Abstract
Schizophrenia is a neurodevelopmental disorder reflecting a convergence of genetic risk and early life stress. The slow progression to first psychotic episode represents both a window of vulnerability as well as opportunity for therapeutic intervention. Here, we consider recent neurobiological insight into the cellular and molecular components of developmental critical periods and their vulnerability to redox dysregulation. In particular, the consistent loss of parvalbumin-positive interneuron (PVI) function and their surrounding perineuronal nets (PNNs) as well as myelination in patient brains is consistent with a delayed or extended period of circuit instability. This linkage to critical period triggers (PVI) and brakes (PNN, myelin) implicates mistimed trajectories of brain development in mental illness. Strategically introduced antioxidant treatment or later reinforcement of molecular brakes may then offer a novel prophylactic psychiatry.
Key words: oxidative stress, GABA, parvalbumin, perineuronal net, myelin, oligodendrocyte, NAC
Introduction
The protracted progression to psychosis1 represents both a window of vulnerability and opportunity for therapeutic intervention. In a general sense, many manifestations of the disease are thoughts, feelings or actions that are normal in childhood or early adolescence which become inadequate in adulthood. This supports a view that neural processes which are normally shaped during various critical periods (CPs) in development fail to stabilize. Various basic affective, intellectual, and social cognitions, which should be consolidated during brain development, thus seem to remain open to fluctuations in adult patients.
In healthy development, convergent multisensory inputs are progressively selected in order to filter the salient ones and focus attention. This process is fundamental to establish “common sense” knowledge and “natural self-evidence” notions (eg, “the sky is blue and above the earth” or “young people will become old”) which are typically settled and confirmed during development. Their deficits lead to basic symptoms2 and disorders of the self believed to be central to the phenomenology of schizophrenia (SZ; see reviews by Parnas3,4). They affect the core of subjective experience constituting the permanent flow of consciousness.
One important aspect of such anomalies concerns agency and ownership, most likely involving mistimed impulse conduction of corollary discharge in central motor-sensory fibers, due to delays in myelination (as documented below). Indeed, the perception of self is in part the result of differences in response to stimuli evoked from external sources and those generated by the subject—differences which are blurred if corollary discharges are deficient.5,6 If a CP were to remain open, perceptual incoherence and instabilities in basic knowledge would lead to symptoms such as loss of common sense. At a more complex level, ambivalence extending toward indecision about actions and unresolved contradictory ideas (frequently observed in patients) may further reflect the failure to develop clear polarities between positive or negative affect evoked by interpersonal relationships.
Poorly filtered, multiple sensory inputs can become overwhelming and stress inducing. Impaired convergence of interoceptive and exteroceptive sensory inputs may lead to a loose perception of “self” as observed in patients. At the same time, patients make strikingly original connections between images, words, or ideas typical of poetic creativity seen in young people, who also tend to make loose associations between unrelated sensory information, leading to unsuspected phantasmal production. As Baudelaire once wrote, “genius is nothing more nor less than childhood recovered at will.” We propose that prolonged windows of plasticity manifest by incomplete CP closure may instead contribute to mental illness.
Here, we consider a modern neurobiological understanding of the cellular and molecular determinants of developmental CP as they relate to the pathophysiology of SZ. A pivotal role for parvalbumin-positive interneuron (PVI) maturation in both CP opening and closure (by their surrounding perineuronal net [PNN] and myelination) on the one hand, and their impairment in SZ patients and animal models on the other, suggest various CP aspects could be perturbed in the disease. The issue is complicated in that synaptic plasticity itself is likely disrupted for genetic reasons7 and that myelin and long-range connectivity fail to develop normally. Redox dysregulation/oxidative stress reflecting complex interaction between genetic and environmental risk factors in the developmental impairment of PVI/PNN and of myelination will be highlighted. A consideration of SZ symptoms from the perspective of impaired developmental trajectory may suggest optimal timing for preventive treatment with redox regulators/antioxidants, thus offering potentially novel strategies for preventive therapies.
Mechanisms of CP Brain Development
Perhaps the best-studied CP model is the enduring loss of responsiveness in primary visual (V1) cortex to a “lazy” or otherwise deprived eye. The behavioral consequence, amblyopia (poor visual acuity), afflicts 2%–5% of the human population and remains without a known cure in adulthood. From the initial discovery by Hubel and Wiesel over 50 years ago, a biological picture has emerged wherein axons serving the two eyes compete with each other upon first converging onto individual neurons in V1. Molecular tools have now begun to unravel the cellular mechanisms which control the onset and closure of such windows for cortical plasticity.8 Overall, three key concepts have emerged (figure 1):
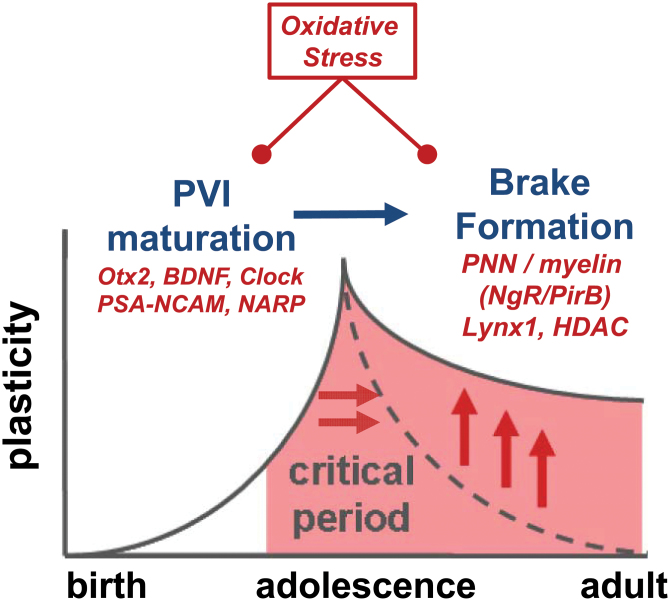
Fig. 1.
Prolonged critical period plasticity as endophenotype. Schizophrenia symptoms may reflect delayed plasticity due to a failure of critical period onset/closure. Our hypothesis is that disease etiologies may dysregulate the expression of molecular brakes which normally follow parvalbumin-positive interneuron (PVI) maturation and extend developmental plasticity. Ultimately, this would destabilize circuit function in the face of undesirable information, as seen in mental illness. A common mechanism impacting PVI/perineuronal nets/myelin is redox dysregulation, which represents a novel target for preventive neurodevelopmental intervention. Alternatively, once PVI functional impairment is detected (eg, mismatch negativity [MMN], γ-oscillations), a supplemental reinforcement of molecular brakes on plasticity may be considered.
1.Excitatory-inhibitory (E-I) circuit balance is a trigger. Specific gamma-aminobutyric acid (GABA) circuit maturation underlies the onset timing of plasticity and is shifted across brain regions consistent with the hierarchical, cascading nature of development.9 Thus, premature gain-of-function by pharmacological agents (benzodiazepines) can trigger precocious onset, whereas genetic (GAD65 deletion) or environmental disruption of GABA circuit function (dark rearing, hearing loss) leads to a delayed plasticity. These manipulations are so powerful that they can determine whether an animal is before, at the peak, or past a plastic window regardless of chronological age. In other words, CP timing per se is plastic.
Among the diversity of inhibitory cell types, it is the PVI large basket cell which serves as the pivotal plasticity switch.9 PVI mature at different rates across brain regions, contributing to the sequential timing of CP. They are dependent upon a variety of extrinsic factors for their health and maintenance, such as brain-derived neurotrophic factor (BDNF), polysialylated-neural cell adhesion molecule (PSA-NCAM), or Otx2 homeoprotein, which appear just ahead of CP onset.8 Notably, PVI networks are interconnected via gap junctions10 and reciprocal GABAergic synapses, capable of synchronizing the excitatory state of large numbers of pyramidal neurons.11 By way of feedback and feed-forward inhibition, these fast-spiking interneurons exert precise temporal control on information flow, favoring summation and transmission of synchronously arriving, convergent input. As such, they allow the binding of information that reaches different pyramidal neurons during a defined and narrow time window,12 as reflected in γ-band oscillations (30–80 Hz)13–17 but can also modulate neuronal activity in the θ-band (4–8 Hz), as well as θ-γ coupling18,19 (figure 2). The maturation of neural synchrony has been suggestively linked to the development of cortical networks.20
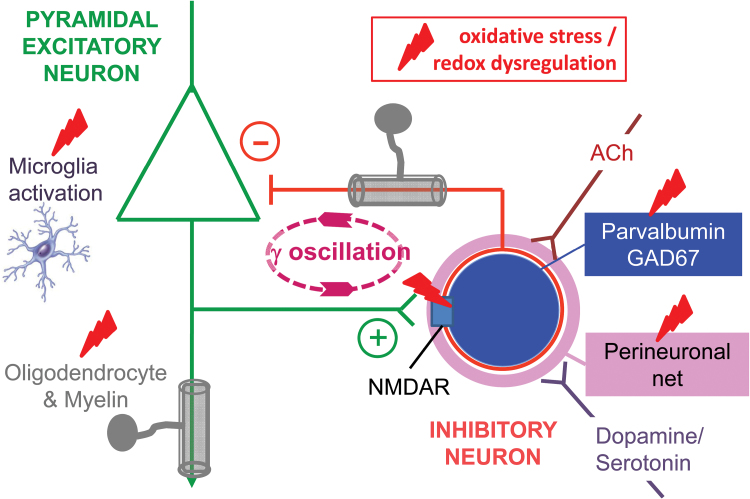
Fig. 2.
Impact of oxidative stress/redox dysregulation on microcircuits. Schematic representation of the impact of oxidative stress/redox dysregulation on cortical microcircuits, including excitatory pyramidal and inhibitory parvalbumin-positive interneuron (PVI) connected reciprocally and supporting γ-oscillations. Oxidative stress/redox dysregulation interacts with inflammatory microglial cells, activating them, and with the N-methyl-d-aspartate receptors, reducing their activity, in both cases leading to a damaging potentiating effect. As a consequence, PVI surrounded by their perineuronal nets and myelin-forming oligodendrocytes are impaired, as manifested by alterations of local oscillations and distant synchronization. These cellular and molecular changes are known to alter critical periods timing. Likewise, microcircuits are affected by cholinergic and by catecholaminergic inputs.
2. Synaptic pruning and homeostasis mediate plasticity. Once PVI enter an optimal state, local circuit rewiring in response to sensory experience is enabled. PVI are the first responders to discordant sensory input, shifting their visual response rapidly.8 Subsequently, a biochemical sequence of synaptic pruning and homeostasis is triggered in pyramidal cells8,9: initially freeing synaptic space through the action of secreted proteases (tissue-type plasminogen activator, tPA) to cleave cell adhesion molecules, synapses, and axons, prior to sprouting new connections through homeostatic growth processes involving tumor necrosis factor alpha (TNFα) and protein synthesis.
3. Molecular “brakes” limit adult plasticity to stabilize neural networks initially sculpted by experience. As PVI mature, they gradually acquire an extracellular coating, called the PNN, which tightly encapsulates the PVI cell body and proximal neurites. In other words, CP closure may reflect an active process on top of the long-held view of declining plasticity factors. A growing number of late-expressing, brake-like factors act to limit excessive circuit rewiring in adulthood. These include structural obstacles which physically prevent neurite pruning and outgrowth, such as PNN or myelin in the extracellular matrix.8,21 Both chondroitin sulfate proteoglycans in the PNN and inhibitory myelin molecules bind to the Nogo receptor (NgR),22 which acts in a complex with immune genes such as PirB to restrict CP plasticity.23,24 In addition, functional brakes (eg, Lynx1)25 can dampen neuromodulatory systems (eg, acetylcholine, serotonin) which endogenously regulate E-I circuit balance.
Windows of plasticity, therefore, arise between the maturation of an optimal E-I balance controlling the machinery of synaptic pruning and a later emerging consolidating set of brake-like factors, which can be reversed (figure 1). Notably, the same principles are repeatedly being observed across brain regions. Adult spatial learning and object recognition reflect bistable PVI states in hippocampal area CA326, focally recapitulating basic CP mechanism throughout life. Adult prefrontal cortex (PFC) encodes acoustic (music) preferences established during a CP early in life and is rendered malleable again later by histone deacetylase (HDAC) inhibitors.27 Interestingly, lifting this epigenetic brake (with valproate) in mice renews prefrontal neuron recruitment and has been successfully applied to healthy human adults learning absolute pitch discrimination.28 The basic cellular principles defined in mouse brain may, therefore, translate to humans.
Relevance of CP Mechanism for SZ
PVI/PNN Impairment
Compelling evidence suggests an imbalance between glutamatergic excitation and GABAergic inhibition in SZ.29,30 Anomalies associated with PVI are a hallmark of the disease, including their reduced density in the hippocampal formation31,32 and alterations at the level of basket and chandelier cells in the dorsolateral prefrontal cortex (DLPFC) of postmortem brains.33 Moreover, the extracellular matrix (PNN) that surrounds most PVI is weakened in the DLPFC,34 entorhinal cortex, and amygdala of SZ patients.35 Current data suggest an impaired PVI maturation rather than a deficit due to the chronic nature of the illness. Therefore, dysfunction of the PVI network may lead to abnormal neuronal activity in patients, including oscillatory activity within θ, β, and γ ranges.36–38 Ultimately, interneuron dysfunction could contribute to altered sensory perception,39 deficits in working memory,18,40 attention,41 and learning.42
Recent studies have revealed anomalies in hippocampal and/or prefrontal PVI in many preclinical animal models aiming to reproduce genetic vulnerabilities43–46 or environmental risk factors47 such as prenatal maternal stress,48 maternal and perinatal immune challenge,49,50 hypoxia,51,52 early-life iron deficiency,53 maternal separation,54 and social isolation.55,56 Similarly, nongenetic developmental models also result in altered prefrontal PVI.57,58
Oligodendrocyte/Myelination Impairment
Convergent evidence points to oligodendrocytes and myelination defects in SZ59–61 both at the neurocytochemical and transcriptomic, as well as neuroimaging levels. Structural alterations of myelinated fibers are reported in gray and white matter of PFC and caudate nucleus of patients.62 Most studies find a decrease in oligodendrocyte density in thalamic nuclei and PFC.63–66 In the latter, an age-related increase in number of mature oligodendrocytes normally observed in control subjects is absent in SZ patients.67 Microarray analysis of patients’ prefrontal and anterior cingulate cortices reveal a reduced expression of several genes related to myelin and oligodendrocytes68–70 and an altered expression of genes coding for cell-cycle maintenance or arrest.71 Altogether, these findings point to impaired oligodendrocyte maturation and myelination.
Such anomalies in SZ could affect axonal integrity and conduction velocity72 with a consequence of disrupting temporal control over long-range brain synchronization. Studies using magnetic resonance techniques such as diffusion tensor imaging (DTI) also suggest abnormal white matter along different fiber tracts, including within and between frontal and temporal areas in SZ.73–75 Although less consistent than in chronic patients, white matter anomalies are also observed in first-episode patients and ultra high-risk subjects.73,75,76 In summary, imaging data indicate that white matter deficits are present before/at illness onset and persist in chronic SZ patients, suggesting a neurodevelopmental component to this impairment.
Vulnerability to Redox Dysregulation/Oxidative Stress
Oxidative stress is defined as an imbalance between pro-oxidants and antioxidants, resulting in macromolecular damage and disruption of redox signaling and control. Recent advances in redox biology show that thiol/disulfide redox systems are regulated under dynamic, nonequilibrium conditions, with distinct redox potentials among subcellular compartments. Apart from traditional “redox signaling” used to describe processes in which a specific oxidative signal is conveyed through a specific redox element to direct a particular cellular response (eg, NF-E2-related factor-2 [Nrf-2] pathway), many general signaling systems including kinases and transmembrane ionopores (eg, N-methyl-D-Aspartate-receptor [NMDA-R]) can be regulated by “redox-sensing” thiols of critical proteins in the pathway.77
Both redox sensing and redox signaling use thiol switches, especially cysteine (Cys) residues in proteins which are sensitive to covalent or noncovalent modification (ie, reversible oxidation, nitrosylation, glutathionylation), leading to structural and functional alteration of target proteins. This has led to the emerging concept of “orthogonal control of signal transduction systems by redox-sensing mechanisms.”78 Moreover, because redox potentials are controlled differently in subcellular compartments, the same signaling mechanism can be differentially controlled by the prevailing local redox environment. This thiol-based redox regulation has crucial importance in nervous tissues known to present complex compartmentalization.
PVI/PNN Vulnerability
To support high-frequency neuronal synchronization, fast-spiking PVI are energy demanding. This requires optimal mitochondrial performance79 with enhanced metabolic activity and oxidative phosphorylation80 leading to elevated mitochondria-generated reactive oxygen species (ROS).81 Consequently, PVI need well-regulated antioxidant systems to neutralize ROS and maintain proper redox state. These cells are vulnerable to redox dysregulation, whether induced by a compromised antioxidant system or ROS overproduction (figure 2).
In a transgenic mouse model (Gclm KO) carrying low levels of the main nonprotein cellular redox regulator and antioxidant, glutathione (GSH), similar to some SZ patients,82–84 a deficit in prefrontal and hippocampal PVI is observed, impairing high-frequency neuronal synchronization.85–87 Compromised GSH synthesis restricted only to PVI is sufficient to affect these cells86 and oxidative stress precedes the PVI deficit.87 Under these conditions of PVI-specific redox dysregulation, CP plasticity (as measured in V1) is notably prolonged concomitant with their loss of PNN.88
PVI can also be affected when antioxidant systems other than GSH are compromised. A reduced number of PVI is observed in mice bearing a deletion of selenoprotein P, a glycoprotein with antioxidant properties89 or for PGC-1α, a transcription factor regulating mitochondria function and ROS metabolism.90 Furthermore, superoxide overproduction by nicotinamide adenine dinucleotide phosphate reduced form (NADPH) oxidase (NOX) is also deleterious to PVI,91 and NOX inhibition prevents the PVI impairment induced by social isolation.56
Most importantly, prefrontal cortical PVI are more vulnerable to a redox dysregulation during postnatal development than later in life. A pharmacologically induced transient postnatal deficit in GSH yields both immediate and long-term decreased PVI density in anterior cingulate cortex (ACC).92–94 In Gclm-KO mice,95 administration of a dopamine reuptake inhibitor (GBR-12909), which partially mimics dopamine release during psychosocial stress96 and produces ROS via the catabolism of dopamine,97,98 permanently decreases PVI density in the ACC when applied during postnatal development, but not in adulthood.85 Thus, immature PVI may have a less robust antioxidant defense system than mature cells.
Alternatively, molecular mechanisms underlying PVI maturation may be highly sensitive to a redox imbalance. Interestingly, the vulnerability of prefrontal immature PVI is associated with the absence of fully mature PNN, which protects these cells against oxidative stress.86 In turn, excess oxidative stress also affects PNN,86 which reciprocally impact PVI. Indeed, the maturation and phenotypic maintenance of PVI requires translocation of a noncell autonomous homeobox protein, Otx2, through its affinity with PNN.99,100
One interesting example of relevance to SZ is the role of Clock genes in the neocortex. Circadian rhythms have been shown to regulate redox homeostasis in the brain, and disruption of circadian genes causes neuronal oxidative damage.101 Aberrant circadian rhythmicity has long been linked to mental illness, and very recent work identifies a postnatal emergence of rhythmic gene expression outside the suprachiasmatic nucleus.102 Maturation of PVI is particularly sensitive to Clock/Bmal gene deletion with the consequence of protracted CP timing into adulthood. Cell-specific transcriptome profiling of PVI by FACS reveals altered expression of genes downstream of CLOCK related to the respiratory chain (eg, Cox and Nduf family genes) and redox regulation (eg, Gpx4). Thus, circadian clock genes may preserve PVI integrity and prevent the manic behaviors observed when they are disrupted.
A role for redox dysregulation/oxidative stress in the developmental impairment of PVI has been further substantiated by recent studies on experimental neurodevelopmental models that do not directly manipulate the redox system. First, the widely studied neonatal ventral hippocampal lesion model also displays oxidative stress and PVI defects, both of which are prevented by juvenile and adolescent treatment with the antioxidant and GSH precursor, N-acetylcysteine (NAC).103 Second, a single injection of the DNA-alkylating agent methylazoxy-methanol acetate (MAM) during pregnancy, which also causes SZ phenotypes in adult rats, leads to anomalies in PVI and neuronal synchronization.57,104 MAM-treated rats show decreased brain GSH levels105 and increased oxidative stress (A. A. Grace and K. Q. Do, unpublished results, 2014).
In the MAM model, a reduction in ventral hippocampal PV expression is sufficient to induce an augmented dopaminergic system function and behavioral hyper-responsivity to amphetamine.106 Moreover, evidence in patients33 and in MAM rats suggest that in the PFC there is a general decrease in PV levels rather than PVI loss, whereas in hippocampus it appears that neuronal loss occurs.107 Third, genetic models (eg, DISC1, PRODH, G71) all exhibit elevated oxidative stress consistent with their PVI abnormalities. In addition, preliminary results in collaboration with the Coyle and La Mantia labs, respectively, reveal oxidative stress-induced PVI/PNN loss in d-serine racemase KO and 22q11 mouse models (K. Q. Do, 44th US Soc for Neuroscience, 2014). Together, these studies demonstrate that redox dysregulation during a critical developmental period can disrupt normal PVI maturation representing one core pathophysiological mechanism in SZ.
Oligodendrocyte Sensitivity to Redox State
Oligodendrocytes are sensitive to redox dysregulation and oxidative stress due to their intrinsic properties and functions. During the myelination process, they have a high metabolic rate to produce and maintain membranes.108–110 High metabolic activity is known to generate copious amounts of ROS,111 whereas oligodendrocytes display surprisingly low GPx activity and intrinsically low GSH levels.112,113
In SZ patients, a direct role for redox control of myelin is seen in the positive correlation between prefrontal GSH levels and functional anisotropy along the cingulum bundle, which connects the ACC to limbic structures.114 The importance of redox control for white matter integrity and oligodendrocyte development is further supported by animal models and in vitro research. Redox state controls oligodendrocyte maturation as well as the switch between proliferation (reduced state) and differentiation (oxidized state).114,115
Abnormal redox control would interfere with oligodendrocyte development. Consistent with this, Gclm-KO mice bearing a 70% GSH deficit within brain and increased oxidative stress marks in the PFC and ventral hippocampus85,87 have lower levels of mature oligodendrocytes and myelin in the peripubertal period114 (figure 2). Although myelination reaches similar levels in adult Gclm-KO and wild-type mice, DTI reveals persistent impairment of white matter integrity and reduced conduction velocity in the fornix and anterior commissure.116
At the cellular level, GSH deficiency in oligodendrocyte progenitors leads to cell-cycle arrest and reduces proliferation which can be reversed by the antioxidant NAC.114,115 At a molecular level, the switch from proliferation to early differentiation is controlled by the platelet-derived growth factor receptor (PDGFR-Fyn) pathway.114,117 Nonreceptor tyrosine kinase, Fyn, is activated by redox dysregulation,117 and interestingly, is impaired in early psychosis patients associated with a vulnerability to redox dysregulation.114 Postmortem studies in the PFC of SZ patients also reveal abnormal Fyn expression (Stanley database).118 Oxidative stress/abnormal redox control during development could therefore contribute to myelin disruptions associated with SZ.
Anomalies of Plasticity in SZ
Pathophysiological changes in SZ are thus consistent with a removal of “brakes” on plasticity, such as the PNN loss, altered E-I balance, or myelin deficits. All these factors are induced by redox dysregulation/oxidative stress among others, which may then yield prolonged network instability.88 Thus, mistimed developmental trajectories of brain plasticity may underlie in part the pathogenesis of SZ. Although limited to date, there is emergent evidence recently reporting dysfunctional plasticity in SZ.119–122 Deficits in long-term potentiation-like plasticity in SZ patients probed by transcranial direct current stimulation are notably restricted to chronic patients, whereas first onset patients do not differ significantly from healthy controls with a trend toward increased plasticity.123 Excessive plastic states likely precede the progressive degenerative process as in other animal models and brain disorders.25,124
Outlook for Preventive Developmental Therapies
Early detection and early intervention in psychotic disorders has become a major focus both in clinical and translational research in psychiatry. The considerations discussed above strongly support this strategy. They highlight mechanisms and drug targets which might modify disease progression or even contribute to prevention, and pave the way for biomarkers needed for early detection and use as efficacy endpoints (apart from clinical symptoms) in clinical trials.
Two noninvasive biomarkers might be worth exploring: (1) anomalies of γ-oscillations as a robust marker of the PVI microcircuit and (2) anomalies of fiber tract connectivity as measured by DTI. As discussed above, oxidative stress or redox dysregulation contribute crucially to PVI and myelin impairment in SZ. Moreover, as reviewed in Steullet et al,125 dysregulation of redox homeostasis is fully reciprocal to neuroinflammation and NMDA-R hypofunction (figure 2). This triad constitutes one central pathophysiological “hub” upon which various genetic and environmental risk factors converge during neurodevelopment, leading to structural and functional connectivity impairments. Drugs targeting the triadic hub of oxidative stress, neuroinflammation, or NMDA-R hypofunction125 would be promising candidates to prevent deleterious effects on cortical and hippocampal PVI and oligodendrocytes/myelin. As such treatments (eg, omega-3, sulforaphane, NAC) should be applied in early phases of the illness, they should be devoid of serious side-effects. Adolescent treatment with atypical antipsychotics (risperidone, clozapine) in the prenatal immune activation model can also prevent hippocampal volume loss and lateral ventricle enlargement as well as behavioral abnormalities.126 However, whether this is mediated through PVI/myelin and CP plasticity is unknown and their serious side effects would temper their use from a preventive perspective.
Converging evidence also points to membrane phospholipid and polyunsaturated fatty acid (PUFA) defects in early course and chronic SZ.127 As membrane PUFAs are highly susceptible to free radical insults, increased oxidative stress may be one of the mechanisms responsible for membrane PUFA reduction. Indeed, oxidative stress in first-episode SZ is associated with decreased PUFA content and increased breakdown products of membrane lipids,128 possibly with a familial basis.129,130 In particular, decreased membrane PUFA levels are associated with increased levels of total lipid peroxides, decreased levels of vitamin E, and increased severity of negative symptoms.131,132 The use of PUFA, particularly omega-3, is a potential alternative and adjunct to current antipsychotics treatments. Omega-3 fatty acids are effective in reducing oxidative stress in preclinical models133,134 and dietary supplementation may be beneficial in psychiatric conditions.135 Omega-3 might be most promising in preventing the transition to psychosis for at-risk mental state subjects.136
Sulforaphane is a dietary isothiocyanate found in broccoli sprouts and has gained attention as a natural, and safe, anticancer compound.137–140 Evidence suggests that sulforaphane is able to reduce oxidative stress by activating the Nrf-2 antioxidant response element pathway, upregulating phase II detoxification enzymes and antioxidant proteins.141 Sulforaphane was shown to protect against antipsychotic-induced oxidative stress in dopaminergic neuroblastoma cells by increasing GSH and quinone oxidoreductase (NQO1) activity.142 In mice injected with phencyclidine, sulforaphane attenuated prepulse inhibition (PPI) deficits in a dose-dependent manner, as well as reducing hyperlocomotion at higher doses.143
NAC, known as a GSH precursor, also has antioxidant and anti-inflammatory properties per se and can regulate glutamatergic neurotransmission. It represents a safe and potential compound for the prevention or treatment of SZ and other psychiatric disorders.144 NAC is deacetylated to form cysteine, the rate-limiting precursor of GSH, and therefore yields upregulation of GSH synthesis when cells face an excess of ROS production. NAC also participates to the control of the intracellular redox state by supplying cysteine into the cystine/cysteine redox couple.145
In Gclm-KO mice, NAC prevents PVI and PNN deficits induced by an oxidative insult during postnatal development85 and normalizes most of the neurochemical profiles, including the glutamine/glutamate ratio known to be altered in a similar way in first-episode SZ patients.146 Likewise, NAC reduces oxidative stress, protects prefrontal PVI, and prevents deficits in MMN and PPI in the developing rat neonatal ventral hippocampal lesion model which is independent of redox manipulation and shows E-I imbalance.103
NAC also prevents myelin impairment following a maternal immune challenge,147 reestablishes normal function of the cystine/glutamate antiporter and GSH levels in MAM-injected rats,148 normalizes extracellular glutamate levels, and attenuates behavioral anomalies in phencyclidine-treated rats.149 It reduces oxidative stress, rescues abnormal behavioral phenotype in G72/G30 transgenic mice,150 and reverses the social isolation-induced changes in corticostriatal monoamine levels.151 Thus, NAC has beneficial effects across a very diverse panel of animal models relevant to SZ.
In a first randomized double-blind placebo-controlled trial, an add-on treatment of NAC in chronic patients diminished negative symptoms and improved global functioning.152 Two additional studies also demonstrate that chronic patients improved with supplemental NAC, particularly in their negative symptoms.153,154 Moreover, NAC normalized neuronal activity and connectivity and improved MMN,155 an auditory-related, NMDA-dependent evoked potential typically impaired in SZ.156 Although not performed during development, these studies can be considered as a proof-of-concept, pointing to the efficacy of an antioxidant and possibly favoring the closure of a pathological CP.
NAC also increased phase synchronization of neuronal activity over the left parieto-temporal, the right temporal, and the bilateral prefrontal regions.157 However, the beneficial effect of NAC has to be taken with caution because the current data are based on only a few studies showing relatively moderate clinical improvement in chronic SZ patients, probably due to the low bioavailability and membrane permeability of NAC which enters the brain at a very modest rate.158 The development of other molecules with better bioavailability and blood-brain barrier permeability is therefore needed.
Interestingly, Du and Grace159 have reported that peripubertal administration of diazepam prevents the increase in dopamine neuron activity and blunts the behavioural hyper-responsivity to amphetamine in the developmental MAM rats. This effect of diazepam may be mediated by normalizing PVI/CP plasticity because CP delay in the GAD65 deletion model can be rescued by enhancing GABA transmission directly with diazepam.160
Because the disruption of PVI maturation and myelination would combine to delay or prolong CP plasticity (figure 1), it may also be useful to strategically introduce well-timed brakes on plasticity or to lift them as needed (as in amblyopia recovery21). Several candidate factors have recently been identified,8 including PNN-promoting transcription factors (Otx2),94 modulators of cholinergic transmission (Lynx1),25 or epigenetic regulators (HDAC).161 An intriguing target may be the NgR/PirB signalling complex which interacts with both chondroitin sulfate proteoglycans in the PNN as well as myelin molecules.22,23 Notably, the plasticity modulating effect of NgR deletion has recently been traced to PVI circuits specifically.162 Further methods to modulate PVI maturational state, ideally from the blood periphery are desirable as therapeutic agents. The peculiar localization of noncell autonomous factors, such as Otx2 synthesis within the accessible choroid plexus,163 is particularly appealing.
Conclusions
Commonly observed abnormalities in the PVI and myelin of SZ patients or associated animal models would predict altered levels of brain plasticity, such as greater perceptual learning in SZ patients.164 Proper timing of redox regulation is crucial to control the proliferation and differentiation of PVI and oligodendrocytes, which in turn contribute to CP timing. Therapeutic approaches aimed at thwarting the emerging redox imbalance may efficiently prevent the impairment of these CP triggers and brakes underlying developmental trajectories of cognitive function. Such approaches, including antioxidants/redox modulators, cognitive interventions in childhood and adolescence, or enhancement of molecular brakes thereafter might become viable strategies toward prophylactic psychiatry.165
Funding
National Institute of Mental Health (NIMH) (1P50MH094271 to T.K.H.); Swiss National Science Foundation (#31-116689, #310030_135736/1, #320030_122419); National Center of Competence in Research “SYNAPSY - The Synaptic Bases of Mental Diseases” (#51AU40_125759); Avina Foundation; Damm-Etienne Foundation; Alamaya Foundation (to K.Q.D.).
Acknowledgments
This review is dedicated to the memory of Prof Pierre Bovet (1946–2014). We thank Drs Hirofumi Morishita for graphics in figure 1 and Philipp Baumann for his helpful comments. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193. [DOI] [PubMed] [Google Scholar]
- 2. Gross G, Huber G, Klosterkotter J, et al. Bonner Skala für die Beurteilung von Basissymptomen. Berlin: Springer; 1987. [Google Scholar]
- 3. Parnas J, Bovet P, Zahavi D. Schizophrenic autism: clinical phenomenology and pathogenetic implications. World Psychiatry. 2002;1:131–136. [PMC free article] [PubMed] [Google Scholar]
- 4. Parnas J, Henriksen MG. Disordered self in the schizophrenia spectrum: a clinical and research perspective. Harv Rev Psychiatry. 2014;22:251–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Picard F, Friston K. Predictions, perception, and a sense of self. Neurology. 2014;83:1112–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ford JM, Mathalon DH. Electrophysiological evidence of corollary discharge dysfunction in schizophrenia during talking and thinking. J Psychiatr Res. 2004;38:37–46. [DOI] [PubMed] [Google Scholar]
- 7. Crabtree GW, Gogos JA. Synaptic plasticity, neural circuits, and the emerging role of altered short-term information processing in schizophrenia. Front Synaptic Neurosci. 2014;6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Takesian AE, Hensch TK. Balancing plasticity/stability across brain development. Prog Brain Res. 2013;207:3–34. [DOI] [PubMed] [Google Scholar]
- 9. Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. [DOI] [PubMed] [Google Scholar]
- 10. Fukuda T, Kosaka T, Singer W, Galuske RA. Gap junctions among dendrites of cortical GABAergic neurons establish a dense and widespread intercolumnar network. J Neurosci. 2006;26:3434–3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. [DOI] [PubMed] [Google Scholar]
- 12. Fries P, Nikolić D, Singer W. The gamma cycle. Trends Neurosci. 2007;30:309–316. [DOI] [PubMed] [Google Scholar]
- 13. Cardin JA, Carlén M, Meletis K, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fuchs EC, Zivkovic AR, Cunningham MO, et al. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53:591–604. [DOI] [PubMed] [Google Scholar]
- 15. Gulyás AI, Szabó GG, Ulbert I, et al. Parvalbumin-containing fast-spiking basket cells generate the field potential oscillations induced by cholinergic receptor activation in the hippocampus. J Neurosci. 2010;30:15134–15145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Massi L, Lagler M, Hartwich K, Borhegyi Z, Somogyi P, Klausberger T. Temporal dynamics of parvalbumin-expressing axo-axonic and basket cells in the rat medial prefrontal cortex in vivo. J Neurosci. 2012;32:16496–16502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J, Monyer H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron. 2010;68:557–569. [DOI] [PubMed] [Google Scholar]
- 19. Wulff P, Ponomarenko AA, Bartos M, et al. Hippocampal theta rhythm and its coupling with gamma oscillations require fast inhibition onto parvalbumin-positive interneurons. Proc Natl Acad Sci U S A. 2009;106:3561–3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Uhlhaas PJ, Roux F, Rodriguez E, Rotarska-Jagiela A, Singer W. Neural synchrony and the development of cortical networks. Trends Cogn Sci. 2010;14:72–80. [DOI] [PubMed] [Google Scholar]
- 21. Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK. Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J Neurosci. 2010;30:14964–14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dickendesher TL, Baldwin KT, Mironova YA, et al. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat Neurosci. 2012;15:703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Atwal JK, Pinkston-Gosse J, Syken J, et al. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–970. [DOI] [PubMed] [Google Scholar]
- 24. Bochner DN, Sapp RW, Adelson JD, et al. Blocking PirB up-regulates spines and functional synapses to unlock visual cortical plasticity and facilitate recovery from amblyopia. Sci Transl Med. 2014;6:258ra140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morishita H, Miwa JM, Heintz N, Hensch TK. Lynx1, a cholinergic brake, limits plasticity in adult visual cortex. Science. 2010;330:1238–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Donato F, Rompani SB, Caroni P. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature. 2013;504:272–276. [DOI] [PubMed] [Google Scholar]
- 27. Yang EJ, Lin EW, Hensch TK. Critical period for acoustic preference in mice. Proc Natl Acad Sci U S A. 2012;109(suppl 2):17213–17220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gervain J, Vines BW, Chen LM, et al. Valproate reopens critical-period learning of absolute pitch. Front Syst Neurosci. 2013;7:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lisman JE, Coyle JT, Green RW, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O’Donnell P. Adolescent onset of cortical disinhibition in schizophrenia: insights from animal models. Schizophr Bull. 2011;37:484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang AY, Lohmann KM, Yang CK, et al. Bipolar disorder type 1 and schizophrenia are accompanied by decreased density of parvalbumin- and somatostatin-positive interneurons in the parahippocampal region. Acta Neuropathol. 2011;122:615–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang ZJ, Reynolds GP. A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res. 2002;55:1–10. [DOI] [PubMed] [Google Scholar]
- 33. Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mauney SA, Athanas KM, Pantazopoulos H, et al. Developmental pattern of perineuronal nets in the human prefrontal cortex and their deficit in schizophrenia. Biol Psychiatry. 2013;74:427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pantazopoulos H, Woo TU, Lim MP, Lange N, Berretta S. Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Arch Gen Psychiatry. 2010;67:155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McNally JM, McCarley RW, Brown RE. Impaired GABAergic neurotransmission in schizophrenia underlies impairments in cortical gamma band oscillations. Curr Psychiatry Rep. 2013;15:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. [DOI] [PubMed] [Google Scholar]
- 38. Uhlhaas PJ, Singer W. Neuronal dynamics and neuropsychiatric disorders: toward a translational paradigm for dysfunctional large-scale networks. Neuron. 2012;75:963–980. [DOI] [PubMed] [Google Scholar]
- 39. Atallah BV, Bruns W, Carandini M, Scanziani M. Parvalbumin-expressing interneurons linearly transform cortical responses to visual stimuli. Neuron. 2012;73:159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Roux F, Wibral M, Mohr HM, Singer W, Uhlhaas PJ. Gamma-band activity in human prefrontal cortex codes for the number of relevant items maintained in working memory. J Neurosci. 2012;32:12411–12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rouhinen S, Panula J, Palva JM, Palva S. Load dependence of beta and gamma oscillations predicts individual capacity of visual attention. J Neurosci. 2013;33:19023–19033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carlén M, Meletis K, Siegle JH, et al. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry. 2012;17:537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Carlson GC, Talbot K, Halene TB, et al. Dysbindin-1 mutant mice implicate reduced fast-phasic inhibition as a final common disease mechanism in schizophrenia. Proc Natl Acad Sci U S A. 2011;108:E962–E970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fazzari P, Paternain AV, Valiente M, et al. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 2010;464:1376–1380. [DOI] [PubMed] [Google Scholar]
- 45. Hikida T, Jaaro-Peled H, Seshadri S, et al. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc Natl Acad Sci U S A. 2007;104:14501–14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wen L, Lu YS, Zhu XH, et al. Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proc Natl Acad Sci U S A. 2010;107:1211–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brown AS. The environment and susceptibility to schizophrenia. Prog Neurobiol. 2011;93:23–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stevens HE, Su T, Yanagawa Y, Vaccarino FM. Prenatal stress delays inhibitory neuron progenitor migration in the developing neocortex. Psychoneuroendocrinology. 2013;38:509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jenkins TA, Harte MK, Stenson G, Reynolds GP. Neonatal lipopolysaccharide induces pathological changes in parvalbumin immunoreactivity in the hippocampus of the rat. Behav Brain Res. 2009;205:355–359. [DOI] [PubMed] [Google Scholar]
- 50. Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 2008;22:469–486. [DOI] [PubMed] [Google Scholar]
- 51. Dell’Anna E, Geloso MC, Magarelli M, Molinari M. Development of GABA and calcium binding proteins immunoreactivity in the rat hippocampus following neonatal anoxia. Neurosci Lett. 1996;211:93–96. [DOI] [PubMed] [Google Scholar]
- 52. Komitova M, Xenos D, Salmaso N, et al. Hypoxia-induced developmental delays of inhibitory interneurons are reversed by environmental enrichment in the postnatal mouse forebrain. J Neurosci. 2013;33:13375–13387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Callahan LS, Thibert KA, Wobken JD, Georgieff MK. Early-life iron deficiency anemia alters the development and long-term expression of parvalbumin and perineuronal nets in the rat hippocampus. Dev Neurosci. 2013;35:427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brenhouse HC, Andersen SL. Nonsteroidal anti-inflammatory treatment prevents delayed effects of early life stress in rats. Biol Psychiatry. 2011;70:434–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Harte MK, Powell SB, Swerdlow NR, Geyer MA, Reynolds GP. Deficits in parvalbumin and calbindin immunoreactive cells in the hippocampus of isolation reared rats. J Neural Transm. 2007;114:893–898. [DOI] [PubMed] [Google Scholar]
- 56. Schiavone S, Sorce S, Dubois-Dauphin M, et al. Involvement of NOX2 in the development of behavioral and pathologic alterations in isolated rats. Biol Psychiatry. 2009;66:384–392. [DOI] [PubMed] [Google Scholar]
- 57. Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29:2344–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tseng KY, Lewis BL, Hashimoto T, et al. A neonatal ventral hippocampal lesion causes functional deficits in adult prefrontal cortical interneurons. J Neurosci. 2008;28:12691–12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chew LJ, Fusar-Poli P, Schmitz T. Oligodendroglial alterations and the role of microglia in white matter injury: relevance to schizophrenia. Dev Neurosci. 2013;35:102–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Davis KL, Stewart DG, Friedman JI, et al. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry. 2003;60:443–456. [DOI] [PubMed] [Google Scholar]
- 61. Takahashi N, Sakurai T, Davis KL, Buxbaum JD. Linking oligodendrocyte and myelin dysfunction to neurocircuitry abnormalities in schizophrenia. Prog Neurobiol. 2011;93:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Uranova NA, Vikhreva OV, Rachmanova VI, Orlovskaya DD. Ultrastructural alterations of myelinated fibers and oligodendrocytes in the prefrontal cortex in schizophrenia: a postmortem morphometric study. Schizophr Res Treatment. 2011;2011:325789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Byne W, Kidkardnee S, Tatusov A, Yiannoulos G, Buchsbaum MS, Haroutunian V. Schizophrenia-associated reduction of neuronal and oligodendrocyte numbers in the anterior principal thalamic nucleus. Schizophr Res. 2006;85:245–253. [DOI] [PubMed] [Google Scholar]
- 64. Hof PR, Haroutunian V, Copland C, Davis KL, Buxbaum JD. Molecular and cellular evidence for an oligodendrocyte abnormality in schizophrenia. Neurochem Res. 2002;27:1193–1200. [DOI] [PubMed] [Google Scholar]
- 65. Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res. 2004;67:269–275. [DOI] [PubMed] [Google Scholar]
- 66. Vostrikov VM, Uranova NA, Orlovskaya DD. Deficit of perineuronal oligodendrocytes in the prefrontal cortex in schizophrenia and mood disorders. Schizophr Res. 2007;94:273–280. [DOI] [PubMed] [Google Scholar]
- 67. Vostrikov V, Uranova N. Age-related increase in the number of oligodendrocytes is dysregulated in schizophrenia and mood disorders. Schizophr Res Treatment. 2011;2011:174689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dracheva S, Davis KL, Chin B, Woo DA, Schmeidler J, Haroutunian V. Myelin-associated mRNA and protein expression deficits in the anterior cingulate cortex and hippocampus in elderly schizophrenia patients. Neurobiol Dis. 2006;21:531–540. [DOI] [PubMed] [Google Scholar]
- 69. Hakak Y, Walker JR, Li C, et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci U S A. 2001;98:4746–4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tkachev D, Mimmack ML, Ryan MM, et al. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. [DOI] [PubMed] [Google Scholar]
- 71. Katsel P, Davis KL, Li C, et al. Abnormal indices of cell cycle activity in schizophrenia and their potential association with oligodendrocytes. Neuropsychopharmacology. 2008;33:2993–3009. [DOI] [PubMed] [Google Scholar]
- 72. Whitford TJ, Ford JM, Mathalon DH, Kubicki M, Shenton ME. Schizophrenia, myelination, and delayed corollary discharges: a hypothesis. Schizophr Bull. 2012;38:486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fitzsimmons J, Kubicki M, Shenton ME. Review of functional and anatomical brain connectivity findings in schizophrenia. Curr Opin Psychiatry. 2013;26:172–187. [DOI] [PubMed] [Google Scholar]
- 74. Kanaan RA, Kim JS, Kaufmann WE, Pearlson GD, Barker GJ, McGuire PK. Diffusion tensor imaging in schizophrenia. Biol Psychiatry. 2005;58:921–929. [DOI] [PubMed] [Google Scholar]
- 75. Kyriakopoulos M, Bargiotas T, Barker GJ, Frangou S. Diffusion tensor imaging in schizophrenia. Eur Psychiatry. 2008;23:255–273. [DOI] [PubMed] [Google Scholar]
- 76. Samartzis L, Dima D, Fusar-Poli P, Kyriakopoulos M. White matter alterations in early stages of schizophrenia: a systematic review of diffusion tensor imaging studies. J Neuroimaging. 2014;24:101–110. [DOI] [PubMed] [Google Scholar]
- 77. Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol. 2008;295:C849–C868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jones DP. Redox sensing: orthogonal control in cell cycle and apoptosis signalling. J Intern Med. 2010;268:432–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kann O, Huchzermeyer C, Kovács R, Wirtz S, Schuelke M. Gamma oscillations in the hippocampus require high complex I gene expression and strong functional performance of mitochondria. Brain. 2011;134:345–358. [DOI] [PubMed] [Google Scholar]
- 80. Harris JJ, Jolivet R, Attwell D. Synaptic energy use and supply. Neuron. 2012;75:762–777. [DOI] [PubMed] [Google Scholar]
- 81. Kann O, Papageorgiou IE, Draguhn A. Highly energized inhibitory interneurons are a central element for information processing in cortical networks. J Cereb Blood Flow Metab. 2014;34:1270–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Do K, Trebesinger A, Kirsten-Kruger M, et al. Schizophrenia: glutathione deficit in cerebro spinal fluid and prefrontal cortex in vivo. Eur J Neurosci. 2000;12:3721–3728. [DOI] [PubMed] [Google Scholar]
- 83. Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol. 2011;14:123–130. [DOI] [PubMed] [Google Scholar]
- 84. Yao JK, Leonard S, Reddy R. Altered glutathione redox state in schizophrenia. Dis Markers. 2006;22:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cabungcal JH, Steullet P, Kraftsik R, Cuenod M, Do KQ. Early-life insults impair parvalbumin interneurons via oxidative stress: reversal by N-acetylcysteine. Biol Psychiatry. 2013;73:574–582. [DOI] [PubMed] [Google Scholar]
- 86. Cabungcal JH, Steullet P, Morishita H, et al. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc Natl Acad Sci U S A. 2013;110:9130–9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Steullet P, Cabungcal JH, Kulak A, et al. Redox dysregulation affects the ventral but not dorsal hippocampus: impairment of parvalbumin neurons, gamma oscillations, and related behaviors. J Neurosci. 2010;30:2547–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Morishita H, Cabungcal JH, Chen Y, Do KQ, Hensch TK. Prolonged period of cortical plasticity upon redox dysregulation in fast-spiking interneurons. Biol Psychiatry. doi: 10.1016/j.biopsych.2014.12.026. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pitts MW, Raman AV, Hashimoto AC, Todorovic C, Nichols RA, Berry MJ. Deletion of selenoprotein P results in impaired function of parvalbumin interneurons and alterations in fear learning and sensorimotor gating. Neuroscience. 2012;208:58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lucas EK, Markwardt SJ, Gupta S, et al. Parvalbumin deficiency and GABAergic dysfunction in mice lacking PGC-1alpha. J Neurosci. 2010;30:7227–7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Behrens MM, Ali SS, Dao DN, et al. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. [DOI] [PubMed] [Google Scholar]
- 92. Cabungcal JH, Nicolas D, Kraftsik R, Cuénod M, Do KQ, Hornung JP. Glutathione deficit during development induces anomalies in the rat anterior cingulate GABAergic neurons: relevance to schizophrenia. Neurobiol Dis. 2006;22:624–637. [DOI] [PubMed] [Google Scholar]
- 93. Kulak A, Steullet P, Cabungcal JH, et al. Redox dysregulation in the pathophysiology of schizophrenia and bipolar disorder: insights from animal models. Antioxid Redox Signal. 2013;18:1428–1443. [DOI] [PubMed] [Google Scholar]
- 94. Steullet P, Cabungcal JH, Kulak A, Cuenod M, Schenk F, Do KQ. Glutathione deficit in animal models of schizophrenia. In: O’Donnell P, ed. Animal Models of Schizophrenia and Related Disorders. New York, NY: Humana Press; 2011:149–188. [Google Scholar]
- 95. Kulak A, Cuenod M, Do KQ. Behavioral phenotyping of glutathione-deficient mice: relevance to schizophrenia and bipolar disorder. Behav Brain Res. 2012;226:563–570. [DOI] [PubMed] [Google Scholar]
- 96. Lataster J, Collip D, Ceccarini J, et al. Psychosocial stress is associated with in vivo dopamine release in human ventromedial prefrontal cortex: a positron emission tomography study using [¹⁸F]fallypride. Neuroimage. 2011;58:1081–1089. [DOI] [PubMed] [Google Scholar]
- 97. Cadet JL, Brannock C. Free radicals and the pathobiology of brain dopamine systems. Neurochem Int. 1998;32:117–131. [DOI] [PubMed] [Google Scholar]
- 98. Rabinovic AD, Hastings TG. Role of endogenous glutathione in the oxidation of dopamine. J Neurochem. 1998;71:2071–2078. [DOI] [PubMed] [Google Scholar]
- 99. Beurdeley M, Spatazza J, Lee HH, et al. Otx2 binding to perineuronal nets persistently regulates plasticity in the mature visual cortex. J Neurosci. 2012;32:9429–9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Miyata S, Komatsu Y, Yoshimura Y, Taya C, Kitagawa H. Persistent cortical plasticity by upregulation of chondroitin 6-sulfation. Nat Neurosci. 2012;15:414–422. [DOI] [PubMed] [Google Scholar]
- 101. Musiek ES, Lim MM, Yang G, et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest. 2013;123:5389–5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kobayashi Y, Ye Z, Hensch TK. Clock genes control cortical critical period timing. Neuron. 2015;86:264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Cabungcal JH, Counotte DS, Lewis EM, et al. Juvenile antioxidant treatment prevents adult deficits in a developmental model of schizophrenia. Neuron. 2014;83:1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Penschuck S, Flagstad P, Didriksen M, Leist M, Michael-Titus AT. Decrease in parvalbumin-expressing neurons in the hippocampus and increased phencyclidine-induced locomotor activity in the rat methylazoxymethanol (MAM) model of schizophrenia. Eur J Neurosci. 2006;23:279–284. [DOI] [PubMed] [Google Scholar]
- 105. Fonnum F, Lock EA. The contributions of excitotoxicity, glutathione depletion and DNA repair in chemically induced injury to neurones: exemplified with toxic effects on cerebellar granule cells. J Neurochem. 2004;88:513–531. [DOI] [PubMed] [Google Scholar]
- 106. Boley AM, Perez SM, Lodge DJ. A fundamental role for hippocampal parvalbumin in the dopamine hyperfunction associated with schizophrenia. Schizophr Res. 2014;157:238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Gill KM, Grace AA. Corresponding decrease in neuronal markers signals progressive parvalbumin neuron loss in MAM schizophrenia model. Int J Neuropsychopharmacol. 2014;17:1609–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bradl M, Lassmann H. Oligodendrocytes: biology and pathology. Acta Neuropathol. 2010;119:37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Cammer W. Carbonic anhydrase in oligodendrocytes and myelin in the central nervous system. Ann N Y Acad Sci. 1984;429:494–497. [DOI] [PubMed] [Google Scholar]
- 110. El Waly B, Macchi M, Cayre M, Durbec P. Oligodendrogenesis in the normal and pathological central nervous system. Front Neurosci. 2014;8:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62:649–671. [DOI] [PubMed] [Google Scholar]
- 112. Baud O, Greene AE, Li J, Wang H, Volpe JJ, Rosenberg PA. Glutathione peroxidase-catalase cooperativity is required for resistance to hydrogen peroxide by mature rat oligodendrocytes. J Neurosci. 2004;24:1531–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Juurlink BH, Thorburne SK, Hertz L. Peroxide-scavenging deficit underlies oligodendrocyte susceptibility to oxidative stress. Glia. 1998;22:371–378. [DOI] [PubMed] [Google Scholar]
- 114. Monin A, Baumann PS, Griffa A, et al. Glutathione deficit impairs myelin maturation: relevance for white matter integrity in schizophrenia patients. Mol Psychiatry. 2014;doi: 10.1038/mp.2014.88. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 115. Noble M, Smith J, Power J, Mayer-Pröschel M. Redox state as a central modulator of precursor cell function. Ann N Y Acad Sci. 2003;991:251–271. [DOI] [PubMed] [Google Scholar]
- 116. Corcoba A SP, Duarte JM, van de Looij Y, Gruetter R, Do KQ. Impaired white matter integrity in fornix and anterior commissure in a schizophrenia mouse model of redox dysregulation. Biol Psychiatry. 2014;75:175S. [Google Scholar]
- 117. Li Z, Dong T, Proschel C, Noble M. Chemically diverse toxicants converge on Fyn and c-Cbl to disrupt precursor cell function. PLoS Biol. 2007;5:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ohnuma T, Kato H, Arai H, McKenna PJ, Emson PC. Expression of Fyn, a non-receptor tyrosine kinase in prefrontal cortex from patients with schizophrenia and its correlation with clinical onset. Brain Res Mol Brain Res. 2003;112:90–94. [DOI] [PubMed] [Google Scholar]
- 119. Daskalakis ZJ, Christensen BK, Fitzgerald PB, Chen R. Dysfunctional neural plasticity in patients with schizophrenia. Arch Gen Psychiatry. 2008;65:378–385. [DOI] [PubMed] [Google Scholar]
- 120. McClintock SM, Freitas C, Oberman L, Lisanby SH, Pascual-Leone A. Transcranial magnetic stimulation: a neuroscientific probe of cortical function in schizophrenia. Biol Psychiatry. 2011;70:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Cavuş I, Reinhart RM, Roach BJ, et al. Impaired visual cortical plasticity in schizophrenia. Biol Psychiatry. 2012;71:512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Mears RP, Spencer KM. Electrophysiological assessment of auditory stimulus-specific plasticity in schizophrenia. Biol Psychiatry. 2012;71:503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Hasan A, Nitsche MA, Rein B, et al. Dysfunctional long-term potentiation-like plasticity in schizophrenia revealed by transcranial direct current stimulation. Behav Brain Res. 2011;224:15–22. [DOI] [PubMed] [Google Scholar]
- 124. Beste C, Wascher E, Dinse HR, Saft C. Faster perceptual learning through excitotoxic neurodegeneration. Curr Biol. 2012;22:1914–1917. [DOI] [PubMed] [Google Scholar]
- 125. Steullet P, Cabungcal JH, Monin A, et al. Redox dysregulation, neuroinflammation, and NMDA receptor hypofunction: a “central hub” in schizophrenia pathophysiology? Schizophr Res. 2014;doi: 10.1016/j.schres.2014.06.021. [Epub ahead of print] Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Piontkewitz Y, Arad M, Weiner I. Tracing the development of psychosis and its prevention: what can be learned from animal models. Neuropharmacology. 2012;62:1273–1289. [DOI] [PubMed] [Google Scholar]
- 127. Yao JK, Keshavan MS. Antioxidants, redox signaling, and pathophysiology in schizophrenia: an integrative view. Antioxid Redox Signal. 2011;15:2011–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Yao JK, Stanley JA, Reddy RD, Keshavan MS, Pettegrew JW. Correlations between peripheral polyunsaturated fatty acid content and in vivo membrane phospholipid metabolites. Biol Psychiatry. 2002;52:823–830. [DOI] [PubMed] [Google Scholar]
- 129. Keshavan MS, Stanley JA, Montrose DM, Minshew NJ, Pettegrew JW. Prefrontal membrane phospholipid metabolism of child and adolescent offspring at risk for schizophrenia or schizoaffective disorder: an in vivo 31P MRS study. Mol Psychiatry. 2003;8:316–323. [DOI] [PubMed] [Google Scholar]
- 130. Keshavan MS PJ, Ward R. Are membrane phospholipid changes in schizophrenia familial? Biol Psychiatry. 1993;33:45A.7678377 [Google Scholar]
- 131. Bentsen H, Solberg DK, Refsum H, et al. Bimodal distribution of polyunsaturated fatty acids in schizophrenia suggests two endophenotypes of the disorder. Biol Psychiatry. 2011;70:97–105. [DOI] [PubMed] [Google Scholar]
- 132. Arvindakshan M, Sitasawad S, Debsikdar V, et al. Essential polyunsaturated fatty acid and lipid peroxide levels in never-medicated and medicated schizophrenia patients. Biol Psychiatry. 2003;53:56–64. [DOI] [PubMed] [Google Scholar]
- 133. Ali HA, Afifi M, Abdelazim AM, Mosleh YY. Quercetin and omega 3 ameliorate oxidative stress induced by aluminium chloride in the brain. J Mol Neurosci. 2014;53:654–660. [DOI] [PubMed] [Google Scholar]
- 134. Zugno AI, Chipindo HL, Volpato AM, et al. Omega-3 prevents behavior response and brain oxidative damage in the ketamine model of schizophrenia. Neuroscience. 2014;259:223–231. [DOI] [PubMed] [Google Scholar]
- 135. Freeman MP, Hibbeln JR, Wisner KL, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry. 2006;67:1954–1967. [DOI] [PubMed] [Google Scholar]
- 136. Amminger GP, Schäfer MR, Papageorgiou K, et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67:146–154. [DOI] [PubMed] [Google Scholar]
- 137. Fahey JW, Haristoy X, Dolan PM, et al. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc Natl Acad Sci U S A. 2002;99:7610–7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Talalay P, Fahey JW, Healy ZR, et al. Sulforaphane mobilizes cellular defenses that protect skin against damage by UV radiation. Proc Natl Acad Sci U S A. 2007;104:17500–17505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Gibbs A, Schwartzman J, Deng V, Alumkal J. Sulforaphane destabilizes the androgen receptor in prostate cancer cells by inactivating histone deacetylase 6. Proc Natl Acad Sci U S A. 2009;106:16663–16668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Li Y, Zhang T, Korkaya H, et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res. 2010;16:2580–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Guerrero-Beltrán CE, Calderón-Oliver M, Pedraza-Chaverri J, Chirino YI. Protective effect of sulforaphane against oxidative stress: recent advances. Exp Toxicol Pathol. 2012;64:503–508. [DOI] [PubMed] [Google Scholar]
- 142. Mas S, Gassó P, Trias G, Bernardo M, Lafuente A. Sulforaphane protects SK-N-SH cells against antipsychotic-induced oxidative stress. Fundam Clin Pharmacol. 2012;26:712–721. [DOI] [PubMed] [Google Scholar]
- 143. Shirai Y, Fujita Y, Hashimoto K. Effects of the antioxidant sulforaphane on hyperlocomotion and prepulse inhibition deficits in mice after phencyclidine administration. Clin Psychopharmacol Neurosci. 2012;10:94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Berk M, Malhi GS, Gray LJ, Dean OM. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol Sci. 2013;34:167–177. [DOI] [PubMed] [Google Scholar]
- 145. Mandal PK, Seiler A, Perisic T, et al. System x©- and thioredoxin reductase 1 cooperatively rescue glutathione deficiency. J Biol Chem. 2010;285:22244–22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. das Neves Duarte JM, Kulak A, Gholam-Razaee MM, Cuenod M, Gruetter R, Do KQ. N-acetylcysteine normalizes neurochemical changes in the glutathione-deficient schizophrenia mouse model during development. Biol Psychiatry. 2012;71:1006–1014. [DOI] [PubMed] [Google Scholar]
- 147. Paintlia MK, Paintlia AS, Contreras MA, Singh I, Singh AK. Lipopolysaccharide-induced peroxisomal dysfunction exacerbates cerebral white matter injury: attenuation by N-acetyl cysteine. Exp Neurol. 2008;210:560–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Cleland JH, Lutgen V, Raddatz N, Qualmann K, Choi S, Baker DA. The potential role for system xc- in schizophrenic pathology. Paper presented at: US Society for Neuroscience, 2013. [Google Scholar]
- 149. Lutgen V, Qualmann K, Resch J, Kong L, Choi S, Baker DA. Reduction in phencyclidine induced sensorimotor gating deficits in the rat following increased system xc⁻ activity in the medial prefrontal cortex. Psychopharmacology (Berl). 2013;226:531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Otte DM, Sommersberg B, Kudin A, et al. N-acetyl cysteine treatment rescues cognitive deficits induced by mitochondrial dysfunction in G72/G30 transgenic mice. Neuropsychopharmacology. 2011;36:2233–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Möller M, Du Preez JL, Viljoen FP, Berk M, Harvey BH. N-acetyl cysteine reverses social isolation rearing induced changes in cortico-striatal monoamines in rats. Metab Brain Dis. 2013;28:687–696. [DOI] [PubMed] [Google Scholar]
- 152. Berk M, Copolov D, Dean O, et al. N-acetyl cysteine as a glutathione precursor for schizophrenia–a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 2008;64:361–368. [DOI] [PubMed] [Google Scholar]
- 153. Bulut M, Savas HA, Altindag A, Virit O, Dalkilic A. Beneficial effects of N-acetylcysteine in treatment resistant schizophrenia. World J Biol Psychiatry. 2009;10:626–628. [DOI] [PubMed] [Google Scholar]
- 154. Farokhnia M, Azarkolah A, Adinehfar F, et al. N-acetylcysteine as an adjunct to risperidone for treatment of negative symptoms in patients with chronic schizophrenia: a randomized, double-blind, placebo-controlled study. Clin Neuropharmacol. 2013;36:185–192. [DOI] [PubMed] [Google Scholar]
- 155. Lavoie S, Murray MM, Deppen P, et al. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology. 2008;33:2187–2199. [DOI] [PubMed] [Google Scholar]
- 156. Umbricht D, Schmid L, Koller R, Vollenweider FX, Hell D, Javitt DC. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Arch Gen Psychiatry. 2000;57:1139–1147. [DOI] [PubMed] [Google Scholar]
- 157. Carmeli C, Knyazeva MG, Cuénod M, Do KQ. Glutathione precursor N-acetyl-cysteine modulates EEG synchronization in schizophrenia patients: a double-blind, randomized, placebo-controlled trial. PLoS One. 2012;7:e29341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Farr SA, Poon HF, Dogrukol-Ak D, et al. The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem. 2003;84:1173–1183. [DOI] [PubMed] [Google Scholar]
- 159. Du Y, Grace AA. Peripubertal diazepam administration prevents the emergence of dopamine system hyperresponsivity in the MAM developmental disruption model of schizophrenia. Neuropsychopharmacology. 2013;38:1881–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282:1504–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Silingardi D, Scali M, Belluomini G, Pizzorusso T. Epigenetic treatments of adult rats promote recovery from visual acuity deficits induced by long-term monocular deprivation. Eur J Neurosci. 2010;31:2185–2192. [DOI] [PubMed] [Google Scholar]
- 162. Stephany CÉ, Chan LL, Parivash SN, et al. Plasticity of binocularity and visual acuity are differentially limited by nogo receptor. J Neurosci. 2014;34:11631–11640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Spatazza J, Lee HH, Di Nardo AA, et al. Choroid-plexus-derived Otx2 homeoprotein constrains adult cortical plasticity. Cell Rep. 2013;3:1815–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Norton DJ, McBain RK, Ongur D, et al. Perceptual training strongly improves visual motion perception in schizophrenia. Brain Cogn. 2011; 77:248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Sawa A, Seidman LJ. Is prophylactic psychiatry around the corner? combating adolescent oxidative stress for adult psychosis and schizophrenia. Neuron. 2014;83:991–993. [DOI] [PMC free article] [PubMed] [Google Scholar]