Abstract
A number of influences have converged that make this Special Theme Issue timely: “A New Direction: Considering Developmentally Sensitive Targets for Very Early Intervention in Schizophrenia”. These factors include: 1. the substantial knowledge about premorbid developmental vulnerabilities to psychosis, especially regarding schizophrenia; 2. the promising results emerging from interventions during the clinical high-risk (CHR) phase of psychosis and; 3. the recognition that the CHR period is a relatively late phase of developmental derailment. These factors have together led to a perspective that even earlier intervention is warranted. This paper briefly summarizes the articles comprising the Special Theme including new data on early neurocognitive development, proposed potential targets for psychosocial and psychopharmacological interventions during the premorbid period as early as pregnancy, and ethical challenges. These thought experiments must be empirically tested, and the ethical challenges overcome as posed by the various interventions, which range from relatively low risk, supportive, psychosocial to higher risk, experimental, pharmacological interventions. All of the interventions proposed require careful study of ethics, safety, potential stigma, feasibility, efficacy and tolerability, and the meaning to the people involved.
Key words: clinical high risk, family high risk, prodrome, premorbid, prenatal, perinatal, primary prevention, endophenotype, critical period, early intervention
The idea of early intervention and prevention for psychotic disorders, has taken hold in the past two decades.1,2 This development likely reflects the current zeitgeist in which similar trends in early interventions for disorders like diabetes or cardiovascular disease,3 dementia4 and a focus on brain plasticity,5 wellness and prolonging healthy aging are dominant. The idea that it might be possible to prevent disorders like schizophrenia has galvanized the field.6 The characterization of a putative prodrome to psychosis, typically called the clinical (or “ultra”) high risk (CHR) state, (or “At Risk Mental State”) consisting primarily of attenuated positive symptoms, has provided a window for early, pre-emptive, interventions.6–8 This syndrome, also named the Attenuated Psychosis Syndrome, is in section 3 of DSM-5, indicating that further study is required.9 Paradoxically, this symptom picture, usually occurring in teenage years or young adulthood, has become recognized as a late stage in the development of psychotic disorders, with a number of earlier stages signaling that the full psychotic manifestations of the disorder could be predicted and perhaps prevented.10,11 Other approaches point to earlier phases. The Basic Symptoms approach identifies internal mental experiences that are thought to characterize an earlier prodromal phase prior to the CHR period.12 The staging perspective10 provides a framework for research and conceptualization of earlier premorbid interventions, perhaps beginning with pregnancy (see figure 1).
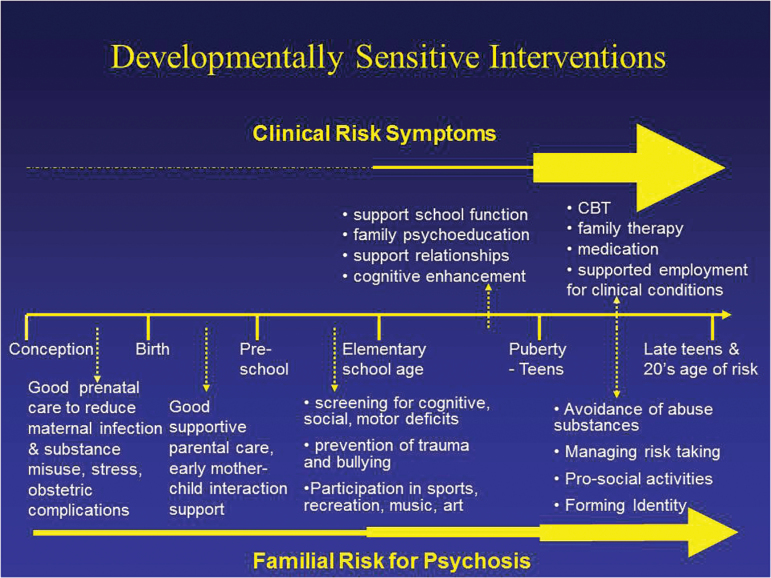
Fig. 1.
Phase specific early intervention & prevention strategies for clinical and familial high risk.
Clinical risk symptoms are contrasted with family risk for psychosis by depicting the greater likelihood of conversion to psychosis to occur in the clinical risk group by the larger yellow arrow. Interventions above the line for the clinical risk group begin around the end of elementary school reflecting the earliest period that prodromal symptoms are typically reported, whereas those below the line begin during pregnancy reflecting more of a primary prevention approach.
In the last decade, five meta-analyses of individuals in the CHR phase have been published regarding interventions to prevent full-blown psychosis, delay its emergence or reduce the liabilities that are associated with its origin and evolution.13–17 We briefly summarize the van der Gaag meta-analysis,17 which is quite comparable in scope and results to the meta-analysis of Stafford et al,16 which was published around the same time in 2013. Van der Gaag et al included five randomized control studies of cognitive behavior therapy (CBT),18–22 two of “integrated psychological therapies”,23,24 three of antipsychotic medication25–29 and one of omega-3 fatty acids.30 According to van der Gaag,17 “overall the risk reduction at 12 months was 54% (RR = 0.463; 95% CI = 0.33–0.64).” After 24 to 48-month follow-up, risk reduction was 37% (RR = .635; 95% CI = 0.44–0.92). They concluded: “early detection and intervention in people at ultra-high risk of developing psychosis can be successful to prevent or delay a first psychosis.” These results are comparable to those for prevention of depression.31 These CHR results illustrate that early intervention can be successful in this late high-risk (HR) phase and set the stage for considering primary preventions.
These promising results give rise to the even more challenging notion, that earlier, pre-teenage interventions might be possible to alter the developmental pathway to schizophrenia. But if so, how would pre-prodromal youth be ethically identified for treatment when they are not seeking help? This is indeed complex. It is likely in the future that biomarkers (and “polygene scores”) will be developed that may identify especially HR youth, that will also generate ethical issues. But for now, the primary viable strategy is to use the family high risk (FHR) approach, even though this approach will only yield roughly 10% of the individuals from these families who will develop psychosis if the sampling strategy is based on selecting offspring of individuals with schizophrenia. Liu et al32 and Ross & Freedman,33 in this issue, make the case, based on the extensive FHR literature that risk factors begin during pregnancy and that impairments which are present from the perinatal period onward can be treatment targets, and that interventions do not need to be limited to “preventing psychosis.” A new generation of FHR studies (FORBOW,34 The Danish High Risk and Resilience Study,35 the Harvard Children’s Development Study (Seidman LJ, Gabrieli J, Keshavan MS, unpublished data), and the Dutch Bipolar Offspring study36) all have early intervention with pre-teen children as a goal.
In essence, young offspring from a family in which schizophrenia is present in a parent (i.e., FHR) are a pre-disorder risk group rather than clinical cases as in CHR/Attenuated Psychosis Syndrome. This approach will only identify a modest number of future cases, because only about 10% of these FHR offspring go on to develop psychosis, and because individuals with schizophrenia with a positive history of schizophrenia in first-degree relatives are a modest minority of all cases of schizophrenia.37 Despite this high false positive rate vis a vis future psychosis, Liu et al argue that there are a number of reasons why these children form a group deserving of low risk interventions: 1.They have a high rate of other behavioral, cognitive and neuromotor problems that could be treated, and 2. There are a variety of family-developmental problems associated with growing up with a parent suffering from schizophrenia.
Liu et al32 propose that the treatment of the parents with illness is ethically appropriate and necessary (and often overlooked), and that proper interventions with the parent provide a window into the treatment of the HR child. These FHR children are at risk not only for a psychotic disorder, but another 50%–60% of them for significant difficulties including socio-emotional, cognitive, neuromotor, and speech-language problems, and various forms of nonpsychotic psychopathology.38–41 Thus, they recommend treating the family system as a unit by focusing on parents and children. While the FHR approach will only identify a modest subset of the HR population, the effects may be significant for that group. The interventions proposed are relevant to the top half of figure 2 and potentially could alter the trajectory to psychosis, CHR states, and other childhood, adolescent, and adult impairments.
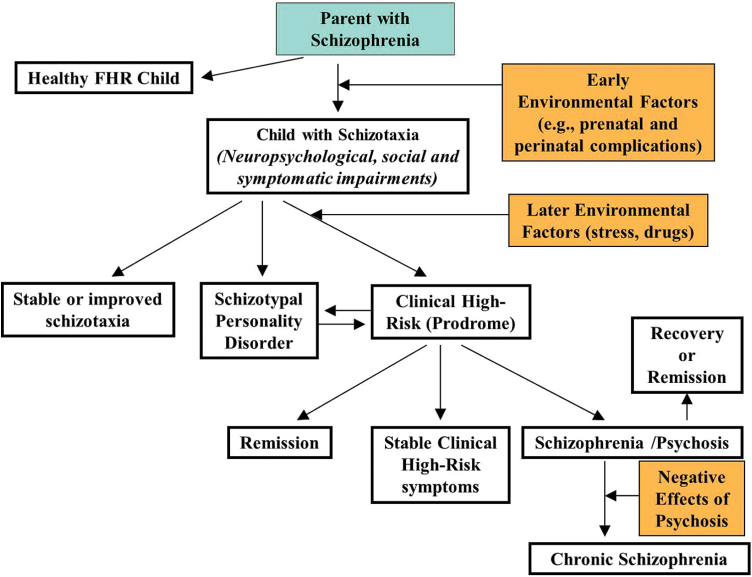
Fig. 2.
Developmental pathway of familial high risk for schizophrenia (adapted from Thermenos et al.59).
This picture depicts the potential development of children who have a parent with schizophrenia. Positive and negative outcomes are shown, as well as the possibility that negative outcomes such as “stable schizotaxia” or schizophrenia can be partially or significantly reversed.
Agnew-Blais et al42 (this issue) illustrates the robustness of premorbid cognitive deficits in schizophrenia in their New England Family cohort studies paper on general intelligence in children who later develop schizophrenia spectrum disorders compared to those with affective psychoses; the latter are not significantly different than controls. The impairments are present at age 4 and 7, and had been found to occur in roughly 40%–45% in age 7 children who later developed schizophrenia spectrum disorder compared to about 7% who did not develop psychosis.43 It’s possible that such cognitive impairments are malleable, as they are milder than in later phases of the illness,32 and that cognitive enhancing treatments designed for CHR adolescents,44 adults with schizophrenia45 or other disorders such as Attention-Deficit Hyperactivity Disorder46 can be implemented with FHR children.
Liu et al32 note that while there is an enormous literature on risk indicators for schizophrenia, beginning with the pioneering work of Barbara Fish in 195247, there is a paucity of research on remediation of these deficits. Liu et al32 address potential psychosocial interventions from pregnancy through the elementary school years. They suggest that parents with psychoses may benefit from: “enhanced prenatal care, social support, parenting skills, reduction of symptoms, and family-centered care across development”. For these children, they suggest a range of early socio-emotional interventions and cognitive remediation.32 Many of these interventions are available in some places but not implemented, nor has their effectiveness been rigorously studied in the FHR population.
Ross and Freedman33 suggest a novel approach toward identifying underlying mechanisms of risk for psychosis through the measurement of endophenotypes during the perinatal period.48 This is innovative because schizophrenia is a neurodevelopmental disorder, whose onset is the end result of brain changes that begin prenatally, but most endophenotype studies have evaluated adolescents or adults who have entered or passed through the age of risk for the disorder. They suggest a treatment approach that targets a well-known schizophrenia endophenotype, P50 sensory gating.49 They based a randomized control trial in 76 healthy pregnant women on the hypothesis that perinatal choline supplementation would increase activation of alpha7 nicotinic receptors and normalize developmental defects associated with receptor deficiencies, including deficits in P50 sensory gating.50,51 Infants whose mothers had received prenatal choline demonstrated improved infant P50 sensory gating, compared to those whose mothers received placebo.51 There was an interaction between choline supplementation with infant genotype on CHRNA7 SNP re3087454 on P50 suppression ratio that increased plausibility. At 40 months follow-up, the children had significantly improved attention, which is often impaired in children who later develop schizophrenia. This preliminary work is promising, and if safe, could be considered for a neurobiologically informed primary prevention intervention for pregnant mothers with schizophrenia. Nevertheless, the idea that choline can prevent schizophrenia is quite speculative, and there are many steps needed to confirm this approach including whether improving P50 or accelerating the maturation of P50 can change the vulnerability to schizophrenia.
Do, Cuenod, and Hensch (this issue),52 invoke developmental neurobiology to provide a framework for understanding the premorbid developmental evolution towards schizophrenia.53 First the concept of “critical periods” (CP) is described as a window of time when a given behavior is especially susceptible to and requires specific environmental influences to develop normally.54 A CP “opens” and should “close” based on environmental input. A potential mechanism for the risk of schizophrenia proposed is paravalbumin-positive interneuron maturation that is involved in contributing to the sequential timing of CP55. The authors propose two potential biomarkers that could be explored as targets for investigation of normalization of CPs: gamma oscillations (measured by event related potentials), and abnormalities of fiber tract connectivity, as measured by diffusion tensor imaging, both of which are associated with oxidative stress.53 They suggest that drugs that target the “hub” of oxidative stress and related dysfunctions (neuroinflammation and NMDAR hypofunction) would be candidates for repairing these developmental anomalies, including Omega 3, sulforaphane and N-acetylcysteine (NAC). Not unlike Ross and Freedman’s model, they consider experimental agents that are neurobiologically informed with respect to developmental risk for schizophrenia.
In the final section of this Special Theme Issue, Dr. Appelbaum evaluates the ethics of the various interventions, which range from low risk, supportive, psychosocial to higher risk, experimental, pharmacological interventions. All of the interventions proposed require careful study of ethics, safety, feasibility, efficacy, and tolerability, and the meaning to the people involved.56,57 Nevertheless, while the idea of primary prevention of schizophrenia will require much study, we believe the time has come to consider this seriously.58
Funding
Supported by the Commonwealth Research Center (SCDMH82101008006) and NIMH R21 MH092840.
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. McGlashan TH, Johannessen JO. Early detection and intervention with schizophrenia: rationale. Schizophr Bull. 1996;22:201–222. [DOI] [PubMed] [Google Scholar]
- 2. Yung AR, McGorry PD, McFarlane CA, Jackson HJ, Patton GC, Rakkar A. Monitoring and care of young people at incipient risk of psychosis. Schizophr Bull. 1996;22:283–303. [DOI] [PubMed] [Google Scholar]
- 3. Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–393. [DOI] [PubMed] [Google Scholar]
- 4. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. [DOI] [PubMed] [Google Scholar]
- 5. Kolb B, Gibb R. Brain plasticity and behaviour in the developing brain. J Can Acad Child Adolesc Psychiatry. 2011;20:265–276. [PMC free article] [PubMed] [Google Scholar]
- 6. Insel TR. The arrival of preemptive psychiatry. Early Interv Psychiatry. 2007;1:5–6. [DOI] [PubMed] [Google Scholar]
- 7. Schultze-Lutter F, Schimmelmann BG, Ruhrmann S. The near Babylonian speech confusion in early detection of psychosis. Schizophr Bull. 2011;37:653–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fusar-Poli P, Borgwardt S, Bechdolf A, et al. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry. 2013;70:107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 10. McGorry PD, Killackey E, Yung A. Early intervention in psychosis: concepts, evidence and future directions. World Psychiatry. 2008;7:148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Keshavan MS, DeLisi LE, Seidman LJ. Early and broadly defined psychosis risk mental states. Schizophr Res. 2011;126:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schutze-Lutter F, Addington J, Ruhrmann S, Klosterkötter J. Schizophrenia Proneness Instrument, Adult Version. Rome, Italy: Giovanni Fioriti; 2007. [Google Scholar]
- 13. Preti A, Cella M. Randomized-controlled trials in people at ultra high risk of psychosis: a review of treatment effectiveness. Schizophr Res. 2010;123:30–36. [DOI] [PubMed] [Google Scholar]
- 14. Marshall M, Rathbone J. Early intervention for psychosis. Cochrane Database Syst Rev. 2011;6:CD004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69:220–229. [DOI] [PubMed] [Google Scholar]
- 16. Stafford MR, Jackson H, Mayo-Wilson E, Morrison AP, Kendall T. Early interventions to prevent psychosis: systematic review and meta-analysis. BMJ. 2013;346:f185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van der Gaag M, Smit F, Bechdolf A, et al. Preventing a first episode of psychosis: meta-analysis of randomized controlled prevention trials of 12 month and longer-term follow-ups. Schizophr Res. 2013;149:56–62. [DOI] [PubMed] [Google Scholar]
- 18. Morrison AP, French P, Walford L, et al. Cognitive therapy for the prevention of psychosis in people at ultra-high risk: randomised controlled trial. Br J Psychiatry. 2004;185:291–297. [DOI] [PubMed] [Google Scholar]
- 19. Morrison AP, French P, Stewart SL, et al. Early detection and intervention evaluation for people at risk of psychosis: multisite randomised controlled trial. BMJ. 2012;344:e2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Addington J, Epstein I, Liu L, French P, Boydell KM, Zipursky RB. A randomized controlled trial of cognitive behavioral therapy for individuals at clinical high risk of psychosis. Schizophr Res. 2011;125:54–61. [DOI] [PubMed] [Google Scholar]
- 21. van der Gaag M, Nieman DH, Rietdijk J, et al. Cognitive behavioral therapy for subjects at ultrahigh risk for developing psychosis: a randomized controlled clinical trial. Schizophr Bull. 2012;38:1180–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McGorry PD, Nelson B, Phillips LJ, et al. Randomized controlled trial of interventions for young people at ultra-high risk of psychosis: twelve-month outcome. J Clin Psychiatry. 2013;74:349–356. [DOI] [PubMed] [Google Scholar]
- 23. Nordentoft M, Thorup A, Petersen L, et al. Transition rates from schizotypal disorder to psychotic disorder for first-contact patients included in the OPUS trial. A randomized clinical trial of integrated treatment and standard treatment. Schizophr Res. 2006;83:29–40. [DOI] [PubMed] [Google Scholar]
- 24. Bechdolf A, Wagner M, Ruhrmann S, et al. Preventing progression to first-episode psychosis in early initial prodromal states. Br J Psychiatry. 2012;200:22–29. [DOI] [PubMed] [Google Scholar]
- 25. McGorry PD, Yung AR, Phillips LJ, et al. Randomized controlled trial of interventions designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Arch Gen Psychiatry. 2002;59:921–928. [DOI] [PubMed] [Google Scholar]
- 26. McGlashan TH, Zipursky RB, Perkins D, et al. Randomized, double-blind trial of olanzapine versus placebo in patients prodromally symptomatic for psychosis. Am J Psychiatry. 2006;163:790–799. [DOI] [PubMed] [Google Scholar]
- 27. Phillips LJ, McGorry PD, Yuen HP, et al. Medium term follow-up of a randomized controlled trial of interventions for young people at ultra high risk of psychosis. Schizophr Res. 2007;96:25–33. [DOI] [PubMed] [Google Scholar]
- 28. Yung AR, Phillips LJ, Nelson B, et al. Randomized controlled trial of interventions for young people at ultra high risk for psychosis: 6-month analysis. J Clin Psychiatry. 2011;72:430–440. [DOI] [PubMed] [Google Scholar]
- 29. McGorry P, van Os J. Redeeming diagnosis in psychiatry: timing versus specificity. Lancet. 2013;381:343–345. [DOI] [PubMed] [Google Scholar]
- 30. Amminger GP, Schäfer MR, Papageorgiou K, et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67:146–154. [DOI] [PubMed] [Google Scholar]
- 31. Cuijpers P, van Straten A, Smit F, Mihalopoulos C, Beekman A. Preventing the onset of depressive disorders: a meta-analytic review of psychological interventions. Am J Psychiatry. 2008;165:1272–1280. [DOI] [PubMed] [Google Scholar]
- 32. Liu CH, Keshavan MS, Tronick E, Seidman LJ. Perinatal risks and childhood premorbid indicators of later psychosis: next steps for early psychosocial interventions. Schizophr Bull. 2015. doi:10.1093/schbul/sbnxxx [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ross RG, Freedman R. Endophenotypes in schizophrenia for the perinatal period: criteria for validation. Schizophr Bull. 2015. doi:10.1093/schbul/sbnxxx [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Uher R, Cumby J, MacKenzie LE, et al. A familial risk enriched cohort as a platform for testing early interventions to prevent severe mental illness. BMC Psychiatry. 2014;14:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ellersgaard D, Thorup A, Jepsen JR, et al. Categorical and dimensional psychopathology among 7-year-old offspring of parents with schizophrenia or bipolar disorder in The Danish High Risk and Resilience Study – VIA 7. Early Interv Psychiatry. 2014;8(suppl S1):1–31. [Google Scholar]
- 36. Mesman E, Nolen WA, Reichart CG, Wals M, Hillegers MH. The Dutch bipolar offspring study: 12-year follow-up. Am J Psychiatry. 2013;170:542–549. [DOI] [PubMed] [Google Scholar]
- 37. Gottesman II, Erlenmeyer-Kimling L. Family and twin strategies as a head start in defining prodromes and endophenotypes for hypothetical early-interventions in schizophrenia. Schizophr Res. 2001;51:93–102. [DOI] [PubMed] [Google Scholar]
- 38. Olin SC, Mednick SA. Risk factors of psychosis: identifying vulnerable populations premorbidly. Schizophr Bull. 1996;22:223–240. [DOI] [PubMed] [Google Scholar]
- 39. Erlenmeyer-Kimling L. Neurobehavioral deficits in offspring of schizophrenic parents: liability indicators and predictors of illness. Am J Med Genet. 2000;97:65–71. [DOI] [PubMed] [Google Scholar]
- 40. Keshavan MS, Kulkarni S, Bhojraj T, et al. Premorbid cognitive deficits in young relatives of schizophrenia patients. Front Hum Neurosci. 2010;3:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Agnew-Blais J, Seidman LJ. Neurocognition in youth and young adults under age 30 at familial risk for schizophrenia: a quantitative and qualitative review. Cogn Neuropsychiatry. 2013;18:44–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Agnew-Blais J, Buka SL, Fitzmaurice GM, Smoller JW, Goldstein JM, Seidman LJ. Early childhood IQ trajectories of adults with schizophrenia and affective psychoses in the New England Family Studies. Schizophr Bull. 2015; doi:10.1093/schbul/sbnxxx [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Seidman LJ, Cherkerzian S, Goldstein JM, Agnew-Blais J, Tsuang MT, Buka SL. Neuropsychological performance and family history in children at age 7 who develop adult schizophrenia or bipolar psychosis in the New England Family Studies. Psychol Med. 2013;43:119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hooker CI, Carol EE, Eisenstein TJ, et al. A pilot study of cognitive training in clinical high risk for psychosis: initial evidence of cognitive benefit. Schizophr Res. 2014;157:314–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Keshavan MS, Vinogradov S, Rumsey J, Sherrill J, Wagner A. Cognitive training in mental disorders: update and future directions. Am J Psychiatry. 2014;171:510–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Diamond A, Lee K. Interventions shown to aid executive function development in children 4 to 12 years old. Science. 2011;333:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fish B, Marcus J, Hans SL, Auerbach JG, Perdue S. Infants at risk for schizophrenia: sequelae of a genetic neurointegrative defect. A review and replication analysis of pandysmaturation in the Jerusalem Infant Development Study. Arch Gen Psychiatry. 1992;49:221–235. [DOI] [PubMed] [Google Scholar]
- 48. Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. [DOI] [PubMed] [Google Scholar]
- 49. Freedman R, Coon H, Myles-Worsley M, et al. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci USA. 1997;94:587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ross RG, Stevens KE, Proctor WR, et al. Research review: cholinergic mechanisms, early brain development, and risk for schizophrenia. J Child Psychol Psychiatry. 2010;51:535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ross RG, Hunter SK, McCarthy L, et al. Perinatal choline effects on neonatal pathophysiology related to later schizophrenia risk. Am J Psychiatry. 2013;170:290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Do KQ, Cuenod M, Hensch TK. Targeting developmental trajectories in the etiology of schizophrenia. Schizophr Bull. 2015; doi:10.1093/schbul/sbnxxx [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Steullet P, Cabungcal JH, Monin A, et al. Redox dysregulation, neuroinflammation, and NMDA receptor hypofunction: a “central hub” in schizophrenia pathophysiology? Schizophr Res. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;26:1003–1017. [DOI] [PubMed] [Google Scholar]
- 55. Takesian AE, Hensch TK. Balancing plasticity/stability across brain development. Prog Brain Res. 2013;207:3–34. [DOI] [PubMed] [Google Scholar]
- 56. Seidman LJ. Listening, meaning and empathy in neuropsychological disorders: Case examples of assessment and treatment. In: Ellison J, Weinstein CS, Hodel–Malinofsky T, eds. Psychotherapist’s Guide to Neuropsychiatric Patients: Diagnostic and Treatment Issues. Washington, DC: American Psychiatric Press, Inc; 1994:1–22. [Google Scholar]
- 57. Warner R. Problems with early and very early intervention in psychosis. Br J Psychiatry Suppl. 2005;187(suppl 48):s104–s107. [DOI] [PubMed] [Google Scholar]
- 58. Sawa A, Seidman LJ. Is prophylactic psychiatry around the corner? combating adolescent oxidative stress for adult psychosis and schizophrenia. Neuron. 2014;83:991–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thermenos HW, Keshavan MS, Juelich RJ, Molokotos E, Whitfield-Gabrieli S, Brent BK, Seidman LJ. A review of neuroimaging studies of young relatives of persons with schizophrenia: a developmental perspective from schizotaxia to schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2013;162:604–635. [DOI] [PubMed] [Google Scholar]