Abstract
Background
In observational studies, low 25-hydroxyvitamin D (25(OH)D) has been associated with increased risk of coronary heart disease (CHD), and this association may vary by race. Racial differences in the frequency of vitamin D binding protein (DBP) single nucleotide polymorphisms (SNPs) might account for similar bioavailable vitamin D in blacks despite lower mean 25(OH)D. We hypothesized that the associations of low 25(OH)D with CHD risk would be stronger among whites and among persons with genotypes associated with higher DBP levels.
Methods
We measured 25(OH)D by mass spectroscopy in 11,945 participants in the ARIC Study (baseline 1990–1992, mean age 57 years, 59% women, 24% black). Two DBP SNPs (rs7041; rs4588) were genotyped. We used adjusted Cox proportional hazards models to examine the association of 25(OH)D with adjudicated CHD events through December 2011.
Results
Over a median of 20 years, there were 1230 incident CHD events. Whites in the lowest quintile of 25(OH)D (<17 ng/ml) compared to the upper 4 quintiles had an increased risk of incident CHD (HR 1.28, 95% CI 1.05–1.56), but blacks did not (1.03, 0.82–1.28), after adjustment for demographics and behavioral/socioeconomic factors (p-interaction with race=0.22). Results among whites were no longer significant after further adjustment for potential mediators of this association (i.e. diabetes, hypertension). There was no statistically significant interaction of 25(OH)D with the DBP SNPs rs4588 (p=0.92) or rs7041 (p=0.87) in relation to CHD risk.
Conclusions
Low 25(OH)D was associated with incident CHD in whites, but no interactions of 25(OH)D with key DBP genotypes was found.
Introduction
Low levels of vitamin D, as measured by serum 25-hydroxyvitamin D [25(OH)D], have been estimated to affect approximately 1 billion people worldwide1 and are associated with increased risk for cardiovascular diseases (CVD)2–5 and specifically for coronary heart disease (CHD).6–7 Suboptimal vitamin D status is thought to influence CVD risk predominantly by acting on established CVD risk factors, namely hypertension, diabetes, dyslipidemia and inflammation (i.e. mediators).8 Whether adequate vitamin D supplementation in those that are deficient can prevent CVD events is still unknown, and clinical trials are in progress to test this question.9
Individuals with darker skin pigmentation are more likely to have low 25(OH)D.1,10 However, research examining the association of low 25(OH)D with CVD outcomes in nonwhites is limited, though there is some evidence that associations may vary by race.7,10 In a recent analysis from the Multi-Ethnic Study of Atherosclerosis (MESA), low 25(OH)D was associated with increased CHD risk among whites and Chinese but not in blacks or Hispanics.7 Similarly, a prior analysis from the National Health and Nutrition Examination Survey (NHANES) found that low 25(OH)D was associated with stroke in whites but not blacks.10
Bioavailable vitamin D may underlie racial differences in associations between total 25(OH)D and CVD outcomes. On average, blacks have lower levels of total 25(OH)D compared to whites.11 However, recent work has shown that blacks and whites have similar concentrations of estimated bioavailable vitamin D because blacks have lower levels of both total 25(OH)D and vitamin D binding protein (DBP) compared to whites.11 Approximately 85–90% of 25(OH)D circulates tightly bound to DBP, which may impair the ability of vitamin D to act on target cells.12 The remainder, referred to as bioavailable vitamin D, circulates mostly bound to albumin, with a small proportion in the free form. There are two common single nucleotide polymorphisms (SNPs) in the DBP gene, rs7041 and rs4588, which are believed to explain ~80% of the variability in serum DBP levels.11 Blacks are more likely than whites to have a T allele at rs7041 and a C allele at rs4588, which both result in lower levels of serum DBP.
Our objective was to examine the association between 25(OH)D and incident CHD in both blacks and whites and to characterize any interplay between 25(OH)D and DBP SNPs with incident CHD occurring over approximately 20 years of follow-up in the community-based Atherosclerosis Risk in Communities (ARIC) Study. We hypothesized that lower concentrations of 25(OH)D would be associated with greater CHD risk, and that these associations would be modified by race (stronger in whites versus blacks) and by rs7041 and rs4588 SNPs (stronger in those with either the rs7041 G allele or the rs4588 A allele).
Methods
Participants
ARIC is an ongoing community-based prospective cohort of 15,792 middle-aged adults recruited from four locations: Forsyth County, North Carolina; Jackson, Mississippi; suburbs of Minneapolis, Minnesota, and Washington County, Maryland.13 Participants have taken part in five main visits: 1987–1989 (Visit 1), 1990–1992 (Visit 2), 1993–1995 (Visit 3), 1996–1998 (Visit 4), and 2011–2013 (Visit 5). Participants provided written consent both for their involvement in the study and for use of their genetic data. The Institutional Review Boards from all four locations and the Coordinating Center have approved the ARIC study.
The sample for this study comprised of participants attending visit 2 (baseline for the present analysis, n=14,348). Exclusion criteria were races other than black or white (n=42), blacks from Minneapolis or Washington County sites (n=49), those with prevalent CHD at visit 2 (n=826) or missing information regarding prevalent CHD at visit 2 (n=298), those with missing 25(OH)D levels (n=1085), those with estimated glomerular filtration rate (eGFR)<15 ml/min/1.73 m2 (n=14), and those missing any covariate information (n=89). After all exclusions, the final sample size was 11,945 participants.
Laboratory Analyses
Serum samples used for measurement of 25(OH)D2 and 25(OH)D3 were collected at visit 2 (1990–1992) and stored at −70°C until 2012–2013, when measurement took place, using liquid chromatography-tandem high-sensitivity mass spectrometry (Waters Alliance e2795, Milford, Massachusetts). Using samples collected in duplicate tubes and stored, the coefficient of variation (processing plus assay variation) for 25(OH)D2 was 20.8% and for 25(OH)D3 was 6.9%. The Pearson correlations from these blind duplicate samples were 0.98 for 25(OH)D2 and 0.97 for 25(OH)D3. 25(OH)D2 and 25(OH)D3 were added together for total 25(OH)D concentration.
Using stored samples from visit 2, in 2012–2013, we also measured calcium, phosphorus, and parathyroid hormone (PTH) as follows: (calcium and phosphorus: Roche Modular P-Chemistry Analyzer [Roche Diagnostics, Indianapolis, Indiana], PTH: Elecsys 2010 [Roche Diagnostics, Indianapolis, Indiana]). Serum magnesium was measured by the Gindler and Heth procedure. High-sensitivity C-reactive protein (hsCRP) was measured using a latex-particle enhanced immunoturbidimetric assay kit (Roche Diagnostics) and read on the Roche Modular P Chemistry analyzer (coefficient of variation 7%). Cystatin C was also measured in 2012–2013 from stored samples collected at visit 2 using the Gentian cystatin C assay on the Roche Modular P Chemistry analyzer. Serum creatinine was measured at visit 2 using a modified kinetic Jaffé reaction. eGFR was estimated using the 2012 chronic kidney disease epidemiology collaboration equation, which incorporates both cystatin C and creatinine.14 Fasting lipids were measured at the time of visit 2, and plasma total cholesterol and triglycerides were determined by enzymatic methods. High-density lipoprotein (HDL)-cholesterol was measured after dextran-magnesium precipitation, and the Friedewald equation was used to calculate low density lipoprotein (LDL)-cholesterol in those with triglyceride levels under 400 mg/dL.
Ascertainment of ARIC CHD
All hospitalizations and deaths in ARIC participants occurring through December 31, 2011 were identified by annual telephone follow-up call, review of hospital discharge lists, and death certificates. Data were abstracted from hospital and death records, next-of-kin interviews, and physician-completed questionnaires. CHD events, defined as definite or probable hospitalized myocardial infarctions or definite fatal CHD, were classified by a combination of computer algorithm and adjudicated physician review, using standardized ARIC criteria.15
Genotyping
Participants’ DNA samples were used to genotype the two SNPs of interest (rs7041 and rs4588), which are found in the coding region of the vitamin DBP gene. A (50K) SNP genotyping array, ITMAT-Broad-CARE Chip, from the Broad Institute of Massachusetts Institute of Technology and Harvard was used for genotyping.16,17 We tested whether rs7041 and rs4588 were in Hardy Weinberg Equilibrium, separately by race. There was no evidence that equilibrium was violated (Chi-square tests p>0.8). We categorized the SNPs as TT versus TG versus GG for rs7041 and CC versus AC versus AA for rs4588.
Covariates
Most of the variables analyzed for this study were measured at visit 2 except for education and physical activity, which were assessed at visit 1. Medication usage, demographical, and behavioral variables were obtained through standard ARIC questionnaires administered by trained interviewers.
The main covariates included: age, race-center (Minneapolis-whites; Washington County-whites; Forsyth County-whites; Forsyth County-blacks; Jackson County-blacks), sex, education, physical activity (measured on a scale of 1–5 based on a modified Baecke Physical Activity questionnaire18), smoking status, body mass index (BMI), diabetes (yes/no; defined as a self-reported physician diagnosis, current diabetes medication use, fasting serum glucose ≥126 mg/dl or non-fasting glucose ≥200 mg/dl), sitting systolic and diastolic blood pressure (continuous; measured in triplicate with random-zero sphygmomanometer; used mean of the second and third measurements in analysis), anti-hypertensive medications, total cholesterol, HDL-cholesterol, hsCRP (modeled as continuous variables), and eGFR (categories of ≥90, 60–89, ≤59 ml/min/1.73m2). In a supplemental model, we also adjusted for the vitamin D related biomarkers of PTH, calcium, phosphate, and magnesium (modeled continuously).
Statistical Analysis
We adjusted serum 25(OH)D concentrations for seasonal variation, since prior data have shown that 25(OH)D levels are affected by season.19 To adjust for seasonal variation, we calculated residuals using a linear regression model such that 25(OH)D was the dependent variable, and the month of blood drawn was the independent variable. Residuals were calculated separately for blacks and whites. The calculated residuals were then added to the total mean, and an estimated annual 25(OH)D value was obtained. Vitamin D levels were divided into quintiles based on the overall study population distribution as done in previous ARIC analyses.20
Baseline characteristics of the study participants were described using means (standard deviations) and proportions stratified by 25(OH)D quintiles and race. We used Cox proportional hazards models to estimate the hazards ratio (95% confidence intervals) for the association of 25(OH)D levels with risk of incident CHD comparing the lowest quintile with the upper 4 quintiles. Study participants contributed follow-up time from the date of the participant’s second visit until the date of incident CHD event, death, loss-to-follow-up, or the end of follow-up (12/31/2011), whichever came first. The proportional-hazards assumption was checked, using log survival curves and interaction terms between 25(OH)D quintiles and time, and was found to be satisfied in all models.
Additionally, to investigate for possible dose-response relationship, we modeled estimated annual 25(OH)D using restricted cubic splines with knots at the 5th, 27.5, 50th, 72.5, & 95th percentiles to show the continuous relationship between 25(OH)D levels and risk of incident CHD.
To account for potential confounders, we used models with increasing degrees of adjustment. We created two primary models: Model 1 adjusted for demographic factors (age, sex, and race/center), and Model 2 further adjusted for potentially confounding behavioral/socioeconomic variables (education, physical activity, smoking status, and BMI). We also tested two additional models. Model 3 further adjusted for potential mediators in the causal pathway between 25(OH)D and CHD including diabetes, systolic and diastolic blood pressure, use of hypertension medication, total and HDL cholesterol, hsCRP, and eGFR. Finally, Model 4 adjusted for the variables in model 3 plus the vitamin D related biomarkers of serum calcium, phosphate, PTH, and magnesium.
Wald tests were used to formally test for two-way multiplicative interactions of 25(OHD) with race, sex, age, or DBP SNPs (rs7041 and rs4588) in relation to incident CHD, by including cross-product terms in the model. We decided a priori that any interactions with p<0.1 would be further explored. Based on prior studies,7,10 we also a priori planned to present the results overall and stratified by race, regardless of whether a race interaction was present.
Since the hazard of low 25(OH)D may potentially attenuate over time due to regression dilution bias, a sensitivity analysis was performed limiting follow-up to only the first 10-years.
Two sided p-values less than or equal to 0.05 were considered statistically significant. We performed statistical analyses using SAS version 9.2.
Results
In the overall study sample, mean age at baseline was 57 years, 59% were female, and 24% were black. Median 25(OH)D concentrations were 18.2 ng/ml among blacks versus 25.6 ng/ml among whites (p<0.0001). The distribution of clinical characteristics of participants stratified by quintiles of 25(OH)D and by race are provided in Table 1 (quintiles 1, 3 and 5, only) and Supplemental Tables 1 and 2 (all quintiles in whites and blacks, respectively). Compared to those with 25(OH)D concentration in the highest quintile, those with 25(OH)D concentration in the lowest quintile were younger, more likely to be female, and had lower levels of physical activity (all p<0.0001). Those in the lowest 25(OH)D quintile were also more likely to have higher BMI and more prevalent hypertension compared to those in the highest quintile (p<0.0001).
Table 1.
Participant characteristics by race and 25(OH)D quintile*, ARIC Visit 2 (1990–1992). [* Note Quintiles 2 and 4 not shown for space, but found in Supplemental Tables 1 and 2]. Data presented as means(SD) or % unless noted.
| Blacks | Whites | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Quintile 1 (n=1261) | Quintile 3 (n=426) | Quintile 5 (n=156) | Quintile 1 (n=1128) | Quintile 3 (n=1963) | Quintile 5 (n=2233) | |
| 25(OH)D (ng/ml), median | 13.4 | 23.5 | 33.8 | 14.2 | 23.8 | 35.2 |
| Vitamin D binding protein gene polymorphisms | ||||||
| rs7041 | ||||||
| TT | 74.0 | 68.9 | 57.6 | 26.9 | 20.3 | 12.0 |
| TG | 23.4 | 27.4 | 38.4 | 48.6 | 50.2 | 47.6 |
| GG | 2.6 | 3.7 | 4.0 | 24.5 | 29.5 | 40.4 |
| rs4588 | ||||||
| CC | 78.2 | 83.9 | 93.3 | 40.3 | 48.6 | 63.7 |
| AC | 20.7 | 14.6 | 6.7 | 45.9 | 42.9 | 33.0 |
| AA | 1.1 | 1.5 | 0.0 | 13.8 | 8.5 | 3.3 |
| Age (years) | 55.3(5.5) | 56.7(6.1) | 57.9(5.8) | 56.6(5.6) | 56.9(5.6) | 57.2(5.7) |
| Female | 75.7 | 61.5 | 40.4 | 72.6 | 52.3 | 52.1 |
| Study Site | ||||||
| Minneapolis, MN | 0.0 | 0.0 | 0.0 | 35.6 | 39.4 | 35.2 |
| Washington County, MD | 0.0 | 0.0 | 0.0 | 36.5 | 33.1 | 30.9 |
| Forsyth County, NC | 12.6 | 9.2 | 10.9 | 27.9 | 27.5 | 33.9 |
| Jackson, MS | 87.4 | 90.8 | 89.1 | 0.0 | 0.0 | 0.0 |
| ** Education | ||||||
| <High school | 32.2 | 44.1 | 48.7 | 15.5 | 13.5 | 15.3 |
| High school or vocational school | 31.6 | 24.9 | 21.2 | 48.4 | 44.0 | 47.1 |
| College, graduate, or professional school | 36.2 | 31.0 | 30.1 | 36.1 | 42.5 | 37.6 |
| Smoking Status | ||||||
| Never | 48.3 | 49.1 | 32.7 | 36.4 | 41.9 | 38.1 |
| Former | 26.0 | 30.0 | 37.8 | 31.8 | 39.1 | 43.5 |
| Current | 25.7 | 20.9 | 29.5 | 31.8 | 19.0 | 18.4 |
| ** Physical activity index | 2.1(0.6) | 2.2(0.7) | 2.3(0.7) | 2.3(0.7) | 2.5(0.8) | 2.8(0.9) |
| Body mass index (kg/m2) | 30.9(7.0) | 29.5(5.8) | 28.1(4.9) | 28.8(6.3) | 27.6(4.8) | 26.0(4.1) |
| Diabetes | 23.6 | 24.2 | 16.7 | 16.2 | 10.6 | 7.5 |
| Diastolic blood pressure (mmHg) | 75.5(10.9) | 75.4(10.9) | 75.1(11.7) | 71.0(10.2) | 71.6(9.9) | 70.9(9.6) |
| Systolic blood pressure (mmHg) | 127.5(20.5) | 125.3(20.5) | 124.9(21.6) | 121.3(18.6) | 119.7(17.7) | 118.4(17.5) |
| Hypertension | 56.1 | 52.6 | 44.9 | 32.4 | 28.7 | 25.1 |
| Antihypertensive medications | 46.0 | 47.7 | 42.3 | 28.8 | 25.7 | 23.4 |
| Total Cholesterol (mg/dl) | 208.9(40.7) | 213.3(42.8) | 208.3(40.2) | 210.2(42.4) | 208.8(37.3) | 210.0(37.4) |
| HDL-cholesterol (mg/dl) | 53.3(17.6) | 53.9(15.5) | 52.0(16.2) | 47.9(16.0) | 48.1(16.0) | 52.4(18.1) |
| Estimated GFR <60 ml/min/1.73 m2 | 3.3 | 3.6 | 6.5 | 2.1 | 1.4 | 2.6 |
| hs-CRP (mg/L), geometric mean (interquartile range) | 3.4(5.8) | 2.9(5.3) | 2.6(4.9) | 2.6(4.5) | 2.0(3.0) | 1.9(2.9) |
| PTH (pg/ml) | 53.6(26.7) | 43.2(18.0) | 41.8(15.8) | 46.1(17.7) | 40.8(14.6) | 36.2(12.1) |
| Calcium (mg/dl) | 9.4(0.5) | 9.5(0.5) | 9.5(0.4) | 9.3(0.4) | 9.3(0.4) | 9.3(0.4) |
| Phosphorus (mg/dl) | 3.6(0.5) | 3.6(0.5) | 3.6(0.5) | 3.5(0.5) | 3.5(0.5) | 3.5(0.5) |
| Magnesium (mEq/L) | 1.6(0.2) | 1.6(0.2) | 1.6(0.2) | 1.6(0.2) | 1.6(0.2) | 1.6(0.2) |
25(OH)D quintiles defined in the overall population. Quintile 1: <17.0; Quintile 2: 17.0–<21.6; Quintile 3: 21.6–<25.9; Quintile 5: 25.9–<31.0; Quintile 5: ≥31.0 ng/ml.
Assessed at ARIC Visit 1 (1987–1989).
Over a median of 19.7 years of follow-up, there were 1230 incident CHD events. In age/sex/race adjusted models (Model 1) for the overall population, those in the lowest quintile of 25(OH)D (<17 ng/ml) compared to the upper 4 quintiles had an increased risk of incident CHD (HR 1.30, 95%CI 1.12–1.51), [Table 2]. There was no significant interaction by age or sex. However, there was a statistically significant interaction by race (Model 1, p-interaction 0.03) with no association seen for blacks but increased CHD risk among whites with low vitamin D [HR 1.53, 1.26–1.85]. In supplemental models exploring alternative 25(OH)D cut-points, there also was no association of low 25(OH)D with CHD risk in blacks when race-specific quintiles (quintile 1 vs quintiles 2–5) were compared [Supplemental Table 3], nor when clinical cut-points of <20 versus 20–30 versus ≥30 ng/ml were compared [Supplemental Table 4]. However, among whites there continued to be an association of low 25(OH)D with CHD risk using these alternate 25(OH)D cut-points (Model 1).
Table 2.
Adjusted* hazard ratios (95% Confidence Intervals) of incident CHD by 25(OH)D quintile, follow-up: 1990–1992 through December 2011.
| Quintiles of 25(OH)D | ||
|---|---|---|
|
| ||
| Quintile 1 (<17.0 ng/ml) | Quintile 2–5 (≥17.0 ng/ml) | |
| Overall: | ||
| Median 25(OH)D (ng/ml) | 13.8 | 25.9 |
| n events/n at risk | 268/2389 | 962/9556 |
| Model 1 | 1.30 (1.12–1.51) | 1 (reference) |
| Model 2 | 1.16 (1.00–1.34) | 1 (reference) |
| Model 3 | 1.10 (0.94–1.28) | 1 (reference) |
| Model 4 | 1.11 (0.95–1.29) | 1 (reference) |
| Blacks: | ||
| Median 25(OH)D (ng/ml) | 13.4 | 22.0 |
| n events/n at risk | 144/1261 | 217/1653 |
| Model 1 | 1.06 (0.85–1.32) | 1 (reference) |
| Model 2 | 1.03 (0.82–1.28) | 1 (reference) |
| Model 3 | 0.98 (0.78–1.23) | 1 (reference) |
| Model 4 | 0.98 (0.78–1.24) | 1 (reference) |
| Whites: | ||
| Median 25(OH)D (ng/ml) | 14.2 | 26.7 |
| n events/n at risk | 124/1128 | 745/7903 |
| Model 1 | 1.53 (1.26–1.85) | 1 (reference) |
| Model 2 | 1.28 (1.05–1.56) | 1 (reference) |
| Model 3 | 1.21 (0.99–1.47) | 1 (reference) |
| Model 4 | 1.22 (1.00–1.50) | 1 (reference) |
Model 1: age, sex, race/center (overall model) or center (race-stratified models).
Model 2: +education, physical activity, smoking status, and BMI.
Model 3: +diabetes, systolic blood pressure, diastolic blood pressure, antihypertensive medications, total cholesterol, HDL-C, hsCRP, and eGFR.
Model 4: +calcium, phosphorous, parathyroid hormone, and serum magnesium.
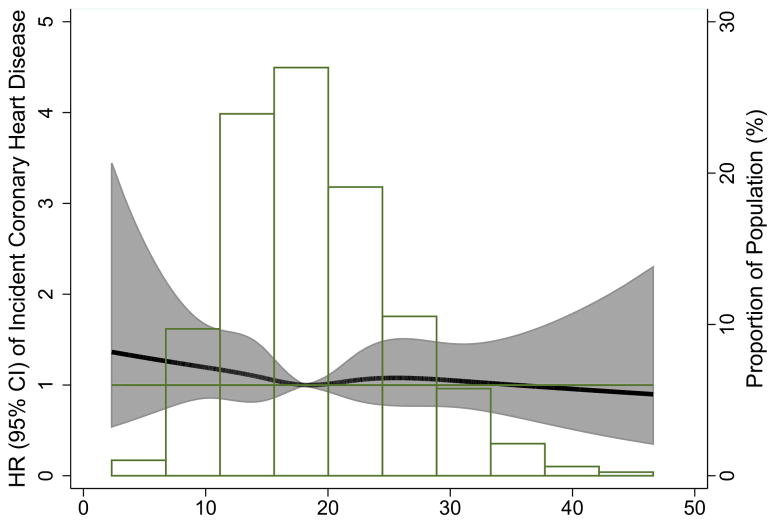
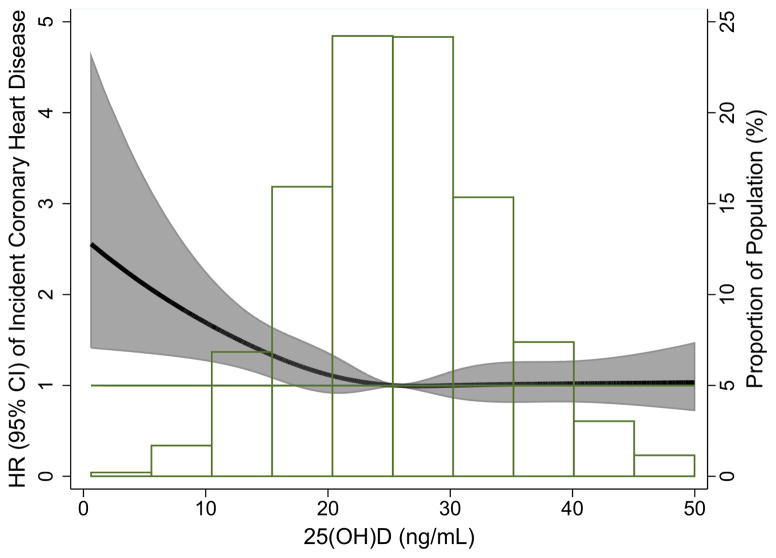
Figure 1 [A: blacks; B: whites] shows the continuous association of 25(OH)D with incident CHD risk (adjusted for Model 1 variables) by race. For whites, similar to the quintile analysis, there was evidence for an association between 25(OH)D and increased risk of CHD primarily among those with low values of 25(OH)D, suggesting a possible threshold effect at approximately 15–20 ng/ml.
Figure.
Adjusted* restricted cubic spline model showing the hazard ratios (95% CI) for the association of 25(OH)D with incident CHD for blacks (Panel A) and whites (Panel B). Adjusted for age, sex, race/center (overall model) or center (race-stratified models). Corresponding knots at the 5th, 27.5, 50th, 72.5, & 95th percentile for each plot: Blacks: 9.37, 14.46, 18.16, 22.15, 31.14; Whites: 13.50, 21.04, 25.56, 30.24, 40.07
After further adjustment for potentially confounding lifestyle factors (Model 2) [Table 2], the associations of low vitamin D and CHD among whites attenuated but remained significant (HR 1.28, 1.05–1.56). The race interaction was no longer significant (Model 2, p-interaction=0.22). Results for whites were no longer statistically significant after further adjustment for potential mediators (HR 1.21, 0.99–1.47, Model 3) and vitamin D related biomarkers (HR 1.22, 1.00–1.50, Model 4).
Frequencies of DBP SNPs varied by race. For rs7041, the G allele frequency was 56% in whites and 16% in blacks. For rs4588, the A allele frequency was 28% in whites and 10% in blacks. There was no main effect between DBP SNPs rs7041 and rs4588 and CHD incidence. Likewise, we found no interaction between DBP SNPs and 25(OH)D in relation to incident CHD in the overall sample (rs7041, p-interaction=0.87; rs4588, p-interaction=0.92, Model 2). Therefore results were not further stratified by DBP genotype.
Since the risk conferred by low 25(OH)D measured at a single time point may attenuate over time, we also looked at the association of 25(OH)D with incident CHD occurring only in the first 10 years of follow-up [Supplemental Table 5]. Results were similar to the 20 year follow-up, but somewhat stronger. Among whites the HR for the lowest quintile of 25(OH)D for incident CHD was 1.43 (1.09–1.88) while for blacks it remained not statistically significant [HR 1.13 (0.82–1.54)], in models adjusted for demographics and lifestyle factors.
Discussion
In this community-based middle-aged sample, as hypothesized, low 25(OH)D was associated with greater risk of incident CHD in whites but not blacks. The association in whites persisted after accounting for behavioral and lifestyle factors but not after considering potential mediators, as might be expected if they were truly mediators. In contrast to our hypothesis, DBP genotypes did not modify the association between low 25(OH)D and incident CHD.
CHD is still the leading cause of death in the United States21 and therefore there is continued great interest in finding potential modifiable factors to reduce this risk. Low vitamin D as a purported CHD risk factor is an attractive candidate because it can be easily screened for, can be treated with supplementation and modest sunlight exposure, and has biologic plausibility in the causal pathway of CHD. Potential mediators of the associations of 25(OH)D and CHD could occur through vitamin D’s effects on blood pressure control22 (vitamin D is an inhibitor of the renin-aldosterone-angiotensin system pathway23), glucose regulation and diabetes risk24,25, and its anti-inflammatory properties26. Activated vitamin D may also retard atherosclerosis by inhibiting macrophage cholesterol uptake and foam cell formation.27 However, it remains unclear whether the observed associations between vitamin D and chronic disease states in epidemiologic studies are due to lower vitamin D status secondary to poorer health or whether a true causal relationship exists. Clinical trials of vitamin D supplementation are ongoing to test this.28
We found that the associations of 25(OH)D with incident CHD among whites were no longer statistically significant after further adjustment for blood pressure, diabetes, inflammation, lipids, and renal function. This could be because these factors may be mediators in the causal pathway between low 25(OH)D and incident CHD, rather than confounding variables. In some other studies, notably MESA7 and the Framingham Heart Study3, an association between low 25(OH)D and incident CHD did remain after adjusting for these potential mediators. Of note, this current study has longer follow-up than the previous studies, but our results were similar when events occurring only in the first 10 years were considered.
Blacks are known to have lower levels of 25(OH)D compared to whites.29–30 However, paradoxically, they are at lower risk of fractures and have higher bone mineral density than whites. The reason for the lack of association of 25(OH)D and incident CHD in blacks, which was seen in both the current ARIC study and the MESA7 cohort, is uncertain, though there are several possible explanations. There is evidence to suggest that blacks have adapted a relative resistance to the adverse effects of 25(OH)D deficiency. For example, despite their low 25(OH)D levels, it is thought that blacks have lower rates of fractures because of a skeletal resistance to the actions of PTH.29 Prior work from NHANES has suggested that the relationship between 25(OH)D, bone mineral density, and PTH may differ by race.30 Also, if it is low bioavailable vitamin D which confers CVD risk, given that blacks have low levels of DBP but similar levels of bioavailable vitamin D relative to whites,11 simply evaluating associations between 25(OH)D and CHD may have been inadequate to detect an effect. We attempted to gauge the association between bioavailable vitamin D and CHD risk by testing for interactions between low 25(OH)D and key DBP SNPs. There was no statistical evidence for interaction; however, the power for testing interactions was quite low.
Certain limitations should be taken into consideration in the interpretation of this study. First, we had only a single measurement of 25(OH)D, but we adjusted 25(OH)D concentrations for seasonal variation. Second, we did not measure serum DBP concentrations and were therefore unable to calculate bioavailable vitamin D. However, we were able to look at associations and effect modification by DBP polymorphisms rs7041 and rs4588, but these analyses have limited power. We only tested two common DBP SNPs, which had been documented to explain 80% variation in bioavailable 25(OH) levels, but other genetic variations exist. There is some data to suggest that some SNPs related to 25(OH)D but not others are associated with subclinical CVD.31
Our study also has a number of strengths, including a large bi-racial sample, comprehensive assessment of confounding variables, physician-adjudicated CHD events using standardized criteria, and approximately 20-years of follow-up. Our study adds to the growing literature suggesting that the risk of low 25(OH)D may vary by race,7,10,20,25 and results in white populations should not necessarily be extrapolated to other race/ethnicities. Also, we newly suggest that two common DBP SNPs are not independently associated with CHD risk, and do not interact with low 25(OH)D to influence CHD risk.
In conclusion, we found that the risk of incident CHD associated with low 25(OH)D varied by race, but it was not explained by interactions of 25(OH)D with key DBP genotypes. Further investigation is warranted into potential mechanisms that can account for this racial difference. Most importantly, randomized clinical trials are needed to determine whether treating low vitamin D status mitigates this risk by reducing CHD events in both whites and blacks.
Supplementary Material
Supplemental Table 1. Participant characteristics by 25(OH)D population-based quintiles in whites*, ARIC Visit 2 (1990–1992).
Supplemental Table 2. Participant characteristics by 25(OH)D population-based quintiles in blacks*, ARIC Visit 2 (1990–1992).
Supplemental Table 3. Adjusted* hazard ratios (95% Confidence Intervals) for incident CHD by race-specific 25(OH)D quintile, follow-up: 1990–1992 through December 2011.
Supplemental Table 4. Adjusted* hazard ratios (95% Confidence Intervals) for incident CHD by 25(OH)D clinical cutpoints, follow-up: 1990–1992 through December 2011.
Supplemental Table 5. Adjusted* hazard ratios (95% Confidence Intervals) for incident CHD by 25(OH)D quintile, follow-up: 1990–1992 through first ten years of follow-up.
HIGHLIGHTS.
Low 25(OH)D status was associated with increased risk of coronary disease in Whites
No association of 25(OH)D with coronary heart disease was found in Blacks
No interaction was found with genetic polymorphisms of vitamin D binding protein
Further investigation into potential mechanisms for this racial difference is needed
Whether treating low vitamin D can reduce coronary events is unknown
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions. Reagents for the C-reactive protein assays were donated by the Roche Diagnostics Corporation.
FUNDING
This research was also supported by grants from NIH/NINDS (R01NS072243 to Dr. Michos), the NIH/NHLBI (R01HL103706 to Dr. Lutsey), the NIH Office of Dietary Supplements (R01HL103706-S1 to Dr. Lutsey) and the NIH/NIDDK (R01DK089174 to Dr. Selvin). Genotyping was supported through the NHLBI CARe (Candidate Gene Resource) grant N01HC65226. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Wang L, Song Y, Manson JE, et al. Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: A meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes. 2012;5:819–829. doi: 10.1161/CIRCOUTCOMES.112.967604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson JL, May HT, Horne BD, et al. Relationship of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general health care population. Am J Cardiol. 2010;106:963–968. doi: 10.1016/j.amjcard.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 5.Fiscella K, Franks P. Vitamin D, race, and cardiovascular mortality: findings from a National US Sample. Ann Fam Med. 2010;8:11–18. doi: 10.1370/afm.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: A prospective study. Arch Intern Med. 2008;168:1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson-Cohen C, Hoofnagle AN, Ix JH, et al. Racial differences in the association of serum 25-hydroxyvitamin D concentration with coronary heart disease events. JAMA. 2013;310(2):179–188. doi: 10.1001/jama.2013.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lutsey PL, Michos ED. Vitamin D, calcium, and atherosclerotic risk – evidence from serum levels and supplementation studies. Curr Atheroscler Rep. 2013;15(1):293. doi: 10.1007/s11883-012-0293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manson JE, Bassuk SS, Lee IM, et al. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33(1):159–171. doi: 10.1016/j.cct.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michos ED, Reis JP, Post WS, et al. 25-hydroxyvitamin D deficiency is associated with fatal stroke among whites but not blacks: The nhanes-iii linked mortality files. Nutrition. 2012;28:367–371. doi: 10.1016/j.nut.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powe CE, Evans MK, Wenger J, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369:1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab. 1986;63:954–959. doi: 10.1210/jcem-63-4-954. [DOI] [PubMed] [Google Scholar]
- 13.The ARIC investigators; The aric investigators, editor. The atherosclerosis risk in communities (aric) study: Design and objectives. Amer J of Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 14.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 16.Musunuru K, Lettre G, Young T, et al. Candidate gene association resource (care): Design, methods, and proof of concept. Circulation Cardiovascular genetics. 2010;3:267–275. doi: 10.1161/CIRCGENETICS.109.882696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keating BJ, Tischfield S, Murray SS, et al. Concept, design and implementation of a cardiovascular gene-centric 50 k SNP array for large-scale genomic association studies. PLoS One. 2008;3(10):e3583. doi: 10.1371/journal.pone.0003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 19.Shoben AB, Kestenbaum B, Levin G, et al. Seasonal variation in 25-hydroxyvitamin D concentrations in the Cardiovascular Health Study. Am J Epidemiol. 2011;174(12):1363–1372. doi: 10.1093/aje/kwr258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lutsey PL, Michos ED, Misialek JR, et al. Race and vitamin D binding protein gene polymorphisms modify the association of 25-hydroxyvitamin D and incident heart failure: The Atherosclerosis Risk in Communities Study (ARIC) JACC Heart Fail. 2015 doi: 10.1016/j.jchf.2014.11.013. Published online April 08, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunutsor S, Apekey T, Steur M. Vitamin D and risk of future hypertension: meta-analysis of 283,537 participants. Eur J Epidemiol. 2013;28:205–221. doi: 10.1007/s10654-013-9790-2. [DOI] [PubMed] [Google Scholar]
- 23.Li YC, Kong J, Wei M, et al. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110(2):229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitri J, Pittas AG. Vitamin D and diabetes. Endocrinol Metab Clin North Am. 2014;43(1):205–232. doi: 10.1016/j.ecl.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reis JP, Michos ED, Pankow JS, Selvin E, Lutsey PL. Race, vitamin D binding protein gene polymorphisms, 25-hydroxyvitamin D, and incident diabetes: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 2015 doi: 10.3945/ajcn.115.107334. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytopkine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83(4):754–759. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 27.Oh J, Weng S, Felton SK, et al. 1,25(OH)2 vitamin D inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation. 2009;120:687–698. doi: 10.1161/CIRCULATIONAHA.109.856070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schnatz PF, Manson JE. Vitamin D and cardiovascular disease: an appraisal of the evidence. Clinical Chemistry. 2014;60(4):600–609. doi: 10.1373/clinchem.2013.211037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris SS. Vitamin D and African Americans. J Nutr. 2006;136:1126–1129. doi: 10.1093/jn/136.4.1126. [DOI] [PubMed] [Google Scholar]
- 30.Gutierrez OM, Farwell WR, Kermah D, Taylor EN. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos Int. 2011;22:1745–1753. doi: 10.1007/s00198-010-1383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strawbridge RJ, Deleskog A, McLeod O, et al. A serum 25-hydroxyvitamin D concentration-associated genetic variant in DHCR7 interacts with type 2 diabetes status to influence subclinical atherosclerosis (measured by carotid intima-media thickness) Diabetologia. 2014;57(6):1159–1172. doi: 10.1007/s00125-014-3215-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Participant characteristics by 25(OH)D population-based quintiles in whites*, ARIC Visit 2 (1990–1992).
Supplemental Table 2. Participant characteristics by 25(OH)D population-based quintiles in blacks*, ARIC Visit 2 (1990–1992).
Supplemental Table 3. Adjusted* hazard ratios (95% Confidence Intervals) for incident CHD by race-specific 25(OH)D quintile, follow-up: 1990–1992 through December 2011.
Supplemental Table 4. Adjusted* hazard ratios (95% Confidence Intervals) for incident CHD by 25(OH)D clinical cutpoints, follow-up: 1990–1992 through December 2011.
Supplemental Table 5. Adjusted* hazard ratios (95% Confidence Intervals) for incident CHD by 25(OH)D quintile, follow-up: 1990–1992 through first ten years of follow-up.