Abstract
Objectives:
Existing treatments for schizophrenia can improve positive symptoms, but it is unclear if they have any impact on negative symptoms. This meta-analysis was conducted to assess the efficacy of available treatments for negative symptoms in schizophrenia.
Methods:
All randomized-controlled trials of interventions for negative symptoms in schizophrenia until December 2013 were retrieved; 168 unique and independent placebo-controlled trials were used. Negative symptom scores at baseline and follow-up, duration of illness, doses of medication, type of interventions, and sample demographics were extracted. Heterogeneity was addressed with the I 2 and Q statistic. Standardized mean difference in values of the Negative Symptom Rating Scale used in each study was calculated as the main outcome measure.
Results:
6503 patients in the treatment arm and 5815 patients in the placebo arm were included. No evidence of publication biases found. Most treatments reduced negative symptoms at follow-up relative to placebo: second-generation antipsychotics: −0.579 (−0.755 to −0.404); antidepressants: −0.349 (−0.551 to −0.146); combinations of pharmacological agents: −0.518 (−0.757 to −0.279); glutamatergic medications: −0.289 (−0.478 to −0.1); psychological interventions: −0.396 (−0.563 to −0.229). No significant effect was found for first-generation antipsychotics: −0.531 (−1.104 to 0.041) and brain stimulation: −0.228 (−0.775 to 0.319). Effects of most treatments were not clinically meaningful as measured on Clinical Global Impression Severity Scale.
Conclusions and Relevance:
Although some statistically significant effects on negative symptoms were evident, none reached the threshold for clinically significant improvement.
Key words: psychosis, schizophrenia, negative symptoms, antipsychotics, treatment
Introduction
Schizophrenia is a heterogeneous clinical syndrome comprising a number of psychopathology domains and patients vary in which pathologies are manifest.1,2 Patients with schizophrenia experience “positive” psychotic symptoms as required by diagnostic criteria and include delusions, hallucinations, and disorganization of thought and behavior. The “negative” symptoms are conceptualized as a deficit or loss of some functions3 and reflect pathology that Kraepelin4 described as a weakening of the well-spring of volition resulting in emotional dullness and loss of drive for vocation. Negative symptoms group in 2 factors, one involving diminished expression of affect and alogia and the second involving avolition including anhedonia and asociality.5 Negative symptoms together with impaired cognition are the major cause of the marked functional disability that is often associated with schizophrenia.6 They underlie impaired vocational7,8 and social functioning9 and place an enormous burden on patients’ carers.10,11 Negative symptoms are thus a key contributor to the enormous costs of schizophrenia to health services and society12 and are identified as an unmet therapeutic need.13
In clinical care, treatment of negative symptoms depends on ascertainment of cause. What appears to be asociality may be a paranoid withdrawal based on fear, or restricted expression of emotion by drug-induced akinesia, or low drive and apathy may be caused by sedating medication.14 Negative symptoms that remain when secondary causes have been addressed tend to be persistent. Currently available treatments for schizophrenia can improve positive symptoms,15 but the extent to which they can impact on negative symptoms is unclear.16 As a result, many patients have persistent negative symptoms, despite optimal clinical care.17 Initial evidence that “second generation” antipsychotics had therapeutic effects on negative as well as positive symptoms18 have not been consistently replicated.19 Thus, although there are approximately 40 different antipsychotic drugs that are licensed to treat symptoms of schizophrenia, no molecule is officially indicated for the treatment of negative symptoms. Some treatment guidelines suggest that other treatments, such as music therapy, may be useful when antipsychotic treatment fails.20 However, the evidence base for these recommendations is generally weak.
The aim of the present study was to clarify whether any available treatments are effective for negative symptoms. We carried out a comprehensive meta-analysis of a range of different treatments, including all randomized placebo-controlled trials of interventions for negative symptoms in schizophrenia published up to December 2013. These studies included pharmacological trials of first- and second-generation antipsychotics (FGA, SGA), glutamatergic medications (Glut), antidepressants (AD), and combinations of these medications (Comb)21–25; brain stimulation (BS) techniques such as repetitive transcranial magnetic stimulation and transcranial direct current stimulation26,27; and psychological interventions (Psych) such as cognitive behavioral therapy, cognitive rehabilitation, and music therapy.28–32 We assessed the efficacy of these treatments relative to placebo, controlling for potentially confounding clinical and sociodemographic modulators and publication bias.
Methods
The full details of the research protocol are appended in supplementary protocol and methods.
Selection Procedures
Search Strategies.
Search strategies are detailed in supplementary protocol and methods.
Selection Criteria.
Articles were included if they: (a) were original articles, written in English, (b) included patients with a DSM or ICD diagnosis of schizophrenia or schizoaffective disorder, (c) included participants aged 18 or above, (d) reported raw data to allow meta-analytical computations, and (e) were randomized placebo-controlled trials of either a double-blind, open-label, or crossover design. We excluded: (a) abstracts, pilot datasets, reviews, articles in language other than English; (b) articles of populations with other psychiatric diagnoses; (c) articles of children and young adolescents; (d) articles failing to report raw data; (e) articles with lack of randomization and control group or observational studies; (f) articles with overlapping datasets. Specifically, in case of multiple publications deriving from the same study population, we selected the articles reporting the most recent or largest dataset. Some of the included articles did not have a placebo group (ie, head-to-head comparison of 2 treatments only) or had several trials, each using different doses. Consequently, the primary efficacy analysis was restricted to unique placebo-controlled trials employing the highest doses. Trials reporting the results of several treatments using the same placebo group were included in the analysis with appropriate corrections of the sample size.
Recorded Variables.
The following variables were recorded from each article: author, year of publication, type of intervention (FGA, SGA, Glut, AD, Comb, BS, Psych); all the above categories of interventions, at the exception of FGA and SGA, were added on interventions on a stable antipsychotic regime (see supplementary protocol and methods for a detailed account), details of intervention (including dosage of medication), design (double-blind, crossover, open-label), quality criterion (A or B, see quality assessment below), type of placebo condition, duration of trial in weeks, epidemiological data of treatment, and placebo samples (baseline and endpoint sample sizes, mean age, male percentage, duration of illness, drop-outs), the Negative Symptom Subscale of the Positive and Negative Syndrome Scale for Schizophrenia (PANSS-NS),33 the Withdrawal-Retardation Subscale of the Brief Psychiatric Rating Scale (BPRS-WR),34 and the Scale for the Assessment of Negative Symptoms (SANS).35 The last 3 variables composed the primary outcome measure and for them the following additional data were extracted: mean value at baseline (and SD of the mean), mean value at endpoint (and SD of the mean), change in means between endpoint and baseline (see below), direction of change (baseline > endpoint or endpoint > baseline), SD and P value of change.36 As secondary outcome measure reflecting the clinicians’ impression on treatment response, we also extracted values for the Clinical Global Impression Scales37: Clinical Global Impression Severity Scale (CGI-S) scores, severity scale (change in means between endpoint and baseline, SD of change) and for Clinical Global Impression Improvement Scale (CGI-I) scores, improvement scale (mean value at endpoint, SD of the mean). To achieve a high standard of reporting, we have adopted the QUORUM guidelines.38 Quality assessment is detailed in supplementary protocol and methods.
Statistical Analysis
The mean difference in the primary outcome measures (PANSS-NS, SANS, BPRS-WR) between treatment and placebo groups was standardized by calculating the difference between the 2 mean changes (difference of post- and pretreatment score) divided by the pooled SD of the difference scores, or, if this was not available, by the pooled baseline SD.39 There were no significant differences in the SD based on change score or baseline for treatment group for the main analyses trials (P = .12 or control group P = .54). A negative change of the standardized mean difference (SMD) indicates a larger reduction in our primary outcome measures at endpoint compared with baseline in the treatment group and thus an improvement in negative symptoms compared with placebo group.
The SE of each study’s standardized effect size was calculated from the estimated effect and the group sizes of the 2 groups.40 Meta-analyses were completed by pooling the standardized effect sizes between trials using a random effects models, which incorporate the between study heterogeneity due to the variety of case mix and settings between trials.39 Our sensitivity analyses also revealed that there were no studies with an unusually large effect on the pooled effect size, which suggests that our effect sizes did not vary considerably. For completeness and clarity, in a supplementary analysis we additionally calculated the percentage of change in the primary outcome measures between treatment and placebo group from pre to follow-up.
Several articles reported trials with 2 or more experimental groups and only 1 placebo group. To avoid counting the placebo patients twice, we followed the recommendation of the Cochrane Collaboration and divided the placebo group equally into 2 (or more) groups with smaller sample size, so that the total numbers of participants add up to the original size of the group. We thereby avoided an inflation of sample size which would lead to an increase of type I errors and thus overoptimistically small SEs.
Sensitivity analyses were conducted to weigh up the relative influence of each individual trial on the pooled effect size using STATA’s user-written function, metainf.41
Homogeneity between the trials was assessed using Cochran’s Q test and by calculating the measure of heterogeneity or inconsistency (I 2).42 I 2 describes the percentage of total variation across trials that is due to heterogeneity rather than sampling error and ranges between 0% (no inconsistency) and 100% (high heterogeneity) with values of 25%, 50%, and 75% suggested as low, moderate, and high heterogeneity.42 Forest plots are used to graphically show the meta-analysis results. The secondary outcome measures (CGI-Sdiff and CGI-I) were then regressed on the primary outcome measures to establish clinically meaningful cutoffs for data interpretation. Such an approach correlating the BPRS, PANSS, SANS scores, and CGI scores has been previously adopted in several studies43–45 and used to better understand the clinical impact of the primary outcome measures. Publication biases were assessed and detailed in supplementary protocol and methods.
Results
Database
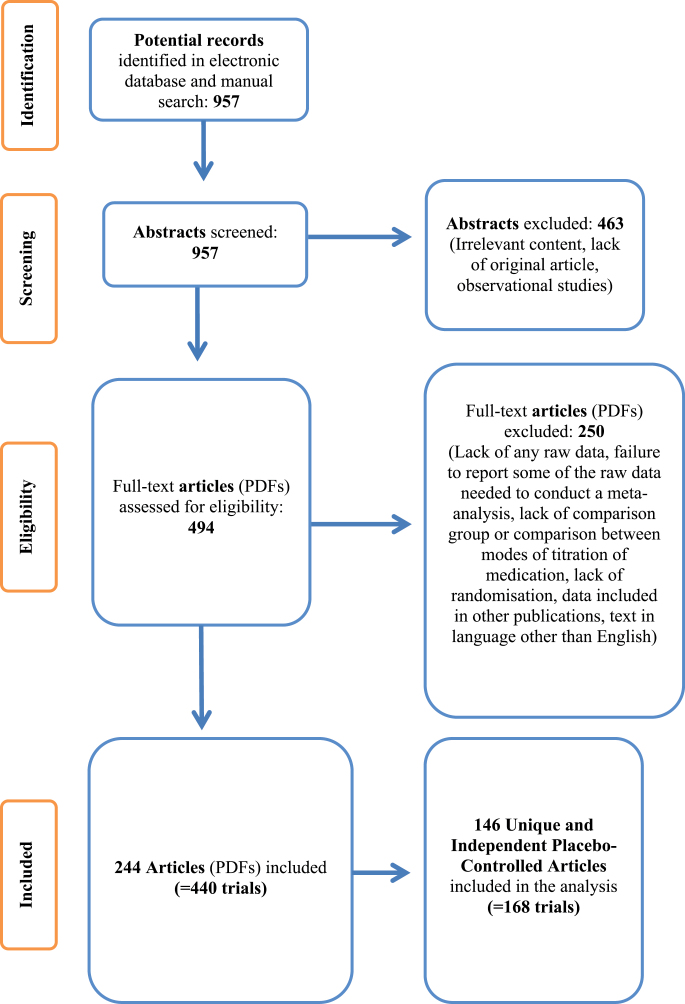
Our literature search uncovered 244 articles (figure 1), which included both placebo-controlled trials and head-to-head comparisons between different molecules or between different doses of the same molecule (for details see supplementary list of articles: tables I and II). On average 1.8 different treatment groups were reported (range 1–6) resulting to a total number of 440 trials; 223 of them lacked a placebo group. Out of the remaining 217 placebo-controlled trials, 27 studies used different doses of the same drugs and only the highest dose was used for the analyses, leaving 168 “unique” trials from 146 published articles for the primary analysis. This dataset was leading to a total population of 6503 patients in the treatment arm and 5815 patients in the placebo arm, for the meta-analysis. Mean age of the participants in 158 of 168 trials was 38.0 (SD = 7.86) years. The mean duration of illness in months was 158.5 (SD = 83.92, N = 95). The mean duration of interventions was 12.4 (SD = 13.86) weeks. The percentage of male patients was 68.9 (SD = 14.59, N = 153). Attrition rates (expressed as % drop-outs) were 10.9 (SD = 17.56, range: 0%–77.8%). Details are appended online (see supplementary results: table I).
Fig. 1.
Prisma diagram54.
Efficacy of Treatments for Negative Symptoms
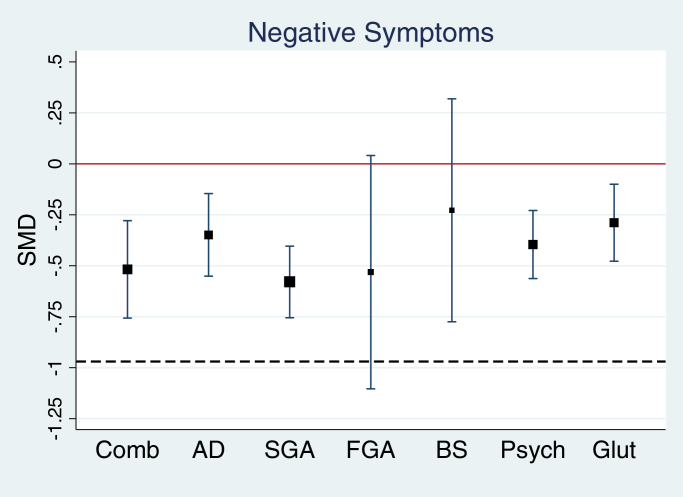
There was a significant heterogeneity across individual trials (I 2 = 77.7%, Q = 748 (167), P < .0001, P < .001, see figure 2). The effect sizes of the 7 different treatment groups varied between −0.23 (BS) and −0.58 (SGA, see table 1). With the exception of BS (P = .41) and FGA (P = .07) all treatment effects were significant. A metaregression with treatment group as a categorical independent variable indicated however no between-group differences F(6, 161) = 0.80, P = .57, all pairwise comparisons not significant (P values ≥ .09). Forest plots for all trials together and for each treatment group separately are appended online (see supplementary results: figures I–X). These effects, even if statistically significant, did not cross the threshold for minimal clinical improvement as detected by clinicians with the CGI (see below). The supplementary analysis (supplementary results: table IV) showed that the mean % change of treatment groups was 16.1% (SD = 12.6%, N = 158), while the control group changed on average by 7.9% (SD = 11.1%, N = 158). Percentage improvement in the treatment group compared with control group between the treatment groups varied between 4.8% (FGA) and 12.7% (psychological treatments; see supplementary results: table IV, figure XXXII).
Fig. 2.
Forest plot showing the pooled standardized mean difference (SMD) (and 95% CI) for the included placebo-controlled trials. The size of the box represents the weight given to the trials of the respective treatment group. A negative effect size corresponds to improvement in the treatment group relative to the placebo group over time. The red line indicates no statistical significant difference from placebo over time. The dashed line indicates minimally detectable clinical improvements over time. The latter line at y = −0.97 is the predicted SMDs of negative symptoms when mean net CGI-S reduction in treatment is 1 (metaregression of negative symptoms on CGI-S: constant = −0.23, β = .735 [95% CI: 0.423 to 1.047], t = 4.70, P < .0001, adjusted r 2 = 42.5%). CGI-S, Clinical Global Impression Severity Scale. For color, see the figure online.
Table 1.
Efficacy of Placebo-Controlled Treatments for Negative Symptoms
| Treatment | N | SMD (95% CI) | z | P | I 2 | Q (df) | P |
|---|---|---|---|---|---|---|---|
| Comb | 33 | −0.518 (−0.757 to −0.279) | 4.24 | <.001 | 80.9% | 167.2 (32) | <.001 |
| AD | 26 | −0.349 (−0.551 to −0.146) | 3.37 | .001 | 56.3% | 57.25 (25) | <.001 |
| FGA | 10 | −0.531 (−1.104 to 0.041) | 1.82 | .069 | 89.8% | 87.97 (9) | <.001 |
| SGA | 38 | −0.579 (−0.755 to −0.404) | 6.47 | <.001 | 84.7% | 241.94 (37) | <.001 |
| Psych | 27 | −0.396 (−0.563 to −0.229) | 4.64 | <.001 | 57.6% | 61.32 (26) | <.001 |
| Glut | 26 | −0.289 (−0.478 to −0.1) | 2.99 | .003 | 66.4% | 74.36 (25) | <.001 |
| BS | 8 | −0.228 (−0.775 to 0.319) | 0.82 | .413 | 73.5% | 26.4 (7) | <.001 |
Note: The table presents for each treatment group and sample size (N), standardized mean differences (SMD) and 95% CI, z test with associated P value, inconsistency I 2, result of Cochran’s Q test of between-group heterogeneity (Q and degrees of freedom [df] and P value). AD, antidepressant; BS, brain stimulation; Comb, combination of pharmacological agents; FGA, first-generation antipsychotics; Glut, glutamatergic medication; Psych, psychological treatments; SGA, second-generation antipsychotics.
Correlation Between Primary Outcome Measures and CGI
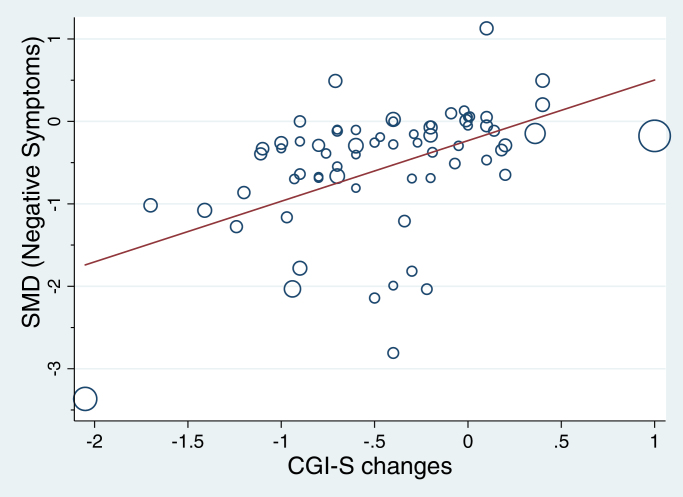
Sixty-eight trials included effect size estimates for CGI-S and negative symptoms. A Pearson’s correlation between the 2 measures demonstrated a relatively large correlation between the 2 variables (r = .46, 95% CI: 0.25 to 0.63, P = .0001). A metaregression showed that the CGI-S score predicted the severity of negative symptoms (β = .735, 95% CI: 0.423 to 1.047, t = 4.7, P < .0001, adjusted r 2 = 42.5%, see figure 3). Including treatment group as a covariate in the model slightly increased the effect to β = .744 (95% CI: 0.41 to 1.079, t = 4.45, P < .0001). On the basis of these results we estimated a threshold for minimally detectable clinical improvements over time: according to this model, a reduction of the mean CGI-S of 1 would correspond to an SMD for negative symptoms of −0.97 (see figures 2 and 3). There were not enough trials to reliable correlate the primary outcomes with CGI-I (N = 17). However, CGI-I effect sizes correlate with CGI-S with r = .79 (P = .0002, N = 17) and the estimated cut-off value of −0.96 when CGI-I = 1 was almost identical.
Fig. 3.
Correlation between negative symptoms and CGI-S (CGI-S: Clinical Global Impression Severity Scale); 68 trials included effect size estimates for CGI-S and negative symptoms. A Pearson’s correlation between the 2 measures demonstrated a medium to large correlation between the 2 variables (r = .46, 95% CI: 0.25 to 0.63, P = .0001). A metaregression showed that with CGI-S predicts negative symptoms (β = .735, 95% CI: 0.423 to 1.047, t = 4.7, P < .0001, adjusted r 2 = 42.5%). Including treatment group as a covariate in the model slightly increased the effect to β = .744 (95% CI: 0.41 to 1.079, t = 4.45, P < .0001). There were no significant treatment group differences (F(5, 61) = 0.11, P = .99). On the basis of these results we estimated a threshold for minimally detectable clinical improvements over time (y = −0.97 is the predicted SMDs of negative symptoms when mean CGI-S reduction is 1 more in treatment group compared with control group). As a sensitivity analysis, the analysis was rerun without the potential influential case at the left bottom corner (de Lucena et al55). The regression coefficient was reduced (β = .48, 95% CI: 0.189 to 0.776, t = 3.28, P = .002) and estimated thresholds for minimal detectable clinical improvement was estimated to be y = −0.78 (95% CI: −1.024 to −0.53). SMD, standardized mean difference.
Effect of Moderators, Quality Assessment, Sensitivity Analysis, and Publication Biases
There was no significant effect for moderators and quality of studies, no evidence of publication biases and the observed findings were robust. Detailed results are appended online (supplementary results).
Discussion
To the best of our knowledge, this is the first comprehensive meta-analysis of the efficacy of available treatments for negative symptoms. We focused on randomized placebo-controlled trials and studied a total of 6503 patients in the treatment arm and 5815 patients in the placebo arm. Almost all the interventions we assessed apart from FGA and BS appeared to have a statistically significant effect in terms of a reduction in either the PANSS-NS, BPRS-WR, or SANS. This is consistent with findings from previous meta-analyses of individual types of treatment. Thus, a meta-analysis of randomized placebo-controlled trials on the efficacy of AD in treating the negative symptoms in chronic schizophrenia, showed a small to medium effect size (−0.48, P < .05), comparable to our results of (−0.349, P = .001).22 A meta-analysis of randomized placebo-controlled trials of cognitive remediation in schizophrenia, showed a small effect size (−0.28, 95% CI: −0.13 to −0.43) for symptoms, without distinguishing between positive and negative ones.46 A meta-analysis of randomized placebo-controlled trials of cognitive behavioral therapy in schizophrenia, showed a small but not significant effect size (−0.21, P = .07), almost half of the effect found by our analysis for psychological treatments (−0.396, P < .001).28 However, a previous meta-analysis of the effects of transcranial magnetic stimulation on negative (and positive) symptoms showed a small and nonsignificant effect (−0.27, P = .41), when a potential placebo effect was considered.26 Our results are also in line with the a recent meta-analysis of social skills training, which concluded that this had no significant effect on negative symptoms in schizophrenia.47
Although these results suggest that a range of interventions had a statistically significant effect, this does not necessarily mean that the effects were clinically meaningful. The first consideration is that observed improvement of negative symptoms in the majority of trials may have been partly based on improvement of secondary negative symptoms—the “pseudospecificity” issue (see below in “Limitations” section). Hence these results may overestimate true efficacy. Second, a small change on a symptom rating scale may not translate into something that impacts on the patient’s level of functioning or quality of life. The clinical meaning of changes in psychotic symptom ratings on the BPRS, PANSS, and SANS has been tested against clinicians’ subjective perception of clinical improvement, as measured with the CGI-I/S.37,43 For example, an improvement on the CGI-S by one “severity step” corresponds to approximately 30%–40% improvement of BPRS scores and approximately 30% improvement in PANSS scores.43 Similarly, the relationship between SANS severity scores and CGI-S ratings also appears to follow a linear trend.44 The introduction of scale-derived clinically meaningful “cut-offs” to define response in antipsychotic drug trials is undoubtedly a complex issue; however, a 25% BPRS/PANSS reduction approximately corresponds to a CGI-I “minimal improvement,” whereas a 50% reduction approximately corresponds to a “much improved” score on the CGI-I.45 Recent studies have recommended translating the standard scores into CGI scores to better understand the clinical relevance of the observed efficacy.48,49
On the basis of the above findings,45 in the present study we considered a CGI-S reduction of one point as the minimum detectable clinical improvement and we estimated a threshold of y = −0.97 as the corresponding SMD. We were thus able to draw a “cut-off” line of minimum detectable “clinical” significance (see figure 2). All the categories of treatment we examined failed to reach this threshold. This was further supported by our supplementary analysis, which indicated a small to modest mean symptom change of 10.1% (BS) to 19.4% (SGA) in the treatment group vs 4.4% BS to 10.1% SGA in the control group. Individual data are appended online (supplementary results: table IV).
This suggests that although most of the treatments appeared to have a statistically significant effect on negative symptoms, this effect was not large enough to be clinically meaningful. The lack of clinically meaningful efficacy is in line with the experiences of clinicians in practice, who do not regard any available treatment as useful for negative symptoms.50 A key factor underlying the current lack of effective treatments for negative symptoms is that their pathophysiological and cognitive basis is still unknown. Whereas there are well-established models for positive psychotic symptoms that can be used as a rational basis for pharmacological and psychological treatments, there is an absence of such models for negative symptoms that have predictive validity for clinical efficacy.
Our second main finding was that there were no significant differences between the effects of the different types of treatment. The results of our analysis were robust as none of the moderators accounted for was identified as a confounder and no publication biases were detected. We considered: the use of different primary outcome measures (PANSS-NS, SANS, and BPRS-WR are different scales being used to measure negative symptoms); the attrition rate (high attrition could be linked to poor efficacy); the duration of the illness (therapeutic effects might vary across various phases of the illness, ie, wearing-off effect); epidemiological data (mean age of participants and percentage of males might variably affect response to treatment); trial duration (longer trials might be need to detect a treatment effect); year of publication (older trials might fail to focus on negative symptomatology, as this concept has attracted attention in the more recent years). After taking into consideration the above confounders, the effect sizes of various treatment groups varied between −0.228 and −0.579 with no significant between-group differences. The nonsignificant results may be partially explained by the large between-group heterogeneity and the small sample sizes in several treatment groups. Nevertheless, the findings challenge some of the recommendations in treatment guidelines about negative symptoms. For example, there was no evidence that SGA were superior to FGA, as suggested by international prescribing guidelines.51 Furthermore, we found no evidence that the combination of psychological treatments (including cognitive behavioral therapy, cognitive rehabilitation, or music therapy) with antipsychotics was more effective than antipsychotics alone, whereas the National Institute of Clinical Excellence (NICE) guidelines suggest that art therapy should be added to antipsychotic medication to treat negative symptoms.20 Similarly, augmentation of antipsychotic treatment with AD or Glut medication was not more effective than antipsychotics employed alone.
Further refining of our analysis, focusing on particular molecules or dose levels did not reveal any significant differences. No significant differences were detected between antipsychotics with 3 or more randomized-controlled trials available. Surprisingly, clozapine was not included in this comparison as there were no placebo-controlled trials of clozapine for the treatment of negative symptoms published in the literature. Higher doses also did not influence the meta-analytical estimates. These results of our analysis contradict recommendations made by the Maudsley Prescribing Guidelines51 and the British Association for Psychopharmacology52 for the management of negative symptoms of schizophrenia such as the preference of SGA over FGA or the addition of AD. Also our results could not provide evidence to support the tendency among prescribers to favor clozapine as an effective treatment for negative symptoms. Our study has clear clinical relevance. It suggests that there are still no clinically effective treatments for negative symptoms, which are—together with cognitive impairment—the most disabling features of schizophrenia. There is thus a major unmet clinical need for the development of novel treatments for these symptoms (see below). Our findings indicate that there is no evidence base for the range of interventions that are currently used to treat negative symptoms, which has implications for patients’ safety and health costs. Current treatment guidelines for schizophrenia need to be updated, such that only treatments that have clinically meaningful efficacy are included. For example, the NICE guidelines for schizophrenia recommend the use of art therapy for negative symptoms,20 yet our analyses indicate that there is no evidence base for this approach.
Some limitations to the present study should be acknowledged. We found an overrepresentation of articles of pharmacological vs psychological treatments. Two main factors contributed to this: (a) our inclusion criteria allowed only for studies with reported PANSS-NS or BPRS-WR or SANS score, resulting in the exclusion of studies of psychological interventions that employed a qualitative design. Furthermore (b) our analysis focused on studies including a placebo arm only (ie, placebo-controlled trials only). This was also reflected by underrepresentation of studies on FGA vs SGA: it appears that trials with no placebo group were the norm at the time FGA were tested, while the research trends moved toward the golden standard of placebo-controlled trials, in more recent years when SGA were introduced. There was a large diversity of clinical and methodological settings which forced us to use a random effects model. This model does not assume a single treatment effect but a distribution of treatment effects. The large between-group heterogeneity could not be explained with any covariates using metaregressions and the estimated effect size needs therefore be interpreted as the average treatment effect.
We did not directly compare psychological vs pharmacological treatments. However, a recent meta-analysis comparing efficacy of pharmacotherapy vs psychotherapy in psychiatry did not show consistent differences.53 As indicated in our methods, studies of effects of antipsychotics are often “pure” in that they evaluate the effect of a single intervention. In contrast, other treatments, such as psychological interventions, may be add-ons to ongoing therapies. The effects of these treatments reflect the combination of 2 therapies.
Other limitations such as the inability to differentiate between primary and secondary negative symptoms, the “pseudospecificity” problem and the short mean duration of follow-up for the included studies have been detailed online (supplementary discussion).
Conclusions
Although available treatments may have statistically significant effects on negative symptoms, these are probably too small to be clinically meaningful. There is a clear clinical need for new treatments for negative symptoms in schizophrenia.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
This work was supported with the resources of the Department of Psychosis Studies ; Institute of Psychiatry, Psychology and Neuroscience; King’s College London, UK. Dr. P.F.P. was supported in part by a 2014 NARSAD Young Investigator Award. Drs. E.P. and D.S. were supported by the National Institute for Health Research Mental Health Biomedical Research Centre at the South London and Maudsley NHS Foundation Trust and King’s College London.
Supplementary Material
Acknowledgments
Prof P.M. conceived the study and worked on the manuscript; Dr P.F.-P. designed the study, searched the literature, and wrote the manuscript; Dr E.P. searched the literature, extracted the data, and wrote the manuscript; Dr D.S. conducted the statistical analysis; Dr M.R. searched the literature and extracted the data; Prof W.C. and Prof S.S. commented and worked on the text of the manuscript. We thank Prof Stefan Leucht for his comments on an early draft of the present manuscript and for his assistance with the CGI analysis. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Buchanan RW, Carpenter WT. Domains of psychopathology: an approach to the reduction of heterogeneity in schizophrenia. J Nerv Ment Dis. 1994;182:193–204. [PubMed] [Google Scholar]
- 2. Carpenter W, Buchanan R. Domains of psychopathology relevant to the study of etiology and treatment of schizophrenia. In: Schulz SC, Tamminga CA, eds. Schizophrenia: Scientific Progress. New York: Oxford University Press; 1989:13–22. [Google Scholar]
- 3. Strauss JS, Carpenter WT, Bartko JJ. Part III. Speculations on the processes that underlie schizophrenic symptoms and signs. Schizophr Bull. 1974;1:61–69. [DOI] [PubMed] [Google Scholar]
- 4. Kraepelin E. Lecture III, Dementia Praecox. Lectures on Clinical Psychiatry. New York: William Wood; 1917:21–29. [Google Scholar]
- 5. Blanchard JJ, Cohen AS. The structure of negative symptoms within schizophrenia: implications for assessment. Schizophr Bull. 2006;32:238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Villalta-Gil V, Vilaplana M, Ochoa S, et al. Neurocognitive performance and negative symptoms: are they equal in explaining disability in schizophrenia outpatients? Schizophr Res. 2006;87:246–253. [DOI] [PubMed] [Google Scholar]
- 7. Ventura J, Hellemann GS, Thames AD, Koellner V, Nuechterlein KH. Symptoms as mediators of the relationship between neurocognition and functional outcome in schizophrenia: a meta-analysis. Schizophr Res. 2009;113:189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Velligan DI, Alphs L, Lancaster S, Morlock R, Mintz J. Association between changes on the Negative Symptom Assessment scale (NSA-16) and measures of functional outcome in schizophrenia. Psychiatry Res. 2009;169:97–100. [DOI] [PubMed] [Google Scholar]
- 9. Lin CH, Huang CL, Chang YC, et al. Clinical symptoms, mainly negative symptoms, mediate the influence of neurocognition and social cognition on functional outcome of schizophrenia. Schizophr Res. 2013;146:231–237. [DOI] [PubMed] [Google Scholar]
- 10. Magliano L, Fadden G, Madianos M, et al. Burden on the families of patients with schizophrenia: results of the BIOMED I study. Soc Psychiatry Psychiatr Epidemiol. 1998;33:405–412. [DOI] [PubMed] [Google Scholar]
- 11. Magliano L, Fadden G, Economou M, et al. Family burden and coping strategies in schizophrenia: 1-year follow-up data from the BIOMED I study. Soc Psychiatry Psychiatr Epidemiol. 2000;35:109–115. [DOI] [PubMed] [Google Scholar]
- 12. Knapp M, Mangalore R, Simon J. The global costs of schizophrenia. Schizophr Bull. 2004;30:279–293. [DOI] [PubMed] [Google Scholar]
- 13. Kirkpatrick B, Fenton WS, Carpenter WT, Jr, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32:214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carpenter WT, Heinrichs DW, Alphs LD. Treatment of negative symptoms. Schizophr Bull. 1985;11:440. [DOI] [PubMed] [Google Scholar]
- 15. Leucht S, Tardy M, Komossa K, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379:2063–2071. [DOI] [PubMed] [Google Scholar]
- 16. Buchanan RW, Gold JM. Negative symptoms: diagnosis, treatment and prognosis. Int Clin Psychopharmacol. 1996;11(suppl 2):3–11. [DOI] [PubMed] [Google Scholar]
- 17. Carpenter WT, Jr, Heinrichs DW, Wagman AM. Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry. 1988;145:578–583. [DOI] [PubMed] [Google Scholar]
- 18. Fleischhacker WW. New drugs for the treatment of schizophrenic patients. Acta Psychiatr Scand Suppl. 1995;92:24–30. [DOI] [PubMed] [Google Scholar]
- 19. Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373:31–41. [DOI] [PubMed] [Google Scholar]
- 20. NICE. Psychological therapy and psychological interventions in the treatment and management of schizophrnenia: arts therapies. In: Schizophrenia: Core Interventions in the Treatment and Management of Schizophrenia in Primary and Secondary Care. Vol National Clinical Guideline Number 82. Updated Edition ed. Leicester, London: The British Psychological Society & The Royal College of Psychiatrists; 2010:251–257. [Google Scholar]
- 21. Darbà J, Minoves A, Rojo E, Jimenez F, Rejas J. Efficacy of second-generation-antipsychotics in the treatment of negative symptoms of schizophrenia: a meta-analysis of randomized clinical trials. Rev Psiquiatr Salud Ment. 2011;4:126–143. [DOI] [PubMed] [Google Scholar]
- 22. Singh SP, Singh V, Kar N, Chan K. Efficacy of antidepressants in treating the negative symptoms of chronic schizophrenia: meta-analysis. Br J Psychiatry. 2010;197:174–179. [DOI] [PubMed] [Google Scholar]
- 23. Rummel C, Kissling W, Leucht S. Antidepressants as add-on treatment to antipsychotics for people with schizophrenia and pronounced negative symptoms: a systematic review of randomized trials. Schizophr Res. 2005;80:85–97. [DOI] [PubMed] [Google Scholar]
- 24. Murphy BP, Chung YC, Park TW, McGorry PD. Pharmacological treatment of primary negative symptoms in schizophrenia: a systematic review. Schizophr Res. 2006;88:5–25. [DOI] [PubMed] [Google Scholar]
- 25. Möller HJ. Management of the negative symptoms of schizophrenia: new treatment options. CNS Drugs. 2003;17:793–823. [DOI] [PubMed] [Google Scholar]
- 26. Freitas C, Fregni F, Pascual-Leone A. Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr Res. 2009;108:11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fitzgerald PB, Daskalakis ZJ. A review of repetitive transcranial magnetic stimulation use in the treatment of schizophrenia. Can J Psychiatry. 2008;53:567–576. [DOI] [PubMed] [Google Scholar]
- 28. Sarin F, Wallin L, Widerlöv B. Cognitive behavior therapy for schizophrenia: a meta-analytical review of randomized controlled trials. Nord J Psychiatry. 2011;65:162–174. [DOI] [PubMed] [Google Scholar]
- 29. Wykes T, Steel C, Everitt B, Tarrier N. Cognitive behavior therapy for schizophrenia: effect sizes, clinical models, and methodological rigor. Schizophr Bull. 2008;34:523–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jones C, Cormac I, Silveira da Mota Neto J, Campbell C. Cognitive behaviour therapy for schizophrenia. Cochrane Database Syst Rev. 2004;4:CD000524. [DOI] [PubMed] [Google Scholar]
- 31. Mossler K, Chen X, Heldal TO, Gold C. Music therapy for people with schizophrenia and schizophrenia-like disorders. Cochrane Database Syst Rev. 2011;12:CD004025. [DOI] [PubMed] [Google Scholar]
- 32. McGrath J, Hayes R. Cognitive rehabilitation for people with schizophrenia and related conditions. Cochrane Database Syst Rev. 2000;3:CD000968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 34. Overall JE, Gorham DR. The Brief Psychiatric Rating-Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 35. Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS). Iowa city: University of Iowa; 1981. [Google Scholar]
- 36. Rosenthal R. Meta-analysis: a review. Psychosom Med. 1991;53:247–271. [DOI] [PubMed] [Google Scholar]
- 37. Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: NIMH Psychopharmacology Research Branch, Department of Health, Education and Welfare; 1976:218–222. [Google Scholar]
- 38. Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999;354:1896–1900. [DOI] [PubMed] [Google Scholar]
- 39. Everitt B. Modern Medical Statistics. London: Arnold; 2003. [Google Scholar]
- 40. Cooper H, Hedges LV, Valentine JC. Handbook of Research Synthesis and Meta-Analysis. New York: Russell Sage Foundation; 2009. [Google Scholar]
- 41. Steichen T. Nonparametric trim and fill analysis of publication bias in meta-analysis. Stata Technical Bulletin, StataCorp LP. 2001;10:57. [Google Scholar]
- 42. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Leucht S, Kane JM, Etschel E, Kissling W, Hamann J, Engel RR. Linking the PANSS, BPRS, and CGI: clinical implications. Neuropsychopharmacology. 2006;31:2318–2325. [DOI] [PubMed] [Google Scholar]
- 44. Levine SZ, Leucht S. Identifying clinically meaningful symptom response cut-off values on the SANS in predominant negative symptoms. Schizophr Res. 2013;145:125–127. [DOI] [PubMed] [Google Scholar]
- 45. Leucht S, Davis JM, Engel RR, Kane JM, Wagenpfeil S. Defining ‘response’ in antipsychotic drug trials: recommendations for the use of scale-derived cutoffs. Neuropsychopharmacology. 2007;32:1903–1910. [DOI] [PubMed] [Google Scholar]
- 46. McGurk S, Twamley E, Sitzer D, McHugo G, Mueser K. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007;164:1791–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kurtz MM, Richardson CL. Social cognitive training for schizophrenia: a meta-analytic investigation of controlled research. Schizophr Bull. 2012;38:1092–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lepping P, Schönfeldt-Lecuona C, Sambhi RS, et al. A systematic review of the clinical relevance of repetitive transcranial magnetic stimulation. Acta Psychiatr Scand. 2014;130:326–341. [DOI] [PubMed] [Google Scholar]
- 49. Leucht S. Measurements of response, remission, and recovery in schizophrenia and examples for their clinical application. J Clin Psychiatry. 2014;75(suppl 1):8–14. [DOI] [PubMed] [Google Scholar]
- 50. Erhart SM, Marder SR, Carpenter WT. Treatment of schizophrenia negative symptoms: future prospects. Schizophr Bull. 2006;32:234–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Taylor D, Paton C, Kapur S. The Maudsley Prescribing Guidelines in Psychiatry. Wiley.com; 2012:57–60. [Google Scholar]
- 52. Barnes TR. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2011;25:567–620. [DOI] [PubMed] [Google Scholar]
- 53. Huhn M, Tardy M, Spineli LM, et al. Efficacy of pharmacotherapy and psychotherapy for adult psychiatric disorders: a systematic overview of meta-analyses. JAMA Psychiatry. 2014;71:706–715. [DOI] [PubMed] [Google Scholar]
- 54. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9, W64. [DOI] [PubMed] [Google Scholar]
- 55. de Lucena D, Fernandes BS, Berk M, et al. Improvement of negative and positive symptoms in treatment-refractory schizophrenia: a double-blind, randomized, placebo-controlled trial with memantine as add-on therapy to clozapine. J Clin Psychiatry. 2009;70:1416–1423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.