Abstract
Individuals with schizophrenia display substantial neurocognitive deficits for which available treatments offer only limited benefits. Yet, findings from studies of animals, clinical and nonclinical populations have linked neurocognitive improvements to increases in aerobic fitness (AF) via aerobic exercise training (AE). Such improvements have been attributed to up-regulation of brain-derived neurotrophic factor (BDNF). However, the impact of AE on neurocognition, and the putative role of BDNF, have not been investigated in schizophrenia. Employing a proof-of-concept, single-blind, randomized clinical trial design, 33 individuals with schizophrenia were randomized to receive standard psychiatric treatment (n = 17; “treatment as usual”; TAU) or attend a 12-week AE program (n = 16) utilizing active-play video games (Xbox 360 Kinect) and traditional AE equipment. Participants completed assessments of AF (indexed by VO2 peak ml/kg/min), neurocognition (MATRICS Consensus Cognitive Battery), and serum-BDNF before and after and 12-week period. Twenty-six participants (79%) completed the study. At follow-up, the AE participants improved their AF by 18.0% vs a −0.5% decline in the TAU group (P = .002) and improved their neurocognition by 15.1% vs −2.0% decline in the TAU group (P = .031). Hierarchical multiple regression analyses indicated that enhancement in AF and increases in BDNF predicted 25.4% and 14.6% of the neurocognitive improvement variance, respectively. The results indicate AE is effective in enhancing neurocognitive functioning in people with schizophrenia and provide preliminary support for the impact of AE-related BDNF up-regulation on neurocognition in this population. Poor AF represents a modifiable risk factor for neurocognitive dysfunction in schizophrenia for which AE training offer a safe, nonstigmatizing, and side-effect-free intervention.
Key words: aerobic fitness, cognition, neurotrophins, brain-derived neurotrophic factor/active-play video games
Introduction
Individuals with schizophrenia display a broad range of neurocognitive deficits1,2 that have been identified as major determinants of poor functioning and disability.1,3,4 To date, treatments approaches to address these deficits have centered on two primary modalities—pharmacotherapy and cognitive remediation. First and second-generation antipsychotics have demonstrated minimal efficacy in improving neurocognition in schizophrenia.5,6 Similarly, results from investigations of novel pharmacological compounds that target neurocognitive deficits have been disappointing.7,8 Cognitive remediation studies provided more promising results, although the benefits have been modest9,10 and the literature has been criticized on a number of methodological grounds.10,11 Thus, there remains an urgent need to identify novel approaches for development of treatments targeting neurocognitive deficits in schizophrenia.
Enhanced neurocognitive functioning is related to the brain’s neuroplasticity, which is dependent on the support of neurotrophins that signal to neurons to survive, differentiate and grow.12,13 Among neurotrophins, brain-derived neurotrophic factor (BDNF) has been found to be particularly relevant to such processes, as BDNF is the most abundant in the growth factor family14 and has a wide repertoire of neurotrophic and neuroprotective properties in the central nervous system.15–18 Among individuals with schizophrenia, reports point to lower serum BDNF,19 including among drug-naïve patients,20,21 with depletions linked to poor memory and smaller hippocampal volumes.22,23
One activity that is known to up-regulate BDNF is aerobic exercise (AE),24–26 with synthesis and release into blood circulation increasing in a dose-response manner.27,28 Findings from animal research strongly support the positive influence of AE on neurocognition29 along with increases in cell proliferation and survival.30,31 Similar associations have been found in humans across the lifespan.32–37 Specifically, in a meta-analysis of 29 randomized clinical trials (n = 2021), AE has been found to significantly improve attention, processing speed, executive functioning, and long-term memory, with a trend for working memory.38 Germane to these literatures, individuals with schizophrenia display a highly sedentary lifestyle39,40 that has been linked to poor neurocognitive and daily dysfunction.39,41 However, only one study to date has examined the impact of enhancing aerobic fitness (AF) on memory in males with schizophrenia, providing preliminary support.42,43
While previous reports are suggestive that AE may improve neurocognition in people with schizophrenia via up-regulation of BDNF, this model has not been examined directly. Specifically, the impact of AE on overall neurocognitive functioning (vs just memory), as well as the feasibility of up-regulating BDNF in people with schizophrenia using AE remain largely unknown. Additionally, previous reports focused solely on male participants and provided limited descriptions of intervention characteristics that may have important implications to outcome. Thus, our aims were to: (1) examine the efficacy of AE training to improve AF and neurocognitive functioning in individuals with schizophrenia; (2) To evaluate the contribution of AF to neurocognitive functioning; and (3) to determine whether AE-related BDNF up-regulation would predict improvement in neurocognitive functioning.
Methods
Participants
Data were obtained from a study conducted at the New York State Psychiatric Institute (NYSPI) at the Columbia University Medical Center (CUMC; ClinicalTrials.gov Identifier NCT01897064). The NYSPI’s Institutional Review Board approved the study and all participants provided written informed consent. The data were collected between May 2012 and July 2014. Forty-one participants were recruited from outpatient mental health clinics in the greater New York City area. The inclusion criteria were a DSM-IV diagnosis of schizophrenia or related disorders; age 18–55 years; English-speaking; taking antipsychotic medication for at least 8 weeks and on current doses for 4 weeks and/or injectable depot antipsychotics with no change in the last 3 months; and medically cleared by a physician to take part in AE training.44 The exclusion criteria were a DSM-IV diagnosis of alcohol/substance abuse within the past month or alcohol/substance dependence within the past 6 months; recent use of street drugs (confirmed by a urine toxicology test); a history of seizures/head trauma with loss of consciousness resulting in cognitive sequelae/rehabilitation; significant clinical abnormalities in physical examination, electrocardiogram, or lab assessments; untreated hyper- or hypothyroidism; extreme obesity (BMI ≥ 40); being pregnant/nursing; having serious suicidal/homicidal risk; presence of moderate or more severe disorganization (SAPS global positive formal thought disorder≥3); more than a mild level of depressive symptoms (BDI > 18); and participation in a study involving neurocognitive assessment in the previous 3 months.
Measures
Diagnostic and Clinical Assessments were established using The Structured Clinical Interview for DSM-IV. Clinical symptoms were assessed using the Scales for Assessment of Positive and Negative Symptoms (SAPS/SANS) and the Beck depression and anxiety inventories (BDI/BAI).
Aerobic fitness was determined by a cardiopulmonary exercise test to establish VO2 peak, an index of the maximum capacity of an individual’s body to transport and use oxygen during incremental AE. All tests were completed on weekdays at ~10 am and were performed on an electronically braked cycle ergometer (Ergometrics 800, SensorMedics Inc., Yorba Linda, CA) with a Viasys Encore metabolic cart (Viasys Corporation, Loma Linda, CA). The equipment was calibrated prior to every test. Continuous 12-lead telemetry was monitored via CardioSoft electrocardiogram software (GE/CardioSoft, Houston, TX). Participants completed measurements of a 5-min resting baseline, 3-min of no-resistance warm-up, ramping exercise protocol of 10–15 watts to peak exercise with a target of exercise for 8–12min. Exercise was terminated when the subject reached maximum capacity44 (VO2 plateau; 85% of maximal heart rate (HRmax; 220-age); respiratory quotient ≥ 1.1; or self-reported exhaustion45). A 3-min active recovery period completed the test. We used VO2 peak (ml/kg/min) scores in all analyses.
For BDNF analyses, a sample of venous blood was collected from participants on a weekday at ~9 am following overnight fasting. The blood samples were collected into serum separating tubes and were allowed to coagulate at room temperature for 30min. Next, serum samples were collected through 10min centrifugation at 4°C using a Clay Adam Compact II Centrifuge (3200rpm). Blood samples were then kept refrigerated at −80°C. At study end, all samples were analyzed for BDNF concentrations using commercially available ELISA kits according to the manufacturer’s protocol (R & D Systems). The intra- and inter-assay precisions were 3.8% and 7.6%, respectively, and the sensitivity was <20 pg/ml.
Neurocognition was assessed using the MATRICS Consensus Cognitive Battery (MCCB).46 The primary variable of interest was the MCCB composite score, with T-scores adjusted for age, gender, and demographics used in all analyses.
Study Procedures and Blinding
We employed a single-blind, parallel assignment, randomized clinical trial design with participants randomized to receive standard psychiatric treatment (“treatment as usual”; TAU) or attend a 12-week AE program in addition to TAU. Participants were randomized in the order they entered the study. After satisfying the inclusion/exclusion criteria, participants completed the diagnostic, clinical, AF, BDNF, and neurocognition baseline assessments. The PI then contacted an independent statistician who allocated participants to treatments based on an a priori computer-generated randomization list. The neurocognitive raters, as well as the technicians conducting the AF assessments and serum-BDNF analyses were all blind to the participants’ treatment status and other collected data. Following the 12-week period, participants completed a follow-up assessment of clinical, AF, BDNF, and neurocognition. Participants received $270 for completing the research assessments. Additionally, those randomized to the AE arm received $5 reimbursement for each AE session they attended (paid weekly) to defray the costs of round-trip public transportation.
Interventions—Description and Fidelity Assessments
All participants receive standard psychiatric care over the course of the study, which included regular meetings with a psychiatrist, as well as as-needed meetings with psychologists, social workers, or psychiatric nurses. Treatment interactions and/or frequency were determined for each participant individually by their respective psychiatrist and no attempts were made to influence treatment. Participants randomized to the AE intervention underwent a 12-week, 3 sessions/week, 1-h AE training program informed by the American College of Sports Medicine and federal guidelines,47 which recommend 150min of moderate-intensity AE per week. Moderate-intensity AE involves activities that expend 3.0–5.9 times the energy expended at rest and are defined as activities in which the participant is able to talk while engaging in the activity. The AE sessions were led by a trainer (Bachelor of Science in Therapeutic Recreation) and opened with a 10-min trainer-led warm-up period, followed by 45-min AE using the equipment, and ended with a 5-min cool-down period. The AE equipment included two active-play video game systems (Xbox 360 Kinect, Microsoft) with whole-body exercise software (Your Shape Fitness Evolved 2012, Ubisoft), two treadmill machines, a stationary bike, and an elliptical machine. The trainer was present during the AE sessions for guidance and support, along with a research assistant who collected AE-related behavioral data.
Training fidelity was indexed by the number of AE sessions attended and in-session AE intensity. The latter was set for participants individually based on his/her maximal heart rate (HRmax), as determined during their baseline VO2 peak assessment. Minimal AE intensity was set to 60% of HRmax in Week 1, 65% in Week 2, 70% in Week 3, and 75% in Weeks 4–12. In-session training intensity was monitored using Polar RS400 HR monitors (a wireless-enabled digital watch and chest strap) that participants wore during each session. The monitors were programmed to emit a soft beep sound when the participant’s HR was lower than the individually targeted AE intensity level. On such occasions, the trainer encouraged the participant to achieve his/her target goal.
Statistical Analyses
Data analyses were conducted using IBM SPSS ver. 22. All tests were 2-tailed and the significance level was α = .05. As the present investigation served as a proof-of-concept study, we focused our analyses on study completers and did not correct P-values for multiple comparisons. For group comparisons, the primary dependent variables were change from baseline to follow-up in neurocognitive functioning (indexed by MCCB overall composite score) and AF (VO2 peak; ml/kg/min). Shapiro–Wilk tests indicated that the primary dependent variables were normally distributed. The AE and TAU intervention groups were compared using multivariate analyses of variance with a repeated-measures design, with time and group designated as within-subject and between-subject factors, respectively. We focused our analyses on study completers, but also present results using an intention-to-treat approach with baseline observations carried forward.
Determination of predictors of change in neurocognition was examined using 2 hierarchical step-wise regression analyses. For evaluation of the impact of change in AF, the MCCB overall composite score was entered as dependent variable; demographic and clinical variables were entered in block 1; and change in AF was entered in block 2. A similar analysis evaluated the impact of change in BDNF. Demographic variables included age and sex. Clinical variables included a number of potential covariates that have been linked to neurocognition or BDNF including changes in antipsychotic, antidepressants, or SSRIs medications, as well as change in phase of menstrual cycle. Changes from baseline to follow-up in the use of antipsychotic medications were indexed by changes in chlorpromazine equivalence.48 Changes in use of antidepressants or SSRI were indexed by a dummy variable indicating reduction in use of same antidepressant (−1), no change in dosage of same antidepressant (0), or increase of same antidepressant/change in antidepressant (1). Associations between variables were examined using partial correlations controlling for depression and antipsychotic medication.
Results
Sample Characteristics
Eight participants signed-up for the study but discontinued participation prior to randomization due to difficulties keeping research appointments (n = 3), psychotic exacerbation (n = 1), diagnosis of cancer (n = 1), discovery of a benign brain tumor (n = 1), BDI > 18 (n = 1), and conflict with changed school schedule (n = 1). The remaining 33 individuals were randomized (AE = 16, TAU = 17) of which 26 completed the study (79%). In the AE group, all 3 non-completers dropped-out during the first week of the AE training after 0, 1, and 3 sessions, respectively due to loss of contact (n = 1), long commute-time to AE site (n = 1), and not liking the AE program (n = 1). In the TAU group, 4 participants dropped-out due to relocation (n = 1), hypomanic episode (n = 1), and protocol violations (n = 2). The status of 3 of the 33 randomized participants became un-blinded to raters (9%)—1 in the TAU group (self-disclosure) and 2 in the AE group (1 self-disclosure, 1 accidental disclosure by clinical staff). Of note, we did not exclude participants who were prescribed beta-blockers or other medications known to influence heart rate out of concern that such exclusion would result in a non-representative sample as many individuals with schizophrenia are prescribed such medications for cardiac, as well as other problems (eg, akathisia). However, only 2 out of the 33 randomized participants were prescribed beta-blockers—one dropped-out prior to randomization and one completed the AE program.
At baseline, there were no significant groups differences with regard to demographic, clinical, and physical health indices (table 1). Changes in neurocognition over the course of the 12-weeks were not associated with changes in antipsychotic, antidepressant, or SSRI medications. Likewise, no changes were reported in smoking status. Additionally, there were no significant differences between the groups in the number of clinical contacts with mental-health professionals (psychiatrists, psychologists, social workers, and psychiatric nurses) during the month prior to the follow-up assessments (AE: mean = 5.00 meetings, SD = 4.91 vs TAU: mean = 4.73, SD = 6.09; t = 0.12, P = .91).
Table 1.
Baseline Demographic and Clinical Information
| Aerobic Exercise (n = 16) | Treatment as Usual (n = 17) | t/X 2 | P | |
|---|---|---|---|---|
| Age | 36.56 (10.37) | 37.24 (9.85) | .19 | .85 |
| Sex (% female) | 37% | 35% | .02 | .89 |
| Ethnicity (% Hispanic) | 43% | 29% | .69 | .39 |
| Race | ||||
| Caucasian | 2 (12%) | 6 (35%) | 2.97 | .40 |
| Black/African-American | 6 (37%) | 6 (35%) | ||
| Asian | 2 (12%) | 2 (12%) | ||
| More than one race | 6 (37%) | 3 (18%) | ||
| Symptoms | ||||
| Positive (SAPS Global Scores Total) | 3.77 (3.37) | 3.46 (2.93) | .25 | .81 |
| Negative (SANS Global Scores Total) | 9.73 (4.24) | 8.54 (4.41) | .67 | .51 |
| Depression (BDI Total) | 7.81 (7.69) | 7.23 (8.57) | .20 | .84 |
| Anxiety (BAI Total) | 4.56 (4.40) | 5.82 (6.83) | .63 | .54 |
| Medications | ||||
| Antipsychotics (Chlorpromazine Equiv.) | 258.85 (232.51) | 439.73 (362.78) | 1.69 | .10 |
| Antidepressants (% yes) | 44% | 35% | .25 | .62 |
| SSRIs (% yes) | 31% | 23% | .25 | .62 |
| Smoking cigarettes (% yes) | 25% | 23% | .02 | .89 |
| If yes, how many per day? | 8.33 (9.27) | 6.50 (6.83) | .39 | .70 |
| Neurocognition (MCCB composite score) | 33.08 (11.21) | 34.23 (12.91) | .34 | .81 |
| Reading ability (WTAR total) | 32.50 (11.56) | 37.59 (7.95) | 1.48 | .15 |
| Aerobic fitness | ||||
| VO2 peak (ml/kg/min) | 21.21 (7.69) | 22.88 (4.41) | .77 | .45 |
| VO2 (L/min) | .34 (.06) | .32 (.06) | .77 | .45 |
| Body mass index | 31.60 (6.57) | 30.75 (5.51) | .40 | .69 |
| Weight (pounds) | 205.23 (44.79) | 204.32 (37.07) | .06 | .95 |
| Heart rate | 85.08 (16.16) | 90.84 (15.93) | .92 | .37 |
| Blood pressure | ||||
| Systolic | 112.92 (14.93) | 112.38 (10.54) | .11 | .92 |
| Diastolic | 70.31 (7.54) | 72.77 (7.86) | .81 | .42 |
| Brain-derived neurotrophic factor (ng/ml) | 28.1 (8.0) | 31.2 (6.5) | .34 | .29 |
Note: n = 33 (schizophrenia = 26; schizoaffective disorder = 7); SAPS, Scale for Assessment of Positive Symptoms; SANS, Scale for Assessment of Negative Symptoms; BDI, Beck Depression Inventory; BAI, Beck Anxiety Inventory; SSRI, Serotonin-Specific Reuptake Inhibitor; MCCB, MATRICS Consensus Cognitive Battery; WTAR, Wechsler Test of Adult Reading.
AE Training—Safety, Attendance and In-Session Engagement
Thirteen of the 16 participants assigned to the AE track successfully completed the training program (81%). There were no adverse events associated with the AE training. Participants attended on average 28.5 of the 36 scheduled AE sessions (79%; range 44%–97%; on average 2.4 sessions per week). Two to three participants typically attended each AE session—they exercised individually and were free to choose which AE equipment they wanted to use, as well as free to take breaks and hydrate as needed (breaks typically lasted 1–2min). During each AE session, participants engaged in active AE training on average for 42.7min/session (SD = 3.51). Thirty-nine percent of this time was spent using the Xbox 360 Kinect, followed by the treadmill (32%), stationary bike (13%), elliptical machine (13%), and trainer-led AE activities (3%). On average, during 25% of the AE sessions time the participants’ HR was between 60% and 69% of their HRmax, 27% between 70% and 79%, 21% between 80% and 89%, and 16% of the sessions at ≥90% of HRmax. During only 11% of the sessions was the participants’ HR below the initial designated training goal of 60% of HRmax (table 2).
Table 2.
Comparison of Changes in Aerobic Fitness, Neurocognition, and Brain-Derived Neurotropic Factor Following Aerobic Exercise or Treatment as Usual
| Treatment | Intention to Treat (n = 33) | Study Completers (n = 26) | Cohen’s d b | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean Δ | SD | F a | P | Mean Δ | SD | F a | P | |||
| Aerobic fitness | Treatment as usual | −0.71 | 2.78 | 3.62 | .001** | −0.48 | 3.07 | 12.24 | .002** | 1.06 |
| Aerobic exercise | 3.11 | 3.26 | 3.82 | 3.21 | ||||||
| Neurocognition | Treatment as usual | 0.35 | 7.16 | 1.34 | .19 | −1.15 | 5.80 | 5.24 | .031* | 0.93 |
| Aerobic exercise | 3.25 | 4.93 | 4.00 | 5.21 | ||||||
| BDNF | Treatment as usual | 1.39 | 4.64 | 0.08 | .94 | 0.17 | 3.50 | 0.76 | .46 | 0.30 |
| Aerobic exercise | 1.26 | 5.02 | 1.55 | 5.56 | ||||||
Note: Aerobic fitness, VO2peak (ml/kg/min); Neurocognition, MATRICS Consensus Cognitive Battery (MCCB) composite score; BDNF, serum BDNF (ng/ml).
aControlling for the impact of sex, age, and changes in antipsychotics, antidepressants and phase of menstrual cycle.
bFor study completers.
*P < .05, **P < .01.
The Impact of AE Training on AF and Indices of Physical Health
We compared the changes in AF over the 12-week period between the AE and TAU groups. Participants in the AE group completed the follow-up AF assessments on average 1.61 days (SD = 1.56) after their last AE session. The VO2 peak improvement in the AE group (18.0%) was significantly greater than the change observed in the TAU group (−0.5%; group × time interaction, F 1,24 = 12.24; P = .002).
Efficacy of AE Training to Improve Neurocognitive Functioning
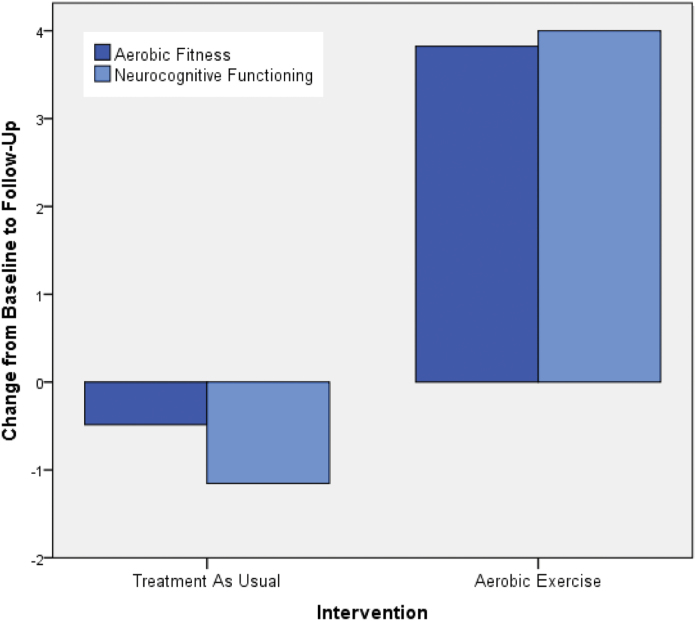
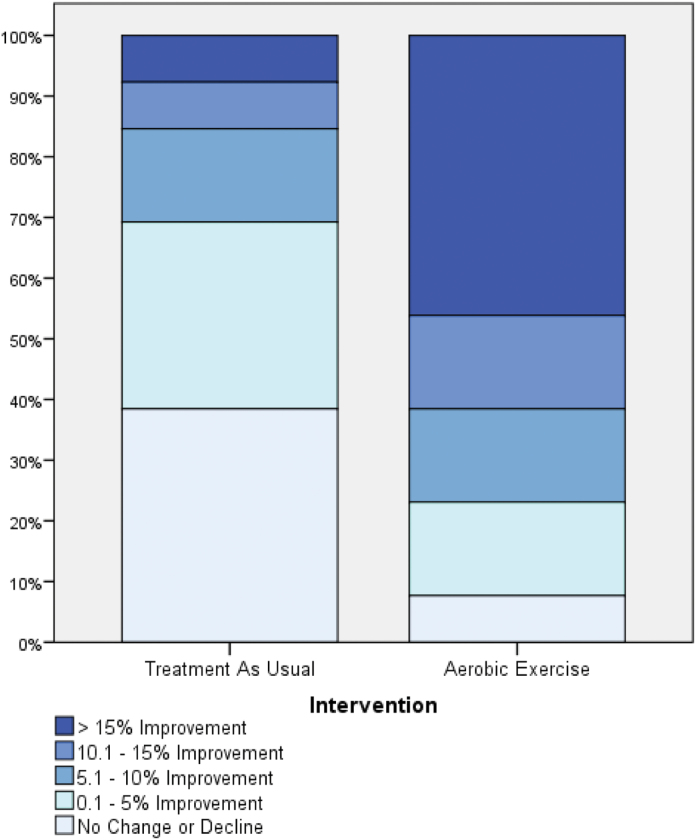
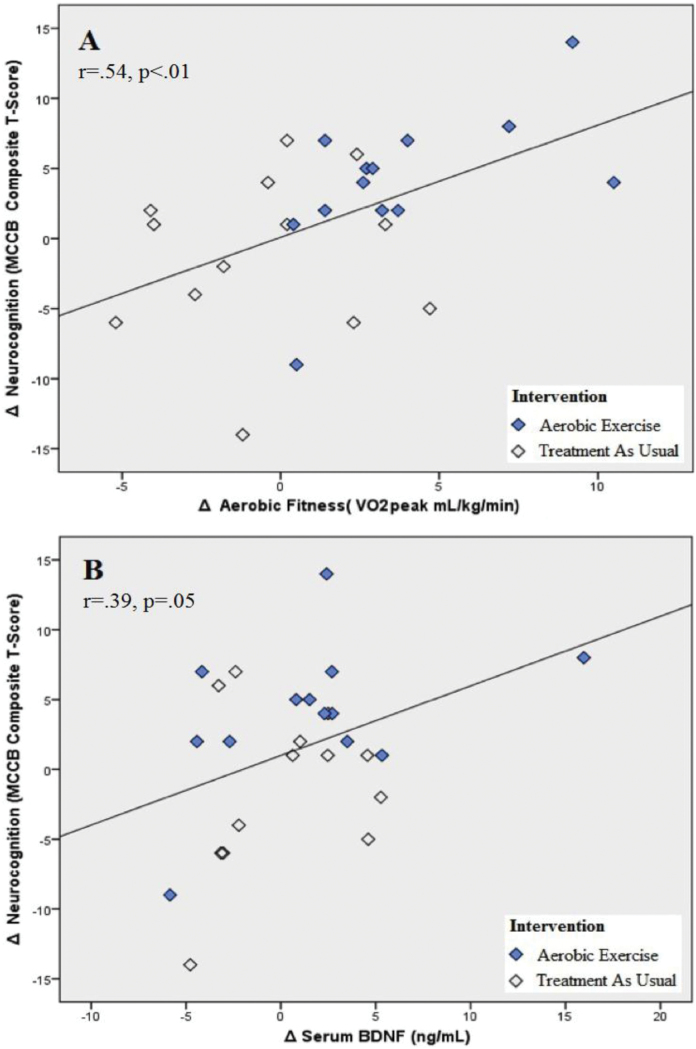
Participants in the AE group completed the follow-up neurocognitive assessments on average 4.61 days (SD = 3.55) after their last AE session. The improvement in neurocognitive functioning in the AE group (15.1%) was significantly greater than the change observed in the TAU group (−2.0%; group × time interaction, F 1,24 = 5.24, P = .031; figure 1). Cohen’s d calculation indicated a large effect size for the AE intervention (d = 0.93), with 77% of participants improving their neurocognitive functioning by more than 5% and 46% by more than 15% (vs 31% and 8% in the TAU group, respectively; figure 2). Improvement in AF was significantly correlated with neurocognition enhancement (r = .54, P < .01; figure 3A). Exploratory analyses indicated that the changes in AF were most strongly associated with changes in the MCCB domains of social cognition (r = .48, P =.02) and visual learning (r = .41, P = .05), with correlations with other domains not reaching significance level.
Fig. 1.
Comparison of changes in aerobic fitness and neurocognitive functioning following aerobic exercise training or treatment as usual. n = 26 (TAU = 13, AE = 13); Changes in aerobic fitness are indexed by VO2 peak (ml/kg/min); Changes in neurocognition are indexed by MATRICS Consensus Cognitive Battery composite T-scores.
Fig. 2.
Distribution of changes in neurocognitive functioning following aerobic exercise training or treatment as usual. n = 26 (TAU = 13, AE = 13); Neurocognitive Functioning—MATRICS Consensus Cognitive Battery composite T-score.
Fig. 3.
Association of changes in aerobic fitness, serum BDNF, and neurocognition. n = 26 (AE = 13, TAU = 13). (A) Association of changes in aerobic fitness and changes in neurocognition. (B) Association of changes in serum-BDNF and changes in neurocognition.
Predictors of Improvement in Neurocognitive Functioning
Using a hierarchical step-wise regression analysis, we evaluated the impact of changes in AF on neurocognition. The regression analysis indicated that, after controlling for age, sex, changes in antipsychotic and antidepressant medications, and changes in phase of menstrual cycle, the model accounted for 25.4% of the explained variance in neurocognitive improvement (F 1,25 = 8.16, P = .009), with AF enhancement contributing uniquely to the model’s validity (b = 0.50, t = 2.86, P = .009).
At follow-up, BDNF increased by 11.0% in the AE group vs a 1.9% in the TAU participants (no significant group difference in BDNF change), with participants in the AE group providing blood samples for serum-BDNF assessments on average 2.38 days (SD = 2.36) after their last AE session. A hierarchical step-wise regression analysis indicated that, after controlling for age, sex, changes in antipsychotic and SSRIs, and changes in phase of menstrual cycle, the model accounted for 14.6% of the explained variance in neurocognitive functioning (F 1,25 = 4.11, P = .05), with BDNF changes contributing uniquely to the model’s validity (b = 0.38, t = 2.06, P = .05; figure 3B).
Discussion
To the best of our knowledge, the present study is the first investigation of the impact of AE on BDNF and neurocognition in individuals with schizophrenia. Our group has previously documented a significant association between AF and neurocognition in individuals with schizophrenia.39 The present results extend these findings by demonstrating that enhancement of AF via AE significantly improves overall neurocognition in this population, with outcome suggesting a large effect size. Previous studies of MCCB-measured neurocognition in schizophrenia reported average overall composite T-scores one to two-and-a-half standard deviation units below healthy population norms.7,8,49,50 Using these findings as a reference, the magnitude of AE-related neurocognitive improvement in the present study (4 T-scores) represents an enhancement equivalent to 16%–40% of the reported neurocognitive functioning deficit, with 24% improvement in our sample. Thus, in addition to its well-documented cardiovascular, weight-management, and other physical health benefits, AE training offer the potential to ameliorate neurocognitive deficits in individuals with schizophrenia via a nonstigmatizing, safe, and side-effect-free intervention.
The AE program led to substantial improvements in AF, consistent with previous reports documenting VO2 peak-indexed AF improvements in first-episode patients,51 as well as more chronic populations.40,42,52,53 Together, these findings suggest AF improvements via AE are feasible in this population. Our results also provide preliminary support for the integration of active-play video games into AE programs for people with schizophrenia. Such technologies promote physical and emotional well-being by making physical activity more fun,54 while minimizing disinterest and boredom, key barriers to programs implementation.55 Reflecting these findings, active-play video games have become mainstream tools for enhancing AF, as evident by their endorsement by the American Heart Association.54 Accordingly, in our study the AE training attendance rate (79%) was substantially higher than the mean rate (64%) reported for similarly structured programs utilizing traditional AE equipment.56 Future studies should confirm these findings and clarify whether the higher attendance rate relates to increased intrinsic motivation to use the active-play video games or may be attributed to other factors.57
Our results provide preliminary support for a model linking AE, BDNF up-regulation, and neurocognitive improvement. While the group difference in changes in BDNF did not reach significance, potentially due to the small sample size, the direction of the results is in agreement with converging animal and human research literatures supporting the positive impact of AE on BDNF. Likewise, a recent report found a significant increase (21%) in serum-BDNF in hospitalized individuals with schizophrenia following completion of a 12-week AE trial,58 with serum-BDNF increases correlating with AF improvements. Yet, the contributions of the changes in BDNF to improvements to neurocognition were rather modest, suggesting that AE-related enhancements in neurocognition may potentially operate via additional pathways (eg, inflammation59). Additionally, while BDNF crosses the brain-blood barrier,60 and decreased cerebrospinal fluid BDNF concentrations were reported in individuals with schizophrenia,61,62 at present there is little evidence directly linking changes in peripheral BDNF and CNS effects. Furthermore, as we did not evaluate healthy controls, we cannot exclude the possibility that the changes in BNDF resulted from other disease factors. Future studies should aim to further elucidate this potential link.
Our sample size did not permit genetic analyses. However, previous reports focusing on the BDNF Val66Met polymorphism have linked BDNFMet carriers to impairment in learning and memory along with lower hippocampal engagement during information encoding/retrieval, including in people with schizophrenia.63–66 Consistent with these findings, in animal studies mice carrying the BDNF Val66Met mutation displayed normal constitutive BDNF secretion, but perturbed physical activity-regulated secretion,63 suggesting compromised physical activity-related BDNF-dependent synaptic modulation. Future studies should determine the relevance of this polymorphism to the impact of AE on BDNF and neurocognition in schizophrenia.
The present study has a number of strengths, first and foremost is the evaluation of in-session AE intensity. While previous reports have linked AE to improvements in neurocognition and hippocampal volume increases,42 these findings are not universal,67 potentially due to less intense AE training programs. As AE training characteristics (eg, frequency, intensity, and duration) may play a critical role in determining outcomes, future studies should characterize AE interventions in detail, allowing comparison of interventions. An additional strength is the inclusion of numerous covariates of neurocognition, AF and BDNF. However, given the relatively modest sample size, our findings should be considered preliminary until replicated by other researchers. Similarly, we did not correct for multiple comparisons, which may have resulted in an increased chance of false-positive findings. Furthermore, while the overall direction of the results is in agreement with previous reports, the nonsignificant results of the intention-to-treat analyses, potentially due to the small sample size, should be noted.
The possibility that AE-related social interactions or other nonspecific care factors influence the outcome deserves consideration. A meta-analysis of cognitive remediation trials in schizophrenia concluded that the amount and nature of clinical attention given to controls did not influence cognitive functioning.68 Likewise, the control group in Pajonk et al’s 42 study was exposed to comparable social contacts but experienced minimal cognitive benefits, suggesting that the AE-related neurocognitive enhancement in our study was not likely due to increased social interactions. Similarly, the lack of group differences in psychiatric clinical care minimizes the likelihood of this potential factor. However, we could not exclude the possibility that the use of video games as part of the AE provided further benefits (in addition to the physical activity), as evidence suggests that even sedentary, passive-play video games may benefit neurocognition.69
In summary, AE is effective in improving overall neurocognition in people with schizophrenia. Such improvements may be attributed, in part, to AE-related up-regulation of BDNF. Poor AF represents a modifiable risk factor for neurocognitive dysfunction in people with schizophrenia for which AE training offer a safe, nonstigmatizing, and side-effect-free intervention.
Funding
The National Institute of Mental Health , Bethesda, MD (1R21MH096132 to D.K).
Acknowledgments
Dr. Ballon has received investigator-initiated research funding from Novartis relating to another project. Dr. Castrén is an advisor of Herantis Pharma. None of the other authors had any conflict of interest relating to this project. Dr. Smith passed away in 2012. His contributions to the manuscript are recognized posthumously.
References
- 1. Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res. 2004;72:41–51. [DOI] [PubMed] [Google Scholar]
- 2. Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29–39. [DOI] [PubMed] [Google Scholar]
- 3. Barch DM, Keefe RS. Anticipating DSM-V: opportunities and challenges for cognition and psychosis. Schizophr Bull. 2010;36:43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lewandowski KE, Cohen BM, Keshavan MS, Sperry SH, Ongür D. Neuropsychological functioning predicts community outcomes in affective and non-affective psychoses: a 6-month follow-up. Schizophr Res. 2013;148:34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Keefe RS, Bilder RM, Davis SM, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64:633–647. [DOI] [PubMed] [Google Scholar]
- 6. Goff DC. Future perspectives on the treatment of cognitive deficits and negative symptoms in schizophrenia. World Psychiatry. 2013;12:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buchanan RW, Keefe RS, Lieberman JA, et al. A randomized clinical trial of MK-0777 for the treatment of cognitive impairments in people with schizophrenia. Biol Psychiatry. 2011;69:442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Javitt DC, Buchanan RW, Keefe RS, et al. Effect of the neuroprotective peptide davunetide (AL-108) on cognition and functional capacity in schizophrenia. Schizophr Res. 2012;136:25–31. [DOI] [PubMed] [Google Scholar]
- 9. Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168:472–485. [DOI] [PubMed] [Google Scholar]
- 10. Vinogradov S, Fisher M, Nagarajan S. Cognitive training in schizophrenia: golden age or wild west? Biol Psychiatry. 2013;73:935–937. [DOI] [PubMed] [Google Scholar]
- 11. Wykes T, Spaulding WD. Thinking about the future cognitive remediation therapy—what works and could we do better? Schizophr Bull. 2011;37(suppl 2):S80–S90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hennigan A, O’Callaghan RM, Kelly AM. Neurotrophins and their receptors: roles in plasticity, neurodegeneration and neuroprotection. Biochem Soc Trans. 2007;35:424–427. [DOI] [PubMed] [Google Scholar]
- 13. Vaynman S, Gomez-Pinilla F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil Neural Repair. 2005;19:283–295. [DOI] [PubMed] [Google Scholar]
- 14. Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22:123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Castrén E, Berninger B, Leingärtner A, Lindholm D. Regulation of brain-derived neurotrophic factor mRNA levels in hippocampus by neuronal activity. Prog Brain Res. 1998;117:57–64. [DOI] [PubMed] [Google Scholar]
- 16. Castrén E, Tanila H. Neurotrophins and dementia–keeping in touch. Neuron. 2006;51:1–3. [DOI] [PubMed] [Google Scholar]
- 17. Castrén E, Rantamäki T. The role of BDNF and its receptors in depression and antidepressant drug action: reactivation of developmental plasticity. Dev Neurobiol. 2010;70:289–297. [DOI] [PubMed] [Google Scholar]
- 18. Knaepen K, Goekint M, Heyman EM, Meeusen R. Neuroplasticity—exercise-induced response of peripheral brain-derived neurotrophic factor: a systematic review of experimental studies in human subjects. Sports Med. 2010;40:765–801. [DOI] [PubMed] [Google Scholar]
- 19. Green MJ, Matheson SL, Shepherd A, Weickert CS, Carr VJ. Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Mol Psychiatry. 2011;16:960–972. [DOI] [PubMed] [Google Scholar]
- 20. Jindal RD, Pillai AK, Mahadik SP, Eklund K, Montrose DM, Keshavan MS. Decreased BDNF in patients with antipsychotic naïve first episode schizophrenia. Schizophr Res. 2010;119:47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rizos EN, Rontos I, Laskos E, et al. Investigation of serum BDNF levels in drug-naive patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1308–1311. [DOI] [PubMed] [Google Scholar]
- 22. Zhang X, Liang J, Chen D, et al. Low BDNF is associated with cognitive impairment in chronic patients with schizophrenia. Psychopharmacology. 2012:1–8. [DOI] [PubMed] [Google Scholar]
- 23. Rizos EN, Papathanasiou M, Michalopoulou PG, et al. Association of serum BDNF levels with hippocampal volumes in first psychotic episode drug-naive schizophrenic patients. Schizophr Res. 2011;129:201–204. [DOI] [PubMed] [Google Scholar]
- 24. Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. [DOI] [PubMed] [Google Scholar]
- 25. van Praag H. Neurogenesis and exercise: past and future directions. Neuromolecular Med. 2008;10:128–140. [DOI] [PubMed] [Google Scholar]
- 26. van Praag H. Exercise and the brain: something to chew on. Trends Neurosci. 2009;32:283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rasmussen P, Brassard P, Adser H, et al. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol. 2009;94:1062–1069. [DOI] [PubMed] [Google Scholar]
- 28. Seifert T, Brassard P, Wissenberg M, et al. Endurance training enhances BDNF release from the human brain. Am J Physiol Regul Integr Comp Physiol. 2010;298:R372–R377. [DOI] [PubMed] [Google Scholar]
- 29. Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. [DOI] [PubMed] [Google Scholar]
- 30. van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. [DOI] [PubMed] [Google Scholar]
- 32. Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci. 2013;17:525–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stroth S, Hille K, Spitzer M, Reinhardt R. Aerobic endurance exercise benefits memory and affect in young adults. Neuropsychol Rehabil. 2009;19:223–243. [DOI] [PubMed] [Google Scholar]
- 34. Themanson JR, Pontifex MB, Hillman CH. Fitness and action monitoring: evidence for improved cognitive flexibility in young adults. Neuroscience. 2008;157:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hansen AL, Johnsen BH, Sollers JJ, III, Stenvik K, Thayer JF. Heart rate variability and its relation to prefrontal cognitive function: the effects of training and detraining. Eur J Appl Physiol. 2004;93:263–272. [DOI] [PubMed] [Google Scholar]
- 36. Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Datab Syst Rev. 2008;2:CD005381. [DOI] [PubMed] [Google Scholar]
- 37. Kimhy D, Crowley OV, McKinley PS, et al. The association of cardiac vagal control and executive functioning—findings from the MIDUS study. J Psychiatr Res. 2013;47:628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smith PJ, Blumenthal JA, Hoffman BM, et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72:239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kimhy D, Vakhrusheva J, Bartels MN, et al. Aerobic fitness and body mass index in individuals with schizophrenia: Implications for neurocognition and daily functioning. Psychiatry Res. 2014;220:784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Strassnig M, Brar JS, Ganguli R. Low cardiorespiratory fitness and physical functional capacity in obese patients with schizophrenia. Schizophr Res. 2011;126:103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leutwyler H, Hubbard EM, Jeste DV, Miller B, Vinogradov S. Associations of schizophrenia symptoms and neurocognition with physical activity in older adults with schizophrenia. Biol Res Nurs. 2014;16:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pajonk FG, Wobrock T, Gruber O, et al. Hippocampal plasticity in response to exercise in schizophrenia. Arch Gen Psychiatry. 2010;67:133–143. [DOI] [PubMed] [Google Scholar]
- 43. Vancampfort D, Probst M, De Hert M, et al. Neurobiological effects of physical exercise in schizophrenia: a systematic review. Disabil Rehabil. 2014;36:1749–1754. [DOI] [PubMed] [Google Scholar]
- 44. American College of Sports Medicine. Clinical exercise testing In: Thompson WR, ed. ACSM’s Guidelines for Exercise Testing and Supervision. 8th ed. Philadeplhia, PA: Woulters Kluwer: Lippincott, Williams and Wilkins; 2009:105–135. [Google Scholar]
- 45. Borg G.Borg’s Perceived exertion and pain scales. Champaign, IL: Human Kinetics; 1998. [Google Scholar]
- 46. Nuechterlein KH, Green MF.Matrics Consensus Battery Manual. Los Angeles, CA: Matrics Assessment, Inc; 2006. [Google Scholar]
- 47.US Department of Health and Human Services. 2008 physical activity guidelines for Americans. 2008. http://www.health.gov/PAGuidelines. Accessed January 15, 2015. [Google Scholar]
- 48. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. [DOI] [PubMed] [Google Scholar]
- 49. Kern RS, Gold JM, Dickinson D, et al. The MCCB impairment profile for schizophrenia outpatients: results from the MATRICS psychometric and standardization study. Schizophr Res. 2011;126:124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. August SM, Kiwanuka JN, McMahon RP, Gold JM. The MATRICS Consensus Cognitive Battery (MCCB): clinical and cognitive correlates. Schizophr Res. 2012;134:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Abdel-Baki A, Brazzini-Poisson V, Marois F, Letendre E, Karelis AD. Effects of aerobic interval training on metabolic complications and cardiorespiratory fitness in young adults with psychotic disorders: a pilot study. Schizophr Res. 2013;149:112–115. [DOI] [PubMed] [Google Scholar]
- 52. Gorczynski P, Faulkner G. Exercise therapy for schizophrenia. Cochrane Datab Syst Rev. 2010;5:CD004412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Heggelund J, Nilsberg GE, Hoff J, Morken G, Helgerud J. Effects of high aerobic intensity training in patients with schizophrenia: a controlled trial. Nord J Psychiatry. 2011;65:269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lieberman DA, Chamberlin B, Medina E, Jr, et al. The power of play: Innovations in Getting Active Summit 2011: a science panel proceedings report from the American Heart Association. Circulation. 2011;123:2507–2516. [DOI] [PubMed] [Google Scholar]
- 55.Bassilios B, Judd F, Pattison P. Why don’t people diagnosed with schizophrenia spectrum disorders (SSDs) get enough exercise? Australas Psychiatry. 2014;22:71–77. [DOI] [PubMed] [Google Scholar]
- 56. Stanton R, Happell B. A systematic review of the aerobic exercise program variables for people with schizophrenia. Curr Sports Med Rep. 2014;13:260–266. [DOI] [PubMed] [Google Scholar]
- 57. Vancampfort D, De Hert M, Vansteenkiste M, et al. The importance of self-determined motivation towards physical activity in patients with schizophrenia. Psychiatry Res. 2013;210:812–818. [DOI] [PubMed] [Google Scholar]
- 58. Kim HJ, Song BK, So B, Lee O, Song W, Kim Y. Increase of circulating BDNF levels and its relation to improvement of physical fitness following 12 weeks of combined exercise in chronic patients with schizophrenia: a pilot study. Psychiatry Res. 2014;220:792–796. [DOI] [PubMed] [Google Scholar]
- 59. Ribeiro-Santos A, Lucio Teixeira A, Salgado JV. Evidence for an immune role on cognition in schizophrenia: a systematic review. Curr Neuropharmacol. 2014;12:273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37:1553–1561. [DOI] [PubMed] [Google Scholar]
- 61. Vasic N, Connemann BJ, Wolf RC, Tumani H, Brettschneider J. Cerebrospinal fluid biomarker candidates of schizophrenia: where do we stand? Eur Arch Psychiatry Clin Neurosci. 2012;262:375–391. [DOI] [PubMed] [Google Scholar]
- 62. Nurjono M, Lee J, Chong SA. A review of brain-derived neurotrophic factor as a candidate biomarker in schizophrenia. Clin Psychopharmacol Neurosci. 2012;10:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Egan MF, Kojima M, Callicott JH, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. [DOI] [PubMed] [Google Scholar]
- 64. Goldberg TE, Iudicello J, Russo C, et al. BDNF val66Met polymorphism significantly affects d’ in verbal recognition memory at short and long delays. Biol Psychol. 2008;77:20–24. [DOI] [PubMed] [Google Scholar]
- 65. Ho BC, Milev P, O’Leary DS, Librant A, Andreasen NC, Wassink TH. Cognitive and magnetic resonance imaging brain morphometric correlates of brain-derived neurotrophic factor Val66Met gene polymorphism in patients with schizophrenia and healthy volunteers. Arch Gen Psychiatry. 2006;63:731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hariri AR, Goldberg TE, Mattay VS, et al. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23:6690–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Scheewe TW, van Haren NE, Sarkisyan G, et al. Exercise therapy, cardiorespiratory fitness and their effect on brain volumes: a randomised controlled trial in patients with schizophrenia and healthy controls. Eur Neuropsychopharmacol. 2013;23:675–685. [DOI] [PubMed] [Google Scholar]
- 68. McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007;164:1791–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kueider AM, Parisi JM, Gross AL, Rebok GW. Computerized cognitive training with older adults: a systematic review. PLoS One. 2012;7:e40588. [DOI] [PMC free article] [PubMed] [Google Scholar]