cross sectional study among hospitalized febrile patients in Northeastern Malaysia between August 2010 and February 2011 found an 8.4% seroprevalence; but among these, only 31% were correctly diagnosed using clinical criteria - the remainder were misdiagnosed usually as dengue/dengue hemorrhagic fever (38%), pneumonia (14%), or typhoid fever (7%) [27]. Among 3,165 sera from acutely febrile patients with suspected dengue in Jamaica over 2007–2008, only 38.4% were confirmed to have dengue antibodies, while 6% were misdiagnosed and had leptospirosis instead, and 1.6% had serologic responses consistent with both infections [28]. Perhaps more directly, Reller et al. showed separately in Sri Lanka and Nicaragua where leptospirosis is often suspected, that clinical diagnosis alone is poorly sensitive, 23% and 11%, respectively [14, 29]. Likewise, in Sri Lanka, clinical diagnosis of other acute febrile illnesses is poor, 14% for dengue virus infection, 3% for rickettsial infections, and 0% for Chikungunya virus infection [13, 30, 31].
The clinical presentation of leptospirosis is highly variable and nonspecific [21, 23], explaining the poor predictive value of clinical assessment for establishing the diagnosis as a guide to appropriate therapy [14, 29, 32]. Severity ranges from subclinical to fatal. Clinical illness usually begins abruptly after an incubation period of 2–26 days. The less severe, anicteric form of leptospirosis resembles an influenza-like illness with fever, rigors, myalgias, headache, abdominal pain, nonproductive cough, and conjunctival suffusion, a distinguishing sign [20, 21]. The severe icteric form, known as Weil’s disease, occurs in a minority of patients and is associated with jaundice, hepatic dysfunction, myocarditis with arrhythmias, hemorrhage, uveitis, and multi-organ failure. Both can occur in two phases: an acute septicemic phase and an immune phase which can immediately follow. Routine laboratory tests are typically nonspecific but can include leukocytosis with a left shift, increased erythrocyte sedimentation rate, mildly elevated transaminases, alkaline phosphatase, and bilirubin, abnormal urinalysis, and thrombocytopenia. Leptospirosis mimics many other tropical diseases and diagnosis requires a high degree of clinical suspicion [14, 17, 33]. The average annual hospitalization rate for leptospirosis in the U.S. from 1998 to 2009 was 0.6/1,000,000 population and the average length of stay and hospital charges were higher in comparison to non-leptospirosis associated hospitalizations [34]. In Brazil, untreated leptospirosis results in significant social costs in years of potential life lost and partial hospitalization costs when compared to early treatment and prevention [35]. Death occurs in 5–15% of infections if untreated, and the proportion increases with age.
Acute disease is the direct result of inflammatory responses to the presence of leptospires in tissues, including hepatitis and cholestasis, interstitial nephritis, and meningoencephalitis [21]. Pulmonary hemorrhage is increasingly observed, and the pathogenesis of this process is not defined, but relates to host immune response, the production of bacterial factors that lead to local coagulation abnormalities, or both [18, 21]. Disease requires the ability of leptospires to enter organs and tissues and spread. Leptospira genome studies show that >1% of genes are dedicated to motility, and the presence of adhesins and invasins, such as the outer membrane protein Loa22 and the immunoglobulin-like LigA could contribute to this [21, 36]. In addition, genes that could affect hemostasis and coagulation are present, including a platelet-activating factor (PAF) acetylhydrolase-14 (LA2144, pafAH) and von Willebrand factor 15 type A domains (LB054 and LB055, vwa) [21, 36]. The development of disseminated intravascular coagulation with multi-organ failure provides some evidence to support an immunological basis for severe leptospirosis including the proinflammatory response to bacterial LPS and lipoproteins that stimulate toll-like receptor-2. Likewise, pulmonary hemorrhage is more frequent in persons with high antibody titers, and there is evidence that immunoglobulin and complement fixation on host cells are related to the expression of specific leptospiral hemostatic proteins [36]. The late occurrence of uveitis correlates with the “immune” phase and with immunoglobulin and complement deposition [37].
Leptospirosis is diagnosed by serology, culture, and molecular tests [18, 19, 38]. Culture is definitive but slow, lacks sensitivity, and requires specialized medium, and therefore not very useful for diagnostic purposes. Immunohistochemistry has been used, but is relatively unavailable. Likewise, PCR is sensitive but requires broad-range primers that despite the high degree of genetic diversity, can amplify all variants. Serological tests are used most often, particularly the reference standard, the microscopic agglutination test (MAT). This is sensitive as early as 5–7 days after onset; a single high titer is acceptable, but seroconversion is preferred. The pitfall of MAT is the requirement for continued growth of multiple serovars, a daunting task for clinical laboratories, and the delay in detection since antibodies develop later in the course of illness.
A recent Cochrane review was unable to discern any significant advantages to the use of antibacterial treatments for active leptospirosis [39]. Yet many believe that antimicrobial therapy shortens illness duration and reduces shedding of leptospires in the urine [33]. Treatment options include oral doxycycline or azithromycin for mild disease and parenteral penicillin, doxycycline, or third generation cephalosporin for severe disease. Supportive care may be required for Jarisch-Herxheimer reactions that result in a systemic inflammatory response after lysis of spirochetes with treatment.
Prevention of leptospirosis focuses on public health efforts, chemoprophylaxis, and vaccination. Public health interventions include identification and modification of risk factors through public education. Human and animal behavioral and environmental risk factors vary by region and include rodent infestations, particularly rats, exposure to contaminated surface water such as in rice paddies, and the presence of skin wounds. A recent study by Samarakoon et al. indicates that in endemic regions the knowledge of leptospirosis prevention is adequate but does not lead to implementation of personal protective measures [40]. Prophylaxis with doxycycline (200 mg once a week) reduces morbidity and mortality during outbreaks and other homeoprophylactic measures are reported to provide preventive benefit, but will require extensive evaluation [41, 42]. There is no human vaccine available but vaccines do exist for animal reservoirs including dogs and cattle.
Relapsing fever borreliosis
Relapsing fever is an arthropod-borne disease caused by pathogenic spirochetes of the genus Borrelia [43, 44]. It occurs in two major forms: tick-borne relapsing fever (TBRF) and louse-borne relapsing fever (LBRF). LBRF is primarily seen in East and Central Africa and is a frequent cause of epidemics in areas of extreme poverty, war, natural disasters, and overcrowding. It is widely perceived that there has been a decline in the incidence of LBRF secondary to improved standards of living and the introduction of the insecticide DDT. However, LBRF is still a major public health issue in East Africa, particularly in Ethiopia and in surrounding regions where louse infestation is commonplace. TBRF is found worldwide and is endemic in many countries including the Americas, Central Asia, the Mediterranean region, and many parts of Africa. In most rural areas of Senegal, Mauritania, and Mali, TBRF is a common cause of fever with incidence comparable to Plasmodium falciparum malaria and influenza [45]. The incidence of tick-borne relapsing fever peaks in summer but infection can occur year round, depending on local climate conditions.
Relapsing fever is caused by bacteria of the genus Borrelia. Borreliae are thin, helical, motile spirochetes that are unique in their ability to change their outer membrane surface proteins by gene conversion to generate antigenic variation [43]. Each relapse is associated with the emergence of a unique antigenic bacterial clone which is subsequently controlled by the immune system, only to select for new antigenic variant clones and another relapse of fever and disease. In fact, some variant clones have a propensity for dissemination into tissues and organs such as the CNS.
LBRF is caused by Borrelia recurrentis, now believed to be a reduced-genome variant of the TBRF Borrelia duttonii [46]. The vector for LBRF is the human body louse (Pediculus humanus), and thus humans are the only known reservoir for this spirochete [44]. TBRF is transmitted to humans through the bite of infected soft ticks of the genus Ornithodoros. There are many Borrelia species that cause TBRF, roughly divided into Old World and New World species, and they are associated with specific tick species. Unlike the human body louse that lives for several weeks, Ornithodoros ticks can live many years between blood meals, harbor spirochetes for prolonged periods, and can transmit the pathogen vertically to offspring [44].
Lice acquire the spirochetes by feeding on infected humans while humans acquire LBRF by scratching infected hemolymph of a crushed louse into the skin [44, 47]. Ticks become infected by feeding on infected wild rodents and then transmit the spirochetes to humans through a tick bite. After entering the blood, the spirochetes disseminate seeding multiple organs including the CNS in the case of TBRF.
Relapsing fever is characterized by two or more episodes of high fever (usually >39°C), myalgias, arthralgias, nausea, vomiting, headache, and nonproductive cough [48, 49]. Signs can include hemodynamic instability, rash, abdominal tenderness, and bleeding. Laboratory studies can reveal mild elevations in bilirubin and aminotransferases, thrombocytopenia, and anemia. Later in illness, jaundice, hepatosplenomegaly, and myocarditis can occur [50]. The initial febrile episode typically lasts 3–6 days and is followed by an afebrile period of 4–14 days, after which fever and symptoms recur. Subsequent relapses are usually less severe and can follow at 1–2 week intervals.
Because the WHO advises a presumptive diagnosis of malaria in endemic regions, or collects “verbal autopsy” reports for deaths, relapsing fever often goes undetected, particularly in parts of Africa where malaria is highly prevalent. A study by Nordstrand et al. in Togo, West Africa between 2002–2004 found that among febrile patients originally diagnosed and treated for malaria, the prevalence of TBRF was 8.8%, compared to 63.1% for malaria; 4.5% of patients had both malaria and TBRF [51]. Similarly, among a cohort of individuals participating in a study of mass treatment to eradicate trachoma in Tanzania, 17% of febrile episodes were caused by either Plasmodium falciparum or P. vivax, whereas 4% were attributed to relapsing fever [52], underscoring how uncorroborated clinical diagnosis can lead to erroneous reporting coupled with inflation of the incidence of reportable infections at the expense of other etiologies.
While the incidence of relapsing fever has declined in the developed world, it continues to be a formidable public health issue in certain regions. In Tanzania, for example, infection with TBRF is associated with significant morbidity and mortality, especially in women and children where it results in high perinatal mortality (436/1000 births) and is frequently listed as one of the top ten causes of mortality in children under 5 years of age [53, 54]. A retrospective study conducted between 2009 and 2012 of patients with LBRF-like symptoms who were admitted to a referral hospital in Ethiopia found the prevalence to be 4.9% and case fatality rates ranged from 2–6% [55]. Relapsing fever can account for up to 27% of hospitalizations in some regions [56]. There still exists the possibility of significant re-emergence, particularly as travel patterns continue to change and imported cases of relapsing fever are described. There is also evidence of a resurgence of louse infestation among certain groups. In Marseille, France, Brouqui et al. found a high prevalence of louse-borne infections in the homeless and a high level of exposure to tick-borne diseases [57].
Relapsing fever is diagnosed by visualization of spirochetes in the blood using darkfield microscopy or Wright or Giemsa staining on thin and thick blood smears [49]. Serologic tests were typically unreliable, but have improved with the development of assays based on detection of antibodies to relapsing fever spirochete-specific GlpQ [58]. Culture requires techniques that are not available in most laboratories. Nucleic acid amplification tests are available in some public health facilities, but not generally accessible to primary health care workers, or accessible in under-resourced regions where relapsing fever is often present.
Few careful studies of in vitro susceptibility for relapsing fever Borrelia species are published to guide treatment. LBRF was shown to be effectively treated with a single dose of oral tetracycline or doxycycline, or IM penicillin G procaine [43, 49], but over one third of patients had recurrent fever suggesting that a prolonged regimen is preferable. Similarly, the relapse rate of TBRF is 20 percent or higher after single dose therapy; thus, the treatment for TBRF is extended to 7–10 days. Since relapses yield as many as 108 bacteria/mL of blood, antibiotic therapy can trigger a Jarisch-Herxheimer reaction, particularly with LBRF, and its occurrence is associated with high mortality. Tetracyclines are highly effective at clearing spirochetes with no or few relapses, but their use is associated with more frequent and severe episodes of Jarisch-Herxheimer reactions [59, 60]. Therefore, preferred treatment employs either single dose doxycycline or tetracycline, or a single dose of procaine penicillin IM followed by 2 or more days of oral doxycycline or tetracycline. Prevention of infection requires good personal hygiene, delousing, laundering at high temperatures and application of insecticides to clothing and bedding to diminish louse infestations for LBRF. TBRF prevention includes exclusion of rodents that host the argasid tick vectors, avoiding habitats that harbor the ticks themselves, and the use of tick repellents.
Scrub typhus, murine typhus, and spotted fever rickettsiosis
Bacteria in the genera Orientia, Rickettsia, Ehrlichia, and Anaplasma are obligate intracellular α2 proteobacteria in the Order Rickettsiales and Families Rickettsiaceae and Anaplasmataceae [61]. Genome sequences illustrate that these agents adapted to an obligatory intracellular lifestyle through the loss of genes and pathways required for extracellular growth [62]. Regardless, each contains DNA, RNA, ribosomes, divides by binary fission and possesses a cell wall with ultrastructural characteristics of Gram negative bacteria. Rickettsiaceae members (Rickettsia and Orientia) target endothelial cells in mammals and reside within the cytosol. In contrast, Anaplasmataceae family members usually reside in hematopoietic-derived cells, such as leukocytes in mammals where they propagate within pathogen-modified endosomes. All Rickettsiales have at least part of their life cycle in vectors, and for Rickettsia, Orientia, Ehrlichia and Anaplasma, these are usually arthropods, often ticks, fleas, lice, or mites.
The genus Rickettsia is divided into spotted fever (SFGR) and typhus groups. Genome studies illustrate diversity with SFGR, yet these cluster separately from typhus group rickettsiae [62]. Several species occupy intermediate positions sometimes called “transitional” or “ancestral”. There are over 25 recognized SFGR species and 2 typhus group species; most are human pathogens, yet at least several “transitional” and “ancestral” rickettsiae are questionably pathogenic or never associated with known human or animal disease. The genus Orientia has a similar degree of genetic diversity as for SFGR, yet has only 2 named species, O. tsutsugamushi and O. chuto, both human pathogens. Although many Anaplasmataceae are animal pathogens, only a limited range are human pathogens.
Among the rickettsial agents, the most neglected for which sufficient evidence exists that there are large global disease burdens include Orientia tsutsugamushi, the agent of scrub typhus [10, 12, 13, 16], Rickettsia typhi, etiologic agent of murine typhus [11–13], and various SFGR, especially R. rickettsii (Rocky Mountain spotted fever [RMSF]) [63, 64], R. conorii (Mediterranean spotted fever), R. africae (African tick bite fever), and likely others in Asia and Australia (R. sibirica, R. heilongjiangensis, R. japonica, R. honei and R. australis) [65, 66]. Although the emergence of ehrlichiosis and anaplasmosis argues for importance as globally-neglected infections [67], for simplicity, only scrub typhus, murine typhus and “generic” SFGR will be considered here.
Scrub typhus
O. tsutsugamushi is transmitted by larval trombiculid mites (genus Leptotrombidium) which are distributed throughout Asia and parts of Australia. The range of scrub typhus extends from northeast Asia to Papua/New Guinea and Northern Australia in the southeast, the Maldives and Reunion Islands in the southwest and to Pakistan and Afghanistan in the northwest [68]. This distribution encompasses regions where over 2 billion people live. Several recent studies provide preliminary evidence that scrub typhus could be endemic outside of the previously established ranges, and extend into Africa and as distant as South America, although much more study is needed [10, 69, 70]. Based on the wide ecological distribution of the pathogen, the density of populations in these geographic regions and the high seroprevalence and incidence of infection, it is conservatively estimated that there are more than 1 million cases of scrub typhus yearly [10].
Scrub typhus usually presents as undifferentiated fever [68, 71]. Patients also often demonstrate headache, myalgias, rash, among other manifestations, and an eschar at the site of the mite bite is a key sign (Table 2). Laboratory studies are usually not revealing, except for a normal leukocyte count and left shift with thrombocytopenia, and moderate increases in serum hepatic transaminase activities (Table 2), all common features of infections described here. The diagnosis of scrub typhus relies on clinical suspicion for early treatment [72]. Diagnostic approaches during acute disease include immunohistochemical demonstration of O. tsutsugamushi in eschar biopsies, and although much less sensitive, PCR amplification of O. tsutsugamushi-specific nucleic acids from blood [72, 73]. Serology is most often employed and includes specific indirect immunofluorescence assays (IFA) for O. tsutsugamushi IgG and IgM, although the latter is associated with false positive tests in the absence of a concurrent IgG seroconversion [60, 74]. Point-of-care assays are not readily available or well validated. Patients with scrub typhus are best treated with doxycycline, and although questions about doxycycline resistance in some Thai strains are not resolved, azithromycin seems to be an effective alternative [75]. Severe outcomes include pneumonia, renal failure, meningoencephalitis, shock, gastrointestinal bleeding, myocarditis, and death [76].
Table 2.
Clinical signs, symptoms and laboratory findings of neglected bacterial zoonoses compared with dengue virus infection (% of cases with finding).
| History, signs, or symptoms | leptospirosis (Leptospira interrogans) | scrub typhus (O. tsutsugamushi) | RMSF (R. rickettsii) | murine typhus (R. typhi) | dengue virus infection |
|---|---|---|---|---|---|
| Clinical findings | |||||
|
| |||||
| Fever | 100 | 100 | 100 | 99 | 100 |
| Headache | 85 | 100 | 91 | 59 | 78 |
| Myalgia | 77 | 32 | 72 | 46 | 77 |
| Rash | 5 | 49* | 90 | 37 | 11–53 |
| Rash on palms and soles | na | na** | 82 | na | na |
| Nausea or vomiting | 45 | 28 | 60 | 40 | 53 |
| Abdominal pain | 33 | na | 43 | 17 | na |
| Conjunctivitis | 61 | 29 | 30 | na | na |
| Pneumonitis | 37 | 28 | 15 | 15 | 35 |
| Any severe neurologic complication | <25 | 10 | 26 | 6 | 1–6 |
|
| |||||
| Laboratory findings | |||||
|
| |||||
| Leukocytosis (WBC) > 10 × 109/L | 39 | 34 | 28 | 22 | 6 |
| Leukopenia (WBC) < 5 × 109/L | 8 | 3 | 24 | 13 | 25 |
| Platelet count < 150 × 109/L | 26 | 25 | 44 | 46 | 59 |
| Elevated ALT or AST | 78 | 70 | 50 | 57 | 64 |
includes eschars;
na – data not available
In some Asia-Pacific regions, scrub typhus can account for up to 23% of all febrile illness in hospitalized patients [77]. A study by Chanyasanha et al. of febrile patients in Thailand who presented to malaria clinics found the seroprevalence of scrub typhus to be close to 60% [78]. A meta-analysis using the search terms “scrub typhus incidence” identified 132 publications that examined acutely febrile subjects between 2009 and 2015, for which 23 contained sufficient details of study design, patient enrollment, diagnostic approach, and individualized data to discern incidence and prevalence (Supplemental Table 1). All studies were conducted in Asia (Lao PDR, Thailand, Vietnam, China, India, Sri Lanka, Indonesia, Bangladesh, and Malaysia). This analysis accumulated data from 10,192 subjects with acute febrile illness, and identified 1,881 incident scrub typhus infections, for a median of 14% (IQR 8–31%) infection rate. One recent study by Varghese et al. conducted between 2005 and 2010 on scrub typhus patients in South India found the overall case fatality rate to be 9% with shock, renal failure, and CNS involvement associated with a higher mortality [79]. The case fatality rate varies in contemporary studies from 0.5% to 24% [80] to historical highs between 30 and 50% [81], such that the mortality among 1 million infections in a single year is likely enormous.
Scrub typhus remains a serious public health issue. In regions such as India, Sri Lanka, and China where it was thought to have declined, scrub typhus has experienced a resurgence, and it is increasingly identified as an emerging infection in new foci [10]. It is unclear whether this is the result of improved diagnostic methods and increased awareness, expansion of farmlands and population growth, or changes in travel and migration patterns. Misdiagnosis and under-diagnosis is also known to occur due to lack of availability of diagnostic tests and the nonspecific nature of symptoms, especially when the characteristic eschar is not present. The economic impact of scrub typhus has only recently begun to be explored in regions of China where the disease has rapidly re-emerged in recent years [82].
Murine typhus and spotted fever rickettsiosis
Murine typhus occurs when the rat flea (Xenopsylla cheopis) or the cat flea (Ctenocephalides felis) transmit R. typhi via infected flea feces into the flea bite wound [83]. The apparent obligatory relationship of rats or domestic pets that harbor fleas with human domiciles makes murine typhus a risk in any location with human activities, and especially in regions with warm weather and year-round flea activity. Murine typhus has been reported on every continent (except Antarctica), and is a particular risk in tropical regions of Southeast Asia, Africa, Central and South America, and even in southern parts of the U.S. and Europe. Well-established prevalence data is lacking in almost all regions owing to neglect of this disease, yet seroprevalence studies continue to demonstrate high rates in many locations and have implicated murine typhus as the causative agent in increasingly large proportions of patients with febrile illness. Hamaguchi et al. studied acute undifferentiated fever in hospitalized patients in Northern Vietnam, where 33% were seropositive for R. typhi. Certain communities, notably, homeless populations, have higher seroprevalence rates, ranging from 9.6% in the U.S. to 22% in Marseille, France, and to 67% in Bangladesh [84] and 21% in Lao PDR [85]. A pilot meta-analysis conducted using the search terms “murine typhus + incidence”, “murine typhus + acute febrile illness”, and “murine typhus” retrieved 880 articles through 1934 and only the 325 articles dating to 2001 were reviewed for adequacy of details and methodology, including geographic location for inclusion in the study, and with sole focus on studies of incidence among prospective acute febrile disease studies (Supplemental Table 2). Selected studies originated from every continent except Antarctica. Of the 325 articles reviewed, 26 were selected for inclusion, comprising a total of 8,642 patients, among which 1,486 (17.2%) of the subjects were deemed to have murine typhus based on serology and/ PCR and culture. The median proportion of murine typhus cases among the studies was 7.9% (IQR 4.2–14.5%).
As with scrub typhus, the clinical presentation is most often undifferentiated fever, but lacking an eschar (Table 2) [86–88]. A rash can develop in half of infected patients. Complications are similar to other rickettsioses. The case fatality rate is variably between 0.5 and 4% in contemporary series and most infections have a benign clinical course; however, morbidity can be significant particularly in cases of delayed diagnosis and in the elderly who, even with proper treatment, suffer a greater number of complications.
Spotted fever group rickettsiosis (SFGR) is best known because of the severity of RMSF (R. rickettsii infection) [89–91]. While RMSF occurs only in the western hemisphere, other SFG rickettsioses occur globally. Individual species vary in pathogenicity, but the underlying pathology for SFGR is endothelial cell infection, vasculitis, and increased systemic and pulmonary vascular permeability [92]. The majority of SFGRs are transmitted by tick bites; for R. rickettsii this includes Dermacentor variabilis, D. andersoni, and R. sanguineus in the North America, and Amblyomma species in Central and South America. R. conorii is vectored by R. sanguineus in the Mediterranean region, while R. africae is transmitted via Amblyomma tick bites in Sub-Saharan Africa. A few SFGR are vectored by other arthropods (mites for rickettsialpox [R. akari]; fleas for cat flea typhus [R. felis]).
SFGR are among the most virulent of all human infections, especially RMSF for which historical case fatality rates of 25–80% are recorded [92]. Contemporary data describe case fatality rates between 0.5 and 8–12% in the US and up to 35% in Brazil. In Beja, Portugal in 1997, the case fatality rate in hospitalized patients with Mediterranean spotted fever was 32.3% and in the United States, the American Indian population is especially affected by RMSF with both incidence and case fatality rates significantly higher than that of other racial groups and this incidence continues to increase at a disproportionate rate. Of particular interest is the high seroprevalence of SFGR in many regions around the world where human SFGR are not known to exist [91, 93]. In southern Taiwan, a region where spotted fever is poorly characterized, an investigation of 413 febrile patients found that 49 (11.9%) were seropositive for spotted fever group rickettsiae. Spotted fever is also increasingly diagnosed in South Asia, including Sri Lanka where spotted fever rickettsiosis occurred in 10% of febrile patients, second in number only to leptospirosis [13], and the seroprevalence was 33% for any rickettsial infection. Despite inadequate data collection, reported cases of SFGR are at historical highs in the US and globally [64, 91]. The reasons for this are not clear, but could include greater clinical suspicion, case definition and diagnostic test changes, or real increases in disease incidence and prevalence. The latter is in part likely given the increasingly defined distribution of infected vectors [91].
Clinical manifestations of RMSF and other SFGRs include fever, and variably headache, myalgias, rash, (macular, maculopapular, or petechial), abdominal pain, nausea, vomiting, diarrhea, and in severe cases, renal failure, non-cardiogenic pulmonary edema, shock and multi-organ failure, and central nervous system involvement (meningoencephalitis, cerebral edema, herniation) (Table 2). Most SFGR do not demonstrate this degree of severity, but it is possible in any single case. Many patients have eschars (except in RMSF), and some develop vesicular rashes. Major factors for ineffective diagnosis and delayed therapy include absence of a typical rash, presentation during non-peak tick activity season, and presentation during first 3 days of illness when a rash may not be present [94].
Murine typhus and SFGR diagnosis requires clinical suspicion since adequate diagnostic tests in the acute phase of disease are not available [95]. If rash is present, rickettsiae can be demonstrated by immunohistochemistry in rash lesion biopsies in SFGR and murine typhus. While PCR sensitivity in blood is low (<25%) accumulating data suggest that skin biopsy PCR could be sensitive [91]. The most sensitive diagnostic test, IFA, is only confirmatory as it requires acute and convalescent serum antibody titers. However, in general IFA cannot distinguish SFGR species or cross-reactions with typhus group rickettsiae. Cultivation is not timely and is generally considered dangerous and unacceptable for clinical laboratories. Perhaps most challenging of all is the fact that serodiagnosis only works well with paired sera separated by at least 14 days, precluding its use for diagnosis and management at the time of active infection. Given the marked but mostly unrecognized incidence of these infections worldwide, and their high case fatality rates, readily available accurate and sensitive point-of-care diagnostic devices are desperately needed.
Both SFGR and murine typhus are treated with doxycycline; however, in a study which examined treatment practices in the US, fewer than 40% of healthcare providers correctly chose doxycycline as the treatment of choice for RMSF for children <8 years of age, a potential cause of the increased case fatality rates in this age category [95]. Controlled clinical trials have not been conducted to adequately define efficacy among antibiotics except for R. conorii but some evidence exists to support the use of fluoroquinolones for murine typhus and forms of SFGR other than RMSF, though their use has become controversial [96]. Currently, no vaccines exist for spotted fever rickettsiosis, murine typhus, or scrub typhus. Prevention requires avoidance of bites by ticks, fleas and chiggers by avoiding infested locations, by wearing clothing that potentially excludes their ability to access the skin, or by use of repellents. Prophylactic antibiotic treatment is not well assessed and currently cannot be advocated.
Conclusions
Other than leptospirosis, relapsing fever, scrub typhus and rickettsioses are not yet recognized as neglected diseases by the WHO, despite data that illustrates their high incidences and prevalence and potentially dramatic morbidity and costs for human life. Future goals should include establishing their incidence and prevalence comparative to other infections that seemingly occur at similar rates. Development of easily deployed point-of-care diagnostics will be critical to assist in accurately collecting these data and in directing appropriate care and treatments. Careful clinical and epidemiological/ecological studies, including the roles of animal or vector reservoirs that increase risk will identify opportunities to prevent infection. Study of human infections could identify new locations where other preventative methods, including vaccination might be used. A basic analysis of DALYs will depend on careful collection of such data and could drive deployment of critically short resources in areas where these infections cause more harm than others commonly believed to have the greatest impact on humans and their environments.
Supplementary Material
Supplemental Table 1. Meta-analysis of scrub typhus incidence among acute febrile illness studies.
Supplemental Table 2. Meta-analysis of murine typhus incidence among prospective acute febrile illness studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Utzinger J, Becker SL, Knopp S, et al. Neglected tropical diseases: Diagnosis, clinical management, treatment and control. Swiss Med, Wkly. 2012;142:w13727. doi: 10.4414/smw.2012.13727. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control. Summary of notifiable diseases - United States, 2012. Morbid Mortal Wkly Rep. 2014;61:1–121. [PubMed] [Google Scholar]
- 3.World Health Organization. Neglected tropical diseases. 2014 http://www.who.int/neglected_diseases/diseases/en/
- 4.Centers for Disease Control. Neglected tropical diseases. 2014 http://www.cdc.gov/globalhealth/ntd/diseases/index.html.
- 5.Bill & Melinda Gates Foundation. Neglected infectious diseases. Seattle, WA: Bill & Melinda Gates Foundation; 2014. http://www.gatesfoundation.org/What-We-Do/Global-Health/Neglected-Infectious-Diseases#bodyregion_0_interiorarticle_0_strategysections_2_strategysubsections45615201ec9a4a6f8b6ec35aec7d7911_3_lnkHeader. [Google Scholar]
- 6.European Commission. Neglected infectious diseases neglected no more. 2014 http://ec.europa.eu/research/health/infectious-diseases/neglected-diseases/pdf/nid-leaflet_en.pdf.
- 7.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joshi R, Kengne AP, Neal B. Methodological trends in studies based on verbal autopsies before and after published guidelines. Bull World Health Organ. 2009;87:678–682. doi: 10.2471/BLT.07.049288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grosse SD, Lollar DJ, Campbell VA, Chamie M. Disability and disability-adjusted life years: Not the same. Pub Health Rpts. 2009;124:197–202. doi: 10.1177/003335490912400206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paris DH, Shelite TR, Day NP, Walker DH. Unresolved problems related to scrub typhus: A seriously neglected life-threatening disease. Am J Trop Med Hyg. 2013;89:301–307. doi: 10.4269/ajtmh.13-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Acestor N, Cooksey R, Newton PN, et al. Mapping the aetiology of non-malarial febrile illness in southeast asia through a systematic review—terra incognita impairing treatment policies. PLoS ONE. 2012;7:e44269. doi: 10.1371/journal.pone.0044269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayxay M, Castonguay-Vanier J, Chansamouth V, et al. Causes of non-malarial fever in laos: A prospective study. Lancet Glob Health. 2013;1:e46–e54. doi: 10.1016/S2214-109X(13)70008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reller ME, Bodinayake C, Nagahawatte A, et al. Unsuspected rickettsioses among patients with acute febrile illness, Sri Lanka, 2007. Emerg Infect Dis. 2012;18:825–829. doi: 10.3201/eid1805.111563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reller ME, Bodinayake C, Nagahawatte A, et al. Leptospirosis as frequent cause of acute febrile illness in southern Sri Lanka. Emerg Infect Dis. 2011;17:1678–1684. doi: 10.3201/eid1709.100915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murdoch DR, Woods CW, Zimmerman MD, et al. The etiology of febrile illness in adults presenting to patan hospital in Kathmandu, Nepal. Am J Trop Med Hyg. 2004;70:670–675. [PubMed] [Google Scholar]
- 16.Chheng K, Carter MJ, Emary K, et al. A prospective study of the causes of febrile illness requiring hospitalization in children in Cambodia. PLoS ONE. 2013;8:e60634. doi: 10.1371/journal.pone.0060634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Punjabi NH, Taylor WR, Murphy GS, et al. Etiology of acute, non-malaria, febrile illnesses in Jayapura, northeastern Papua, Indonesia. Am J Trop Med Hyg. 2012;86:46–51. doi: 10.4269/ajtmh.2012.10-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bharti AR, Nally JE, Ricaldi JN, et al. Leptospirosis: A zoonotic disease of global importance. The Lancet infectious diseases. 2003;3:757–771. doi: 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 19.Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14:296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartskeerl RA, Collares-Pereira M, Ellis WA. Emergence, control and re-emerging leptospirosis: Dynamics of infection in the changing world. Clin Microbiol Infect. 2011;17:494–501. doi: 10.1111/j.1469-0691.2011.03474.x. [DOI] [PubMed] [Google Scholar]
- 21.Ko AI, Goarant C, Picardeau M. Leptospira: The dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat Rev Microbiol. 2009;7:736–747. doi: 10.1038/nrmicro2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brenner DJ, Kaufmann AF, Sulzer KR, Steigerwalt AG, Rogers FC, Weyant RS. Further determination of DNA relatedness between serogroups and serovars in the family leptospiraceae with a proposal for Leptospira alexanderi sp. nov. and four new Leptospira genomospecies. Int J Syst Bacteriol. 1999;49(Pt 2):839–858. doi: 10.1099/00207713-49-2-839. [DOI] [PubMed] [Google Scholar]
- 23.Schreier S, Doungchawee G, Chadsuthi S, Triampo D, Triampo W. Leptospirosis: Current situation and trends of specific laboratory tests. Expert Rev Clin Immunol. 2013;9:263–280. doi: 10.1586/eci.12.110. [DOI] [PubMed] [Google Scholar]
- 24.Katz AR, Ansdell VE, Effler PV, Middleton CR, Sasaki DM. Assessment of the clinical presentation and treatment of 353 cases of laboratory-confirmed leptospirosis in Hawaii, 1974–1998. Clin Infect Dis. 2001;33:1834–1841. doi: 10.1086/324084. [DOI] [PubMed] [Google Scholar]
- 25.Pappas G, Papadimitriou P, Siozopoulou V, Christou L, Akritidis N. The globalization of leptospirosis: Worldwide incidence trends. Int J Infect Dis. 2008;12:351–357. doi: 10.1016/j.ijid.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Organization WH. Leptospirosis burden epidemiology reference group (lerg) 2015 http://www.who.int/zoonoses/diseases/lerg/en/index2.html.
- 27.Rafizah AA, Aziah BD, Azwany YN, et al. A hospital-based study on seroprevalence of leptospirosis among febrile cases in northeastern Malaysia. Int J Infect Dis. 2013;17:e394–397. doi: 10.1016/j.ijid.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Lindo J, Brown PD, Vickers I, Brown M, Jackson ST, Lewis-Fuller E. Leptospirosis and malaria as causes of febrile illness during a dengue epidemic in Jamaica. Pathog Glob Health. 2013;107:329–334. doi: 10.1179/2047773213Y.0000000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reller ME, Wunder EA, Jr, Miles JJ, et al. Unsuspected leptospirosis is a cause of acute febrile illness in Nicaragua. PLoS Negl Trop Dis. 2014;8:e2941. doi: 10.1371/journal.pntd.0002941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reller ME, Bodinayake C, Nagahawatte A, et al. Unsuspected dengue and acute febrile illness in rural and semi-urban southern Sri Lanka. Emerg Infect Dis. 2012;18:256–263. doi: 10.3201/eid1802.110962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reller ME, Akoroda U, Nagahawatte A, et al. Chikungunya as a cause of acute febrile illness in southern Sri Lanka. PLoS ONE. 2013;8:e82259. doi: 10.1371/journal.pone.0082259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rafizah AA, Aziah BD, Azwany YN, et al. Leptospirosis in northeastern Malaysia: Misdiagnosed or coinfection? Int J Collab Res Intern Med Public Health. 2012;4:1419–1427. [Google Scholar]
- 33.Suputtamongkol Y, Niwattayakul K, Suttinont C, et al. An open, randomized, controlled trial of penicillin, doxycycline, and cefotaxime for patients with severe leptospirosis. Clin Infect Dis. 2004;39:1417–1424. doi: 10.1086/425001. [DOI] [PubMed] [Google Scholar]
- 34.Traxler RM, Callinan LS, Holman RC, Steiner C, Guerra MA. Leptospirosis-associated hospitalizations, United States, 1998–2009. Emerg Infect Dis. 2014;20:1273–1279. doi: 10.3201/eid2008.130450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Souza VM, de Arsky ML, Castro AP, Araujo WN. Years of potential life lost and hospitalization costs associated with leptospirosis in Brazil. Revista de saude publica. 2011;45:1001–1008. doi: 10.1590/s0034-89102011005000070. [DOI] [PubMed] [Google Scholar]
- 36.Adler B, Lo M, Seemann T, Murray GL. Pathogenesis of leptospirosis: The influence of genomics. Vet Microbiol. 2011;153:73–81. doi: 10.1016/j.vetmic.2011.02.055. [DOI] [PubMed] [Google Scholar]
- 37.Verma A, Stevenson B. Leptospiral uveitis - there is more to it than meets the eye! Zoonoses Public Health. 2012;59 (Suppl 2):132–141. doi: 10.1111/j.1863-2378.2011.01445.x. [DOI] [PubMed] [Google Scholar]
- 38.Bajani MD, Ashford DA, Bragg SL, et al. Evaluation of four commercially available rapid serologic tests for diagnosis of leptospirosis. J Clin Microbiol. 2003;41:803–809. doi: 10.1128/JCM.41.2.803-809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brett-Major DM, Coldren R. Antibiotics for leptospirosis. Cochrane Database Syst Rev. 2012;2:CD008264. doi: 10.1002/14651858.CD008264.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samarakoon YM, Gunawardena N. Knowledge and self-reported practices regarding leptospirosis among adolescent school children in a highly endemic rural area in sri lanka. Rural Remote Health. 2013;13:2360. [PubMed] [Google Scholar]
- 41.Sehgal SC, Sugunan AP, Murhekar MV, Sharma S, Vijayachari P. Randomized controlled trial of doxycycline prophylaxis against leptospirosis in an endemic area. Int J Antimicrob Agents. 2000;13:249–255. doi: 10.1016/s0924-8579(99)00134-x. [DOI] [PubMed] [Google Scholar]
- 42.Bracho G, Varela E, Fernandez R, et al. Large-scale application of highly-diluted bacteria for leptospirosis epidemic control. Homeopathy. 2010;99:156–166. doi: 10.1016/j.homp.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Barbour AG, Hayes SF. Biology of Borrelia species. Microbiol Rev. 1986;50:381–400. doi: 10.1128/mr.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cutler SJ. Relapsing fever – a forgotten disease revealed. J Appl Microbiol. 2010;108:1115–1122. doi: 10.1111/j.1365-2672.2009.04598.x. [DOI] [PubMed] [Google Scholar]
- 45.Vial L, Diatta G, Tall A, et al. Incidence of tick-borne relapsing fever in West Africa: longitudinal study. Lancet. 2006;368:37–43. doi: 10.1016/S0140-6736(06)68968-X. [DOI] [PubMed] [Google Scholar]
- 46.Lescot M, Audic S, Robert C, et al. The genome of Borrelia recurrentis, the agent of deadly louse-borne relapsing fever, is a degraded subset of tick-borne Borrelia duttonii. PLoS Genet. 2008;4:e1000185. doi: 10.1371/journal.pgen.1000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Houhamdi L, Raoult D. Excretion of living Borrelia recurrentis in feces of infected human body lice. J Infect Dis. 2005;191:1898–1906. doi: 10.1086/429920. [DOI] [PubMed] [Google Scholar]
- 48.Larsson C, Andersson M, Bergstrom S. Current issues in relapsing fever. Curr Op Infect Dis. 2009;22:443–449. doi: 10.1097/QCO.0b013e32832fb22b. [DOI] [PubMed] [Google Scholar]
- 49.Dworkin MS, Schwan TG, Anderson DE, Jr, Borchardt SM. Tick-borne relapsing fever. Infect Dis Clin North Am. 2008;22:449–468. viii. doi: 10.1016/j.idc.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mayegga E, Ljøstad U, Mygland Å, Monstad P. Absence of focal neurological involvement in tick-borne relapsing fever in northern Tanzania. Eur J Neurol. 2005;12:449–452. doi: 10.1111/j.1468-1331.2005.01003.x. [DOI] [PubMed] [Google Scholar]
- 51.Nordstrand A, Bunikis I, Larsson C, et al. Tickborne relapsing fever diagnosis obscured by malaria, togo. Emerg Infect Dis. 2007;13:117–123. doi: 10.3201/eid1301.060670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reller ME, Chen WH, Dalton J, Lichay MA, Dumler JS. Multiplex 5′ nuclease quantitative real-time pcr for clinical diagnosis of malaria and species-level identification and epidemiologic evaluation of malaria-causing parasites, including Plasmodium knowlesi. J Clin Microbiol. 2013;51:2931–2938. doi: 10.1128/JCM.00958-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McConnell J. Tick-borne relapsing fever under-reported. Lancet Infect Dis. 2003;3:604. doi: 10.1016/s1473-3099(03)00787-4. [DOI] [PubMed] [Google Scholar]
- 54.Barclay AJ, Coulter JB. Tick-borne relapsing fever in central Tanzania. Trans R Soc Trop Med Hyg. 1990;84:852–856. doi: 10.1016/0035-9203(90)90106-o. [DOI] [PubMed] [Google Scholar]
- 55.Yimer M, Mulu W, Ayalew W, Abera B. Louse-borne relapsing fever profile at Felegehiwot referral hospital, Bahir Dar City, Ethiopia: A retrospective study. BMC Res Notes. 2014;7:250. doi: 10.1186/1756-0500-7-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cutler SJ, Abdissa A, Trape JF. New concepts for the old challenge of African relapsing fever borreliosis. Clin Microbiol Infect. 2009;15:400–406. doi: 10.1111/j.1469-0691.2009.02819.x. [DOI] [PubMed] [Google Scholar]
- 57.Brouqui P, Stein A, Dupont HT, et al. Ectoparasitism and vector-borne diseases in 930 homeless people from Marseilles. Medicine (Baltimore) 2005;84:61–68. doi: 10.1097/01.md.0000152373.07500.6e. [DOI] [PubMed] [Google Scholar]
- 58.Porcella SF, Raffel SJ, Schrumpf ME, Schriefer ME, Dennis DT, Schwan TG. Serodiagnosis of louse-borne relapsing fever with glycerophosphodiester phosphodiesterase (Glpq) from Borrelia recurrentis. J Clin Microbiol. 2000;38:3561–3571. doi: 10.1128/jcm.38.10.3561-3571.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seboxa T, Fekade D. Borrelia species (relapsing fever) In: Yu VL, Weber R, Raoult D, editors. Antimicrobial therapy and vaccines. 2. New York, New York: Apple Trees Productions, LLC; 2002. pp. 117–120. [Google Scholar]
- 60.Blacksell SD, Jenjaroen K, Phetsouvanh R, et al. Accuracy of rapid IgM-based immunochromatographic and immunoblot assays for diagnosis of acute scrub typhus and murine typhus infections in Laos. Am J Trop Med Hyg. 2010;83:365–369. doi: 10.4269/ajtmh.2010.09-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dumler JS, Barbet AF, Bekker CP, et al. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: Unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol. 2001;51:2145–2165. doi: 10.1099/00207713-51-6-2145. [DOI] [PubMed] [Google Scholar]
- 62.Gillespie JJ, Williams K, Shukla M, et al. Rickettsia phylogenomics: Unwinding the intricacies of obligate intracellular life. PLoS ONE. 2008;3:e2018. doi: 10.1371/journal.pone.0002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dahlgren FS, Moonesinghe R, McQuiston JH. Short report: Race and rickettsiae: A United States perspective. Am J Trop Med Hyg. 2011;85:1124–1125. doi: 10.4269/ajtmh.2011.11-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Openshaw JJ, Swerdlow DL, Krebs JW, et al. Rocky Mountain spotted fever in the United States, 2000–2007: Interpreting contemporary increases in incidence. Am J Trop Med Hyg. 2010;83:174–182. doi: 10.4269/ajtmh.2010.09-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aung AK, Spelman DW, Murray RJ, Graves S. Rickettsial infections in Southeast Asia: Implications for local populace and febrile returned travelers. Am J Trop Med Hyg. 2014;91:451–460. doi: 10.4269/ajtmh.14-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang L, Shan A, Mathew B, et al. Rickettsial seroepidemiology among farm workers, tianjin, People’s Republic of China. Emerg Infect Dis. 2008;14:938–940. doi: 10.3201/eid1406.071502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walker DH, Paddock CD, Dumler JS. Emerging and re-emerging tick-transmitted rickettsial and ehrlichial infections. Med Clin North Am. 2008;92:1345–1361. x. doi: 10.1016/j.mcna.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 68.Kelly DJ, Fuerst PA, Ching WM, Richards AL. Scrub typhus: The geographic distribution of phenotypic and genotypic variants of Orientia tsutsugamushi. Clin Infect Dis. 2009;48 (Suppl 3):S203–230. doi: 10.1086/596576. [DOI] [PubMed] [Google Scholar]
- 69.Thiga JW, Mutai BK, Eyako WK, et al. High seroprevalence of antibodies against spotted fever and scrub typhus bacteria in patients with febrile illness, Kenya. Emerg Infect Dis. 2015;21:688–691. doi: 10.3201/eid2104.141387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Balcells ME, Rabagliati R, Garcia P, et al. Endemic scrub typhus-like illness, Chile. Emerg Infect Dis. 2011;17:1659–1663. doi: 10.3201/eid1709.100960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Phongmany S, Rolain JM, Phetsouvanh R, et al. Rickettsial infections and fever, Vientiane, Laos. Emerg Infect Dis. 2006;12:256–262. doi: 10.3201/eid1202.050900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koh GC, Maude RJ, Paris DH, Newton PN, Blacksell SD. Diagnosis of scrub typhus. Am J Trop Med Hyg. 2010;82:368–370. doi: 10.4269/ajtmh.2010.09-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Paris DH, Blacksell SD, Stenos J, et al. Real-time multiplex pcr assay for detection and differentiation of rickettsiae and orientiae. Trans Royal Soc Trop Med Hyg. 2008;102:186–193. doi: 10.1016/j.trstmh.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 74.Phetsouvanh R, Thojaikong T, Phoumin P, et al. Inter- and intra-operator variability in the reading of indirect immunofluorescence assays for the serological diagnosis of scrub typhus and murine typhus. Am J Trop Med Hyg. 2013;88:932–936. doi: 10.4269/ajtmh.12-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jang MO, Jang HC, Kim UJ, et al. Outcome of intravenous azithromycin therapy in patients with complicated scrub typhus compared with that of doxycycline therapy using propensity-matched analysis. Antimicrob Agents Chemother. 2014;58:1488–1493. doi: 10.1128/AAC.01996-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jang MO, Kim JE, Kim UJ, et al. Differences in the clinical presentation and the frequency of complications between elderly and non-elderly scrub typhus patients. Arch Gerontol Geriatr. 2014;58:196–200. doi: 10.1016/j.archger.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 77.Brown GW, Robinson DM, Huxsoll DL, Ng TS, Lim KJ. Scrub typhus: A common cause of illness in indigenous populations. Trans Royal Soc Trop Med Hyg. 1976;70:444–448. doi: 10.1016/0035-9203(76)90127-9. [DOI] [PubMed] [Google Scholar]
- 78.Chanyasanha C, Kaeburong K, Chenchittikul M, Sujirarat D. Seroprevalence of scrub typhus infection in patients with pyrexia at some malaria clinics in three western provinces of Thailand. Asian Pac J Allergy Immunol. 1998;16:119–125. [PubMed] [Google Scholar]
- 79.Varghese GM, Trowbridge P, Janardhanan J, et al. Clinical profile and improving mortality trend of scrub typhus in South India. Int J Infect Dis. 2014;23:39–43. doi: 10.1016/j.ijid.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 80.Griffith M, Peter JV, Karthik G, et al. Profile of organ dysfunction and predictors of mortality in severe scrub typhus infection requiring intensive care admission. Indian J Crit Care Med. 2014;18:497–502. doi: 10.4103/0972-5229.138145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kawamura A, Jr, Tanaka H. Rickettsiosis in Japan. Jpn J Exp Med. 1988;58:169–184. [PubMed] [Google Scholar]
- 82.Yang LP, Liang SY, Wang XJ, Li XJ, Wu YL, Ma W. Burden of disease measured by disability-adjusted life years and a disease forecasting time series model of scrub typhus in Laiwu, China. PLoS Negl Trop Dis. 2015;9:e3420. doi: 10.1371/journal.pntd.0003420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gillespie JJ, Ammerman NC, Beier-Sexton M, Sobral BS, Azad AF. Louse- and flea-borne rickettsioses: Biological and genomic analyses. Vet Res. 2009;40:12. doi: 10.1051/vetres:2008050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maude RR, Maude RJ, Ghose A, et al. Serosurveillance of Orientia tsutsugamushi and Rickettsia typhi in Bangladesh. Am J Trop Med Hyg. 2014;91:580–583. doi: 10.4269/ajtmh.13-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vallee J, Thaojaikong T, Moore CE, et al. Contrasting spatial distribution and risk factors for past infection with scrub typhus and murine typhus in Vientiane city, Lao PDR. PLoS Negl Trop Dis. 2010;4:e909. doi: 10.1371/journal.pntd.0000909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gikas A, Doukakis S, Pediaditis J, Kastanakis S, Psaroulaki A, Tselentis Y. Murine typhus in Greece: Epidemiological, clinical, and therapeutic data from 83 cases. Trans Royal Soc Trop Med Hyg. 2002;96:250–253. doi: 10.1016/s0035-9203(02)90090-8. [DOI] [PubMed] [Google Scholar]
- 87.Silpapojakul K, Chayakul P, Krisanapan S, Silpapojakul K. Murine typhus in Thailand: clinical features, diagnosis and treatment. Q J Med. 1993;86:43–47. [PubMed] [Google Scholar]
- 88.Dumler JS, Taylor JP, Walker DH. Clinical and laboratory features of murine typhus in South Texas, 1980 through 1987. JAMA. 1991;266:1365–1370. [PubMed] [Google Scholar]
- 89.Walker DH, Ismail N. Emerging and re-emerging rickettsioses: Endothelial cell infection and early disease events. Nat Rev Microbiol. 2008;6:375–386. doi: 10.1038/nrmicro1866. [DOI] [PubMed] [Google Scholar]
- 90.Cazorla C, Socolovschi C, Jensenius M, Parola P. Tick-borne diseases: Tick-borne spotted fever rickettsioses in Africa. Infectious disease clinics of North America. 2008;22:531–544. ix–x. doi: 10.1016/j.idc.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 91.Parola P, Paddock CD, Socolovschi C, et al. Update on tick-borne rickettsioses around the world: A geographic approach. Clin Microbiol Rev. 2013;26:657–702. doi: 10.1128/CMR.00032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ismail N, Walker DH, Ghose P, Tang YW. Immune mediators of protective and pathogenic immune responses in patients with mild and fatal human monocytotropic ehrlichiosis. BMC Immunol. 2012;13:26. doi: 10.1186/1471-2172-13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Susilawati TN, McBride WJ. Acute undifferentiated fever in Asia: A review of the literature. SE Asian J Trop Med Public Health. 2014;45:719–726. [PubMed] [Google Scholar]
- 94.Kirkland KB, Wilkinson WE, Sexton DJ. Therapeutic delay and mortality in cases of Rocky Mountain spotted fever. Clin Infect Dis. 1995;20:1118–1121. doi: 10.1093/clinids/20.5.1118. [DOI] [PubMed] [Google Scholar]
- 95.Chapman AS, Bakken JS, Folk SM, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis--united states: A practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep. 2006;55:1–27. [PubMed] [Google Scholar]
- 96.Botelho-Nevers E, Rovery C, Richet H, Raoult D. Analysis of risk factors for malignant mediterranean spotted fever indicates that fluoroquinolone treatment has a deleterious effect. J Antimicrob Chemother. 2011;66:1821–1830. doi: 10.1093/jac/dkr218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Meta-analysis of scrub typhus incidence among acute febrile illness studies.
Supplemental Table 2. Meta-analysis of murine typhus incidence among prospective acute febrile illness studies.