Abstract
Children with sickle cell disease (SCD), including those without evidence for cerebral infarcts, are at increased risk for cognitive deficits that may contribute to difficulties in academic and social functioning. Chronic inflammatory processes are endemic to SCD and are apparent in common comorbidities including asthma. Cytokines mediating inflammatory processes may influence cognition. We examined the relationship between plasma levels of cytokines commonly associated with asthma and cognitive functioning using standardized neuropsychological measures in 25 children with SCD with normal MRI studies of the brain. Children with SCD performed significantly below the normative mean on tests of cognitive function. Pearson correlations indicated significant negative relations between cytokines (IL-4, IL-5, IL-8, and IL-13) and standardized tests of executive function (r = −.54 to −.74). Preliminary evidence suggests an association between cytokine levels and executive function in children with SCD, indicating a potential role for inflammatory processes in cognitive outcomes in these children.
Keywords: Sickle cell disease, executive function, cytokines, asthma, inflammation
Introduction
Sickle cell disease (SCD) is a progressive genetic disorder, occurring in approximately 1 in 2,400 American births and 1 in 400 African American births1. All major organ systems, including the central nervous system (CNS) are affected2.
Related to impairment in CNS development, children with SCD are at increased risk for cognitive deficits that are associated with difficulties in academic and social functioning3. For example, Hijmans et al.4 found significant impairments in overall IQ and domains of executive functioning in children with SCD compared to matched controls. While this neuropsychological profile is related to overt and silent strokes, children with SCD with normal MRI study of the brain often display similar deficits5. Despite evidence that cognitive deficits are present with and without cerebral infarcts, little is known about the biological pathways that impact cognitive functioning in children with SCD.
Chronic inflammatory processes are endemic to SCD6 and are apparent in common comorbidities including asthma. These inflammatory processes are one possible pathway underlying cognitive deficits associated with SCD, particularly for those without evidence of cerebral infarcts. Cytokines mediating inflammatory processes play an important role in cognition through effects on synaptic plasticity, neurogenesis, and neuromodulation7. Direct cytokine administration negatively impacts cognition8, suggesting that immunologic responses to inflammation may be an additional mechanism for cognitive impairments in children with SCD.
Given the well established observation that individuals with SCD have an increased level of plasma cytokines compared to individuals without SCD9, using a convenience sample from two studies with this population, we performed analyses to determine if there is preliminary support for the hypothesis that cognitive functioning in children with SCD and normal MRI examinations of the brain would be associated with cytokine levels commonly elevated in asthma. These cytokines (IL-4, 5, 8, and 13) mediate T helper type 2 (Th2) response known to be associated with vascular inflammation that extends to neural tissues, promotes brain aging, and negatively impacts cognition. 10,11 This study thus represents the first examination, to our knowledge, of a possible link between inflammatory markers and cognitive abilities and suggests a potential mechanism for deficits that have remained unexplained in this population.
Methods
Protocol
The Washington University School of Medicine Human Research Protection Office approved the protocol for this study. Written informed consent was obtained from all participating children and parents. Neurocognitive testing was conducted by a trained examiner, and tests were administered in a fixed order to all participants. Participants in the study consented to participate in Sleep and Asthma Cohort Study at Washington University designed to assess the influence of asthma and sleep disordered breathing on SCD related morbidity. Participants were also enrolled in Cognition of Children with Sickle Cell Anemia, a study at Washington University School of Medicine designed to assess the cognitive functioning of individuals with a normal MRI study of the brain. Thus, the participants represent a convenience sample of individuals that participated in both studies simultaneously.
EDTA plasma containing Sigma P-8340 protease inhibitor (10 uL P-8340 per ml of plasma) was analyzed for interleukins-4, 5, 8, and 13 (IL-4, 5, 8, 13). Cytokine analyses were performed using Luminex xMAP technology (Luminex Corporation, Austin TX, USA). Plasma concentrations of IL-8 were determined using the Milliplex MAP Human Cardiovascular Disease 3 panel (Millipore, Billerica, MA, USA). IL-4, IL-5 and IL-13 were measured using the Milliplex MAP High Sensitivity Human Cytokine panel (Millipore). All analyses were performed according to the manufacturer's recommendations. These cytokines were selected because they have been implicated in inflammatory processes in children with SCD and other comorbidities, including asthma12 and mediate a T Helper 2 response involved in neurovascular inflammation and cognitive decline10,11. MRI studies of the brains were performed as part of routine care prior to cognitive testing.
Measures
Full-scale, verbal, and non-verbal IQ were obtained using the Wechsler Abbreviated Scale of Intelligence13, a well-validated measure of verbal, performance, and full-scale IQ for individuals age 6-89 years. Verbal IQ was estimated using the Vocabulary and Similarities subtests and non-verbal IQ was estimated using the Block Design and Matrix Reasoning subtests. These four subtests have adequate reliability for estimates of FSIQ (α = .98).
Four tests of the Delis-Kaplan Executive Function System (D-KEFS)14 were used to assess several aspects of executive function, including mental flexibility, inhibition, and fluency. In the Trailmaking Letter-Number Sequencing Test, a child must manually connect letters and numbers printed on a page in order while switching between numbers and letters (e.g., A-1-B-2-C, etc.). In the Color Word Interference Test, children must state the ink color of words printed in incongruously colored ink. The Card Sorting Test requires a child to sort a set of objects into as many different categories as possible in a given time period. The Verbal Fluency Test requires a child to verbally state as many words beginning with either a particular letter or from a particular category in a limited time.
Sample scores on neurocognitive tests were compared to published normative data. For the WASI, the standardization sample includes 1,100 children age 6 to16 years proportionate to the U.S. census population by geographic region, gender, educational level, and race/ethnicity13. For the D-KEFS, the standardization sample includes 875 children age 8 to 19 years demographically and regionally matched to the U.S. census population14.
Results
Data from 25 children (Males = 13; Mean age = 11.4 years, SD = 2.7 years) with SCD were analyzed. All were African American. Mean yearly family household income was 39,100 USD (SD = 30,290 USD), and mean level of formal education attained by the head of the household was 14.8 years (SD = 2.4 years). Sixteen participants (64%) had a diagnosis of asthma. All participants’ SCD condition was stable at the time all neurocognitive assessments were conducted. Participants with asthma did not differ significantly from participants without asthma diagnoses on any demographic variable measured.
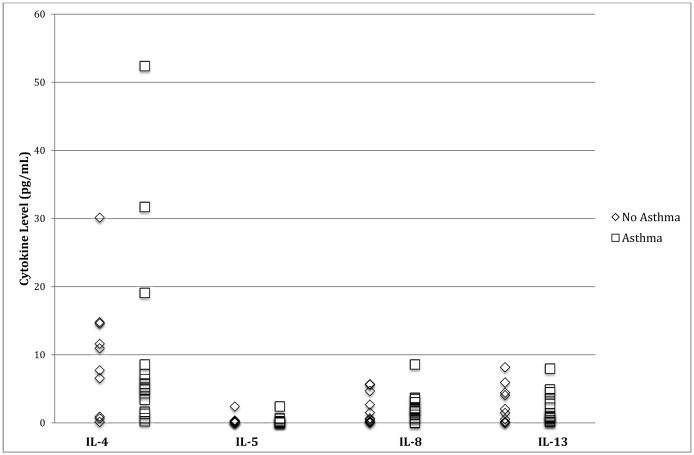
As expected in individuals with SCD, levels indicate mild to moderate increases in plasma cytokine levels when compared to normal controls (see Table 1). Analyses revealed no significant differences between participants with and without asthma diagnoses in plasma cytokine levels measured (see Figure 1). As such, use of the total sample of children with SCD (n = 25) was justified for all further analyses. WASI scores indicate that the sample was significantly below the normative population mean and exhibited adequate variability on this cognitive measure (see Table 2). Scores on tests of executive function indicate that the sample was significantly below the normative population mean on select tasks, but exhibited adequate variability on all cognitive measures (see Table 2). These results are consistent with previous studies indicating deficits in overall cognitive and executive function abilities in children with SCD4.
Table 1.
Summary of Plasma Cytokine Levels in pg/mL (n = 25)
| Cytokine | Mean (SD) | Median | Range | Normative Levels |
|---|---|---|---|---|
| IL-4 | 10.2 (12.5) | 6.41 | .13-52.4 | <1.215 |
| IL-5 | 0.3 (0.5) | .11 | 0-2.4 | <3.015 |
| IL-8 | 2.1 (2.1) | 1.92 | 0-8.6 | <5.816 |
| IL-13 | 2.3 (2.5) | 1.44 | 0-8.2 | <8.015 |
Reference for normative values for IL-4, IL-5, IL-13;
Reference for normative values for IL-8
Figure 1.
Cytokine levels (pg/mL) by asthma diagnosis
Table 2.
Summary of Cognitive Results Compared to Population Normative Data (n = 25)
| Measure | Mean (SD) | Cohen’s d |
|---|---|---|
| WASI FSIQ | 91.2 (12.2) * | .59 |
| WASI Verbal IQ | 93.4 (12.9) * | .44 |
| WASI Performance IQ | 90.7 (12.6) * | .62 |
| D-KEFS Trailmaking Number-Letter Sequencing | 39.9 (13.8) * | .67 |
| D-KEFS Color Word Interference | 44.6 (11.3) * | .36 |
| D-KEFS Card Sorting - Total Correct Sorts | 48.6 (10.2) | .09 |
| D-KEFS Verbal Fluency - Total Correct | 51.8 (10.3) | .12 |
Abbreviations: WASI, Wechsler Abbreviated Scale of Intelligence, results presented as standard scores; D-KEFS, Delis-Kaplan Executive Function System, results presented as T scores
Significantly below normative sample mean with Bonferroni correction for multiple (i.e. seven) comparisons, p < .05
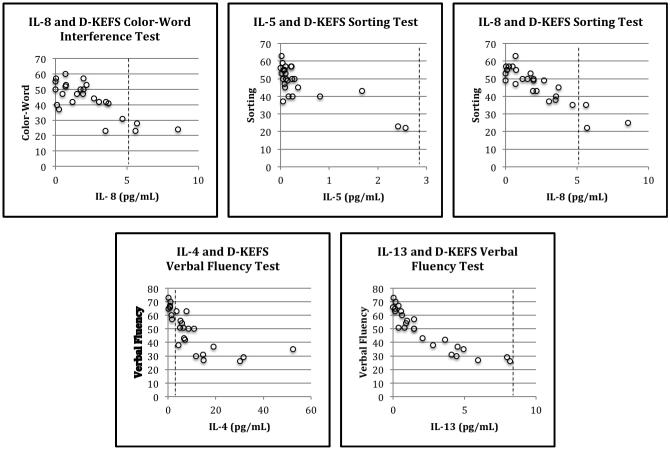
An overall pattern of negative associations was observed between performance on cognitive measures and plasma cytokine levels (see Table 3). Significant (p < .05) associations were found between plasma cytokine levels and measures of executive function (see Figure 2). IL-8 was significantly negatively correlated with performance on the Color Word Interference test (r = −.59, p = .027) and confirmed correct sorts on the Card Sorting test (r = −.54, p = .045). IL-5 was significantly negatively correlated with the confirmed correct sorts on the Card Sorting test (r = −.74, p = .002). Additionally, IL-4 (r = −.61, p = .02) and IL-13 (r = −.59, p = .028) were significantly negatively correlated with performance on the Verbal Fluency test. No other significant correlations between cytokine levels and scores on measures of executive function were obtained.
Table 3.
Pearson correlations between plasma cytokine levels and performance on cognitive measures
| FSIQ | VIQ | PIQ | Trails. | Color-Word | Sorting | Fluency | |
|---|---|---|---|---|---|---|---|
|
|
|||||||
| IL-4 | −0.30 | −0.35+ | −0.14 | −0.07 | −0.07 | −0.14 | −.61* |
| IL-5 | −0.32 | −0.38+ | −0.14 | −0.47+ | −0.47+ | −0.74** | −0.28 |
| IL-8 | −0.28 | −0.33+ | −0.13 | −0.1 | −0.59* | −.54* | −0.21 |
| IL-13 | −0.29 | −0.35+ | −0.12 | 0.03 | 0.03 | −0.12 | −.59* |
Abbreviations: FSIQ, WASI Full Scale IQ; VIQ, WASI Verbal IQ; PIQ, WASI Performance IQ; Trails, D-KEFS Trailmaking Letter-Number Sequencing Test; Color-Word, D-KEFS Color-Word Interference Test; Sorting, D-KEFS Card Sorting Test Total Correct Sorts; Fluency, D-KEFS Verbal Fluency Test
p < .10;
p < .05;
p < .01
(remains significant with Bonferroni correction)
Figure 2.
Scatterplots of cytokine levels against cognitive test scores. Dashed lines indicate upper limit of normative reference ranges.
Discussion
Despite having no evidence of cerebral infarcts, many children with SCD have lower global IQ and cognitive function when compared to sibling controls without SCD, which can negatively impact school performance and psychosocial function17. Several explanations can be offered to explain these deficits, such as injury below the resolution of the MRI scanner or chronic anemia13; however, no study has explored that possibility that chronic inflammatory processes related to SCD and common comorbidities such as asthma may be associated with poor cognitive performance. Behavioral measures of cognitive and executive function, including verbal and non-verbal abilities, mental flexibility, inhibition, and verbal fluency, were examined in relation to plasma levels of IL-4, 5, 8, and 13. As hypothesized, significant negative correlations were found between levels of cytokines measured in this study and several indices of executive function abilities.
While this study was limited by a relatively small sample size, it provides initial evidence for one potential mechanism for the diminished cognitive abilities that have been noted in children with SCD who have not experienced overt or silent infarcts. That is, neurovascular inflammation related to interleukin-derived Th2 activation may negatively impact cognition10,11. Although the observed trends suggest an association, several cytokines measured (i.e., IL-5, IL-8 and IL-13) were not significantly elevated with respect to the normative reference range for these markers. While IL-4 was more clearly elevated, it is of note that this study depended on published normative reference ranges, and future work should ideally employ cytokine control ranges from childhood disease populations to optimize contextualization of findings. Additional research is necessary in order to determine the biological importance of the IL-4 elevation observed and its unique relation to cognitive status. While this elevation is potentially causally involved in the noted cognitive alterations, it is also therapeutically conceivable that cytokine elevations could be secondary to neuronal damage, vascular damage, or even involved in neural repair. Additional work that more clearly elucidates the function of these inflammatory markers will be required to fully understand the associations observed in this study. As such, clear conclusions cannot be made, but the trends of results are unique and hypothesis-generating for future studies, which may examine other inflammatory markers more strongly linked to cognition (e.g., IL-6).
The specific cytokine levels signifying inflammatory responses measured in this study have been associated with asthma, a frequent comorbid condition in children with SCD. Although a significant relation between asthma diagnosis and cytokine levels was not found in the current study, the limited sample size may have lacked the required power to detect such an association given expected overall elevations in cytokine levels in SCD. In addition, cytokine levels were measured in plasma, not CSF, and may thus have been affected by comorbid medical issues endemic to the population studied (e.g., systemic disease, bacterial infection). However, the consistent pattern of negative associations between cytokine levels and cognitive performance observed despite limited power underscores the potential role of inflammatory processes endemic to SCD and its comorbidities in deleterious neurocognitive sequelae. Medical approaches currently in use may be further developed to prevent vaso-occlusive episodes and other physiological events directly related to inflammation. In addition, mental health interventions may also be indicated to improve patients’ abilities to cope with the stress of chronic illness and psychosocial difficulties common to this population.
Acknowledgements
Data collection performed as part of the Sleep and Asthma Cohort Study at Washington University and the Cognition of Children with Sickle Cell Anemia at Washington University School of Medicine, St. Louis, MO.
Funding Sources:
This study was supported by funding from Burroughs Wellcome Foundation, the Doris Duke Charitable Foundation, and grant R01-HL079937 from the National Institutes of Health.
Footnotes
Declaration of Conflicting Interests:
The authors have no conflicts of interest to declare.
Ethical Approval:
The Washington University School of Medicine Human Research Protection Office approved the protocol for this study. Written informed consent was obtained from all participating children and parents.
References
- 1.Ashley-Koch A, Yang Q, Olney RS. Sickle hemoglobin (HbS) allele and sickle cell disease: HuGE review. Am J Epidemiol. 2000;151:839–45. doi: 10.1093/oxfordjournals.aje.a010288. [DOI] [PubMed] [Google Scholar]
- 2.Adams RJ, Ohene-Frempong K, Wang W. Sickle cell and the brain. Am Soc Hematol Educ Program. 2001:31–46. doi: 10.1182/asheducation-2001.1.31. [DOI] [PubMed] [Google Scholar]
- 3.Trzepacz AM, Vannatta K, Gerhardt G, et al. Emotional, social, and behavioral functioning of children with sickle cell disease and comparison peers. J Pediatr Hematol Onc. 2004;26:642–648. doi: 10.1097/01.mph.0000139456.12036.8d. [DOI] [PubMed] [Google Scholar]
- 4.Hijmans CT, Grootenhuis MA, Oosterlaan J, et al. Neurocognitive deficits in children with sickle cell disease are associated with the severity of anemia. Pediatr Blood Cancer. 2011;57:297–302. doi: 10.1002/pbc.22892. [DOI] [PubMed] [Google Scholar]
- 5.Schatz J, White DA, Moinuddin A, et al. Lesion burden and cognitive morbidity in children with sickle cell disease. J Child Neurol. 2002;17:891–895. [PubMed] [Google Scholar]
- 6.Field JJ, DeBaun MR. Asthma and sickle cell disease: two distinct diseases or part of the same process. Hematology Am Soc Hematol Educ Program. 2009:45–53. doi: 10.1182/asheducation-2009.1.45. [DOI] [PubMed] [Google Scholar]
- 7.McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev. 2009;33:355–366. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Casamenti F, Prosperi C, Scali C, et al. Interleukin-1B activates forebrain ?glial cells and increases nitric oxide production and cortical glutamate and GABA release in vivo: Implications for Alzheimer’s disease. Neurosci. 1999;91:831–842. doi: 10.1016/s0306-4522(98)00680-0. [DOI] [PubMed] [Google Scholar]
- 9.Brittain JE, Parise LV. Cytokines and plasma factors in sickle cell disease. Curr Opin Hematol. 2007;14:438–43. doi: 10.1097/MOH.0b013e3282a4a673. [DOI] [PubMed] [Google Scholar]
- 10.Elenkov IJ. Neurohormonal-cytokine interactions: Implications for inflammation, common human diseases and well-being. Neurochem Int. 2008;52:40–51. doi: 10.1016/j.neuint.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 11.Baruch K, Ron-Harel N, Gal H, et al. CNS-specific immunity at the choroid plexus shifts toward destructive Th2 inflammatio in brain aging. PNAS. 2013;110:2264–2269. doi: 10.1073/pnas.1211270110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung KF, Barnes PJ. Cytokines in asthma. Thorax. 1999;54:825–857. doi: 10.1136/thx.54.9.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wechsler, D. Wechsler Abbreviated Scale of Intelligence (WASI) Harcourt Assessment; San Antonio: 1999. [Google Scholar]
- 14.Delis DC, Kaplan E, Kramer JH. The Delis- Kaplan Executive Function System: Examiner’s Manual. The Psychological Corporation; San Antonio: 2001. [Google Scholar]
- 15.Pukelsheim K, Stoeger T, Kutschke D, et al. Cytokine profiles in asthma families depend on age and phenotype. PLoS ONE. 2010;5(12):e14299. doi: 10.1371/journal.pone.0014299. doi: 10.1371/journal.pone.0014299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang YH, Lai HJ, Huang CM, et al. Sera from children with active Henoch-Schonlein purpura can enhance the production of interleukin 8 by human umbilical venous endothelial cells. Ann Rheum Dis. 2004;63:1511–1513. doi: 10.1136/ard.2003.016196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schatz J, McClellan CB. Sickle cell disease as a neurodevelopmental disorder. Ment Retard Dev D R. 2006;12:200–207. doi: 10.1002/mrdd.20115. [DOI] [PubMed] [Google Scholar]