Abstract
Shigellosis is the major global cause of dysentery. Shigella sonnei, which has historically been more commonly isolated in developed countries, is undergoing an unprecedented expansion across industrializing regions in Asia, Latin America, and the Middle East. The precise reasons underpinning the epidemiological distribution of the various Shigella species and this global surge in S. sonnei are unclear but may be due to three major environmental pressures. First, natural passive immunization with the bacterium Plesiomonas shigelloides is hypothesized to protect populations with poor water supplies against S. sonnei. Improving the quality of drinking water supplies would, therefore, result in a reduction in P. shigelloides exposure and a subsequent reduction in environmental immunization against S. sonnei. Secondly, the ubiquitous amoeba species Acanthamoeba castellanii has been shown to phagocytize S. sonnei efficiently and symbiotically, thus allowing the bacteria access to a protected niche in which to withstand chlorination and other harsh environmental conditions in temperate countries. Finally, S. sonnei has emerged from Europe and begun to spread globally only relatively recently. A strong selective pressure from localized antimicrobial use additionally appears to have had a dramatic impact on the evolution of the S. sonnei population. We hypothesize that S. sonnei, which exhibits an exceptional ability to acquire antimicrobial resistance genes from commensal and pathogenic bacteria, has a competitive advantage over S. flexneri, particularly in areas with poorly regulated antimicrobial use. Continuing improvement in the quality of global drinking water supplies alongside the rapid development of antimicrobial resistance predicts the burden and international distribution of S. sonnei will only continue to grow. An effective vaccine against S. sonnei is overdue and may become one of our only weapons against this increasingly dominant and problematic gastrointestinal pathogen.
Introduction
Shigellosis, caused by members of the bacterial genus Shigella, is a severe and occasionally life-threatening diarrheal infection. Worldwide, Shigella spp. are the most common cause of acute, bloody diarrhea (dysentery) and are responsible for a significant proportion of the burden of morbidity and mortality associated with diarrheal disease [1,2]. In Asia alone, it is estimated that there are 125 million infections and 14,000 deaths due to shigellosis annually [3]. As a result of the considerable global burden, low infectious dose [4], clinical severity, and frequent reports of emerging antimicrobial resistance against first- and, more recently, second-line therapies [5,6], a vaccine against Shigella infections is a growing necessity. Yet, more than a century after the discovery of the agent of bacillary dysentery, there is still neither a licensed vaccine nor agreement on the precise mechanisms that induce Shigella immunity [7]. Vaccine development is further complicated by the probable need for a multivalent combination of O polysaccharide antigens to protect against a variety of heterogeneously distributed serotypes [8].
The genus Shigella incorporates four species. Shigella dysenteriae was historically responsible for large epidemics [9] yet is now rarely identified [8]. Similarly, S. boydii is also infrequently isolated. S. flexneri, however, is common globally and traditionally isolated most frequently in resource-poor countries [10]. S. flexneri has 15 different serotypes distributed heterogeneously across different regions, with predominant serotypes including S. flexneri 2a, 3a, and 6 [8,11]. Finally, S. sonnei is also prevalent globally, although traditionally most commonly detected in high-income regions [10,12]. S. sonnei has only one serotype.
S. sonnei: An Emergent Pathogen
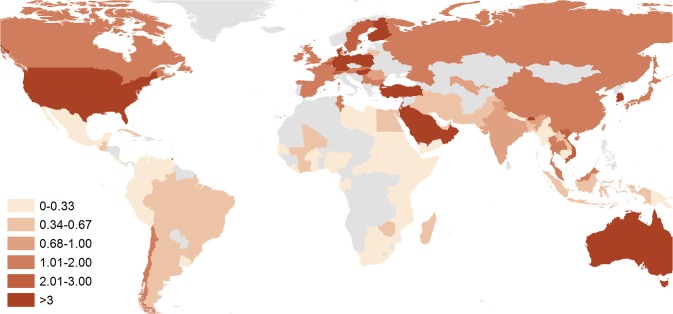
Reasons behind the conventional dominance of S. sonnei in industrialized countries remain unclear [13]. However, an increasing proportion of shigellosis due to S. sonnei generally correlates with improving economic prosperity [12], which in the context of many rapidly developing countries, has led to a proportional decrease in S. flexneri and the simultaneous emergence of S. sonnei [14]. This shift toward S. sonnei has been documented in many regions in Asia, Latin America, and the Middle East (Fig 1) [15–20], with proven explanations behind such an epidemiological phenomenon lacking. This review aims to summarize the existing evidence as to why S. sonnei may predominate in high-income countries and why it is now emerging in regions traditionally dominated by S. flexneri and explores the implications of the growing threat of this increasingly antimicrobial-resistant pathogen for public health globally.
Fig 1. The ratio of S. sonnei to S. flexneri isolated from 100 countries, 1990–2014.
The darker the color, the higher the proportion of S. sonnei isolated from each country; the lighter the color, proportionally higher the proportion of S. flexneri isolated. Countries colored grey indicate no data on species were identified. To generate this map, we performed an extensive literature review in PubMed using the term “Shigella” followed by the name of 178 countries. The most recent publication that included species information that was nonoutbreak and nontravel associated was included as representative of each country. If country data were pre-1990 or were not available on PubMed, the Gideon Infectious Disease encyclopedia as well as national reference laboratory data were referenced where possible. References are listed by country in the supplementary material (S1 Table).
Plesiomonas shigelloides: Passive Environmental Immunization?
One of the principal theories regarding the lack of S. sonnei in industrializing areas focuses on the Gram-negative bacteria P. shigelloides, which like Shigella falls within the large eubacterial family of the Enterobacteriaece. P. shigelloides and S. sonnei share an identical lipopolysaccharide (LPS) O-side chain (confirmed by nuclear magnetic resonance [NMR] and mass spectrometry) that is thought to be the major surface antigen targeted by the adaptive immune system during Shigella infection [21,22]. These surface antigens are cross-reactive, and vaccines prepared from O-antigen derived from P. shigelloides have been shown to be reasonably effective in preventing infection with S. sonnei in humans [23,24]. The O-antigen gene cluster is located on the S. sonnei invasion plasmid and is essential for penetration of host epithelial cells [25]. Evidence suggests not only that S. sonnei acquired the O-antigen gene cluster from P. shigelloides but also that this acquisition was the defining event in the emergence of S. sonnei [26].
Due to the cross-reactive nature of the S. sonnei/P. shigelloides O-antigens, Sack and colleagues suggested that exposure to P. shigelloides serotype O17 leads to protection against infection with S. sonnei [27]. In areas with poor-quality water supplies, the authors postulated that exposure to P. shigelloides occurs frequently and thus disease due to S. sonnei is rare, as the population is effectively naturally immunized [27]. Although P. shigelloides is found in water and environmental samples in both industrialized and industrializing countries [28,29], water treatment practices are likely to prevent frequent exposure in regions with adequate sanitation. Highlighting this, outbreaks of diarrheal disease thought to be due to P. shigelloides occurred after a lapse in water chlorination in Japan in the mid-1970s [30]. Although the serotypic distribution of P. shigelloides has not been well described [31], serotype O17 has been reported in both water and stool samples from patients admitted for diarrhea in industrializing regions [32,33], lending credence to the hypothesis of water-driven immunization at the population level.
The phenomenon of passive immunization in low-income countries would explain, at least in part, why S. sonnei is proportionally more commonly isolated in industrialized countries. Accordingly, an increase in the proportion of Shigella episodes due to S. sonnei would occur concurrently with economic development and improved water supplies [27]. Ram and colleagues confirmed this economic trend by identifying a strong positive correlation between country-level GDP and proportion of isolated Shigella due to S. sonnei from 56 studies conducted from 1984–2005 (R = 0.55, p < 0.0001) [12]. Therefore, the combination of improving economic outlook and fulfillment of the Millennium Development Goals (MDGs) will lead to an improvement of drinking water supply, a drop in population-level cross protection against S. sonnei, and potentially to a global increase in S. sonnei infections in heavily populated regions currently undergoing such transitions [34].
Acanthamoeba: An Environmental Host?
Acanthamoeba is the most common amoeba found globally, with a wide distribution in both aquatic and nonaquatic environments [35]. Amoeba such as Acanthamoeba are known to act as environmental hosts for a variety of intracellular pathogens including Helicobacter pylori, Vibrio cholerae, and also various Shigella spp. [36–38]. The uptake of bacteria into amoebic cysts allows the bacteria to persist in adverse environmental conditions, including desiccation, starvation, and a variety of chemical and physical agents [39]. Acanthamoeba cysts, which form when triggered by nutritional or osmotic stress, are particularly resistant to chlorine treatment [40]. Found commonly through environmental sampling [41], Acanthamoeba has been identified in public water supplies in developed countries with appropriate chlorination levels [42] and in hospital water supplies [43] and can also be isolated from drinking water supplies in industrializing regions [44].
Recent evidence confirms that S. sonnei, S. dysenteriae, and S. flexneri can be taken up by the species Acanthamoeba castellanii when grown under laboratory conditions [36,45,46]. Once phagocytized, Shigella spp. are localized in A. castellanii vacuoles and eventually in the cysts [36,45] and can survive for over three weeks [45]. Notable differences in symbiotic growth were recorded with respect to amoebic uptake between Shigella species. S. sonnei has been shown to be efficiently taken up and maintained by A. castellanii at temperatures between 18–30°C [36,46]; indeed, growth of S. sonnei in the presence of Acanthamoeba was found to exceed that of S. sonnei cultured alone [46]. S. flexneri, however, was found to significantly inhibit A. castellanii growth in the laboratory at 30°C [46]. Inhibition and killing of A. castellanii by S. flexneri is due to activation of invasion genes, which may induce apoptosis through the secretion of effector proteins into the host cell via the type three secretion system [47,48].
The growth and survival rates of Shigella in the cytoplasm of the amoeba resemble their pattern of growth and survival in mammalian macrophages [45,49]. In fact, it has been suggested that growth in the amoebic intracellular niche may have influenced the ability of Shigella to survive in the mammalian phagocytic cell environment [50]. Additionally, as free-living amoeba feed on bacteria, fungi, and algae, lateral gene transfer within the amoeba phagolysosome may have facilitated genetic adaptations that allow for the expression of pathogenic or symbiotic phenotypes based on impact on the host cell [51,52]. It has been further suggested that amoebae themselves represent an important genetic reservoir for internalized microbes [51]. For example, there has been an observed increase in resistance to various antimicrobials and biocides in Legionella pneumophila grown within free-living amoeba [53], which may be due to selection within the amoeba itself [51].
All aspects considered, available evidence suggests that A. castellanii may contribute to transmission of S. sonnei in temperate regions by phagocytizing S. sonnei, thus allowing the bacteria to circumvent the effects of chlorination and good sanitation [54]. As S. flexneri has been shown to inhibit growth of A. castellani [46], the amoeba may not be a viable reservoir for S. flexneri in either developed or industrializing countries. Protozoa appear to play an important role in the transition of bacteria from the environment to mammals and as such may be the source of emerging pathogenic bacteria [50], and may play an increasing role in the epidemiology of S. sonnei in industrializing regions as the prevalence increases.
Antimicrobial Resistance: A New Defense Strategy?
Phylogeographical analyses of a large number of S. sonnei isolates spanning several continents and several decades in a publication by Holt et al. demonstrated that all contemporary S. sonnei infections are due to a small number of clones that dispersed globally from Europe within the last 500 years [34]. Four distinct lineages of S. sonnei were identified, with lineage III the most prevalent globally, becoming dominant in Asia, Africa, and South America [34]. S. sonnei belonging to lineage III are characterized by the presence of distinct class II integron (In2), which confers resistance to trimethoprim, streptothricin, and streptomycin [55]. Many lineage III isolates were also found to harbor a genetic locus on a small plasmid conferring resistance to a variety of additional antimicrobials including tetracycline and sulphonamides. Holt and colleagues indicated that In2 was likely acquired during the mid-20th century, after which the clone spread internationally, undergoing contemporary global dispersal and localized clonal expansions [34].
This localized microevolution of S. sonnei appears to be largely driven by selection pressure induced by antimicrobials [56]. The determinants for antimicrobial resistance in Shigella are generally located on mobile genetic elements such as plasmids, transposons, and integrons [55]. Horizontal gene transfer (HGT) of such elements is now recognized to be an important driver of bacterial evolution [57,58]. One study, for example, estimated 18% of the 4,288 genes of Escherichia coli strain MG1655 were acquired laterally since the species diverged from the Salmonella lineage 100 million years (Myr) ago, a rate of 16 kb/Myr/lineage [59]. Transfer of mobile genetic elements between members of the Enterobacteriaceae is known to be responsible for the dissemination of antimicrobial resistance genes and the emergence of a variety of multidrug-resistant (MDR) Gram-negative bacteria globally [55,60,61].
S. sonnei can acquire advantageous chromosomal and plasmid-mediated resistance genes through HGT from both commensal and pathogenic bacteria circulating locally, enhancing its ability to establish infection, prolonging shedding, and, presumably, outcompeting antimicrobial-susceptible bacteria [56]. A study of >250 S. sonnei isolates collected over 15 years in Vietnam documented the rapid emergence and dominance of successful clones of S. sonnei after the acquisition and fixation of plasmids conferring colicin production/immunity and resistance to third-generation cephalosporins in two separate genetic bottleneck events [56]. In areas with unregulated antimicrobial use, S. sonnei may have abundant opportunity to acquire locally derived resistance genes [62]. In countries with restricted antimicrobial usage, for example, S. sonnei are generally more susceptible to quinolones [5], presumably because of lower selective pressure combined with reduced availability of resistance genes in the circulating accessory gene pool. Such a phenomenon is thought to be leading to increasingly successful clones in areas of unregulated antimicrobial use and could lead to a rapidly growing and increasingly challenging public health problem in many industrializing areas [56].
In 2005, the WHO published guidelines recommending ciprofloxacin to be used as the first-line treatment for dysentery [63]. The late 2000s saw the first documented resistance in S. sonnei against fluoroquinolones [5]. Phylogeographical data from Holt and colleagues indicate marked differences in the global prevalence of gyrA (DNA gyrase) mutations, which confer resistance to quinolones and reduced susceptibility to fluoroquinolones. Strong selection for quinolone resistance was identified, as the facilitating mutations have occurred independently on multiple occasions in several different lineages and genetic locations [34]. Unless the use of fluoroquinolones becomes regulated in areas of current unrestricted use, ciprofloxacin will likely become ineffective for treating Shigella infections in the near future [5]. Yet pivmecillinam, ceftriaxone, or azithromycin may be effective alternatives [64,65], depending on local resistance patterns.
Does Shigella sonnei Have a Competitive Advantage?
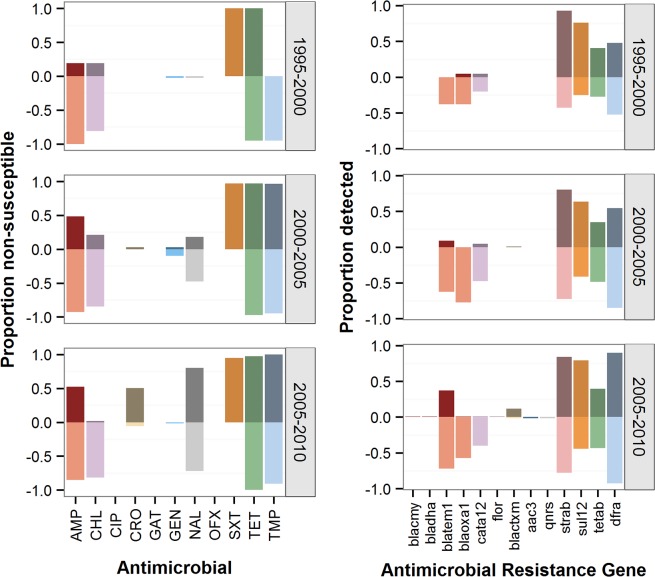
Taken together, evidence suggests that the global burden of S. sonnei may be growing compared to that of S. flexneri. This phenomenon may not only be due to the global improvements in water quality and an ability of S. sonnei to grow successfully within Acanthamoeba but may also be due to a potential, but as yet unproven, ability to acquire and/or maintain a wider array of antimicrobial resistance genes. Indeed, it has been speculated that the plasmid composition and resistance profiles may differ between the Shigella species isolated from contemporaneous patient populations in the same locations (Fig 2) [15,66–71]. Although S. sonnei can acquire extended-spectrum beta-lactamase (ESBL)-mediated resistance from other Enterobacteriaceae, particularly E. coli and Klebsiella spp. [72], it is not currently known whether S. flexneri and S. sonnei acquire resistance genes from each other. Toro et al. suggested that there is a greater restriction barrier for conjugal plasmids between S. sonnei and S. flexneri than between other Gram-negative donors and recipients [67]. A differential ability to acquire and/or maintain plasmids between S. flexneri and S. sonnei from other bacterial donors may also explain discrepant resistance profiles between contemporaneous species, although such a phenomenon has yet to be explicitly investigated.
Fig 2. Antimicrobial resistance and presence of resistance-conferring genes in S. sonnei and S. flexneri.
Plots on the left show proportion of antimicrobial resistance determined by minimum inhibitory concentration (MIC) amongst isolates collected from Vietnam over a 15-year period (n = 231 for S. sonnei and 136 for S. flexneri) [34,56]. S. sonnei are shown by darker colors on the top of each graph, and S. flexneri is shown by lighter colors at the bottom. Plots on the right show the proportion of S. sonnei (dark color, top of each graph) and S. flexneri (light color, bottom of each graph) found to have varying resistance genes present on either plasmids or the chromosome. See Supporting Information (SI) S1 Text for a description of procedures. The color of the gene corresponds with the color of the antimicrobial to which it confers resistance on the left. AMP: ampicillin; CHL: chloramphenicol; CIP: ciprofloxacin; CRO: ceftriaxone; GAT: gatifloxacin; GEN: gentamicin; NAL: nalidixic acid; OFX: ofloxacin; SXT: cotrimoxazole; TET: tetracycline; TMP: trimethoprim.
Levels of inflammation in the gut during infection could explain differences in the ability to acquire mobile genetic elements of resistance between the species. Stecher et al. demonstrated that inflammatory responses in the gut during infection may facilitate conjugative transfer and reassortment of plasmid-encoding genes between pathogens and commensal organisms [73]. Although it was shown recently that S. flexneri is able to modify its LPS structure to dampen the inflammatory innate immune response to allow it to successfully evade detection in the initial phases of infection [74], it is not yet known whether this occurs during S. sonnei infection. Investigations into differential inflammatory response between the Shigella species during infection and their relationship to HGT within the human gastrointestinal tract are warranted.
Finally, S. sonnei has been shown to be genetically more similar to its ancestor E. coli than to other Shigella species [75,76]. Gene retention from E. coli potentially imbues S. sonnei with a higher likelihood for survival in the environment or an environmental adaptive host [75], such as Acanthamoeba. S. flexneri, however, loses genes faster than any other Shigella species and is the most genetically distant of the Shigellae from E. coli [76]. Hershberg and colleagues suggest a potential “point of no return” for Shigella in that once it undergoes enough purifying selection, it cannot regain enough of its lost functionality to escape niche limitation [76]. Does enhanced capacity for genomic plasticity explain the hypothesized increased ability of S. sonnei to acquire or maintain plasmids from other bacterial donors [77]? Experimental evidence of differences in gene acquisition and retention between the two species is needed.
Next Steps
We predict that the combination of improving water supplies and rapid acquisition and maintenance of mobile elements conferring advantageous resistance genes is accelerating a Shigella species shift toward S. sonnei dominance, which traditionally has been shown to occur over a period of decades in individual countries (Table 1, S1 Table) [78,79]. To counter this rapid species replacement, many questions regarding the epidemiology of S. sonnei and, crucially, vaccine development need to be addressed. Identifying prominent transmission routes of S. sonnei and S. flexneri in resource-poor countries should remain a primary goal. Indeed, experiments to determine the relative fitness of each species in varying environmental conditions and investigating antimicrobial fitness [80] would provide information on potential niche preferences and add insight into which accessory gene pools each species samples from. Finally, longitudinal monitoring of water supplies for the presence of both P. shigelloides and A. castellanii would help to verify the hypotheses presented in this review in regard to both a reduction of population immunity against the S. sonnei O-antigen as well as an environmental amoeba niche for these bacteria.
Table 1. Summary of factors behind the traditional and current epidemiological distribution of S. sonnei and S. flexneri.
| Factor | Region | |||
|---|---|---|---|---|
| Industrializing | Industrialized | |||
| Explanation of traditional geographical distribution | 1. | S. sonnei is not present because of population immunity due to cross protection from exposure to P. shigelloides found in contaminated water supplies [26] | 1. | S. sonnei is present because of a lack of cross protection from exposure to P. shigelloides due to clean water supplies |
| 2. | S. flexneri is not able to grow within the common amoeba A. castellanii [46] | 2. | S. sonnei is symbiotically phagocytosed by A. castellanii and can withstand chlorination and other harsh environmental conditions [36] | |
| Why is the burden of S. sonnei growing? | 1. | Improving water supplies may lead to a decrease in the prevalence of P. shigelloides, resulting in lack of cross protection against S. sonnei [27] | ||
| 2. | Expansion of S. sonnei from Europe in the last 500 years and subsequent microevolution due largely to local antimicrobial use [34,56] | |||
| 3. | Proposed competitive advantage of S. sonnei against S. flexneri due to an enhanced ability to acquire and maintain mobile resistance genes from other bacterial species | |||
Furthermore, research on the genetic structure of global S. flexneri populations is warranted in order to help further understand the global species shift and to explore the global and localized microevolution of this pathogen over time. Such analyses would help to predict the role of S. flexneri in the context of improving sanitation and growing prevalence of S. sonnei worldwide. Finally, although plagued with many setbacks [11], the development of a sufficiently safe and effective S. sonnei vaccine may be feasible in the coming decade. However, in order to carry out properly designed vaccine trials in the future, outstanding questions regarding correlates of immunity, incidence in the community, seroconversion rates, and the role of maternal antibody in the first years of life will need to be answered.
In conclusion, S. sonnei represents an emerging threat to public health globally. With continuing efforts for improvements in water and sanitation worldwide, population-level immunization against S. sonnei due to exposure to P. shigelloides is declining. Additionally, environmental hosts such as A. castellani represent an important yet potentially overlooked reservoir of S. sonnei and may explain in part the persistence of S. sonnei in regions with a reasonably good standard of sanitation. Finally, the incredible ability of S. sonnei to acquire resistance to a variety of widely used antimicrobials may endow the pathogen with a competitive advantage over sensitive bacterial competitors and predicts its emergence in areas with unregulated antimicrobial use. Combined, this evidence suggests alarming increases in global prevalence of S. sonnei and unprecedented levels of resistance, demanding a vaccine in the near future that can be administered to the most vulnerable populations, particularly young children in rapidly industrializing countries.
Boxes
Box 1. Key Learning Points
Traditionally, the various species of the bacterial genus Shigella have a distinct geographical distribution. S. sonnei is most commonly isolated in industrialized countries, whereas S. flexneri is more commonly isolated in industrializing regions. However, S. sonnei is now becoming recognized as a common enteric pathogen in many industrializing regions. The exact mechanisms catalyzing this shift in the epidemiological distribution are unclear.
Improving the quality of drinking water supplies in industrializing regions is likely to reduce cross protection against S. sonnei derived from the bacterium P. shigelloides, which is commonly found in contaminated water.
S. sonnei may be efficiently phagocytized by the ubiquitous amoeba species A. castellani, thereby providing it with a reservoir in which to withstand chlorination and other harsh environmental conditions.
In comparison to S. flexneri, S. sonnei has a greater ability to develop resistance to broad-spectrum antimicrobials. We suggest that S. sonnei is more likely to accept and maintain horizontally transferred DNA, which gives it a competitive advantage against S. flexneri, particularly in areas with unregulated antimicrobial use.
With ongoing improvements in the international quality of water supplies and rapid development of antimicrobial resistance, the burden of S. sonnei is likely to grow substantially. A vaccine against S. sonnei is increasingly necessary.
Box 2. Top Five Papers
Sack D, Hoque A, Huq A, Etheridge M (1994) Is Protection against Shigellosis Induced by Natural Infection with Plesiomonas shigelloides? Lancet 343: 1413–1415.
Saeed A, Johansson D, Sandström G, Abd H (2012) Temperature Depended Role of Shigella flexneri Invasion Plasmid on the Interaction with Acanthamoeba castellanii. Int J Microbiol 2012: 917031.
Holt KE, Baker S, Weill F-X, Holmes EC, Kitchen A, et al. (2012) Shigella sonnei Genome Sequencing and Phylogenetic Analysis Indicate Recent Global Dissemination from Europe. Nat Genet 44: 1056–1059.
Holt KE, Thieu Nga TV, Thanh DP, Vinh H, Kim DW, et al. (2013) Tracking the Establishment of Local Endemic Populations of an Emergent Enteric Pathogen. Proc Natl Acad Sci 110: 17522–17527.
Levine MM, Kotloff KL, Barry EM, Pasetti MF, Sztein MB (2007) Clinical Trials of Shigella Vaccines: Two Steps Forward and One Step Back on a Long, Hard Road. Nat Rev Microbiol 5: 540–553.
Supporting Information
(DOCX)
Data are derived from analyses of 367 Shigella isolates collected between 1995 and 2010 from Vietnam (136 S. flexneri and 231 S. sonnei). Resistance was determined by MIC and gene content analysis from Illumina genome sequencing data.
(DOCX)
Funding Statement
The authors received no specific funding for this work.
References
- 1. Thapar N, Sanderson IR (2004) Diarrhoea in children: an interface between developing and developed countries. Lancet 363: 641–653. [DOI] [PubMed] [Google Scholar]
- 2. Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, et al. (2013) Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382: 209–222. 10.1016/S0140-6736(13)60844-2 [DOI] [PubMed] [Google Scholar]
- 3. Bardhan P, Faruque A, Naheed A, Sack DA (2010) Decrease in shigellosis-related deaths without Shigella spp.-specific interventions, Asia. Emerg Infect Dis 16: 1718–1723. 10.3201/eid1611.090934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DuPont HL, Levine MM, Hornick RB, Formal SB (1989) Inoculum Size in Shigellosis and Implications for Expected Mode of Transmission. J Infect Dis 159: 1126–1128. [DOI] [PubMed] [Google Scholar]
- 5. Gu B, Cao Y, Pan S, Zhuang L, Yu R, et al. (2012) Comparison of the prevalence and changing resistance to nalidixic acid and ciprofloxacin of Shigella between Europe-America and Asia-Africa from 1998 to 2009. Int J Antimicrob Agents 40: 9–17. 10.1016/j.ijantimicag.2012.02.005 [DOI] [PubMed] [Google Scholar]
- 6. Vinh H, Baker S, Campbell J, Hoang NVM, Loan HT, et al. (2009) Rapid emergence of third generation cephalosporin resistant Shigella spp. in Southern Vietnam. J Med Microbiol 58: 281–283. 10.1099/jmm.0.002949-0 [DOI] [PubMed] [Google Scholar]
- 7. Germani Y, Sansonetti PJ (2011) Replicating Vaccines In: Dormitzer PR, Mandl CW, Rappuoli R, editors. Replicating Vaccines. Basel: Springer Basel; pp. 99–117. [Google Scholar]
- 8. Livio S, Strockbine NA, Panchalingam S, Tennant SM, Barry EM, et al. (2014) Shigella isolates from the global enteric multicenter study inform vaccine development. Clin Infect Dis 59: 933–941. 10.1093/cid/ciu468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tuttle J, Ries A, Chimba R, Perera C, Bean N, et al. (1995) Antimicrobial-resistant epidemic Shigella dysenteriae type 1 in Zambia: modes of transmission. J Infect Dis 171: 371–375. [DOI] [PubMed] [Google Scholar]
- 10. Kotloff K, Winickoff J, Ivanoff B, Clemens J, Swerdlow D, et al. (1999) Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ 77: 651–666. [PMC free article] [PubMed] [Google Scholar]
- 11. Levine MM, Kotloff KL, Barry EM, Pasetti MF, Sztein MB (2007) Clinical trials of Shigella vaccines: two steps forward and one step back on a long, hard road. Nat Rev Microbiol 5: 540–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ram P, Crump J, Gupta S, Miller M, Mintz E (2008) Part II. Analysis of data gaps pertaining to Shigella infections in low and medium human development index countries, 1984–2005. Epidemiol Infect 136: 577–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Keusch GT (2009) Bacterial Infections of Humans. Brachman PS, Abrutyn E, editors Boston, MA: Springer US. [Google Scholar]
- 14. Feil E (2012) The emergence and spread of dysentery. Nat Genet 44: 964–965. 10.1038/ng.2389 [DOI] [PubMed] [Google Scholar]
- 15. Vinh H, Nhu NTK, Nga TVT, Duy PT, Campbell JI, et al. (2009) A changing picture of shigellosis in southern Vietnam: shifting species dominance, antimicrobial susceptibility and clinical presentation. BMC Infect Dis 9: 204–216. 10.1186/1471-2334-9-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qu F, Bao C, Chen S, Cui E, Guo T, et al. (2012) Genotypes and antimicrobial profiles of Shigella sonnei isolates from diarrheal patients circulating in Beijing between 2002 and 2007. Diagn Microbiol Infect Dis 74: 166–170. 10.1016/j.diagmicrobio.2012.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fullá N, Prado V, Durán C, Lagos R, Levine MM (2005) Surveillance for antimicrobial resistance profiles among Shigella species isolated from a semirural community in the northern administrative area of Santiago, Chile. Am J Trop Med Hyg 72: 851–854. [PubMed] [Google Scholar]
- 18. Sousa MÂB, Mendes EN, Collares GB, Péret-Filho LA, Penna FJ, et al. (2013) Shigella in Brazilian children with acute diarrhoea: prevalence, antimicrobial resistance and virulence genes. Memorias Inst Oswaldo Cruz 108: 30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tajbakhsh M, García Migura L, Rahbar M, Svendsen CA, Mohammadzadeh M, et al. (2012) Antimicrobial-resistant Shigella infections from Iran: an overlooked problem? J Antimicrob Chemother 67: 1128–1133. 10.1093/jac/dks023 [DOI] [PubMed] [Google Scholar]
- 20. Ashkenazi S, Levy I, Kazaronovski V, Samra Z (2003) Growing antimicrobial resistance of Shigella isolates. J Antimicrob Chemother 51: 427–429. [DOI] [PubMed] [Google Scholar]
- 21. Van de Verg L, Herrington D, Boslego J, Lindberg A, Levine M (1992) Age-specific prevalence of serum antibodies to the invasion plasmid and lipopolysaccharide antigens of Shigella species in Chilean and North American populations. J Infect Dis 166: 158–161. [DOI] [PubMed] [Google Scholar]
- 22. Kubler-Kielb J, Schneerson R, Mocca C, Vinogradov E (2008) The elucidation of the structure of the core part of the LPS from Plesiomonas shigelloides serotype O17 expressing O-polysaccharide chain identical to the Shigella sonnei O-chain. Carbohydr Res 343: 3123–3127. 10.1016/j.carres.2008.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cohen D, Ashkenazi S, Green M, Gdalevich M, Robin G, et al. (1997) Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet 349: 155–159. [DOI] [PubMed] [Google Scholar]
- 24. Passwell JH, Ashkenazi S, Harlev E, Miron D, Ramon R, et al. (2003) Safety and immunogenicity of Shigella sonnei-CRM 9 and Shigella flexneri type 2a-rEPA succ conjugate vaccines in one- to four-year-old children. Pediatr Infect Dis J 22: 701–706. [DOI] [PubMed] [Google Scholar]
- 25. Watanabe H, Nakamura A (1986) Identification of Shigella sonnei form I plasmid genes necessary for cell invasion and their conservation among Shigella species and enteroinvasive Escherichia coli. Infect Immun 53: 352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shepherd JG, Wang L, Reeves P (2000) Comparison of O-antigen gene clusters of Echerichia coli (Shigella) Sonnei and Plesiomonas shigelloides O17: Sonnei gained its current plasmid-borne O-antigen genes from P. shigelloides in a recent event. Infect Immun 68: 6056–6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sack D, Hoque A, Huq A, Etheridge M (1994) Is protection against shigellosis induced by natural infection with Plesiomonas shigelloides? Lancet 343: 1413–1415. [DOI] [PubMed] [Google Scholar]
- 28. Krovacek K, Eriksson LM, González-Rey C, Rosinsky J, Ciznar I (2000) Isolation, biochemical and serological characterisation of Plesiomonas shigelloides from freshwater in Northern Europe. Comp Immunol Microbiol Infect Dis 23: 45–51. [DOI] [PubMed] [Google Scholar]
- 29. Kwaga J, Adesiyun A, Bello C, Abdullahi S (1988) Occurrence of Plesiomonas shigelloides in humans and water in Zaria, Nigeria. Microbiologica 11: 165–167. [PubMed] [Google Scholar]
- 30. Tsukamoto T, Kinoshita Y, Shimada T, Sakazaki R (1978) Two epidemics of diarrhoeal disease possibly caused by Plesiomonas shigelloides. J Hyg (Lond) 80: 275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. González-Rey C, Svenson SB, Bravo L, Siitonen A, Pasquale V, et al. (2004) Serotypes and anti-microbial susceptibility of Plesiomonas shigelloides isolates from humans, animals and aquatic environments in different countries. Comp Immunol Microbiol Infect Dis 27: 129–139. [DOI] [PubMed] [Google Scholar]
- 32. Bravo LF, Correa Y, Clausell JF, Fernandez A, Ramírez M, et al. (2009) Caracterización de factores de virulencia y susceptibilidad antimicrobiana en cepas de Plesiomonas shigelloides aisladas de pacientes con diarrea aguda en Cuba. Microbiol Clin 26: 233–238. [PubMed] [Google Scholar]
- 33. Aldova E (1987) Serotyping of Plesiomonas shigelloides strains with our own antigenic scheme: an attempted epidemiology study. Zentralblatt fur Bakteriol Mikrobiol un Hyg 265: 253–262. [DOI] [PubMed] [Google Scholar]
- 34. Holt KE, Baker S, Weill F-X, Holmes EC, Kitchen A, et al. (2012) Shigella sonnei genome sequencing and phylogenetic analysis indicate recent global dissemination from Europe. Nat Genet 44: 1056–1059. 10.1038/ng.2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Trabelsi H, Dendana F, Sellami A, Sellami H, Cheikhrouhou F, et al. (2012) Pathogenic free-living amoebae: epidemiology and clinical review. Pathol Biol 60: 399–405. 10.1016/j.patbio.2012.03.002 [DOI] [PubMed] [Google Scholar]
- 36. Jeong HJ, Jang ES, Han BI, Lee KH, Ock MS, et al. (2007) Acanthamoeba: could it be an environmental host of Shigella? Exp Parasitol 115: 181–186. [DOI] [PubMed] [Google Scholar]
- 37. Abd H, Weintraub A, Sandström G (2005) Intracellular survival and replication of Vibrio cholerae O139 in aquatic free-living amoebae. Environ Microbiol 7: 1003–1008. [DOI] [PubMed] [Google Scholar]
- 38. Winiecka-Krusnell J, Wreiber K, von Euler A, Engstrand L, Linder E (2002) Free-living amoebae promote growth and survival of Helicobacter pylori. Scand J Infect Dis 34: 253–256. [DOI] [PubMed] [Google Scholar]
- 39. Aksozek A, Mcclellan K, Howardt K, Niederkornt JY, Alizadehtt H (2002) Resistance of Acanthamoeba castellanii cysts to physical, chemical and radiological conditions. J Parasitol 88: 621–623. [DOI] [PubMed] [Google Scholar]
- 40.Dupuy M, Berne F, Herbelin P, Binet M, Berthelot N, et al. (2013) Sensitivity of free-living amoeba trophozoites and cysts to water disinfectants. Int J Hyg Environ Health 12711. [DOI] [PubMed]
- 41. Marciano-Cabral F, Cabral G (2003) Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev 16: 273–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang H, Edwards M, Falkinham JO, Pruden A (2012) Molecular survey of the occurrence of Legionella spp., Mycobacterium spp., Pseudomonas aeruginosa, and amoeba hosts in two chloraminated drinking water distribution systems. Appl Environ Microbiol 78: 6285–6294. 10.1128/AEM.01492-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ovrutsky AR, Chan ED, Kartalija M, Bai X, Jackson M, et al. (2013) Cooccurrence of free-living amoebae and nontuberculous Mycobacteria in hospital water networks, and preferential growth of Mycobacterium avium in Acanthamoeba lenticulata. Appl Environ Microbiol 79: 3185–3192. 10.1128/AEM.03823-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tanveer T, Hameed A, Muazzam AG, Jung S-Y, Gul A, et al. (2013) Isolation and molecular characterization of potentially pathogenic Acanthamoeba genotypes from diverse water resources including household drinking water from Khyber Pakhtunkhwa, Pakistan. Parasitol Res 112: 2925–2932. 10.1007/s00436-013-3465-5 [DOI] [PubMed] [Google Scholar]
- 45. Saeed A, Abd H, Edvinsson B, Sandström G (2009) Acanthamoeba castellanii an environmental host for Shigella dysenteriae and Shigella sonnei. Arch Microbiol 191: 83–88. 10.1007/s00203-008-0422-2 [DOI] [PubMed] [Google Scholar]
- 46.Saeed A, Johansson D, Sandström G, Abd H (2012) Temperature Depended Role of Shigella flexneri Invasion Plasmid on the Interaction with Acanthamoeba castellanii. Int J Microbiol: 917031. [DOI] [PMC free article] [PubMed]
- 47. Zychlinsky A, Prevost MC, Sansonetti P (1992) Shigella flexneri induces apoptosis in infected macrophages. Nature 358: 167–169. [DOI] [PubMed] [Google Scholar]
- 48. Fernandez-Prada CM, Hoover DL, Tall BD, Hartman AB, Kopelowitz J, et al. (2000) Shigella flexneri IpaH(7.8) facilitates escape of virulent bacteria from the endocytic vacuoles of mouse and human macrophages. Infect Immun 68: 3608–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ismail N, Olano JP, Feng H-M, Walker DH (2002) Current status of immune mechanisms of killing of intracellular microorganisms. FEMS Microbiol Lett 207: 111–120. [DOI] [PubMed] [Google Scholar]
- 50. Harb OS, Gao L, Kwaik YA (2000) From protozoa to mammalian cells: a new paradigm in the life cycle of intracellular bacterial pathogens. Environ Microbiol 2: 251–265. [DOI] [PubMed] [Google Scholar]
- 51. Greub G, Raoult D (2004) Microorganisms resistant to free-living amoebae. Clin Microbiol Rev 17: 413–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Goebel W, Gross R (2001) Intracellular survival strategies of mutualistic and parasitic prokaryotes. Trends Microbiol 9: 267–273. [DOI] [PubMed] [Google Scholar]
- 53. Barker J, Scaife H, Brown MR (1995) Intraphagocytic growth induces an antibiotic-resistant phenotype of Legionella pneumophila. Antimicrob Agents Chemother 39: 2684–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. King CH, Shotts EB, Wooley RE, Porter KG (1988) Survival of coliforms and bacterial pathogens within protozoa during chlorination. Appl Environ Microbiol 54: 3023–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ke X, Gu B, Pan S, Tong M (2011) Epidemiology and molecular mechanism of integron-mediated antibiotic resistance in Shigella. Arch Microbiol 193: 767–774. 10.1007/s00203-011-0744-3 [DOI] [PubMed] [Google Scholar]
- 56. Holt K, Thieu Nga T, Thanh D, Vinh H, Kim D, et al. (2013) Tracking the establishment of local endemic populations of an emergent enteric pathogen. Proc Natl Acad Sci 110: 17522–17527. 10.1073/pnas.1308632110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ochman H, Lawrence JG, Groisman E a (2000) Lateral gene transfer and the nature of bacterial innovation. Nature 405: 299–304. [DOI] [PubMed] [Google Scholar]
- 58. Juhas M (2015) Horizontal gene transfer in human pathogens. Crit Rev Microbiol 7828: 101–108. [DOI] [PubMed] [Google Scholar]
- 59. Lawrence JG, Ochman H (1998) Molecular archaeology of the Escherichia coli genome. Proc Natl Acad Sci U S A 95: 9413–9417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. White PA, Iver CJMC, Rawlinson WD (2001) Integrons and Gene Cassettes in the Enterobacteriaceae. Antimicrob Agents Chemother 45: 2658–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cergole-Novella MC, Pignatari ACC, Castanheira M, Guth BEC (2011) Molecular typing of antimicrobial-resistant Shiga-toxin-producing Escherichia coli strains (STEC) in Brazil. Res Microbiol 162: 117–123. 10.1016/j.resmic.2010.09.022 [DOI] [PubMed] [Google Scholar]
- 62. Le TMV, Baker S, Le TPT, Le TPT, Cao TT, et al. (2009) High prevalence of plasmid-mediated quinolone resistance determinants in commensal members of the Enterobacteriaceae in Ho Chi Minh City, Vietnam. J Med Microbiol 58: 1585–1592. 10.1099/jmm.0.010033-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.World Health Organization: Guidelines for the control of shigellosis, including epidemics due to Shigella dysenteriae type 1 (2005). Geneva.
- 64. Traa BS, Walker CLF, Munos M, Black RE (2010) Antibiotics for the treatment of dysentery in children. Int J Epidemiol 39 Suppl 1: i70–4. 10.1093/ije/dyq024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zaidi MB, Estrada-Garcia T (2014) Shigella: A Highly Virulent and Elusive Pathogen. Curr Trop Med Reports 1: 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nandy S, Mitra U, Rajendran K, Dutta P, Dutta S (2010) Subtype prevalence, plasmid profiles and growing fluoroquinolone resistance in Shigella from Kolkata, India (2001–2007): a hospital-based study. Trop Med Int Heal 15: 1499–1507. 10.1111/j.1365-3156.2010.02656.x [DOI] [PubMed] [Google Scholar]
- 67. Toro CS, Farfán M, Contreras I, Flores O, Navarro N, et al. (2005) Genetic analysis of antibiotic-resistance determinants in multidrug-resistant Shigella strains isolated from Chilean children. Epidemiol Infect 133: 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dutta S, Rajendran K, Roy S, Chatterjee A, Dutta P, et al. (2002) Shifting serotypes, plasmid profile analysis and antimicrobial resistance pattern of shigellae strains isolated from Kolkata, India during 1995–2000. Epidemiol Infect 129: 235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Von Seidlein L, Kim DR, Ali M, Lee H, Wang X, et al. (2006) A multicentre study of Shigella diarrhoea in six Asian countries: disease burden, clinical manifestations, and microbiology. PLoS Med 3: e353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ghosh S, Pazhani GP, Chowdhury G, Guin S, Dutta S, et al. (2011) Genetic characteristics and changing antimicrobial resistance among Shigella spp. isolated from hospitalized diarrhoeal patients in Kolkata, India. J Med Microbiol 60: 1460–1466. 10.1099/jmm.0.032920-0 [DOI] [PubMed] [Google Scholar]
- 71. Replogle M, Fleming D, Cieslak P (2000) Emergence of antimicrobial-resistant shigellosis in Oregon. Clin Infect Dis 30: 515–519. [DOI] [PubMed] [Google Scholar]
- 72. Pai H, Choi E, Lee H, Yun J, Jacoby GA (2001) Identification of CTX-M-14 Extended-Spectrum β -Lactamase in Clinical Isolates of Shigella sonnei, Escherichia coli, and Klebsiella pneumoniae in Korea. J Clin Microbiol 39: 3747–3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Stecher B, Denzler R, Maier L, Bernet F, Sanders MJ, et al. (2012) Gut inflammation can boost horizontal gene transfer between pathogenic and commensal Enterobacteriaceae. Proc Natl Acad Sci U S A 109: 1269–1274. 10.1073/pnas.1113246109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Paciello I, Silipo A, Lembo-Fazio L, Curcurù L, Zumsteg A, et al. (2013) Intracellular Shigella remodels its LPS to dampen the innate immune recognition and evade inflammasome activation. Proc Natl Acad Sci U S A: 4345–4354. [DOI] [PMC free article] [PubMed]
- 75. Balbi KJ, Rocha EPC, Feil EJ (2009) The temporal dynamics of slightly deleterious mutations in Escherichia coli and Shigella spp. Genome Biol 26: 345–355. [DOI] [PubMed] [Google Scholar]
- 76. Hershberg R, Tang H, Petrov DA (2007) Reduced selection leads to accelerated gene loss in Shigella. Genome Biol 8: R164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Thomas CM, Nielsen KM (2005) Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat Rev Microbiol 3: 711–721. [DOI] [PubMed] [Google Scholar]
- 78. Green M, Block C, Cohen D, Slater P (1991) Four decades of shigellosis in Israel: epidemiology of a growing public health problem. Rev Infect Dis 13: 248–253. [DOI] [PubMed] [Google Scholar]
- 79. Rosenberg ML, Weissman JB, Gangarosa EJ, Reller LB, Beasley RP, et al. (1976) Shigellosis in the United States: Ten-year review of nationwide surveillance, 1964–1973. Am J Epidemiol 104: 543–551. [DOI] [PubMed] [Google Scholar]
- 80. Baker S, Duy PT, Nga TVT, Dung TTN, Phat VV, et al. (2013) Fitness benefits in fluoroquinolone-resistant Salmonella Typhi in the absence of antimicrobial pressure. Elife 2: e01229 10.7554/eLife.01229 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data are derived from analyses of 367 Shigella isolates collected between 1995 and 2010 from Vietnam (136 S. flexneri and 231 S. sonnei). Resistance was determined by MIC and gene content analysis from Illumina genome sequencing data.
(DOCX)