Abstract
Immunology has traditionally focused on the lymphocytes circulating among primary lymphoid organs while the large reservoir of tissue-resident T cells have received relatively less attention. In epithelia, these populations are comprised of significant, and sometimes exclusive, subsets of γδ T cells that are highly specialized in promoting tissue homeostasis. As the epithelial layers of the skin and gut are permanently exposed to the environment, they are continually subject to injury and therefore require highly efficient repair processes to maintain barrier functions. Here, we review the role of γδ T cells in promoting wound healing, a critical and complex process occurring in the skin and other barrier sites.
Keywords: Epithelial γδ T cell, dendritic epidermal T cell, DETC, skin wound repair
Introduction
As the interface between an organism and its environment, epithelial surfaces provide essential protection against water and nutrient loss, pathogen entry and physical damage. Mechanisms to efficiently detect breaches in barrier integrity and promote return to homeostasis are therefore critical for host survival. γδ T cells are found in the surface-contacting epithelia of all mammals [1]. These lymphocytes have evolved to meet the site-specific challenges encountered at barrier tissues: anti-microbial defense, tumor surveillance, tissue homeostasis and repair after damage [2–5]. Indeed, by regulating inflammation after wounding and epithelial cell growth and survival, epidermal-resident γδ T cells appear to have specialized roles in promoting the repair of damaged skin [6–8].
The skin is constantly exposed to physical, chemical and radiant energy damage from the environment. After cutaneous injury, a complex intercellular crosstalk regulates distinct but overlapping phases of wound repair [9]. Critically, keratinocytes at wound edges migrate and proliferate to fill wounded areas and reestablish a protective barrier [9]. Reepithelialization and other wound repair responses are highly dependent upon resident γδ T cells, evidenced by the delayed wound closure observed in the skin of mice lacking γδ T cells [6]. Importantly, the wound healing functions of epithelial resident T cells extends beyond the skin, as γδ T cells resident to the epithelia of the gut, lungs and eyes also contribute to damage repair at these sites [10–15].
While we now appreciate the importance of epithelial γδ T cells in the maintenance of tissues, we are only beginning to dissect the mechanisms regulating the function of these cells and the pathways they use to promote homeostasis. A more complete understanding of how epithelial-resident γδ T cells are functionally controlled would have a clear impact on the treatment of a number of human disease conditions.
Distribution of epithelial-resident γδ T cells
In contrast to conventional T cells, γδ TCR-bearing cells are rare in lymphoid organs but abundant in surface epithelia [1]. This tropism is associated with highly restricted TCR γδ gene rearrangements, in some tissues resulting in oligo- or mono-clonal populations [1]. γδ T cells also differ from conventional T cells in that they do not require priming to elaborate effector activity and appear partially activated in the resting state [16]. Collectively, these properties have led to the notion that epithelial-resident γδ T cells recognize and respond to tissue-specific distress. The vanguard nature of tissue-resident γδ T cells is highlighted by the fact that they are the first T cells to develop during ontogeny; the initial wave of progenitors to emerge from the embryonic thymus are Vγ3Vδ1+ (Garman nomenclature [17]) cells that home to the epidermis followed by Vγ4Vδ1+ progenitors that seed the reproductive tract [18] Subsequently, waves of γδ T cell progenitors emerge from the thymus and migrate to the lung, lymphoid sites and intestines and that coincide with the first appearance of cells bearing diverse αβ TCRs [18].
The skin is the largest barrier organ and the first site colonized by γδ T cells [19]. The most superficial skin layer, the epidermis, is 3–5 cells deep in mice (6–10 cell layers in humans) and overlays a much thicker dermal layer. While keratinocytes comprise the majority of cells in the epidermis and serve as the primary physical barrier to the environment, resident T cells, myeloid-derived cells and melanocytes comprise functionally integral components of epidermal tissue [9]. In the mouse, the epidermis is home to a single prototypic γδ T cell population. Termed dendritic epidermal T cells (DETC), these cells are the sole epidermal-resident T cell population in wildtype mice and uniformly express a Vγ3Vδ1 (Vγ5Vδ1, alternative nomenclature [20]) TCR gene rearrangement [17, 21, 22]. DETC form a dense stationary network of cells amongst basal keratinocytes and display a highly dendritic morphology. The majority of these dendrites are long and sessile extensions, polarized toward the apical epidermal layer, which coordinate with multiple surrounding keratinocytes while shorter, basally oriented projections frequently extend and retract [23]. While the significance of this morphology is unclear, it likely facilitates the tissue surveillance function of DETC.
In contrast to mice, the distribution of T cells in human skin appears much more complex. The epidermis contains a mixture of both αβ and γδ-expressing compartments, the latter being enriched for Vδ1+ T cells [7, 24, 25]. While there is not a human T cell population with a dendritic morphology, analysis of clinical skin samples has revealed that both αβ and γδ epidermal T cells have the capacity to regulate epidermal homeostasis by producing insulin-like growth factor 1 (IGF-1), a critical growth and survival factor for epithelial cells [7]. Importantly, T cells isolated from chronic skin wound samples lack the capacity to produce IGF-1 and appear functionally anergic [7]. Dysfunction of epidermal T cells could therefore be a factor in the development of non-healing skin wounds.
Similar to the epidermis, the epithelium of the gut is a large barrier tissue and home to vast numbers of γδ T cells, in some species accounting for half of all the T cells in the organism. Often referred to as intraepithelial lymphocytes (IEL), these highly motile gut-resident cells are comprised of both αβ and γδ T cell populations, the latter being biased toward Vγ1 and Vγ5 TCR expression in mice and Vδ1 usage in humans [26, 27]. While the functions of intestinal-resident T cell populations has been enigmatic, studies using models of intestinal injury repair have revealed that γδ, but not αβ, IELs in the gut can secrete epithelial cell growth factors, akin to epidermal-resident T cells, that maintain intestinal epithelia and promote repair after injury [11, 28, 29].
Responses of γδ T cells to wounding
In normal healthy skin, keratinocyte proliferation, maturation, migration, terminal differentiation and corneocyte shedding are all perfectly balanced to continually replenish and maintain the epidermis. In this steady state, DETC critically regulate keratinocyte survival via low but constitutive production of IGF-1 [30, 31]. After skin trauma, a complex multi-cellular tissue response is orchestrated to cleanse and repair damaged areas. Almost immediately after injury, coagulation and vasoconstriction occur to prevent blood loss [9]. DETC are one of the first cellular components of the skin to respond following cutaneous damage [32]. Within hours of injury, DETC bordering the wound edge begin to retract their dendrites and become rounded [33]. This striking change in morphology is accompanied by upregulation of the activation marker, CD69, and the release of soluble factors that regulate various aspects of tissue repair: recruitment of inflammatory cells, deposition of matrix proteins and production of epithelial cell mitogens [34]. Importantly, DETC activation and responses to injury are completely dependent upon Vγ3Vδ1 TCR engagement of an unknown ligand on wound-proximal keratinocytes [33–35].
Nearly concurrent to DETC activation, myeloid-derived cells, attracted by soluble factors released by damaged tissue, begin to enter wound sites. Neutrophils are the first circulating leukocytes to arrive and begin to phagocytose bacteria and debris as well as produce reactive-oxygen species to sterilize damaged tissue [36, 37]. Within 24 hours, circulating monocytes have entered damaged tissue and differentiate into functional macrophages, the predominant inflammatory cell 3 days after injury [37]. Macrophages have a pivotal role in wound repair by removing dead cells, particularly spent neutrophils, and secreting factors that promote angiogenesis and tissue growth [36, 37]. In the absence of DETC, neutrophils appear to infiltrate wounds normally but macrophage accumulation is significantly delayed [38]. As DETC are able to produce CCL3 and CCL4, chemokines important for macrophage homing, this defect could be do to an inability to attract inflammatory cells to the wound sites [39]. In addition to providing chemotactic signals, DETC are able to promote macrophage recruitment by regulating production of hyaluronan, a glycosoaminocglycan that accumulates in damaged tissues and is required for inflammatory cell motility [38]. DETC do not produce hyaluronan but production can be induced in keratinocytes via DETC-derived keratinocyte growth factor (KGF) signaling [38, 40]. DETC therefore posses multiple pathways to promote macrophage recruitment, a critical component in the early repair process.
In addition to the antimicrobial activities of inflammatory cells, epithelial cells are able to upregulate production of antimicrobial peptides and proteins (AMP) upon wounding to prevent infection and promote repair [41]. β-defensin 3, S100A8 and RegIIIγ are three AMPs that are induced in keratinocytes after damage [42–44]. A subset of DETC appears to possess the ability to regulate keratinocyte AMP production via IL-17 release [45]. While murine epidermal γδ T cells have previously been shown to produce this proinflammatory cytokine, the ability of human epidermal γδ T cells to release IL-17 is unknown [46]. However, both human and mouse dermis contains significant populations of γδ T cells that are able to release IL-17 and therefore may impact AMP production [47, 48].
Beyond regulating the early and inflammatory wound repair phases, DETC regulate the reestablishment of a physical protective barrier by keratinocytes. TCRδ-deficient mice display reduced hyperepithelial thickening at wound sites and delayed reepithelialization, defects that can be attributed to reduced cellular proliferation and/or increased keratinocyte apoptosis in the tissue [30]. As epithelial cell mitogens, KGF-1 and KGF-2 can potently induce keratinocyte proliferation. While resting DETC do not produce these factors, the genes for KGF-1 and KGF-2 become highly transcribed in DETC upon activation [28]. The importance of DETC-derived KGF in mediating the proliferative response of keratinocytes is apparent during in vitro skin wound cultures, in which the defective healing response of TCRδ-deficient skin samples can be rescued by introduction of activated DETC or addition of exogenous KGF-1[6].
Tissue maintenance by γδ T cells extends beyond the skin as gut-resident γδ+, but not as αβ+, IELs appear to posses the ability to regulate intestinal homeostasis via KGF production [28]. The functional importance of γδ+ intestinal IELs is observed in the DSS mouse model of ulcerative colitis, which replicates the repair of epithelial erosion found in human inflammatory bowel disease. In this experimental system, mice given dextran sulfate sodium (DSS) in drinking water develop intestinal epithelial lesions that heal several weeks after DSS treatment is stopped [49]. In animals recovering from DSS exposure, γδ IELs accumulate near epithelial gut ulcers and become activated for local KGF-1 production [28]. Compared to wildtype mice, TCRδ-deficient mice develop a more severe colitis and the rate of epithelial cell proliferation is severely reduced, due in part to the lack KGF-1 production [11]. These findings indicate a gut-protective role for γδ IELs in humans and raises an interesting possibility that dysregulation of gut-resident γδ IELs could be a contributing factor for the development of inflammatory bowel diseases and underscores the specialization of epithelial-resident γδ cells for barrier tissue maintenance.
In addition to influencing keratinocyte proliferation, DETC promote the survival of epithelial cells in wounds by upregulating IGF-1 production. DETC are the primary source of IGF-1 in the epidermis and, when this hormone is absent, the epidermis appears underdeveloped and an increased spontaneous rate of keratinocyte apoptosis is observed [30]. Interestingly, TCR stimulation also triggers upregulated expression of the IGF-1 receptor on DETC, suggesting that autocrine survival signaling might promote a positive feedback loop that enhances DETC potency during repair [30].
Epithelial signals of damage
In addition to activation by physical cutaneous injury, DETC are also responsive to keratinocyte tumors [6, 50]. Given this dual sensitivity towards injured or transformed cells, universal expression of an invariant TCR and exclusive epidermal localization, DETC have been postulated to recognize a stress-induced self-antigen [35]. Indeed, by using a modified DETC TCR as a staining reagent (a Vγ3Vδ1 TCR tetramer), this stress antigen appears to be transiently expressed by keratinocytes bordering skin wounds in vivo whereas undamaged keratinocytes do not appear to express stress ligand [33].
Interestingly, in vivo imaging studies indicate that the apical dendrites of steady state DETC form immunological synapse-like contacts, enriched in TCR clusters and phosphorylated CD3ζ, with keratinocyte tight junctions [23]. These findings suggest that resting DETC normally engage ligand and receive constitutive TCR signals, which could explain the semi-activated state of these T cells. In this model, reorganization of a signaling complex caused by tissue injury, rather than changes in TCR signal strength, would control DETC activation. While identification of a keratinocyte stress-induced antigen will allow the possibility of constitutive TCR ligand engagement to be examined, defining the stress molecule activating DETC has proved challenging; the few known ligands for γδ TCRs appear highly diverse in chemical nature, leaving little insight into the identity of the molecules seen by specific populations of γδ T cells.
In humans, some skin-homing αβ subsets have been identified that respond to the MHC class I-like molecule, CD1a [51]. CD1a is expressed on antigen presenting cells and, like other CD1 family molecules, is able to bind and present lipids for T cell activation [52]. The stimulatory potential of CD1a is dependent on the hydrophobic characteristics of bound lipids; apolar lipids appear stimulatory whereas polar oils are inhibitory to CD1a-reactive αβ T cells [53]. This dichotomy may be due to disruption of antigenic TCR binding to CD1a by the bulky headgroups of polar lipids. Interestingly, while the extracellular apolar lipid content of most tissues is relatively low, sebaceous gland secretions coat the epidermal surface with highly hydrophobic lipids, such as squalene and wax esters, that contribute to the waterproofing of the stratum corneum. As CD1a expression in the skin is restricted to dendritic cells found in the basal layers of the epidermis, the spatial separation of stimulatory lipids on the surface of the skin from deeper cutaneous antigen presenting cells might normally only allow for CD1a presentation of inhibitory polar lipids. However, damage to the skin by injury or infection could allow dendritic cell access to apolar lipids for CD1a presentation. In this model, oily skin antigen would therefore serve as a cutaneous damage signal for T cells [53]. While some γδ T cell populations have been shown to recognize a variety of endogenous and exogenous lipid antigens, further work is necessary to determine whether activation by CD1-presented lipids extends to γδ T cells in the skin [54–59].
The Vδ2- subset of human blood γδ cells has been demonstrated to respond to CMV-infected cells or intestinal epithelial tumors [60]. By using a Vγ4Vδ5+ clone to typify this population, the dual reactivity of these cells was revealed to be TCR-mediated recognition of the MHC I-like protein, endothelial protein C receptor (EPCR) [61]. Upregulated on virally infected endothelial cells and transformed intestinal epithelia, EPCR may serve a broad stress signal in these tissues recognized by some blood γδ T cell populations. Interestingly, EPCR is also expressed on human keratinocytes and is implicated in cutaneous wound repair via a critical role in the cytoprotective protein C pathway [62]. While epidermal γδ T cell recognition of EPCR is unknown, it is interesting to speculate whether this molecule may also regulate skin wound healing by serving as a cutaneous stress signal recognized by skin resident γδ T cells.
In humans, up to 90% of gut γδ IELs are Vδ1+ cells and it has been reported that a subset of these recognize the MHC Class I-like molecules, MICA and MICB [63]. These proteins are inducibly expressed by damaged enterocytes and target epithelial tumors for killing by Vδ1+ T cells. MICA and MICB have therefore been proposed to be stress molecules signaling gut epithelial injury or dysregulation to T cells. However, Vδ1+ populations also express NKG2D, an NK cell activating receptor, which engages MICA and may compete with γδ TCR binding. [64]. The role of MICA-TCR interactions in regulating gut γδ IELs therefore remains unclear.
Interestingly, NKG2D also activates epidermal T cells in the mouse. Expression of the murine NKG2D ligand, H60c, is upregulated on keratinocytes after damage or transformation and, in concert with TCR signaling, can stimulate DETC killing of stressed cells or mediate DETC production of KGF1 [50, 65, 66]. However, whether NKG2D acts as a costimulatory receptor, requiring simultaneous TCR signaling, or is a primary immunoreceptor that drives DETC activation is unclear; RAE1 is another NKG2D ligand upregulated by stress in the skin and is able to stimulate DETC in the absence of TCR engagement [67, 68]. It may be that alternative skin ligands for NKG2D are expressed depending on the form or severity of the epidermal stressor and that differential expression of these molecules gives context for the DETC response.
Interactions modulating T cell responses
While the damage signals detected by γδ T cells are mostly unknown, the necessity of TCR signaling in DETC function is clear; the Vγ3Vδ1 TCR is necessary for detection of epithelial stress ligand and ectopic expression of this TCR can transfer reactivity against distressed keratinocytes [35]. For αβ T cells, TCR signals alone are insufficient for cellular activation as costimulatory signals are needed to elaborate effector functions and prevent T cell anergy [69]. γδ T cells do not express the classic receptors known to mediate αβ costimulation, leaving the γδ T cell requirement for a “second signal” unresolved [25, 35]. However, recent studies using epidermal T cells as a model system clearly indicate that secondary signals, in addition to TCR signaling, are required to stimulate γδ T cell functions.
The primary example of an epithelial γδ T cell-specific costimulatory receptor is junctional adhesion molecule-like (JAML). Low levels of JAML are constitutively expressed on DETC and gut γδ IEL, but not other γδ T cell populations, and stimulation of epithelial γδ T cells results in upregulation of JAML [70]. In the epidermis, JAML engages a ligand, coxsackie and adenovirus receptor (CAR), expressed on keratinocytes [70]. Upon wounding, CAR expression is upregulated by keratinocytes proximal to damage and concomitant with DETC activation [70]. JAML-CAR interactions are required for efficient KGF-1 production by DETC and, importantly, a severe in vivo defect in wound closure is observed when this interaction is disrupted [70]. While regulation of IEL wound responses by JAML-CAR interactions in the gut remains to be tested, the responsiveness of intestinal γδ T cells to JAML costimulatory signals makes this an interesting prospect.
Upon wounding, one of the first observable DETC responses is cellular rounding [33]. This morphological change is controlled by CD100 signaling on DETC after engagement of its ligand, plexin B2, expressed by keratinocytes [71]. While the functional significance of this response is unknown, failure to round is associated with defects in wound closure. In vivo, DETC at wound borders of CD100-deficient mice remain dendritic after injury and wound closure is delayed by 1–2 days compared to wildtype mice [71]. In contrast to DETC, resident T cells in the gut do not display a dendritic morphology and are highly motile [27]. However, CD100-plexin B2 interactions on respective gut-resident γδ IELs and intestinal epithelial cells also appears involved in the repair processes of gut injury as CD100-deficient mice also display delayed healing and exacerbated colitis when given DSS compared to wildtype mice [29].
Conclusions
The maintenance of epithelial barrier tissues is necessary for the critical separation of an organism and the environment. Epithelial-resident γδ T cells promote barrier tissue homeostasis by producing factors that regulate the growth and survival of epithelia in the skin and gut. In the mouse, DETC and intestinal γδ IELs function as sentinels that detect breaches in barrier integrity caused by injury or disease. DETC regulate tissue level responses to injury by promoting inflammatory cell recruitment, keratinocyte growth and epithelial cell survival (Summarized in Figure 1). γδ IELs in the gut play a similar tissue protective role by releasing KGFs that regulate tissue repair after intestinal injury. While less studied, resident γδ T cells found in the epithelia of the lungs, urogenital tracts, and eyes also appear to possess tissue repair capacity. This distribution, coupled with conservation in the epithelia of all mammals, highlights the evolutionary specialization of these lymphocytes in barrier tissue homeostasis.
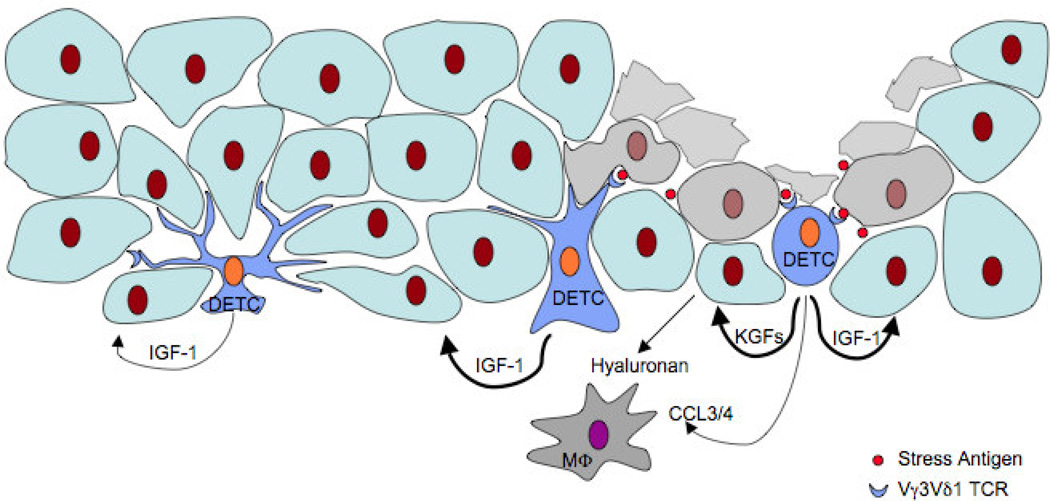
Figure 1.
Dendritic epidermal T cells (DETC) respond to skin injury by detecting an unknown self-antigen (Stress Antigen) expressed on distressed keratinocytes at wound sites. Activated DETC regulate inflammatory cell recruitment directly, by producing CCL3 and CCL4, and indirectly, by controlling hyaluronan deposition by keratinocytes. DETC stimulate keratinocyte proliferation via release of keratinocyte growth factors (KGFs) and promote epithelial cell survival by upregulating production of insulin-like growth factor-1 (IGF-1).
Human diseases of barrier sites such as chronic non-healing skin wounds, skin cancers and inflammatory bowel diseases are becoming increasingly common and take a heavy toll on both afflicted patients and society. Work aimed at understanding the signals regulating the functions of epithelial-resident γδ T cells and the pathways used by these lymphocytes to promote tissue repair might one day be used to clinically modulate γδ T cell functions for disease therapies.
Epithelial-resident γδ T cells respond to stress-induced self-antigens
Epithelial-resident γδ T cells promote repair of tissue injury
Molecules that regulate tissue-resident γδ T cell and αβ T cell functions differ
Acknowledgements
This work was prepared by support from NIH AI064811, AI036964 and AI107506.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allison JP, Havran WL. The immunobiology of T cells with invariant γδ antigen receptors. Annu Rev Immunol. 1991;9:679–705. doi: 10.1146/annurev.iy.09.040191.003335. [DOI] [PubMed] [Google Scholar]
- 2.Girardi M. Immunosurveillance and immunoregulation by γδ T cells. J Invest Dermatol. 2006;126(1):25–31. doi: 10.1038/sj.jid.5700003. [DOI] [PubMed] [Google Scholar]
- 3.Hayday AC. γδ T cells and the lymphoid stress-surveillance response. Immunity. 2009;31(2):184–196. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Jameson J, Havran WL. Skin γδ T-cell functions in homeostasis and wound healing. Immunol Rev. 2007;215:114–122. doi: 10.1111/j.1600-065X.2006.00483.x. [DOI] [PubMed] [Google Scholar]
- 5.Macleod AS, Havran WL. Functions of skin-resident γδ T cells. Cell Mol Life Sci. 2011;68(14):2399–2408. doi: 10.1007/s00018-011-0702-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jameson J, et al. A role for skin γδ T cells in wound repair. Science. 2002;296(5568):747–749. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 7.Toulon A, et al. A role for human skin-resident T cells in wound healing. J Exp Med. 2009;206(4):743–750. doi: 10.1084/jem.20081787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Girardi M, et al. Resident skin-specific γδ T cells provide local, nonredundant regulation of cutaneous inflammation. J Exp Med. 2002;195(7):855–867. doi: 10.1084/jem.20012000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu YC, L Li, Fuchs E. Emerging interactions between skin stem cells and their niches. Nat Med. 2014;20(8):847–856. doi: 10.1038/nm.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann JC, et al. Role of T lymphocytes in rat 2,4,6-trinitrobenzene sulphonic acid (TNBS) induced colitis: increased mortality after γδ T cell depletion and no effect of αβT cell depletion. Gut. 2001;48(4):489–495. doi: 10.1136/gut.48.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, et al. Protection of the intestinal mucosa by intraepithelial γδ T cells. Proc Natl Acad Sci U S A. 2002;99(22):14338–14343. doi: 10.1073/pnas.212290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathews JA, et al. γδ T cells are required for pulmonary IL-17A expression after ozone exposure in mice: role of TNFα. PLoS One. 2014;9(5):e97707. doi: 10.1371/journal.pone.0097707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pociask DA, et al. γδ T cells attenuate bleomycin-induced fibrosis through the production of CXCL10. Am J Pathol. 2011;178(3):1167–1176. doi: 10.1016/j.ajpath.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byeseda SE, et al. ICAM-1 is necessary for epithelial recruitment of γδ T cells and efficient corneal wound healing. Am J Pathol. 2009;175(2):571–579. doi: 10.2353/ajpath.2009.090112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z, et al. γδ T cells are necessary for platelet and neutrophil accumulation in limbal vessels and efficient epithelial repair after corneal abrasion. Am J Pathol. 2007;171(3):838–845. doi: 10.2353/ajpath.2007.070008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boismenu R, Havran WL. Intraepithelial γδ T cells exposed by functional genomics. Genome Biol. 2001;2(11) doi: 10.1186/gb-2001-2-11-reviews1031. REVIEWS1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garman RD, Doherty PJ, Raulet DH. Diversity, rearrangement, and expression of murine T cell γ genes. Cell. 1986;45(5):733–742. doi: 10.1016/0092-8674(86)90787-7. [DOI] [PubMed] [Google Scholar]
- 18.Havran WL, Jameson JM, Witherden DA. Epithelial cells and their neighbors. III. Interactions between intraepithelial lymphocytes and neighboring epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2005;289(4):G627–G630. doi: 10.1152/ajpgi.00224.2005. [DOI] [PubMed] [Google Scholar]
- 19.Havran WL, Allison JP. Developmentally ordered appearance of thymocytes expressing different T-cell antigen receptors. Nature. 1988;335(6189):443–445. doi: 10.1038/335443a0. [DOI] [PubMed] [Google Scholar]
- 20.Hayday AC, et al. Structure, organization, and somatic rearrangement of T cell γ genes. Cell. 1985;40(2):259–269. doi: 10.1016/0092-8674(85)90140-0. [DOI] [PubMed] [Google Scholar]
- 21.Asarnow DM, et al. Limited diversity of γδ antigen receptor genes of Thy-1+ dendritic epidermal cells. Cell. 1988;55(5):837–847. doi: 10.1016/0092-8674(88)90139-0. [DOI] [PubMed] [Google Scholar]
- 22.Havran WL, et al. Limited diversity of T-cell receptor γ-chain expression of murine Thy-1+ dendritic epidermal cells revealed by Vγ3-specific monoclonal antibody. Proc Natl Acad Sci U S A. 1989;86(11):4185–4189. doi: 10.1073/pnas.86.11.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chodaczek G, et al. Body-barrier surveillance by epidermal γδ TCRs. Nat Immunol. 2012;13(3):272–282. doi: 10.1038/ni.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holtmeier W, et al. The TCR-δ repertoire in normal human skin is restricted and distinct from the TCR-δ repertoire in the peripheral blood. J Invest Dermatol. 2001;116(2):275–280. doi: 10.1046/j.1523-1747.2001.01250.x. [DOI] [PubMed] [Google Scholar]
- 25.Hayday A, et al. Intraepithelial lymphocytes: exploring the Third Way in immunology. Nat Immunol. 2001;2(11):997–1003. doi: 10.1038/ni1101-997. [DOI] [PubMed] [Google Scholar]
- 26.Pang DJ, et al. Understanding the complexity of γδ T-cell subsets in mouse and human. Immunology. 2012;136(3):283–290. doi: 10.1111/j.1365-2567.2012.03582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edelblum KL, et al. Dynamic migration of γδ intraepithelial lymphocytes requires occludin. Proc Natl Acad Sci U S A. 2012;109(18):7097–7102. doi: 10.1073/pnas.1112519109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boismenu R, Havran WL. Modulation of epithelial cell growth by intraepithelial γδ T cells. Science. 1994;266(5188):1253–1255. doi: 10.1126/science.7973709. [DOI] [PubMed] [Google Scholar]
- 29.Meehan TF, et al. Protection against colitis by CD100-dependent modulation of intraepithelial γδ T lymphocyte function. Mucosal Immunol. 2014;7(1):134–142. doi: 10.1038/mi.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharp LL, et al. Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat Immunol. 2005;6(1):73–79. doi: 10.1038/ni1152. [DOI] [PubMed] [Google Scholar]
- 31.Taylor KR, Costanzo AE, Jameson JM. Dysfunctional gammadelta T cells contribute to impaired keratinocyte homeostasis in mouse models of obesity. J Invest Dermatol. 2011;131(12):2409–2418. doi: 10.1038/jid.2011.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jameson J, Witherden D, Havran WL. T-cell effector mechanisms: γδ and CD1d-restricted subsets. Curr Opin Immunol. 2003;15(3):349–353. doi: 10.1016/s0952-7915(03)00045-1. [DOI] [PubMed] [Google Scholar]
- 33.Komori HK, et al. Cutting edge: dendritic epidermal γδ T cell ligands are rapidly and locally expressed by keratinocytes following cutaneous wounding. J Immunol. 2012;188(7):2972–2976. doi: 10.4049/jimmunol.1100887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jameson JM, et al. A keratinocyte-responsive γδ TCR is necessary for dendritic epidermal T cell activation by damaged keratinocytes and maintenance in the epidermis. J Immunol. 2004;172(6):3573–3579. doi: 10.4049/jimmunol.172.6.3573. [DOI] [PubMed] [Google Scholar]
- 35.Havran WL, Chien YH, Allison JP. Recognition of self antigens by skinderived T cells with invariant γδ antigen receptors. Science. 1991;252(5011):1430–1432. doi: 10.1126/science.1828619. [DOI] [PubMed] [Google Scholar]
- 36.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 37.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127(3):514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 38.Jameson JM, et al. γδ T cell-induced hyaluronan production by epithelial cells regulates inflammation. J Exp Med. 2005;201(8):1269–1279. doi: 10.1084/jem.20042057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boismenu R, et al. Chemokine expression by intraepithelial γδ T cells. Implications for the recruitment of inflammatory cells to damaged epithelia. J Immunol. 1996;157(3):985–992. [PubMed] [Google Scholar]
- 40.Karvinen S, et al. Keratinocyte growth factor stimulates migration and hyaluronan synthesis in the epidermis by activation of keratinocyte hyaluronan synthases 2 and 3. J Biol Chem. 2003;278(49):49495–49504. doi: 10.1074/jbc.M310445200. [DOI] [PubMed] [Google Scholar]
- 41.Schauber J, Gallo RL. Expanding the roles of antimicrobial peptides in skin: alarming and arming keratinocytes. J Invest Dermatol. 2007;127(3):510–512. doi: 10.1038/sj.jid.5700761. [DOI] [PubMed] [Google Scholar]
- 42.Ahrens K, et al. Mechanical and metabolic injury to the skin barrier leads to increased expression of murine β-defensin-1-3, and -14. J Invest Dermatol. 2011;131(2):443–452. doi: 10.1038/jid.2010.289. [DOI] [PubMed] [Google Scholar]
- 43.Thorey IS, et al. The Ca2+-binding proteins S100A8 and S100A9 are encoded by novel injury-regulated genes. J Biol Chem. 2001;276(38):35818–35825. doi: 10.1074/jbc.M104871200. [DOI] [PubMed] [Google Scholar]
- 44.Lai Y, et al. The antimicrobial protein REG3A regulates keratinocyte proliferation and differentiation after skin injury. Immunity. 1999;37(1):74–84. doi: 10.1016/j.immuni.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacLeod AS, et al. Dendritic epidermal T cells regulate skin antimicrobial barrier function. J Clin Invest. 2013;123(10):4364–4374. doi: 10.1172/JCI70064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mabuchi T, Takekoshi T, Hwang ST. Epidermal CCR6+ γδ T cells are major producers of IL-22 and IL-17 in a murine model of psoriasiform dermatitis. J Immunol. 2011;187(10):5026–5031. doi: 10.4049/jimmunol.1101817. [DOI] [PubMed] [Google Scholar]
- 47.Cai Y, et al. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity. 2011;35(4):596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gray EE, Suzuki K, Cyster JG. Cutting edge: Identification of a motile IL-17-producing γδ T cell population in the dermis. J Immunol. 2011;186(11):6091–6095. doi: 10.4049/jimmunol.1100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chassaing B, et al. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014;104 doi: 10.1002/0471142735.im1525s104. p. Unit 15 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Girardi M, et al. Regulation of cutaneous malignancy by γδ T cells. Science. 2001;294(5542):605–609. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- 51.de Jong A, et al. CD1a-autoreactive T cells are a normal component of the human αβT cell repertoire. Nat Immunol. 2010;11(12):1102–1109. doi: 10.1038/ni.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moody DB. The surprising diversity of lipid antigens for CD1-restricted T cells. Adv Immunol. 2006;89:87–139. doi: 10.1016/S0065-2776(05)89003-0. [DOI] [PubMed] [Google Scholar]
- 53.de Jong A, et al. CD1a-autoreactive T cells recognize natural skin oils that function as headless antigens. Nat Immunol. 2014;15(2):177–185. doi: 10.1038/ni.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vincent MS, et al. CD1a-, b-, and c-restricted TCRs recognize both self and foreign antigens. J Immunol. 2005;175(10):6344–6351. doi: 10.4049/jimmunol.175.10.6344. [DOI] [PubMed] [Google Scholar]
- 55.Russano AM, et al. Recognition of pollen-derived phosphatidyl-ethanolamine by human CD1d-restricted γδ T cells. J Allergy Clin Immunol. 2006;117(5):1178–1184. doi: 10.1016/j.jaci.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 56.Dieude M, et al. Cardiolipin binds to CD1d and stimulates CD1d-restricted γδ T cells in the normal murine repertoire. J Immunol. 2011;186(8):4771–4781. doi: 10.4049/jimmunol.1000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bai L, et al. The majority of CD1d-sulfatide-specific T cells in human blood use a semiinvariant Vδ1 TCR. Eur J Immunol. 2012;42(9):2505–2510. doi: 10.1002/eji.201242531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luoma AM, et al. Crystal structure of Vδ1 T cell receptor in complex with CD1d-sulfatide shows MHC-like recognition of a self-lipid by human γδ T cells. Immunity. 2013;39(6):1032–1042. doi: 10.1016/j.immuni.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uldrich AP, et al. CD1d-lipid antigen recognition by the γδ TCR. Nat Immunol. 2013;14(11):1137–1145. doi: 10.1038/ni.2713. [DOI] [PubMed] [Google Scholar]
- 60.Halary F, et al. Shared reactivity of Vδ2neg γδ T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J Exp Med. 2005;201(10):1567–1578. doi: 10.1084/jem.20041851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Willcox CR, et al. Cytomegalovirus and tumor stress surveillance by binding of a human γδ T cell antigen receptor to endothelial protein C receptor. Nat Immunol. 2012;13(9):872–879. doi: 10.1038/ni.2394. [DOI] [PubMed] [Google Scholar]
- 62.Xue M, et al. Endothelial protein C receptor and protease-activated receptor-1 mediate induction of a wound-healing phenotype in human keratinocytes by activated protein C. J Invest Dermatol. 2005;125(6):1279–1285. doi: 10.1111/j.0022-202X.2005.23952.x. [DOI] [PubMed] [Google Scholar]
- 63.Groh V, et al. Recognition of stress-induced MHC molecules by intestinal epithelial γδ T cells. Science. 1998;279(5357):1737–1740. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 64.Bauer S, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285(5428):727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 65.Yoshida S, et al. Involvement of an NKG2D ligand H60c in epidermal dendritic T cell-mediated wound repair. J Immunol. 2012;188(8):3972–3979. doi: 10.4049/jimmunol.1102886. [DOI] [PubMed] [Google Scholar]
- 66.Whang MI, Guerra N, Raulet DH. Costimulation of dendritic epidermal γδ T cells by a new NKG2D ligand expressed specifically in the skin. J Immunol. 2009;182(8):4557–4564. doi: 10.4049/jimmunol.0802439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nitahara A, et al. NKG2D ligation without T cell receptor engagement triggers both cytotoxicity and cytokine production in dendritic epidermal T cells. J Invest Dermatol. 2006;126(5):1052–1058. doi: 10.1038/sj.jid.5700112. [DOI] [PubMed] [Google Scholar]
- 68.Strid J, et al. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat Immunol. 2008;9(2):146–154. doi: 10.1038/ni1556. [DOI] [PubMed] [Google Scholar]
- 69.Fathman CG, Lineberry NB. Molecular mechanisms of CD4+ T-cell anergy. Nat Rev Immunol. 2007;7(8):599–609. doi: 10.1038/nri2131. [DOI] [PubMed] [Google Scholar]
- 70.Witherden DA, et al. The junctional adhesion molecule JAML is a costimulatory receptor for epithelial γδ T cell activation. Science. 2010;329(5996):1205–1210. doi: 10.1126/science.1192698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Witherden DA, et al. The CD100 receptor interacts with its plexin B2 ligand to regulate epidermal γδ T cell function. Immunity. 2012;37(2):314–325. doi: 10.1016/j.immuni.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]