Abstract
Objective: The current study examined the efficacy and safety of rasagiline, a selective MAO-B inhibitor, for the treatment of persistent negative symptoms. Methods: Sixty people with Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, schizophrenia or schizoaffective disorder, who met a priori criteria for persistent negative symptoms, were randomized to receive rasagiline, 1mg/d (n = 31) or placebo (n = 29) in a 12-week, double-blind, placebo-controlled clinical trial. The Scale for the Assessment of Negative Symptoms (SANS) total score was used to assess change in negative symptoms. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), N-Back test, a probabilistic learning task, and a delayed discounting task were used to assess cognition. Results: In a mixed model analysis of covariance (MM-ANCOVA), with time as a continuous variable, there was a significant treatment × time effect for SANS total score (F = 5.61(df = 1,40.3), P = .023). The treatment × time interaction effect was also significant for the SANS avolition subscale score (F(1,40.2) = 10.41, P = .002). In a post hoc MM-ANCOVA analyses, with time as a categorical variable, group differences were significant at week 12 for SANS total score (t(37.3) = 2.15; P = .04; d = −0.41) and SANS avolition subscale score (t(49.0) = 3.06; P = .004; d = −0.46). There was a significant difference in number of participants with a ≥20% reduction in SANS avolition score (χ2(1) = 10.94; P = .0009), but not in SANS total score (χ2(1) = 1.11; P = .29). There were no significant group differences on the RBANS, N-Back, probabilistic learning, or delayed discounting tasks. Conclusions: Study results support future studies of the utility of rasagiline for the treatment of negative symptoms, including avolition (clinicaltrials.gov trial number: NCT00492336).
Key words: randomized clinical trial, monoamine oxidase, dopamine, cognition
Introduction
Negative symptoms and cognitive impairments are major determinants of poor functional outcome in people with schizophrenia.1–5 First-generation antipsychotics (FGAs) and second-generation antipsychotics (SGAs) have limited benefit for these illness dimensions.6,7 The lack of effective treatments for negative symptoms and cognitive impairments represents a major shortfall in the treatment of schizophrenia.6,8
A number of recent studies suggest a link between negative symptoms and disturbances in dopamine and reward system function.9–11 Schultz et al12,13 demonstrated that changes in tonic and phasic dopamine release play an important role in brain reward mechanisms. If people with schizophrenia have decreased dopamine release in response to the appearance of environmental cues predictive of potential rewards or reward receipt, then one would expect to observe decreased goal-directed, reward-seeking behaviors. This failure to spontaneously initiate goal-directed behaviors would lead to the type of behavioral inertia that is typically considered to reflect avolition, a characteristic of people with negative symptoms. Abnormalities in cortical dopamine transmission have also been implicated in the production of cognitive impairments, in particular, working memory deficits.14,15 The development of a treatment that enhances dopaminergic function could have significant clinical benefit for the treatment of negative symptoms and cognitive impairments.
Monoamine oxidase (MAO) metabolizes several neurotransmitters, including dopamine, serotonin, and norepinephrine. Two types of MAO have been identified: MAO-A and MAO-B, with MAO-B being more predominant (~80%) in the human brain. Nonselective MAO inhibitors have been effectively used as antidepressants. They may also improve negative symptoms in people with schizophrenia.16 However, drugs that inhibit both forms of the MAO can produce severe cardiovascular reactions and hypertensive crisis due to the inability to metabolize tyramine and other indirectly acting sympathomimetic amines found in foods and medications. Agents that selectively inhibit MAO-B (MAO-B inhibitors) do not cause this tryamine-induced hypertensive crisis because MAO-A remains available to metabolize tyramine. MAO-B inhibitors increase dopamine levels in several areas of the brain, including the prefrontal cortex, substantia nigra, and basal ganglia.17–19
Because MAO-B inhibition increases endogenous dopamine levels, these agents may be useful in the treatment of negative symptoms and cognitive impairments. Several studies have evaluated the selective MAO-B inhibitor selegiline in the treatment of negative symptoms.20–25 Bodkin et al24 found that selegiline was significantly more effective than placebo for the treatment of predominant negative symptoms. However, Jungerman et al23 failed to find a significant selegiline effect on negative symptoms. In that study, both placebo and selegiline significantly improved negative symptoms compared with baseline. None of these studies examined the effect of selegiline on cognitive impairments.
Rasagiline is a new, selective MAO-B inhibitor, which is up to 15 times more potent than selegiline.26 Rasagiline is expected to have a number of clinical benefits over selegiline. Both are propargylamine derivatives and exhibit neuroprotective properties.27 However, the primary metabolites of selegiline are l-methamphetamine and l-amphetamine, which may be neurotoxic and interfere with the neuroprotective effects of the parent compound.28,29 In contrast, the major metabolite of rasagiline is aminoindan, which may have neuroprotective properties similar to the parent compound.29
The present study is designed to examine the efficacy and safety of rasagiline for the treatment of both persistent negative symptoms and cognitive impairments in people with schizophrenia.
Methods
Participants
Inpatients or outpatients, between 18 and 64 years, who met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), criteria for schizophrenia or schizoaffective disorder were selected for study entry. Participants were diagnosed using a best-estimate diagnostic approach, which utilized information from the Structured Clinical Interview for DSM-IV.30 Participants were required to be clinically stable, in the nonacute phase of their illness, and meet retrospective and prospective criteria for persistent moderate to severe negative symptoms.31,32 The retrospective determination of persistence was based on the best-estimate diagnosis and/or therapist report. The prospective definition of persistence used negative symptom assessments completed at the beginning and end of the 4-week lead-in phase. Participants were required to demonstrate a minimum level of negative symptoms, defined as a modified Scale for the Assessment of Negative Symptoms (SANS)32 total score greater than 20 and the affective flattening or alogia global item ≥ 3. In addition, participants were required to not exceed specified levels of positive symptoms (ie, none of the 4 Brief Psychiatric Rating Scale [BPRS]33; positive symptom item scores ≥ 5), affective symptoms (ie, none of the BPRS Anxiety/Depression factor items ≥ 5), and extrapyramidal symptoms (ie, Simpson-Angus Extrapyramidal Symptom Rating Scale [SAS] total score ≤ 6).6 The use of persistent negative symptoms to define the study cohort is designed to (1) maximize the likelihood that study participants are characterized by the presence of primary negative symptoms and those secondary negative symptoms that have not responded to previous treatments for these symptoms and (2) minimize potential improvement in negative symptoms secondary to improvement in positive, depressive, or extrapyramidal symptoms.31
Participants could be treated with a FGA or a SGA, but if treated with an FGA, they could not be receiving concomitant antiparkinsonian treatment. The participants were required to be on the same antipsychotic for at least 8 weeks and to be on the same dose for at least 30 days. If prescribed other psychotropic medications, they were required to be on the same drug and dose for at least 30 days. Participants who met DSM-IV criteria for current alcohol or substance dependence (except nicotine) within the last 6 months or DSM-IV criteria for alcohol or substance abuse (except nicotine) within the last month were excluded. Participants with intermittent alcohol or substance use were not excluded. Participants with mental retardation, or a medical condition, whose pathology or treatment could alter the presentation or treatment of schizophrenia or significantly increase the risk associated with the proposed treatment protocol were excluded. Pregnant and lactating female participants were excluded.
The University of Maryland School of Medicine, the State of Maryland Department of Health and Mental Hygiene, and the National Institute on Drug Abuse institutional review boards approved the study protocol and informed consent procedures. Written informed consent was obtained from all participants after full explanation of study procedures and prior to study participation. Participant ability to provide valid informed consent was documented using study-specific procedures.
Clinical Assessments
The modified SANS total score was used to assess negative symptom change.32 The BPRS positive symptom item (ie, conceptual disorganization, hallucinatory behavior, unusual thought content, and suspiciousness) total score was used to assess positive symptom change. The Clinical Global Impression (CGI) severity of illness item was used to assess global changes. The Calgary Depression Scale (CDS)34 total score was used to assess depressive symptom change. The SANS, BPRS, CDS, and CGI were obtained at lead-in phase weeks 0 and 4 and every 4 weeks during the 12-week double-blind treatment phase. Intraclass correlation coefficients for these instruments ranged from 0.76 to 0.90. All raters were blind to treatment assignment.
Neurocognitive Assessments
Participants were administered 4 cognitive assessments: (1) the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), (2) the N-Back test, (3) a probabilistic learning (PL) task, and (4) a delayed discounting task. The RBANS provides for a screening assessment of episodic memory, attention, visual-constructional, and language performance; there are 2 alternate forms.35 The N-Back is a sequential letter working memory task, with 3 different working memory load conditions.36 d′ was used to measure accuracy on the 0-back, 1-back, and 2-back conditions. A PL task, in which participants used performance feedback to choose the most frequently rewarded item in 3 pairs of stimuli (reward probabilities: 80 vs 20, 70 vs 30, and 60 vs 40),37 was used to assess reward learning. “Win-stay” and “lose-shift” frequencies were calculated to assess the use of positive and negative feedbacks. The 27-item Monetary Choice Questionnaire for hypothetical monetary rewards was used to assess delayed discounting.38 Participants choose between a smaller, immediate reward and a larger, delayed reward (LDR). There are 3 LDR sizes: small ($25–35), medium ($50–60), and large ($75–85). The delayed discounting parameter, k, was estimated independently for the small, medium, and large LDRs.39 The neurocognitive assessments were conducted at the end of the lead-in phase and at the end of the double-blind phase.
Safety Assessments
A standard blood chemistry panel, complete blood count, urinalysis, and electrocardiogram were obtained in the lead-in phase and at the end of the double-blind treatment phase. The SAS40 and the Barnes Akathisia Scale (BAS),41 used to assess extrapyramidal symptoms and akathisia, were administered at the beginning and end of the lead-in phase and every 4 weeks during the double-blind treatment phase. The Side Effect Checklist (SEC) was used to assess side effects and monitor vital signs. The SEC is composed of common medication side effects, which are rated on a 1 (none) to 4 (severe) scale. The SEC ratings were conducted at the beginning, then weekly throughout the 12-week double-blind treatment phase by a nonblinded pharmacist.
Study Design
Participants who met inclusion criteria for persistent negative symptoms entered the 4-week lead-in phase, during which they underwent medical screening and baseline symptom, safety, and cognitive assessments. Participants who continued to meet inclusion criteria entered the 12-week double-blind treatment phase and were randomly assigned to rasagiline (1mg tablet) or placebo (matching tablet) using a permuted block randomization system. The rasagiline 1mg dose was chosen because it is selective for the MAO-B enzyme and has been shown to be effective for people with Parkinson’s disease.42 Randomization was stratified by inpatient vs outpatient status and clozapine user vs nonuser. If a participant complained of any side effect, then the blinded psychiatrist was allowed to omit the next dose of study medication and then continue the participant on the target dose. If, despite this intervention, the participant was still unable to tolerate the study medication, then they were withdrawn from the study. Medication compliance was assessed by weekly pill count. All participants who received 75% or more of their assigned study medication were considered compliant.
Statistical Analysis
In the primary analysis, a mixed model for unbalanced repeated measures analysis of covariance (MM-ANCOVA): follow-up score = baseline score + treatment + follow-up week + treatment × follow-up week was used to examine treatment effects on SANS total score. All baseline and postrandomization SANS total scores were included in the analysis; time (ie, follow-up week) was treated as a continuous variable. In this model, the treatment × week interaction term tests for treatment differences in the linear trends (slopes) over the duration of the study and allows for the linear estimation of end of study treatment differences. To further explore the pattern of SANS total score changes over time, we fitted an MM-ANCOVA model, with time as a categorical variable, which allows the magnitude of treatment differences to vary freely rather than assuming a linear change; this model was used to generate post hoc estimates of the size of treatment differences at the 3 postrandomization symptom assessments. Finally, we used chi-square analyses to examine the number of participants who completed the study who exhibited a ≥20% improvement in SASNS total and subscale scores.
The MM-ANCOVA model was also used to examine treatment effects on BPRS total and positive item scores and CDS total scores. The Mantel-Haenszel chi-square test, stratified by individual, was used to assess within-group CGI changes. ANCOVA was used to assess treatment group differences in RBANS total and PL test scores at week 12 (adjusted for baseline scores). A mixed model ANCOVA model was used to evaluate whether treatment differences on the N-Back and Monetary Choice Questionnaire varied across conditions and to perform post hoc estimates of rasagiline − placebo differences for each of the conditions. In the Monetary Choice Questionnaire, discount rates, summarized by the parameter k, were estimated for the 3 LDR reward sizes (small, medium, and large) for each treatment group. The distributions of k within each reward size were highly skewed; therefore, log(k) was used in the analysis of discount rates. MM-ANCOVA was used to examine whether there was a significant change with treatment in the group difference in delayed discounting.
For each SEC item, Fisher exact test was used to compare treatments on the number of participants who, at any point during follow-up, had new or worsened (compared with baseline) side effect severity. The Wilcoxon rank sum test for differences in change scores was used to compare treatments on changes in SAS total score from baseline to end of study. The Mantel-Haenszel test for difference in change score was used to compare treatments on changes in BAS total score from baseline to end of study. ANCOVA was used to examine mean changes in laboratory measures and vital signs.
Results
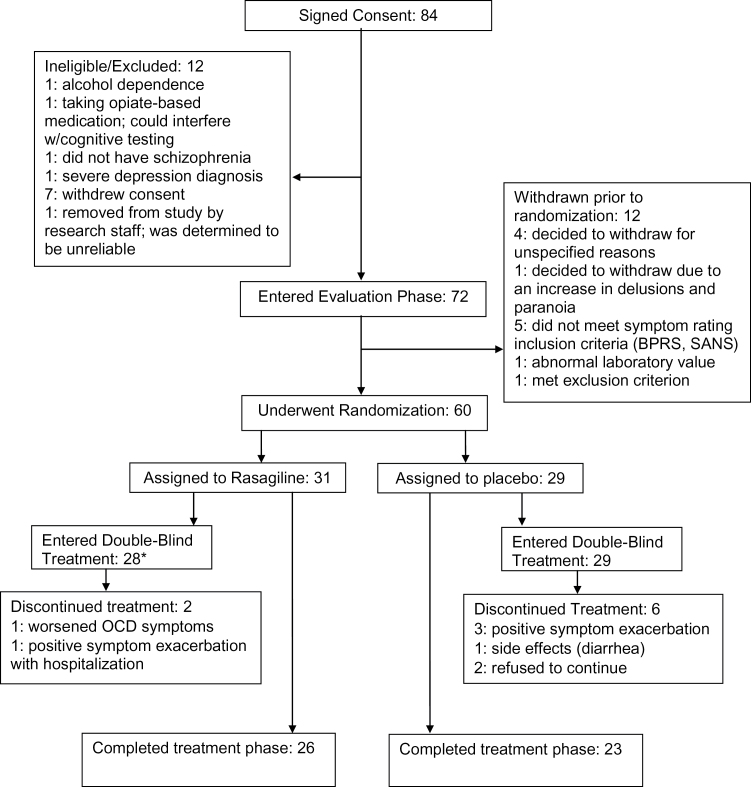
Sixty participants underwent randomization, with 31 participants assigned to rasagiline and 29 assigned to placebo (see figure 1 for CONSORT flow chart). There was 1 inpatient participant in each treatment group. Three rasagiline participants withdrew prior to receiving study medication: 2 were considered medically unstable and 1 discontinued at the recommendation of their treating clinician. Forty-nine participants completed the study. Demographic and baseline clinical characteristics are presented in table 1. The 2 groups were comparable with respect to age, race, gender, and educational level.
Fig. 1.
Participant flow through study. *Three rasagiline participants withdrew prior to receiving study medication and 2 withdrew before first scheduled symptom outcome assessment. Therefore, 26 rasagiline participants are available for symptom efficacy analyses and 28 rasagiline participants are available for safety analyses.
Table 1.
Demographic and Baseline Clinical Characteristics
| Rasagiline | Placebo | |
|---|---|---|
| N | 28 | 29 |
| Age (y; ±SD) | 46.3 (12.2) | 45.9 (11.1) |
| Gender (N; % male) | 24; 85.7 | 22; 75.9 |
| Race (N) | ||
| White | 18; 64.3% | 17; 68.6% |
| Black | 8; 28.7% | 12; 41.4% |
| Other | 2; 7.1% | 0 |
| Education (y; ±SD) | 12.9 (2.5) | 12.1 (2.4) |
| Marital status | ||
| Never married | 23; 82.1% | 27; 93.1% |
| Presently married | 2; 7.1% | 0 |
| Divorced | 3; 10.7% | 2; 6.9% |
| Diagnosis (N; % schizophrenia) | 20 (71.4) | 25 (86.2) |
| Age of onset (y; ±SD) | 19.7 (7.9)a | 20.5 (5.1)b |
| SANS total score (±SD) | 32.5 (8.5) | 33.5 (7.8) |
| BPRS total score (±SD) | 33.7 (6.9) | 33.3 (6.4) |
| BPRS positive symptom item score (±SD) | 8.7 (3.2) | 9.1 (4.0) |
| CDS total score (±SD) | 2.3 (2.1) | 2.0 (2.4) |
| CGI severity of illness (±SD) | 4.0 (0.4) | 4.3 (0.5) |
Note: BPRS, Brief Psychiatric Rating Scale; CDS, Calgary Depression Scale; CGI, Clinical Global Impression; SANS, Scale for the Assessment of Negative Symptoms.
a N = 27.
b N = 28.
Thirty-two percent of the participants assigned to rasagiline and 21% of participants assigned to placebo were treated with antipsychotic polypharmacy; 39% of rasagiline participants and 38% of placebo participants were treated with clozapine; the remainder of the participants received monotherapy with other SGAs or FGAs (2 rasagiline participants). Adjunctive mood stabilizers were prescribed to 14% of rasagiline participants and 31% of participants in the placebo group, while antidepressants were given to 64% of participants in the rasagiline group and 45% of participants in the placebo group.
Symptom Measures
Two rasagiline and 1 placebo participant withdrew from the study prior to their first postrandomization efficacy assessment; thus, symptom efficacy analyses were based on 26 rasagiline and 28 placebo participants (table 2).
Table 2.
Symptom Scores by Week and Treatment Group
| Week | Rasagiline Mean (±SD) | Placebo Mean (±SD) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | SANS Total Score | BPRS Total Score | BPRS Positive Symptom Item Score | CDS Total Score | N | SANS Total Score | BPRS Total Score | BPRS Positive Symptom Item Score | CDS Total Score | |
| 0 | 26 | 32.7 (8.5) | 33.7 (6.9) | 8.8 (3.2) | 2.3 (2.1) | 28 | 33.5 (7.8) | 33.3 (6.4) | 9.1 (4.0) | 2.0 (2.4) |
| 4 | 26 | 31.2 (9.4) | 33.9 (7.7) | 9.2 (3.7) | 2.2 (2.1) | 28 | 32.3 (8.2) | 32.3 (6.7) | 9.4 (3.9) | 2.0 (2.3) |
| 8 | 26 | 31.1 (8.1) | 33.7 (7.3) | 9.2 (3.5) | 2.3 (1.9) | 25 | 34.1 (8.6) | 33.3 (6.5) | 9.8 (4.2) | 1.5 (1.9) |
| 12 | 26 | 29.9 (9.3) | 33.2 (6.9) | 8.3 (3.3) | 2.6 (2.6) | 23 | 34.3 (8.6) | 31.8 (6.9) | 8.9 (3.7) | 1.5 (2.2) |
| Week 12 change from baseline | 26 | −2.8 (5.8) | −0.5 (6.4) | −0.5 (2.4) | 0.3 (2.1) | 23 | 0.6 (4.2) | -0.8 (3.1) | 0.0 (1.8) | 0.0 (1.6) |
Note: Abbreviations are explained in the first footnote to table 1. In the mixed model analysis of covariance, with time treated as a continuous variable, there was a significant treatment × time interaction for SANS total score (F(1,40.3) = 5.61, P = .023), but not for BPRS total score (F(1,44.9) = 1.15, P = .29), BPRS positive symptom item score (F(1,48.2) = 2.73, P = .10), or CDS total score (F(1,44.8) = 0.37, P = .54).
Negative Symptoms.
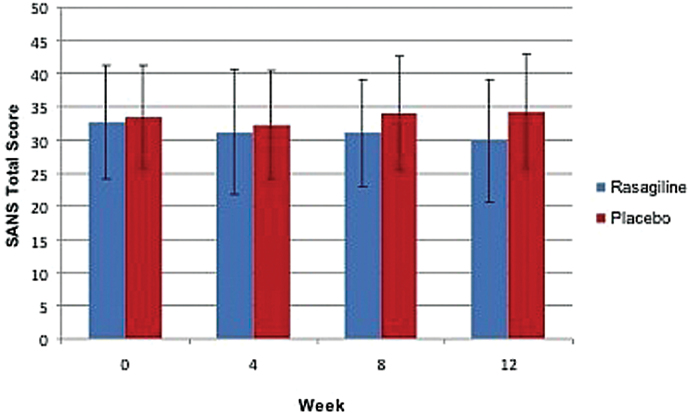
In the MM-ANCOVA, with time treated as a continuous variable (MM-ANCOVA-CON), there was a significant treatment × time interaction for SANS total score (F(1,40.3) = 5.61, P = .023; figure 2). The increase in group differences over time was largely driven by a reduction in the SANS avolition score in the participants randomized to rasagiline (treatment × time interaction: F(1,40.2) = 10.41, P = .002), which continued to be significant even after allowing for multiple trend tests for the 4 SANS subscales. The treatment × time effects for the other SANS subscale scores were not significant.
Fig. 2.
Scale for the Assessment of Negative Symptoms total score, by treatment and week. The mixed model analysis of covariance treatment × week interaction, with time treated as a continuous variable, was statistically significant (F = 5.61, df = 1,40.3, P = .023), supporting the existence of an increasingly large treatment difference favoring rasagiline. In the rasagiline group, n = 26 participants for all weeks. In the placebo group, n = 28 at weeks 0 and 4, n = 25 at week 8, and n = 23 at week 12.
A similar pattern of results was observed in the MM-ANCOVA, with time treated as a categorical variable (MM-ANCOVA-CAT). The treatment × time interaction approached significance for SANS total score (F(2,39.9) = 2.85, P = .07). In the post hoc analyses of the treatment size differences at the 3 postrandomization symptom assessments, the estimated magnitude of the group treatment differences increased over time, with a significant group difference for SANS total score at week 12 (3.42±1.59; t(37.3) = 2.15, P = .04; effect size: d = 0.41). The group difference at week 12 was primarily due to the reduction in SANS avolition score in the participants randomized to rasagiline (0.46±0.15; t(49.0) = 3.06, P = .004; effect size: d = 0.50; supplementary figure 1A). Rasagiline treatment was also associated with a reduction in the SANS Alogia score, with a significant group difference at week 4, but not at week 8 or 12. There was no evidence of a rasagiline effect on the SANS Anhedonia or Blunted Affect scores (supplementary figures 1B–D).
There was no significant group difference in the number of participants who completed the study with a 20% or greater reduction in SANS total score (rasagiline: 5/26; placebo: 2/23; χ2(1) = 1.11; P = .29). However, there was a significant difference in the number of participants with a 20% or greater reduction in SANS avolition score (rasagiline: 12/26; placebo: 1/23; χ2(1) = 10.94; P = .0009). There were no significant group responder differences on the other subscale scores.
Other Symptom Measures.
In the MM-ANCOVA-CON, there were no significant treatment × time interactions for BPRS total score (F(1,44.9) = 1.15, P = .29), BPRS positive symptom item score (F(1,48.2) = 2.73, P = .10), or CDS total score (F(1,44.8) = 0.37, P = .54). A similar pattern of results was observed with the MM-ANCOVA-CAT; there were no significant group differences on any of these 3 measures at weeks 4, 8, or 12 (data not shown). There was no significant change in the CGI severity of illness item within either group: rasagiline: χ2(1) = 0.03, P = .87; placebo: χ2(1) = 0.46, P = .50.
Neuropsychological Measures
Repeatable Battery for the Assessment of Neuropsychological Status.
Both treatment groups exhibited small improvements on the RBANS total score (table 3); the group difference was not significant (F(1,41.8) = 0.63, P = .43). There were no significant group differences in the RBANS individual domain scores (table 3).
Table 3.
RBANS Total and Domain Scores
| Week | Rasagiline | Placebo | Mixed Model ANCOVA Estimates of Treatment Differences | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean (±SD) | N | Mean (±SD) | |||||||
| RBANS total scorea | 0 | 26 | 76.8 (12.5) | 23 | 74.2 (13.7) | |||||
| 12 | 26 | 78.7 (14.1) | 23 | 75.3 (14.5) | ||||||
| Change | 26 | 1.9 (6.9) | 23 | 1.1 (7.5) | ||||||
| RBANS Domainsb | Week | N | Mean (±SD) | N | Mean (±SD) | Diff. | SE | t | df | P value |
| Attention | 0 | 26 | 82.4 (15.2) | 23 | 76.8 (18.5) | |||||
| 12 | 26 | 84.0 (15.9) | 23 | 78.3 (17.2) | ||||||
| Change | 26 | 1.6 (10.6) | 23 | 1.6 (10.8) | 1.75 | 2.93 | −0.60 | 46.8 | 0.55 | |
| Delay memory | 0 | 26 | 77.6 (17.0) | 23 | 79.9 (18.7) | |||||
| 12 | 26 | 81.0 (17.5) | 23 | 79.2 (21.2) | ||||||
| Change | 26 | 3.4 (16.5) | 23 | −0.7 (13.0) | 3.40 | 4.07 | −0.83 | 47.0 | 0.41 | |
| Immediate memory | 0 | 26 | 79.8 (19.4) | 23 | 77.9 (19.4) | |||||
| 12 | 26 | 81.3 (20.6) | 23 | 78.5 (18.3) | ||||||
| Change | 26 | 1.4 (13.4) | 23 | 0.6 (11.6) | 1.40 | 3.48 | −0.40 | 46.1 | 0.69 | |
| Language | 0 | 26 | 88.2 (11.1) | 23 | 83.4 (8.4) | |||||
| 12 | 26 | 84.8 (14.3) | 23 | 85.7 (13.4) | ||||||
| Change | 26 | −3.4 (12.6) | 23 | 2.2 (15.4) | −4.18 | 3.79 | 1.10 | 47.1 | 0.28 | |
| Visuospatial | 0 | 26 | 81.6 (15.9) | 23 | 81.2 (19.0) | |||||
| 2 | 26 | 86.2 (16.0) | 23 | 80.5 (20.7) | ||||||
| Change | 26 | 4.6 (13.1) | 23 | −0.7 (13.8) | 5.44 | 3.67 | −1.48 | 46.8 | 0.14 | |
Note: RBANS, Repeatable Battery for the Assessment of Neuropsychological Status.
aRBANS total score was analyzed in a separate ANCOVA model (F = 0.63; df = 1,41.8; P = .43).
bRBANS domain scores were analyzed with the mixed model ANCOVA model: week 12 change = baseline score + domain + treatment + treatment × domain.
N-Back Test.
The 2 groups had similar baseline d′ values for 0-back, 1-back, and 2-back (supplementary table 1). The treatment main effect (F(1,140) = 0.83, P = .36), averaged over all 3 conditions, and the treatment by condition interaction (F(2,140) = 0.92, P = .40) were not significant. Post hoc estimates of rasagiline − placebo group differences within each condition were also not significant.
PL Test.
There were no group differences in acquisition scores for any of the 3 pairs of stimuli (80 vs 20: t 46 = 0.26, P = .79; 70 vs 30: t 46 = 0.37, P = .71; 60 vs 40: t 46 = 0.12, P = .90) (supplementary table 2). Rasagiline did not alter the use of positive reinforcement, as reflected in the lack of significant change in win-stay frequencies (t 46 = 0.33, P = .74) or negative reinforcement, as reflected in the lack of significant change in lose-shift frequencies (t 46 = 0.14, P = .89).
Delayed Discounting Test.
There was no significant relative difference in discounting between the 2 groups (F(1,140) = 1.43, P = .23; data not shown). Post hoc estimates of rasagiline − placebo group differences within each reward size were also not significant.
Safety Measures
There was 1 serious adverse event: a rasagiline participant was hospitalized due to a panic attack, accompanied by command hallucinations to commit suicide. This event was considered possibly related to study drug although the participant had a prior history of multiple hospitalizations for panic attacks. The suicidal behavior resolved with hospitalization and discontinuation of the study medication.
The 2 groups did not differ significantly on the SAS total score (rasagiline—week 0: 1.8 ± 2.3 and week 12: 0.96 ± 1.3, change: −0.8 ± 1.9; placebo—week 0: 1.9 ± 2.1 and week 12: 1.9 ± 2.7, change: 0.0 ± 2.2; χ2(1) = 1.51, P = .22). There was no significant group difference in the BAS global akathisia categorical ratings (χ2(1) = 1.53, P = .22).
On the SEC, the only significant group difference was a higher incidence of new onset or worsening of tremor in the placebo group (rasagiline: 3/28 [10.7%] vs placebo: 11/28 [39.3%]; P = .02).
There were no significant treatment differences in changes in body mass index (t(53.7) = −0.81, P = .42) or systolic (2.3±2.4 mmHg; t 54 = 0.96, P = .44) or diastolic (2.7±1.5 mmHg; t(54.4) = 1.806, P = .08) blood pressure (data available upon request from the authors).
The only laboratory measure for which there was a significant group difference was serum glutamic oxaloacetic transaminase (SGOT) (P = .05; data not shown). There was also a trend toward a group difference in serum glutamic pyruvic transaminase (SGPT) (P = .07; data not shown). There were no significant group differences for any of the other laboratory measures.
Discussion
The study results suggest that rasagiline may be of clinical benefit for persistent negative symptoms. The effect of rasagiline on SANS total and avolition subscale scores increased over time, with estimated between-group differences for these measures reaching statistical significance at week 12. There was a significant group difference in the number of participants with a 20% or greater reduction in the SANS avolition subscale score. The beneficial effect of rasagiline on avolition is consistent with its hypothesized mechanism of action.
The magnitude of the effect on SANS total score was small to medium, whereas a medium effect was observed for the SANS avolition subscale. The group differences on both measures resulted from small to medium decreases in the rasagiline group and small increases in the placebo group. The worsening of symptoms in the placebo group is difficult to understand, because placebo should be pharmacologically inert, but suggests the possibility that symptom worsening may be related to some element(s) of study participation. If both treatment groups would be exposed to these elements, then any improvement in the active treatment arm would represent a decrease in symptoms that takes into account the small worsening that occurred in the placebo-treated participants.
Although the effects of rasagiline on dopamine levels occurs relatively quickly, the full effect of rasagiline for negative symptoms was not observed until 12 weeks, which suggests that the observed benefits do not merely reflect acute changes in dopamine levels. The delay in full negative symptom benefit suggests that a more prolonged time period may be required to reverse the neurobiological impairment(s) (eg, altered dopamine receptor density or sensitivity) that underlie diminished volition.
There were no significant group differences in global psychopathology, positive symptoms, or depressive symptoms. In light of the study requirement that eligible participants have low levels of positive and depressive symptoms, the lack of beneficial effect for these symptom domains is not surprising. There were also no significant group differences in extrapyramidal symptoms. The observed benefit for negative symptoms, in the absence of concurrent changes in positive, depressive, or extrapyramidal symptoms, suggests that the beneficial effect of rasagiline for negative symptoms is not secondary to its effects on other symptom complexes.
In the most comparable previous study of MAO-B inhibitors, Bodkin et al24 reported a significant benefit of selegiline for negative symptoms. They also found the greatest therapeutic effect to be observed with the SANS avolition subscale score.24 The results of these studies suggest that MAO-B inhibitors could be used to effectively augment the therapeutic effects of psychosocial treatments, which focus on negative symptoms and functional outcomes.43 The combined use of pharmacological and psychosocial treatments may provide a more pronounced impact on this disabling component of the illness.
The other major efficacy finding was the lack of beneficial effect of rasagiline for cognition. The lack of effect was observed with general neuropsychological performance and neurocognitive measures, which were specifically selected for their modulation by the cortical or subcortical dopaminergic system. We hypothesized that rasagiline would improve performance on the neurocognitive measures through an increase in dopaminergic activity at cortical and/or striatal D1 receptors.37 However, the D1 antagonist properties of the antipsychotic medications may have blocked this potential mechanism although the observed benefit of rasagiline for negative symptoms, which would have also been mediated through increased D1 receptor activity, argues against this explanation. Alternatively, the lack of efficacy suggests that MAO-B enzyme inhibition effects on dopaminergic activity are not sufficient to enhance cognition and modulation of other neurotransmitter systems may be required to enhance performance on these measures. However, the possibility remains that alternative approaches to dopaminergic augmentation, eg, inhibition of catecholamine-O-methyltransferase,44 may enhance cognition.
Rasagiline was relatively well tolerated. There were differential effects of rasagiline and placebo on SGOT and SGPT liver enzymes; however, post-treatment changes observed with rasagiline were still well within the normal values for these measures. Rasagiline was not associated with greater side effects than placebo. In fact, the only side effect for which there was a significant group difference was tremor, which was more likely to develop or worsen in the placebo group. There were no significant group differences in vital signs.
In summary, the results of the current study support further study of the utility of MAO-B inhibitors for the treatment of negative symptoms, especially avolition. The use of other nonpharmacological interventions may be able to further enhance the therapeutic benefit of such agents.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
Stanley Medical Research Institute; P30 068580 to R.W.B.); National Institute of Drug Abuse (NIDA) (N01DA59909 to D.L.K.); NIDA Intramural Research Program.
Supplementary Material
Acknowledgments
R.W.B.: DSMB member: Pfizer; Consultant: Abbott, Amgen, Bristol Myers Squibb, EnVivo, Janssen Pharmaceuticals Inc, Omeros, NuPathe Inc, Pfizer, Roche, and Takeda; DSMB member for Otsuka and Pfizer; D.L.K.: Consultant: Bristol Myers Squibb, Lundbeck; Grant Support: Bristol Myers Squibb; J.M.G.: Consultant: Amgen, Pfizer, and Roche and receives royalty payments from the BACS; J.A.W.: Consultant: Hoffman-LaRoche. E.W., W.R.K., R.P.M., and D.A.G. have no conflicts to report.
References
- 1. Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. [DOI] [PubMed] [Google Scholar]
- 2. Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res. 2004;72:41–51. [DOI] [PubMed] [Google Scholar]
- 3. Greenwood KE, Landau S, Wykes T. Negative symptoms and specific cognitive impairments as combined targets for improved functional outcome within cognitive remediation therapy. Schizophr Bull. 2005;31:910–921. [DOI] [PubMed] [Google Scholar]
- 4. Kurtz MM, Moberg PJ, Ragland JD, Gur RC, Gur RE. Symptoms versus neurocognitive test performance as predictors of psychosocial status in schizophrenia: a 1- and 4-year prospective study. Schizophr Bull. 2005;31:167–174. [DOI] [PubMed] [Google Scholar]
- 5. Milev P, Ho BC, Arndt S, Andreasen NC. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162:495–506. [DOI] [PubMed] [Google Scholar]
- 6. Buchanan RW. Persistent negative symptoms in schizophrenia: an overview. Schizophr Bull. 2007;33:1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Keefe RS, Bilder RM, Davis SM, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64:633–647. [DOI] [PubMed] [Google Scholar]
- 8. Marder SR, Fenton W. Measurement and treatment research to improve cognition in schizophrenia: NIMH MATRICS initiative to support the development of agents for improving cognition in schizophrenia. Schizophr Res. 2004;72:5–9. [DOI] [PubMed] [Google Scholar]
- 9. Simon JJ, Biller A, Walther S, et al. Neural correlates of reward processing in schizophrenia—relationship to apathy and depression. Schizophr Res. 2010;118:154–161. [DOI] [PubMed] [Google Scholar]
- 10. Waltz JA, Schweitzer JB, Ross TJ, et al. Abnormal responses to monetary outcomes in cortex, but not in the basal ganglia, in schizophrenia. Neuropsychopharmacology. 2010;35:2427–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Waltz JA, Kasanova Z, Ross TJ, et al. The roles of reward, default, and executive control networks in set-shifting impairments in schizophrenia. PLoS One. 2013;8:e57257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. [DOI] [PubMed] [Google Scholar]
- 13. Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. [DOI] [PubMed] [Google Scholar]
- 14. Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6:348–357. [DOI] [PubMed] [Google Scholar]
- 15. Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Williams GV. Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology (Berl). 2004;174:3–16. [DOI] [PubMed] [Google Scholar]
- 16. Silver H, Aharon N, Hausfater N, Jahjah N. The effect of augmentation with moclobemide on symptoms of schizophrenia. Int Clin Psychopharmacol. 1999;14:193–195. [PubMed] [Google Scholar]
- 17. Riederer P, Konradi C, Schay V, et al. Localization of MAO-A and MAO-B in human brain: a step in understanding the therapeutic action of L-deprenyl. Adv Neurol. 1987;45:111–118. [PubMed] [Google Scholar]
- 18. Wayment HK, Schenk JO, Sorg BA. Characterization of extracellular dopamine clearance in the medial prefrontal cortex: role of monoamine uptake and monoamine oxidase inhibition. J Neurosci. 2001;21:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Youdim MB, Riederer PF. A review of the mechanisms and role of monoamine oxidase inhibitors in Parkinson’s disease. Neurology. 2004;63:S32–S35. [DOI] [PubMed] [Google Scholar]
- 20. Perenyi A, Goswami U, Frecska E, Arató M, Bela A. L-deprenyl in treating negative symptoms of schizophrenia. Psychiatry Res. 1992;42:189–191. [DOI] [PubMed] [Google Scholar]
- 21. Bodkin JA, Cohen BM, Salomon MS, Cannon SE, Zornberg GL, Cole JO. Treatment of negative symptoms in schizophrenia and schizoaffective disorder by selegiline augmentation of antipsychotic medication. A pilot study examining the role of dopamine. J Nerv Ment Dis. 1996;184:295–301. [DOI] [PubMed] [Google Scholar]
- 22. Gupta S, Droney T, Kyser A, Keller P. Selegiline augmentation of antipsychotics for the treatment of negative symptoms in schizophrenia. Compr Psychiatry. 1999;40:148–150. [DOI] [PubMed] [Google Scholar]
- 23. Jungerman T, Rabinowitz D, Klein E. Deprenyl augmentation for treating negative symptoms of schizophrenia: a double-blind, controlled study. J Clin Psychopharmacol. 1999;19:522–525. [DOI] [PubMed] [Google Scholar]
- 24. Bodkin JA, Siris SG, Bermanzohn PC, Hennen J, Cole JO. Double-blind, placebo-controlled, multicenter trial of selegiline augmentation of antipsychotic medication to treat negative symptoms in outpatients with schizophrenia. Am J Psychiatry. 2005;162:388–390. [DOI] [PubMed] [Google Scholar]
- 25. Amiri A, Noorbala AA, Nejatisafa AA, et al. Efficacy of selegiline add on therapy to risperidone in the treatment of the negative symptoms of schizophrenia: a double-blind randomized placebo-controlled study. Hum Psychopharmacol. 2008;23:79–86. [DOI] [PubMed] [Google Scholar]
- 26. Youdim MB, Gross A, Finberg JP. Rasagiline [N-propargyl-1R(+)-aminoindan], a selective and potent inhibitor of mitochondrial monoamine oxidase B. Br J Pharmacol. 2001;132:500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Youdim MB, Weinstock M. Molecular basis of neuroprotective activities of rasagiline and the anti-Alzheimer drug TV3326 [(N-propargyl-(3R)aminoindan-5-YL)-ethyl methyl carbamate]. Cell Mol Neurobiol. 2001;21:555–573. [DOI] [PubMed] [Google Scholar]
- 28. Abu-Raya S, Tabakman R, Blaugrund E, Trembovler V, Lazarovici P. Neuroprotective and neurotoxic effects of monoamine oxidase-B inhibitors and derived metabolites under ischemia in PC12 cells. Eur J Pharmacol. 2002;434:109–116. [DOI] [PubMed] [Google Scholar]
- 29. Bar Am O, Amit T, Youdim MB. Contrasting neuroprotective and neurotoxic actions of respective metabolites of anti-Parkinson drugs rasagiline and selegiline. Neurosci Lett. 2004;355:169–172. [DOI] [PubMed] [Google Scholar]
- 30. First MB, Spitzer RL, Gibbon M, Williams J. Structural Clinical Interview for DSM-IV Axis Disorders (SCID-IV). New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1997. [Google Scholar]
- 31. Buchanan RW. Persistent negative symptoms in schizophrenia: an overview. Schizophr Bull. 2007;33:1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buchanan RW, Javitt DC, Marder SR, et al. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am J Psychiatry. 2007;164:1593–1602. [DOI] [PubMed] [Google Scholar]
- 33. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 34. Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3:247–251. [DOI] [PubMed] [Google Scholar]
- 35. Gold JM, Queern C, Iannone VN, Buchanan RW. Repeatable battery for the assessment of neuropsychological status as a screening test in schizophrenia I: sensitivity, reliability, and validity. Am J Psychiatry. 1999;156:1944–1950. [DOI] [PubMed] [Google Scholar]
- 36. Cohen JD, Perlstein WM, Braver TS, et al. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–608. [DOI] [PubMed] [Google Scholar]
- 37. Waltz JA, Frank MJ, Robinson BM, Gold JM. Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biol Psychiatry. 2007;62:756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128:78–87. [DOI] [PubMed] [Google Scholar]
- 39. Kirby KN. Instructions for Inferring Discount Rates From Choices Between Immediate and Delayed Rewards. Williamstown, MA: Williams College; 2000:7. [Google Scholar]
- 40. Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. [DOI] [PubMed] [Google Scholar]
- 41. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–676. [DOI] [PubMed] [Google Scholar]
- 42. Parkinson Study Group. A controlled trial of rasagiline in early Parkinson disease: the TEMPO Study. Arch Neurol. 2002;59:1937–1943. [DOI] [PubMed] [Google Scholar]
- 43. Grant PM, Huh GA, Perivoliotis D, Stolar NM, Beck AT. Randomized trial to evaluate the efficacy of cognitive therapy for low-functioning patients with schizophrenia. Arch Gen Psychiatry. 2012;69:121–127. [DOI] [PubMed] [Google Scholar]
- 44. Apud JA, Mattay V, Chen J, et al. Tolcapone improves cognition and cortical information processing in normal human subjects. Neuropsychopharmacology. 2007;32:1011–1020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.