Abstract
Objective
Patients with type 2 diabetes (T2DM) are at increased risk of fracture. High prevalence of chronic kidney disease (CKD) in T2DM may contribute to bone fragility, but whether dynamic change in kidney function is associated with fracture risk is unclear.
Research design and methods
To evaluate the association of pre-randomization baseline estimated glomerular filtration (eGFR) and its change over time with subsequent fracture risk in the Bone substudy of Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial, we conducted an observational study of 2262 women and 4737 men with T2DM and with at least 2 eGFR values.
Results
During a mean follow-up of 4.40±1.54 years, 235 women and 223 men sustained a new non-vertebral fracture. In multivariable adjusted sex-specific models, pre-randomization baseline eGFR was not a significant predictor of fracture risk in either men or women. However, a steeper decline in eGFR was associated with greater risk of fracture in women (hazard ratio [HR] per standard deviation [SD] decrement in eGFR slope, 1.30; 95%CI 1.17–1.44) but not men (HR per SD decrement in eGFR slope, 0.97; 95%CI 0.82–1.13). Accounting for competing risks of death modestly attenuated the association in women (HR per SD decrement in eGFR slope, 1.19; 95%CI 1.04–1.37), with the relationship in men remaining non-significant (HR per SD decrement in eGFR slope, 0.96; 95%CI 0.77–1.18).
Conclusions
Declining kidney function predicts fracture risk in women but not in men with T2DM. Future studies should investigate the mechanisms for these associations.
Introduction
Type 2 diabetes (T2DM) is a leading cause of blindness, amputations, kidney failure, and cardiovascular disease (1). These microvascular and macrovascular complications impair functional status, shorten lifespan and increase healthcare expenditures (2). Although T2DM is associated with significantly higher bone mass compared to the general population, it increases fracture risk, further contributing to morbidity, mortality and costs (3-6). Incomplete understanding of the pathogenesis of bone fragility in T2DM and limited utility of bone densitometry in this population that experiences excess fracture rates in the setting of normal or high bone mass has hampered fracture risk stratification (7, 8). An enhanced understanding of clinical factors that promote decreased bone strength in T2DM could facilitate identification of individuals who are most at risk for fractures and who could be targeted for proven osteoporosis preventative measures.
The high prevalence of chronic kidney disease (CKD) in individuals with T2DM may contribute to increased bone fragility in T2DM. CKD, even early in its course, is frequently complicated by disordered bone and mineral metabolism that may promote reduced bone strength (9). Epidemiologic studies suggest that compared to the general population, the presence of CKD, defined as reduced estimated glomerular filtration rate (eGFR) at a single point in time, confers nearly a 2-fold greater risk of hip fracture (10, 11). However, it remains uncertain if similar relationships hold true among individuals with T2DM, for whom cross-sectional eGFR assessments may carry greater imprecision compared to the general population (12). Given that a dynamic decline over time in eGFR is a clinically-accepted and evidence-based surrogate for loss of kidney function in both diabetic and non-diabetic populations (13, 14), we studied the relationship between longitudinal change in eGFR and the incidence of non-vertebral fracture in men and women with T2DM who participated in the Bone sub-study of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial. We hypothesized that a decrease in eGFR over time is an independent risk factor for non-spine fractures in patients with T2DM and that sequential change in eGFR would outperform pre-randomization baseline eGFR as a predictor of fracture risk.
Materials and Methods
Study Population
The ACCORD Trial was a randomized, multi-center, double 2 x 2 factorial design study that compared the effects of intensive vs. standard glycemic control, fibrates versus placebo, and intensive versus standard blood pressure (BP) control on major cardiovascular disease events in 10,251 patients with T2DM. The study design, entry criteria, and results have been published (15, 16). Randomization occurred from 2001 to 2005. Participants in the intensive glycemic control arm achieved a median HbA1C of 6.4%, whereas the median HbA1C in the standard glycemic control arm was 7.5% (16). After a mean treatment period of 3.7 years, the intensive glycemia intervention was stopped in 2008 due to increased all-cause mortality (16).
The ACCORD-BONE study evaluated the effect of intensive versus standard glycemic control on fracture risk. Five of the seven clinical center networks, including 54 of 77 clinical sites and 7,287 of the 10,251 ACCORD participants, participated in the BONE sub-study. Participants were asked annually about the occurrence of any non-spine fractures. All fractures were confirmed by blinded central adjudication, based on radiology records (17). The protocol was approved by the institutional review board at each study site, and all participants provided written informed consent.
We analyzed data from 6,609 of the ACCORD-BONE study participants who had a month 4 serum creatinine value and at least one subsequent serum creatinine measured prior to occurrence of fracture or end of follow-up.
Exposure
The primary exposures were pre-randomization baseline eGFR and post-randomization change in eGFR expressed as eGFR “slope” (change over time). GFR was estimated from serum creatinine, measured by the Roche Creatinine Plus enzymatic method (Roche Diagnostics, Basel, Switzerland), using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (18). The month 4 serum creatinine was used as the first point in calculating eGFR change because introduction of some ACCORD interventions (including fenofibrate for those Lipid Trial participants randomized to active treatment) affected serum creatinine levels early (19). The eGFR slope was calculated as eGFR at last visit before fracture (last visit for censored observations) minus eGFR at month 4 visit divided by time from month 4 visit to fracture or censoring. The mean time from month 4 to the last (pre-event or follow-up) serum creatinine measurement was 4.37 ± 1.57 years (median 4.35 years).
Outcome
The primary outcome was a non-vertebral fracture, which included upper extremity (hand, distal forearm, proximal humerus), lower extremity (foot, ankle, leg, hip), or axial (rib, chest/sternum, face) fracture. Reported fracture events were centrally adjudicated, based on radiology records (17). Confirmed pathologic fractures (periprosthetic fractures or fractures occurring secondary to neoplasm, necrosis or sepsis) were excluded, but all other fractures, including those following trauma, were included. We calculated follow-up time for events and censored observations from the date of the month 4 visit rather than randomization. The mean time to fracture (or last follow-up for censored observations) was 4.40 ± 1.54 years (median 4.37 years).
Assessment of Pre-randomization Baseline Covariates
Demographic characteristics, diabetes duration, smoking status, medical and medication history were determined at the pre-randomization baseline visit using standardized questionnaires. Height, weight and systolic BP were measured according to a standardized protocol. All pre-randomization baseline laboratory values were obtained centrally at the University of Washington Northwest Lipid Metabolism and Diabetes Research Laboratory. Urine creatinine was determined enzymatically on a Roche Double Modular P Analytics automated analyzer. Urine albumin was determined by immunonephelometry on a Siemens BN II nephelometer. HbA1c was measured by an automated high-performance liquid chromatography (Tosoh Bioscience, South San Francisco, CA). Microalbuminuria was defined as a urinary albumin to creatinine ratio of ≥30 mg/g, and macroalbuminuria was defined as urinary albumin to creatinine ratio of ≥300 mg/g.
Statistical Analysis
Descriptive statistics of pre-randomization baseline characteristics were calculated overall and stratified by sex due to greater fracture risk in T2DM in women compared to men (4). We used Cox proportional-hazards regression models to examine in sex-specific analyses the associations of pre-randomization baseline eGFR and eGFR slope with time to first non-vertebral fracture. We evaluated pre-randomization baseline eGFR as a continuous variable, with increases in risk calculated per one sex-specific standard deviation decrement in eGFR. We adjusted for the following pre-randomization baseline covariates: age, white race, living status (with others or alone), education less than high school graduate, uninsured or Medicaid patient, smoking status (ever), body mass index, systolic BP, HbA1c, diabetes duration, history of clinical cardiovascular disease (myocardial infarction, stroke, coronary, carotid or peripheral arterial revascularization procedure, positive stress test), microalbuminuria, macroalbuminuria, glycemia trial treatment assignment, second trial (BP or Lipid) treatment assignment, and use of inhaled steroids, thiazolidinediones, insulin, thiazide diuretics, loop diuretics, statins, and anti-psychotic medications. We examined models incorporating interaction terms to evaluate variation by gender in the effect of change in eGFR on the risk of fracture. Since death precludes the occurrence of fracture, we used the method of Fine and Gray to account for the competing risk of death (20). Magnitudes of associations were assessed by hazard ratios and associated 95% confidence limits. Appropriateness of the proportional hazards assumption was assessed via examination of Martingale-based residuals (21). All tests of significance were performed at the two-sided 5% alpha-level and analyses performed using SAS version 9.3 software (SAS Institute, Cary, NC). Proportional hazards analyses with competing risks were performed utilizing the “%PSHREG” SAS macro of Kohl and Heinze (22).
Results
The pre-randomization baseline characteristics of the 6609 ACCORD-Bone participants (Table 1) are qualitatively similar to those of the overall ACCORD, which was composed of middle-aged and older patients with poorly controlled T2DM and multiple complications (16). The mean age was 62.5 ± 6.7 years. The mean pre-randomization baseline eGFR was 78.5 ± 18.2 ml/min/1.73m2, and 17% of participants had pre-randomization baseline eGFR <60 ml/min/1.73m2. Nearly 30% of participants had microalbuminuria, and the prevalence of macroalbuminuria was 6%. The median annualized decline in eGFR during follow up was −1.2 ml/min/1.73 m2/year (IQR, −3.5, 0.1).
Table 1.
Pre-randomization baseline characteristics
| All Participants | Women | Men | |||||
|---|---|---|---|---|---|---|---|
| N=6609 | All women N=2262 |
Fracture N=245 |
No Fracture N=2017 |
All men N=4347 |
Fracture N=228 |
No Fracture N=4119 |
|
| Age, years | 62.5 ± 6.7 | 62.1 ± 6.5 | 62.7 ± 6.4 | 62.0 ± 6.5 | 62.8 ± 6.8 | 63.4 ± 7.3 | 62.8 ± 6.7 |
| Black, % | 20.0 | 26.4 | 12.7 | 28.1 | 16.6 | 12.7 | 16.8 |
| Hispanic, % | 2.3 | 1.6 | 0.8 | 1.7 | 2.7 | 3.1 | 2.6 |
| Previous CVD event, % | 35.2 | 24.9 | 26.1 | 24.8 | 40.6 | 41.7 | 40.5 |
| Smoking, % | 63.1 | 46.8 | 46.1 | 46.8 | 71.5 | 72.4 | 71.5 |
| BMI, kg/m2 | 32.5 ± 5.3 | 33.7 ± 5.6 | 33.1 ± 5.7 | 33.8 ± 5.6 | 31.9 ± 4.9 | 31.7 ± 4.8 | 31.9 ± 4.9 |
| Median diabetes duration, years | 10 (5, 15) | 10 (5, 15) | 11 (6, 17) | 10 (5, 15) | 10 (5, 15) | 11 (6, 16) | 10 (5, 15) |
| HbAlC, % | 8.3 ± 1.0 | 8.4 ± 1.1 | 8.4 ± 1.0 | 8.3 ± 1.1 | 8.3 ± 1.0 | 8.3 ± 1.0 | 8.3 ± 1.0 |
| SEP, mm Hg | 135.3 ± 17.0 | 136.3 ± 18.2 | 137.2 ± 17.7 | 136.2 ± 18.3 | 134.8 ± 16.4 | 134.0 ± 16.0 | 134.9 ± 16.4 |
| eGFR, ml/min/1.73 m2 | 78.5 ± 18.2 | 79.3 ± 20.6 | 79.3 ± 19.5 | 79.3 ± 20.7 | 78.1 ± 16.8 | 76.5 ± 18.9 | 78.2 ± 16.7 |
| Annual eGFR decline, ml/min/1.73 m2/year | −1.2 (−3.5, 0.1) | −1.3 (−3.9, 0.3) | −1.9 (−5.1, −0.2) | −1.3 (−3.7, 0.3) | −1.1 (−3.4, 0.0) | −0.70 (−2.9, 0.7) | −1.10 (−3.4, −0.0) |
| eGFR <60 ml/min/1.73m2 (%) | 17.2 | 19.6 | 17.6 | 19.8 | 16.0 | 21.1 | 15.7 |
| Microalbuminuria, % | 30.4 | 25.5 | 26.9 | 25.3 | 33.0 | 32.9 | 33.0 |
| Macroalbuminuria, % | 6.0 | 5.8 | 5.3 | 5.9 | 6.1 | 7.5 | 6.0 |
| Intensive glycemia arm, % | 49.6 | 49.4 | 48.6 | 49.5 | 49.7 | 46.1 | 49.9 |
| Thiazolidinediones, % | 19.5 | 18.0 | 22.4 | 17.5 | 20.4 | 19.7 | 20.4 |
| Insulin, % | 34.5 | 38.0 | 40.7 | 37.7 | 32.7 | 37.7 | 32.4 |
Abbreviations: CVD, cardiovascular disease; BMI, body mass index; HbAlC, hemoglobin AlC; SBP, systolic blood pressure; eGFR, estimated glomerular filtration rate.
Values are %, means ± standard deviation, medians (interquartile range).
During a mean follow-up of 4.40 ± 1.54 years, 458 participants (15.9/1000 person-years, 95% CI 14.5 – 17.3) sustained a new non-vertebral fracture. Compared to participants without a fracture, those who experienced a fracture were more likely to be white, non-Hispanic and have longer diabetes duration. Among women, the use of thiazolidinediones was greater in those that sustained a fracture than in women without a fracture. Among both sexes, the use of insulin was more common in fracture cases compared to non-cases. The unadjusted incidence rate of fracture did not differ in patients with pre-randomization baseline eGFR <60 ml/min/1.73m2 (18.9/1000 person-years, 95% CI 15.6 – 22.8) versus those with preserved kidney function (15.2/1000 person-years, 95% CI 13.8 – 16.8; P = 0.05).
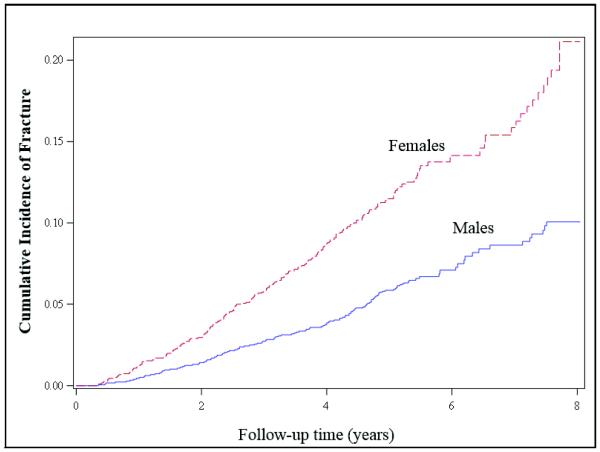
The unadjusted incidence rate of fractures in women (235 events, 23.8/1000 person-years, 21.1 – 26.9) was higher than in men (223 events, 11.8/1000 person-years, 95% CI 10.4 – 13.3). The cumulative incidence plots also demonstrated greater fracture risk in women compared to men (Figure 1). A marginally significant interaction term in the fully adjusted fracture model supported the possibility of effect modification by gender on the relationship of change in eGFR with fracture risk (P value for gender X eGFR slope = 0.07). Therefore, we conducted sex-specific analyses.
Figure 1.
Cumulative incidence plots of time to first non-vertebral fracture stratified by sex.
In crude and adjusted models, pre-randomization baseline eGFR and presence of micro- or macroalbuminuria was not associated with risk of fracture in either men or women (Table 2).
Table 2.
Risk of incident non-spinal fracture in men and women according to pre-randomization baseline eGFR and albuminuria
| Unadjusted1 | MV-adjusted2 | ||||
|---|---|---|---|---|---|
| Variable | HR | 95% CI | HR | 95% CI | |
|
Women
(N=2203, 235 events) |
eGFR (per 20 mg/dL/1.73 m2 decrease) | 1.01 | 0.89 – 1.15 | 0.93 | 0.79 – 1.08 |
| microalbuminuria | 1.17 | 0.85 – 1.60 | 1.14 | 0.82 – 1.58 | |
| macroalbuminuria | 0.91 | 0.49 – 1.68 | 1.11 | 0.60 – 2.08 | |
|
Men
(N=4255, 223 events) |
eGFR (per 17 mg/dL/1.73m2 decrease) | 0.97 | 0.83 – 1.12 | 0.86 | 0.72 – 1.03 |
| microalbuminuria | 0.93 | 0.69 – 1.27 | 0.78 | 0.70 – 1.31 | |
| macroalbuminuria | 1.38 | 0.79 – 2.41 | 1.44 | 0.82 – 2.53 | |
Abbreviations: eGFR, estimated glomerular filtration rate; HR, hazard ratio; CI, confidence; MV, multivariable.
Unadjusted models include pre-randomization baseline eGFR and micro- and macro-albuminuria
MV-adjusted models include eGFR variables and the following pre-randomization baseline covariates included in adjusted models: age, white race, Hispanic ethnicity, living status (with others or alone), education less than high school graduate, uninsured or Medicaid patient, smoking, body mass index, systolic blood pressure, hemoglobin AlC, diabetes duration, history of clinical cardiovascular disease, microalbuminuria, macroalbuminuria, glycemia trial treatment assignment, second trial (BP or Lipid) treatment assignment, and use of inhaled steroids, thiazolidinediones, insulin, thiazide diuretics, loop diuretics, statins, and antipsychotic medications.
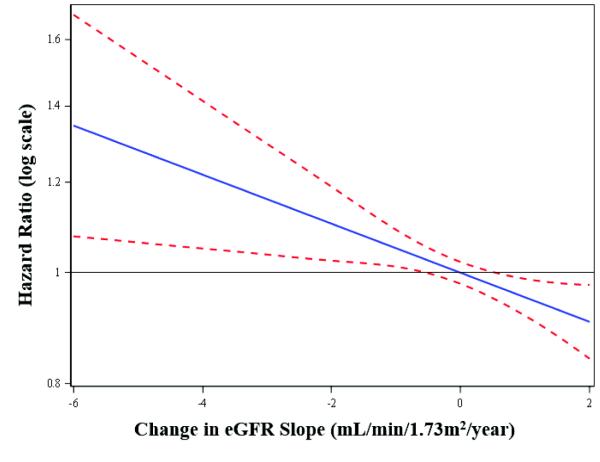
Change in eGFR was associated with a greater risk of fracture in women. The median change in eGFR among women who sustained a fracture was greater than in women who did not (−1.9 vs. −1.3 ml/min/1.73m2/year). In unadjusted models, every 3.6 ml/min/1.73m2/year decrease in eGFR slope was associated with HR of 1.29 (95% CI, 1.17 – 1.42) for risk of fracture. Results were similar after adjusting for clinical characteristics, including pre-randomization baseline eGFR, and after accounting for competing risk of death (Table 3, Figure 2).
Table 3.
Risk of incident non-spinal fracture in men and women for one standard deviation decrease (3.6 ml/min/1.73m2/year) in eGFR slope
| Unadjusted1 | MV-adjusted2 | MV-adjusted + competing risk3 | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Events | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Women | 2203 | 235 | 1.29 | 1.17 – 1.42 | 1.30 | 1.17 – 1.44 | 1.19 | 1.04 – 1.37 |
| Men | 4255 | 223 | 0.97 | 0.83 – 1.12 | 0.97 | 0.82 – 1.13 | 0.96 | 0.77 – 1.18 |
Abbreviations: eGFR, estimated glomerular filtration rate; HR, hazard ratio; CI, confidence; MV, multivariable.
Unadjusted models include pre-randomization baseline eGFR and eGFR slope only
MV-adjusted models include eGFR variables and the following pre-randomization baseline covariates included in adjusted models: age, white race, Hispanic ethnicity, living status (with others or alone), education less than high school graduate, uninsured or Medicaid patient, smoking, body mass index, systolic blood pressure, hemoglobin AlC, diabetes duration, history of clinical cardiovascular disease, micro- or macroalbuminuria, glycemia trial treatment assignment, second trial (BP or Lipid) treatment assignment, and use of inhaled steroids, thiazolidinediones, thiazide diuretics, loop diuretics, statins, and anti-psychotic medications.
MV-adjusted + competing risks models include all covariates listed in MV-adjusted models and correction for competing risk of death
Figure 2.
Hazard ratios for risk of non-vertebral fracture across values of eGFR slope in women. From multivariable proportional hazards regression model adjusted for competing risks and covariates.
For men, there was no significant association between eGFR slope and fracture risk in any model (Table 3). This finding was unchanged when death was treated as a competing risk (HR 0.97; 95% CI, 0.77 – 1.18).
Discussion
Prior studies that examined decreased kidney function as a risk factor for fracture yielded conflicting results, with some but not all studies reporting a significant association (10, 11, 23-25). In this prospective analysis of data from the ACCORD-BONE study participants with mean pre-randomization baseline eGFR of 78.5 ml/min/1.73 m2/year, neither pre-randomization baseline eGFR nor presence of albuminuria was associated with subsequent risk of fracture in either sex. However, the results demonstrated an independent association between decline in eGFR during 4 years of follow up and risk of fracture in women but not in men. Although the hazard ratios for fracture in men were not significant in all analyses, the finding in women remained robust with multivariable adjustment and with accounting for competing risk of death. Thus, our results suggest that reduction in eGFR is an independent risk factor for non-spine fracture in women with T2DM.
Most studies that have investigated the association between eGFR and bone fragility have relied on single creatinine-based GFR estimates to define baseline kidney function (10, 11, 23-25). Other studies have used cystatin C-based GFR estimates, but they also obtained only baseline assessments (26-30). In contrast, a recent secondary analysis of data from two clinical trials employed repeated measures of eGFR. Although sex-specific analyses were not performed, the investigators found that rapid eGFR loss over 2 years was independently associated with increased fracture risk despite absence of a significant relationship between baseline eGFR and risk of fracture (31). Our findings in female participants of the ACCORD-BONE study are in line with this report and add to the growing body of literature that supports the use of serial assessments of kidney function for superior risk prediction (32, 33).
Consistent with prior reports of sexual differences in bone strength, fracture rates and risk factors for bone fragility in T2DM (4, 34-37), we did not detect a significant relationship between change in eGFR and risk of fracture in men. This finding probably indicates a decreased contribution of reduced kidney function to bone fragility in men with T2DM, who may be more protected from adverse effects of reduced kidney function on bone as a result of higher levels of estradiol or testosterone. Alternatively, differences by gender may stem from diminished ability to detect an association in men compared to women due to lower frequency of fractures and reduced longevity. Recent studies demonstrate the importance of addressing excess mortality in analyses of fracture (38, 39). However, our results in men did not change in models that accounted for competing risk of death, suggesting that lack of an effect in this group was not driven by sexual differences in longevity.
Although additional studies are needed to confirm our findings, the robust results in women support the possibility that a decline in kidney function is an important contributor to bone fragility in T2DM. Disordered bone and mineral metabolism that accompanies CKD and develops early in its course could be one mechanistic mediator of bone fragility associated with newly reduced kidney function (40, 41). Interestingly, circulating levels of sclerostin, an osteocyte-derived inhibitor of bone formation, are elevated in patients with T2DM, bone sclerostin production is induced in animal models of early CKD, and elevated sclerostin levels have been linked with risks of vertebral and non-vertebral fractures (42-45). Frequent falls, perhaps due to impaired physical performance and frailty commonly observed in CKD (46), may also have played a role in excess fracture risk in ACCORD participants with declining eGFR. Finally, increased inflammation, metabolic acidosis and microvascular disease that accompany CKD have also been implicated as contributors to bone fragility (47-49).
Several limitations need to be considered. Although we lacked of information on vertebral fractures, our study was strengthened by a large number of centrally adjudicated non-vertebral fracture cases. Bone mineral density, biomarkers of bone and mineral metabolism, including sclerostin, and acid-base balance were also not obtained. However, we were able to control for other important clinical risk factors, including BMI, medications and diabetes duration. We used the creatinine-based CKD-EPI equation to estimate kidney function. While this method may be imprecise (12), its widespread use affords clinical applicability to our results. Another limitation is our inability to definitively exclude the possibility that the change in eGFR immediately prior to fracture event was due to acute changes in kidney function or a result of changes in non-GFR determinants of serum creatinine, such as changes in creatinine generation (50). However, mean eGFR declined progressively during follow up in ACCORD, suggesting that the change in eGFR ascertained in our analyses likely reflects true decline in kidney function over time (13, 51). Finally, the generalizability of our findings is limited to middle-aged and older patients with T2DM and multiple complications.
Current guidelines acknowledge the increased risk of fracture in T2DM and advise clinicians to screen for osteoporosis, using the same tools as in the general population (52). However, the standard for fracture risk assessment is the measurement of bone mineral density by dual-energy X-ray absorptiometry, which does not accurately stratify fracture risk in T2DM (53). Our results indicate that decline in kidney function is associated with fracture risk in patients with T2DM, suggesting that awareness of longitudinal eGFR trajectory may enhance clinical assessment of bone fragility in this high-risk group. Future studies are needed to confirm our findings in other populations and to investigate potential causal mechanisms.
Acknowledgements
TI was supported by K23DK087858 and by the Carl W. Gottschalk Research Scholar Grant from the American Society of Nephrology Foundation for Kidney Research. JS was supported by K23DK095949.
The ACCORD trial was supported by National Heart Lung and Blood Institute contracts N01-HC-95178, N01-HC-95179, N01-HC-95180, N01-HC-95181, N01-HC-95182, N01-HC-95183, N01-HC-95184, and IAA #Y1-HC-9035 and IAA#Y1-HC-1010.
T.I. and T.E.C. wrote the manuscript and researched the data. J.J.S., T.L.N., A.S., J.B, and A.V.S. contributed to data analyses, results presentation, discussion, and reviewed and edited the manuscript. T.I. is the guarantor of this work and, as such, had full access to all the data outputs in the study and takes responsibility for the integrity of the data.
Parts of this study were presented in abstract form at the American Society of Nephrology Kidney Week, Atlanta, Georgia, November 5 – 10, 2013.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
TI has received honoraria from Bayer and consulting fees from Guidepoint Global and Daiichi Sankyo.
All the other authors declared no competing interests.
References
- 1.Nathan DM. Long-term complications of diabetes mellitus. N Engl J Med. 1993;328:1676–85. doi: 10.1056/NEJM199306103282306. [DOI] [PubMed] [Google Scholar]
- 2.Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36:1033–46. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz AV, Sellmeyer DE, Ensrud KE, et al. Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab. 2001;86:32–8. doi: 10.1210/jcem.86.1.7139. [DOI] [PubMed] [Google Scholar]
- 4.Oei L, Zillikens MC, Dehghan A, et al. High bone mineral density and fracture risk in type 2 diabetes as skeletal complications of inadequate glucose control: the rotterdam study. Diabetes Care. 2013;36:1619–28. doi: 10.2337/dc12-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de L, II, van der Klift M, de Laet CE, van Daele PL, Hofman A, Pols HA. Bone mineral density and fracture risk in type-2 diabetes mellitus: the Rotterdam Study. Osteoporos Int. 2005;16:1713–20. doi: 10.1007/s00198-005-1909-1. [DOI] [PubMed] [Google Scholar]
- 6.Liao CC, Lin CS, Shih CC, et al. Increased risk of fracture and postfracture adverse events in patients with diabetes: two nationwide population-based retrospective cohort studies. Diabetes Care. 2014;37:2246–52. doi: 10.2337/dc13-2957. [DOI] [PubMed] [Google Scholar]
- 7.Leslie WD, Rubin MR, Schwartz AV, Kanis JA. Type 2 diabetes and bone. J Bone Miner Res. 2012;27:2231–7. doi: 10.1002/jbmr.1759. [DOI] [PubMed] [Google Scholar]
- 8.Moseley KF. Type 2 diabetes and bone fractures. Curr Opin Endocrinol Diabetes Obes. 2012;19:128–35. doi: 10.1097/MED.0b013e328350a6e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malluche HH, Porter DS, Pienkowski D. Evaluating bone quality in patients with chronic kidney disease. Nat Rev Nephrol. 2013;9:671–80. doi: 10.1038/nrneph.2013.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nickolas TL, McMahon DJ, Shane E. Relationship between moderate to severe kidney disease and hip fracture in the United States. J Am Soc Nephrol. 2006;17:3223–32. doi: 10.1681/ASN.2005111194. [DOI] [PubMed] [Google Scholar]
- 11.Ensrud KE, Lui LY, Taylor BC, et al. Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med. 2007;167:133–9. doi: 10.1001/archinte.167.2.133. [DOI] [PubMed] [Google Scholar]
- 12.Silveiro SP, Araujo GN, Ferreira MN, Souza FD, Yamaguchi HM, Camargo EG. Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation pronouncedly underestimates glomerular filtration rate in type 2 diabetes. Diabetes Care. 2011;34:2353–5. doi: 10.2337/dc11-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Padala S, Tighiouart H, Inker LA, et al. Accuracy of a GFR estimating equation over time in people with a wide range of kidney function. Am J Kidney Dis. 2012;60:217–24. doi: 10.1053/j.ajkd.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Boer IH, Sun W, Cleary PA, et al. Longitudinal changes in estimated and measured GFR in type 1 diabetes. J Am Soc Nephrol. 2014;25:810–8. doi: 10.1681/ASN.2013050557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buse JB, Bigger JT, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol. 2007;99:21i–33i. doi: 10.1016/j.amjcard.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz AV, Margolis KL, Sellmeyer DE, et al. Intensive glycemic control is not associated with fractures or falls in the ACCORD randomized trial. Diabetes Care. 2012;35:1525–31. doi: 10.2337/dc11-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mychaleckyj JC, Craven T, Nayak U, et al. Reversibility of fenofibrate therapy-induced renal function impairment in ACCORD type 2 diabetic participants. Diabetes Care. 2012;35:1008–14. doi: 10.2337/dc11-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 21.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of Martingale-based residuals. Biometrika. 1993;80:557–72. [Google Scholar]
- 22.PSHREG: A SAS® macro for proportional and nonproportional subdistribution hazards regression for survival analyses with competing risks Medical University of Vienna, Center for Medical Statistics, Informatics, and Intelligent System, Section for Clinical Biometrics. Techincal Report 06/2013. http://www.meduniwien.ac.at/user/maria.kohl/tr08_2012-PSHREG.pdf. accessed on 04/21/14.
- 23.Elliott MJ, James MT, Quinn RR, et al. Estimated GFR and fracture risk: a population-based study. Clin J Am Soc Nephrol. 2013;8:1367–76. doi: 10.2215/CJN.09130912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jassal SK, von Muhlen D, Barrett-Connor E. Measures of renal function, BMD, bone loss, and osteoporotic fracture in older adults: the Rancho Bernardo study. J Bone Miner Res. 2007;22:203–10. doi: 10.1359/jbmr.061014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naylor KL, McArthur E, Leslie WD, et al. The three-year incidence of fracture in chronic kidney disease. Kidney Int. 2014;86:810–8. doi: 10.1038/ki.2013.547. [DOI] [PubMed] [Google Scholar]
- 26.Fried LF, Biggs ML, Shlipak MG, et al. Association of kidney function with incident hip fracture in older adults. J Am Soc Nephrol. 2007;18:282–6. doi: 10.1681/ASN.2006050546. [DOI] [PubMed] [Google Scholar]
- 27.LaCroix AZ, Lee JS, Wu L, et al. Cystatin-C, renal function, and incidence of hip fracture in postmenopausal women. J Am Geriatr Soc. 2008;56:1434–41. doi: 10.1111/j.1532-5415.2008.01807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ensrud KE, Barbour K, Canales MT, et al. Renal function and nonvertebral fracture risk in multiethnic women: the Women's Health Initiative (WHI) Osteoporos Int. 2012;23:887–99. doi: 10.1007/s00198-011-1667-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ensrud KE, Parimi N, Cauley JA, et al. Cystatin C and risk of hip fractures in older women. J Bone Miner Res. 2013;28:1275–82. doi: 10.1002/jbmr.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ensrud KE, Parimi N, Fink HA, et al. Estimated GFR and risk of hip fracture in older men: comparison of associations using cystatin C and creatinine. Am J Kidney Dis. 2014;63:31–9. doi: 10.1053/j.ajkd.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barzilay JI, Gao P, Clase CM, et al. Albuminuria and rapid loss of GFR and risk of new hip and pelvic fractures. Clin J Am Soc Nephrol. 2013;8:233–40. doi: 10.2215/CJN.06640712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsushita K, Selvin E, Bash LD, Franceschini N, Astor BC, Coresh J. Change in estimated GFR associates with coronary heart disease and mortality. J Am Soc Nephrol. 2009;20:2617–24. doi: 10.1681/ASN.2009010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rifkin DE, Shlipak MG, Katz R, et al. Rapid kidney function decline and mortality risk in older adults. Arch Intern Med. 2008;168:2212–8. doi: 10.1001/archinte.168.20.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moseley KF, Chia CW, Simonsick EM, Egan JM, Ferrucci L, Sellmeyer DE. Sex-specific differences in progressive glucose intolerance and hip geometry: the Baltimore Longitudinal Study of Aging. Osteoporos Int. 2015 doi: 10.1007/s00198-015-3027-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz AV, Vittinghoff E, Margolis KL, et al. Intensive glycemic control and thiazolidinedione use: effects on cortical and trabecular bone at the radius and tibia. Calcif Tissue Int. 2013;92:477–86. doi: 10.1007/s00223-013-9703-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loke YK, Singh S, Furberg CD. Long-term use of thiazolidinediones and fractures in type 2 diabetes: a meta-analysis. CMAJ. 2009;180:32–9. doi: 10.1503/cmaj.080486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz AV, Sellmeyer DE, Vittinghoff E, et al. Thiazolidinedione use and bone loss in older diabetic adults. J Clin Endocrinol Metab. 2006;91:3349–54. doi: 10.1210/jc.2005-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berry SD, Ngo L, Samelson EJ, Kiel DP. Competing risk of death: an important consideration in studies of older adults. J Am Geriatr Soc. 2010;58:783–7. doi: 10.1111/j.1532-5415.2010.02767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perez-Saez MJ, Prieto-Alhambra D, Barrios C, et al. Increased hip fracture and mortality in chronic kidney disease individuals: the importance of competing risks. Bone. 2015;73:154–9. doi: 10.1016/j.bone.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 40.Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79:1370–8. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nickolas TL, Stein EM, Dworakowski E, et al. Rapid cortical bone loss in patients with chronic kidney disease. J Bone Miner Res. 2013;28:1811–20. doi: 10.1002/jbmr.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gennari L, Merlotti D, Valenti R, et al. Circulating sclerostin levels and bone turnover in type 1 and type 2 diabetes. J Clin Endocrinol Metab. 2012;97:1737–44. doi: 10.1210/jc.2011-2958. [DOI] [PubMed] [Google Scholar]
- 43.Sabbagh Y, Graciolli FG, O'Brien S, et al. Repression of osteocyte Wnt/beta-catenin signaling is an early event in the progression of renal osteodystrophy. J Bone Miner Res. 2012;27:1757–72. doi: 10.1002/jbmr.1630. [DOI] [PubMed] [Google Scholar]
- 44.Arasu A, Cawthon PM, Lui LY, et al. Serum sclerostin and risk of hip fracture in older Caucasian women. J Clin Endocrinol Metab. 2012;97:2027–32. doi: 10.1210/jc.2011-3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ardawi MS, Rouzi AA, Al-Sibiani SA, Al-Senani NS, Qari MH, Mousa SA. High serum sclerostin predicts the occurrence of osteoporotic fractures in postmenopausal women: The center of excellence for osteoporosis research study. J Bone Miner Res. 2012;27:2592–602. doi: 10.1002/jbmr.1718. [DOI] [PubMed] [Google Scholar]
- 46.Odden MC, Chertow GM, Fried LF, et al. Cystatin C and measures of physical function in elderly adults: the Health, Aging, and Body Composition (HABC) Study. Am J Epidemiol. 2006;164:1180–9. doi: 10.1093/aje/kwj333. [DOI] [PubMed] [Google Scholar]
- 47.Burkhardt R, Kettner G, Bohm W, et al. Changes in trabecular bone, hematopoiesis and bone marrow vessels in aplastic anemia, primary osteoporosis, and old age: a comparative histomorphometric study. Bone. 1987;8:157–64. doi: 10.1016/8756-3282(87)90015-9. [DOI] [PubMed] [Google Scholar]
- 48.Manolagas SC. Role of cytokines in bone resorption. Bone. 1995;17:63S–7S. doi: 10.1016/8756-3282(95)00180-l. [DOI] [PubMed] [Google Scholar]
- 49.Chen W, Melamed ML, Abramowitz MK. Serum bicarbonate and bone mineral density in US adults. Am J Kidney Dis. 2014 doi: 10.1053/j.ajkd.2014.07.007. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Levey AS, Inker LA, Coresh J. GFR estimation: from physiology to public health. Am J Kidney Dis. 2014;63:820–34. doi: 10.1053/j.ajkd.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Isakova T, Craven TE, Lee J, et al. Fibroblast Growth Factor 23 and Incident CKD in Type 2 Diabetes. Clin J Am Soc Nephrol. 2015;10:29–38. doi: 10.2215/CJN.06190614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Standards of medical care in diabetes--2013. Diabetes Care. 2013;36(Suppl 1):S11–66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwartz AV, Vittinghoff E, Bauer DC, et al. Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA. 2011;305:2184–92. doi: 10.1001/jama.2011.715. [DOI] [PMC free article] [PubMed] [Google Scholar]