Abstract
Arbuscular mycorrhizal fungi (AMF) have a major impact on plant nutrition, defence against pathogens, a plant’s reaction to stressful environments, soil fertility, and a plant’s relationship with other microorganisms. Such effects imply a broad reprogramming of the plant’s metabolic activity. However, little information is available regarding the role of AMF and their relation to other soil plant growth—promoting microorganisms in the plant metabolome, especially under realistic field conditions. In the present experiment, we evaluated the effects of inoculation with AMF, either alone or in combination with plant growth–promoting rhizobacteria (PGPR), on the metabolome and changes in metabolic pathways in the roots of durum wheat (Triticum durum Desf.) grown under N-limited agronomic conditions in a P-rich environment. These two treatments were compared to infection by the natural AMF population (NAT). Soil inoculation with AMF almost doubled wheat root colonization by AMF and decreased the root concentrations of most compounds in all metabolic pathways, especially amino acids (AA) and saturated fatty acids, whereas inoculation with AMF+PGPR increased the concentrations of such compounds compared to inoculation with AMF alone. Enrichment metabolomics analyses showed that AA metabolic pathways were mostly changed by the treatments, with reduced amination activity in roots most likely due to a shift from the biosynthesis of common AA to γ-amino butyric acid. The root metabolome differed between AMF and NAT but not AMF+PGPR and AMF or NAT. Because the PGPR used were potent mineralisers, and AMF can retain most nitrogen (N) taken as organic compounds for their own growth, it is likely that this result was due to an increased concentration of mineral N in soil inoculated with AMF+PGPR compared to AMF alone.
Introduction
Most land plants live in symbiosis with AMF. This mutualistic symbiosis plays a crucial role in the uptake of nutrients by the host plant and its defence against pathogens and frequently increases plant growth and stress resistance [1,2]. From a nutritional perspective, AMF can dominate the uptake of phosphorus (P) and other nutrients with low mobility in soil even in the absence of any plant growth response [3]. Yet their role in the uptake of nitrogen (N) and its importance for plant N nutrition is still unclear [4] and can vary according to the nutrient status of the host plant [5,6]; the type of nutrient supplied to the soil [7,8] and its availability; and interactions among the plant, AMF, and soil bacteria [8–10]. In addition, the arbuscular mycorrhizal (AM) fungal partner can influence important plant transcriptomic pathways related to plant N uptake and metabolism [11–13] and other metabolic pathways [14]. Thus, AMF can influence a wide range of plant metabolic processes. Such effects can be due to the effects of AMF on resource availability, which has direct major implications for the regulation of metabolic networks [15–18]; to the presence of the AM fungal partner itself [19]; to variations in the energy cost of the uptake of nutrients and feeding of plant-associated microbes [20]; and to their interactions with the plant and other soil microbes [21,22]. Among the soil microorganisms different than AMF, plant growth promoting rhizobacteria (PGPR) are likely to influence both plant growth and plant-AMF relationships mainly through indirect mechanisms including an increase in soil nutrient availability [23] whereas their direct effect on plant growth are still under debate [24]. However, few studies have evaluated the effects of either AMF or other plant growth–promoting microorganisms on plant metabolic pathways, and most that have been conducted under controlled greenhouse conditions in sterilised uninoculated media as a control [24–26] or have analysed only target compounds [27,28]. Metabolomics has improved at elucidating complex metabolic pathways thanks to increasing standardization of extraction and analytic procedures, especially gas chromatography (GC)-electronic ionization (EI)-mass spectrometry (MS)–based techniques, and increasing compound annotation in databases [29–32]. The aim of the present experiment was to evaluate the effects of AMF field inoculation, alone or in combination with a consortium of PGPR efficient at mineralising organic matter, on the metabolome and changes in metabolic pathways in the roots of durum wheat (Triticum durum Desf.) grown under N-limited, P-rich field conditions. Such condition was chosen since it has been shown that N limitation can reduce the AM benefit for the plant, especially under high P availability [6]. We hypothesized that a competition occurs between AM fungi and plants for nitrogen coming from the soil organic matter and that this should rearrange the metabolome of the plant root. An additional hypothesis is that the ability of PGPR to release N from the native organic matter should consist in an increase of N availability and thus reduce the competition for N between plant and AM fungus.
Materials and Methods
Ethics Statement
No specific permits were required for the described field study. The location is not protected in any way. The experiment did not involve endangered or protected species.
Experimental design
A field trial was performed in 2011–2012 in a typical semiarid Mediterranean area (37°33’ N–13°31’E, 178 m a.s.l.) on a deep, well-structured soil classified as a Vertic Xerochrept. Soil characteristics (0–0.60 m layer) were as follows: 52% clay, 25% sand, pH 8.2 (1:2.5 H2O), 16.8 g kg−1 total carbon (C; Walkley—Black), 1.78 g kg−1 total N (Kjeldahl), 92 mg kg−1 available P2O5 (Olsen), 1.37 g kg−1 total P2O5, 35 cmol kg−1 cation exchange capacity, 37.2% water content at field capacity, and 19.6% at the permanent wilting point. The climate at the experimental site is semiarid Mediterranean. From September to March, mean rainfall is 490 mm and mean air temperature ranges from 9.2°C to 11.9°C. During the cropping season, total rainfall (513 mm) was well distributed, whereas air temperature was 1.3°C lower than the long-term average. Weather data were collected from a weather station located within 500 m of the experimental site. Soil was cropped in the previous growing season with durum wheat. Before the experiment started, soil was ploughed at a depth of 30 cm in the summer and then shallow harrowed twice to control weeds and prepare suitable seedbed conditions. The natural AM spore population in the field measured before sowing by the wet-sieving method consisted of mainly by Glomus-group AM species and Acaulospora at an overall AM spore density of 5 spores per 100 g air-dried soil.
The experiment included three treatments (replicated six times): uninoculated control (NAT), inoculation with AMF alone (AMF), and inoculation with both AMF and PGPR (AMF+PGPR). Plots were arranged according to a randomised block design. Inoculation with AMF included the application of a commercial polispecies inoculum (Micronised Endo Mycorrhizae; Symbio, Wormley, Surrey, Great Britain) at a rate of 1.55 g m−2 at the time of sowing. The inoculum was composed of the following AM species: Scutellospora calospora, Acaulospora laevis, Glomus aggregatum, Rhizophagus irregulare (syn G. intraradices), Funneliformis mosseae (syn G. mosseae), G. fasciculatum, G. etunicatum e G. deserticola, and Gigaspora margarita. The inoculum was composed of 95% AM spores and 5% organic material. Total spore density in the inoculum was 25 g−1 per species. Inoculation with AMF+PGPR was performed by applying to the soil both 1.55 g m−2 AM inoculum as previously described and 1.55 g m−2 of a commercial PGPR inoculum. The PGPR inoculum was also purchased from Symbio (Bacillus Sp. on bran; Symbio, Wormley, Surrey, Great Britain) and was composed of Bacillus amyloliquefaciens, B. brevis, B. circulans, B. coagulans, B. firmus, B. halodenitrificans, B. laterosporus, B. licheniformis, B. megaterium, B. mycoides, B. pasteurii, B. polymyxa, and B. subtilis, each at a density of 2 billion cfu g−1. Durum wheat (cv. Anco Marzio, 1000-seed weight 47.4 g, 95% germination) was sown on 16 December 2012 at a rate of 350 seeds m−2 in rows 18.75 cm apart. The experimental plot consisted of eight rows, each 6 m long. Each experimental plot was spaced 0.5 m out from the next to avoid cross inoculation among treatments and 0.5-m wide corridors were tilled once per month to avoid AMF and PGPR movements across plots. Weeds were controlled by hand during the experiment. At wheat tillering (110 days after sowing), the aboveground biomass of a subplot (six rows 75 cm long) was harvested and weighed and a subsample of 1 kg fresh matter was taken and oven dried at 70°C until a constant weight. Dry mass was determined and further analysed for total N (Kjieldhal) and P (Bertramson), the latter after 48 h of heating at 550°C and no addition of magnesium nitrate. Roots (0–0.30 m layer) from five random plants from each plot were also sampled and two root subsamples of about 3 g were taken. The first subsample was immediately freeze dried in liquid N to stop metabolic activity and stored at—80°C for further analysis. The other subsample was stained with 0.05% trypan blue in lactic acid according to [33] and root colonization by AM fungi was measured using the grid intersect method as described in [34].
Metabolite extraction and derivatization
Roots were lyophilised and ground in liquid N, and a 20-mg aliquot was processed as follows according to [35,36]. Samples were added to 0.75 ml methanol:chloroform:water (5:2:2), agitated at 4°C for 5 min, vortexed briefly, and then centrifuged at 6000 rpm for 2 min. After centrifugation, 0.60 ml supernatant was collected and dried in a SpeedVac. The dried extract was added to 2 ml of an internal retention index (from C8 to C16 at 0.8 mg/ml and from C18 to C30 at 0.4 mg/ml) and 5 μl of a methoxyamine hydrochloride solution (20 mg/ml in pyridine) and then shaken at 30°C for 90 min. Then 45 μl N-Methyl-N-(trimethylsilyl) trifluoroacetamide (MSTFA) with 1% trimethylchlorosilane (TMCS) for trimethylsilylation was added and the solution was shaken at 37°C for 30 min.
GC-TOF-MS analysis and spectra processing
GC-MS analysis was performed on an Agilent 6890 gas chromatograph coupled with a Leco Pegasus III TOF mass spectrometer controlled by Leco ChromaTOF software 2.32. The gas chromatograph was equipped with an Rtx-5Sil MS column (Restek, Bellefonte, PA; 30 m long, 0.25 mm i.d. with 0.25 μm 95%-dimethyl-5% diphenyl polysiloxane film) and an additional 10 m integrated guard column. GC-time of flight (TOF)-MS analysis was performed according to [37]. Briefly, helium (99.99% purity) was used as a carrier gas at a constant flow of 1 ml/min. Oven temperature was held at 50°C for 1 min, then increased 10°C/min to 330°C, after which it was held at 330°C for 5 min. The transfer line was held at 280°C and the ion source at 250°C. Ionization was performed at 70 eV. A 290-s solvent delay was set. The mass range was set at 85–600 mass units acquired at 10 spectra s−1 and 1800 V detector voltage with no mass defect option. Resolution was 1 ppm with 2% error for isotopic abundance patterns. Spectra were compared to those present in the Fiehn Library (fiehnlab.ucdavis.edu/projects/FiehnLib, including 1,013 metabolites on 2014) and NIST 2005 with at least 95% matching.
HILIC-Q-TOF-MS analysis and spectra processing
HILIC-Q-TOFMS analysis (hydrophilic interaction chromatography time of flight mass spectrometry) was performed on an Agilent 1290 UHPLC equipped with a Waters Acuity 1.7 μm BEH HILIC 2.1 × 150 mm column for separation and coupled with an Agilent G6530A accurate-mass QTOF equipped with an Agilent ESI Jet Stream ion source. Mobile phases were Solvent A (5mM ammonium acetate with 0.2% acetic acid) and Solvent B (9:1 acetonitrile:water with 5mM ammonium acetate and 0.2% acetic acid). Dry samples were resuspended in 100 μl solvent B and then injected in the column under the following gradient conditions: from 0 to 4 min, isocratic 100% B; from 4 to 12 min, B linearly reduced to 45%; from 12 to 20 min, isocratic 45% B. After the run, there was a 20-min re-equilibration phase before the next sample was injected. The following Source/MS conditions were applied: electrospray ionization (ESI) set in positive mode, 3000 V capillary voltage; source gas temperature set to 350°C and gas flow to 10 L/min, mass range acquisition 50–1700 Da at 4 scans per second. After deconvolution, raw data were aligned and adducts [M+H+] searched against METLIN, NIST MS, and MZmine software. Metabolite intensities were identified as the peak height for the largest precursor ion (not normalized).
Annotation and statistical analyses
One way analysis of variance (ANOVA) [38] was performed for biomass, N and P content and uptake, and percent root colonization by AMF. When treatments were significant, least significant differences were computed to separate means. Enrichment metabolomics analyses were performed on Zea mays precompiled background sets in MBRole [39], and analyses of metabolic pathways were performed using Pathos [40] with minimum variation set at 20%. The P value of annotation for Biological Role in MBRole was adjusted for multiple testing using the false discovery rate according to [41].
All data for annotated and unannotated identified GC and LC peaks were standardised by setting the mean to 0 and the standard deviation to 1. This allowed the computation of means of different compounds with the same biological role without algebraic distortions due to a different actual concentration—to—MS signal ratio. Biological role means of standardised data from annotated and unannotated identified GC peaks were computed and ANOVAs were run on these means to highlight average variations in compounds in the same biological group. Canonical discriminant analysis (CDA) [38] was run using biological group means as vectors to summarize between-treatments variation. The percentage of compounds not varying or varying at either >+20% or <−20% of the base condition was drawn from Pathos results for each metabolic pathway significantly annotated in MBRole.
Results
Plant growth
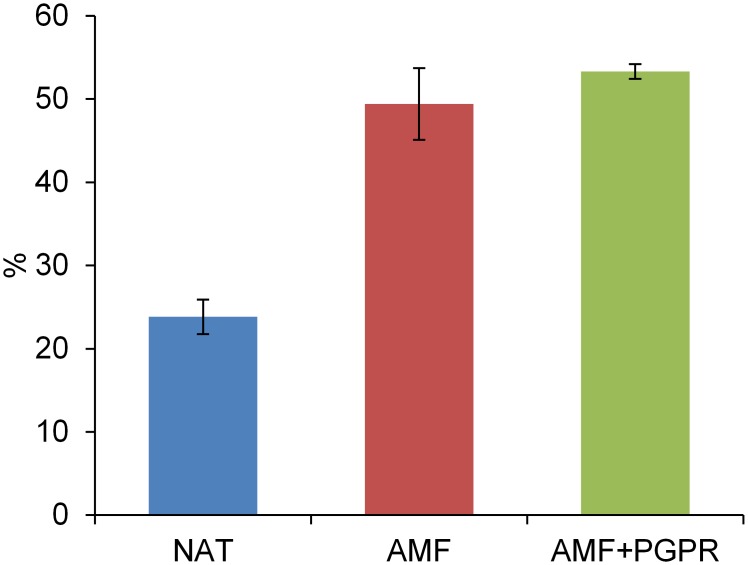
Inoculation with AMF (either alone or in combination with PGPR) increased by 23.3% the aboveground biomass of durum wheat, although this change was not significant (Table 1). It also slightly decreased both N (−7.8%) and P (−8.0%) content. Soil inoculation with AMF almost doubled wheat root colonization by AM fungi (Fig 1). No effects of treatment on wheat above ground biomass, grain yield and yield components were observed at maturity (Table A in S1 File).
Table 1. Aboveground biomass, its N and P and root infection by AM fungi at tillering of durum wheat grown in the field.
| Aboveground biomass | N content | N uptake | P content | P uptake | |
|---|---|---|---|---|---|
| Mg ha–1 | mg N g–1 biomass | kg N ha–1 | mg P g–1 biomass | kg P ha–1 | |
| NAT | 1.52±0.177 | 18.78±0.970 | 28.39±2.441 | 3.60±0.117 | 5.40±0.260 |
| AMF | 1.85±0.240 | 16.88±0.383 | 31.30±4.176 | 3.37±0.173 | 6.17±0.733 |
| AMF+PGPR | 1.89±0.223 | 17.78±0.868 | 34.25±5.574 | 3.25±0.075 | 6.17±0.793 |
| P-value | 0.106 | 0.236 | 0.253 | 0.153 | 0.525 |
NAT = wheat with natural arbuscular mycorrhizal inoculum; AMF = wheat inoculated with spores of AM fungi; AMF+PGPR = wheat inoculated with both spores of AM fungi and plant growth—promoting rhizobacteria.
Fig 1. Root colonization by AMF in roots of durum wheat.
Wheat with natural arbuscular mycorrhizal inoculum (NAT), inoculated with AM fungi spores (AMF), or inoculated with both AMF and plant growth—promoting rhizobacteria (AMF+PGPR). Data are means±S.E. (n = 6).
Biological classes
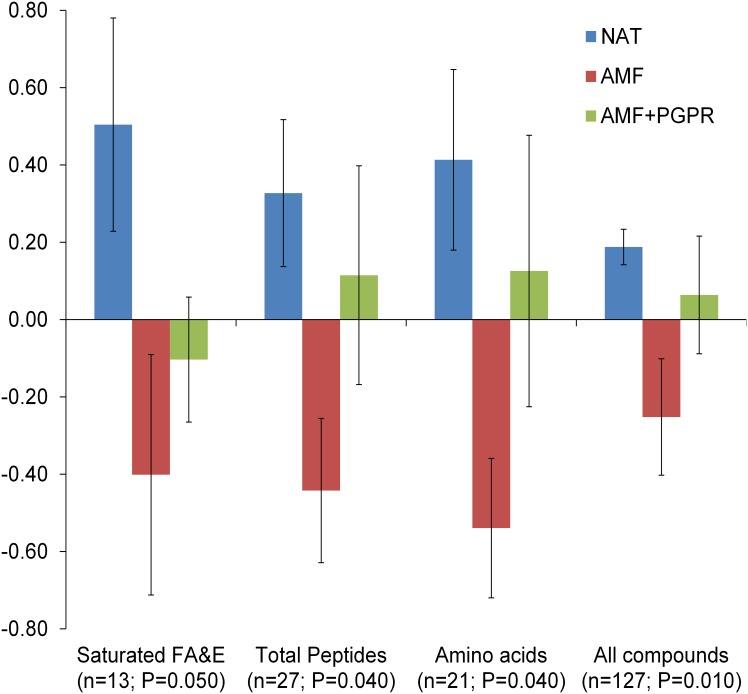
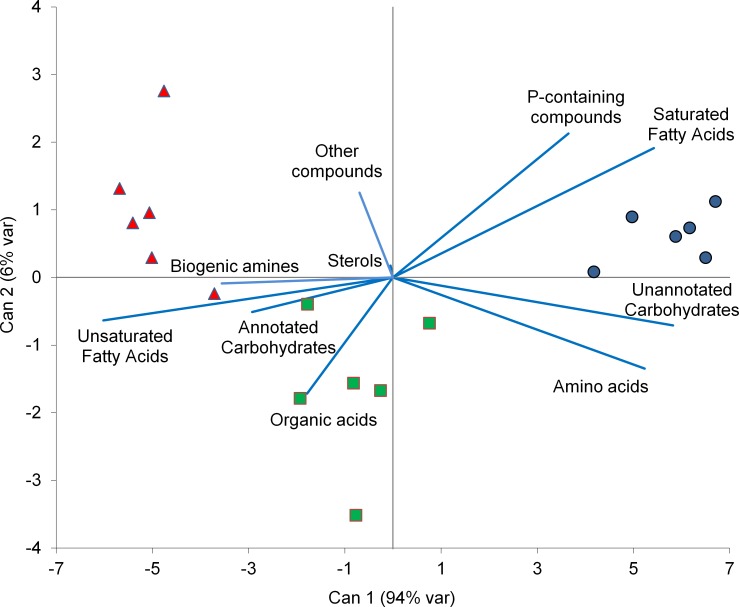
A total of 315 GC peaks were found, 118 of which clearly identified and 9 of which were assigned to a carbohydrate chemical structure. A Kegg ID was given to only 83 compounds and used for an enrichment metabolomics analysis against Z. mays precompiled background sets in MBRole, which annotated only 73, 38, 44, 64, and 28 Kegg IDs in Pathways, Enzyme interactions, Biological role, Chemical groups, and Other interactions, respectively (Tables A, B, C, D, and F in S1 File, respectively). In general, AMF showed a lower concentration of most GC-separated compounds than NAT, especially saturated fatty acids (SaFA) and AA (Fig 2 and Table G in S1 File). No differences were observed in the concentrations of any compounds between AMF+PGPR and either NAT or AMF (Fig 2). CDA run using classes of compounds from GC only separated AMF from NAT (Fig 3). Canonical Variable 1 accounted for 94% of the total variance and mostly depended on unsaturated fatty acids (UnFA) (standardized canonical coefficient, SCORE = −6.02), unannotated carbohydrates (SCORE = +5.82), SaFA (SCORE = +5.42) and AA (SCORE = +5.23). Few correlations were found between Canonical Variable 2 and the concentrations of different classes of compounds included in the CDA. When root extract was separated by HILIC, 516 peaks were found and 52 compounds were assigned a simple empirical formula (Table H in S1 File). Among them, 80% contained P in their structures. HILIC-separated compounds included carnitines, glycerophosphocholine, lysophosphatidylcholines, phosphatidylcholines, lysophosphatidylethanolamines, and phosphatidylethanolamines. In particular, lysophosphatidylcholine 16:0 was lower in AMF than NAT, whereas concentrations of the other compounds, both singly or grouped by chemical class, did not vary significantly by treatment. CDA run using HILIC-separated compounds did not discriminate among treatments (data not shown).
Fig 2. Group means ± S.E. across standardised data for identified GC peaks grouped per biological group significantly varying according to the treatments.
n indicates the number of compounds contributing to the relative mean, and P is the P value of the ANOVA for that group. Carbohydrates were analysed separately according to KEGG annotation. All compounds includes both annotated and unannotated compounds. GC was run with methanol:chloroform:water (5:2:2) extracts from roots of durum wheat grown in the field with natural arbuscular mycorrhizal inoculum (NAT), inoculation with AM fungi (AMF), or inoculation with both AMF and plant growth—promoting rhizobacteria (PGPR). FA&E, fatty acids and their esters. Please see Table G in S1 File for non-significantly varying classes of compounds.
Fig 3. CDA run using biological group means of standardised data from identified GC peaks as vectors.
The percentage of the total variance explained by each canonical axis is shown in parentheses. NAT, blue circles; AMF, red triangles; AMF+PGPR, green squares. Please note that CDA vectors do not represent perpendicular directions through the space of the original variables. Fatty acids vectors include both fatty acids and their esters.
Metabolic pathways
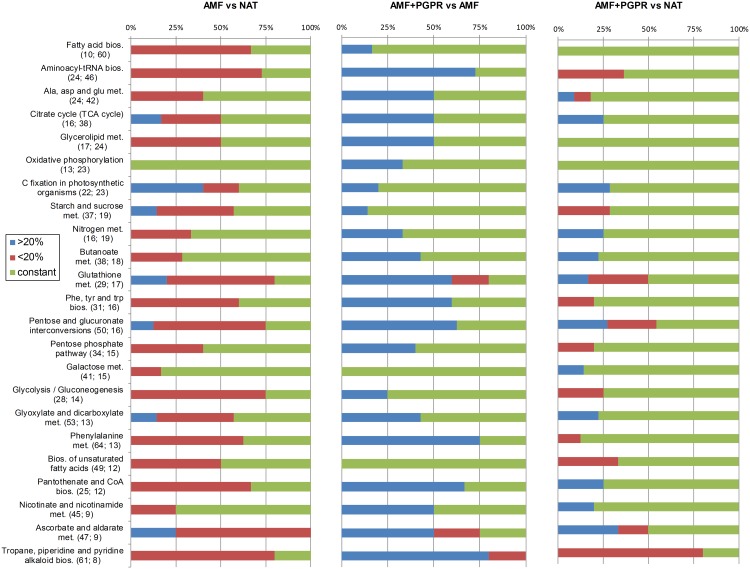
A total of 25 metabolic pathways were significantly annotated on Z. mays background in MBRole, and 23 of these were analysed in Pathos on Arabidopsis thaliana background. Symbiosis with AM fungi reduced the concentrations of most compounds in all metabolic pathways. No changes were observed for oxidative phosphorylation. AMF mostly upregulated metabolites involved in carbon fixation in photosynthetic organisms (Fig 4). AMF+PGPR mostly increased the concentrations of compounds in all metabolic pathways except for galactose metabolism and the biosynthesis of unsaturated fatty acids.
Fig 4. The percentage of annotated compounds included in Pathos in each metabolic pathway.
Compounds are displayed as unchanged (green bars), or significantly increased (blue bars), or significantly decreased (red bars) at 20% minimum variation. The first number in parentheses indicates the total number of annotated compounds in the pathway, and the second number indicates the percentage of annotated compounds from the present data set in each metabolic pathway. Only those pathways that were significantly annotated in MBRole are shown.
Metabolic enrichment analysis showed that AMF down-regulated key pathways belonging to the backbone of primary metabolism, such as amino acid biosynthesis and interconversions, especially those involving alanine, glutamine, asparagine, and phenylalanine (Table C in S1 File); TCA cycle and glycolysis; and carbohydrate biosynthesis and metabolism (sugar, starch, pentose phosphate, galactose; Table B in S1 File). Lipid-related pathways, such as fatty acid biosynthesis and glicerolipid metabolism, were also down-regulated.
Peptide metabolic pathways were more depressed in AMF and AMF+PGPR compared to NAT. In particular, a reduction in products formed by asparagine synthase (glutamine-hydrolysing; EC 6.3.5.4) and glutamate-ammonia ligase (EC 6.3.1.2) was observed in different metabolic pathways, such as alanine, aspartate, and glutamate metabolism (map 00250) and arginine and proline metabolism (map 00330). In addition, a lower abundance of compounds included in the urea cycle (map 00330), especially ornithine and its direct product, putrescine (data not shown), was found in AMF than NAT. Gamma-amino butyric acid (GABA) was slightly higher in AMF than NAT. Only one metabolic pathway leading to GABA formation was higher in AMF than NAT (glutamate decarboxylase, EC 4.1.1.15). Because all other GABA-forming pathways showed reduced concentrations of the other metabolites directly leading to GABA (namely, succinic acid in butanoate metabolism [map 00650] and putrescine [map 00330]) and an increase in some precursors of these metabolites (maleate in map 00650), it is likely that AMF reduced amination activity in roots compared to NAT. Compared to AMF, AMF+PGPR reduced both GABA and one of its direct precursors, glutamic acid, and increased other GABA precursors, the compounds in the urea cycle, and glutamine, which is formed from glutamic acid in competition with GABA formation. Finally, AMF+PGPR reduced the concentration of pipecolic acid compared to either AMF or NAT, although no differences were observed between AMF and NAT.
AMF increased the concentration of xilitol and reduced its derivatives (D-xilose, D-arabitol, and L-arabinose) in pentose and the glucuronate interconversions pathway (map 00040) compared to NAT. This led to a reduction in 2-oxoglutarate and pyruvate. In contrast, AMF+PGPR increased D-arabitol, 2-oxoglutarate, pyruvate, and L-arabinose compared to AMF but not NAT. D-xilose (map 00040) can also derive from the metabolism of starch and sucrose (map 00050), where few compounds were annotated (7 out of 37), and none of these were directly related to D-xilose. This allows for little elucidation of the latter pathway. Finally, AMF and AMF+PGPR increased the concentration of malic acid compared to NAT in carbon fixation in photosynthetic organisms (map 00710). On the one hand, malic acid is directly converted to pyruvate by means of a malate dehydrogenase (EC 1.1.1.40) under light conditions in map 00710. On the other hand, pyruvate can undergo different transformations in pyruvate metabolism (map 00620): it can be indirectly converted to malic acid to feed the glycolysis/glucogenesis, glyoxylate and citrate cycles; it can be indirectly or directly interconverted with lactic acid by means of an L-lactate dehydrogenase (EC 1.1.1.27); or it can be transferred to the biosynthesis of leucine, lysine, or fatty acid or the metabolism of propanoate, butanoate, and ketone bodies. We found that pyruvate decreased in AMF compared to NAT and increased in AMF+PGPR compared to AMF, whereas lactic acid was lower in AMF and AMF+PGPR than in NAT. Finally, annotation indicated that two out of 4 sterols (namely zymosterol, Kegg: C05437; and ergosterol, Kegg: C01694) were indicated as potentially deriving from the fungi. However, no differences in zymosterol concentration among treatments were found, and ergosterol was 36.5% and 41.5% lower in AMF and AMF+PGPR, respectively, if comparing to NAT, thus we exclude that these sterols were deriving from the AMF. In general, steroid biosynthesis (map 00100, adjusted P annotation = 0.054 in MBRole) was slightly depressed by AMF and AMF+PGPR compared to NAT. No metabolite potentially belonging to bacteria was found.
Discussion
Above ground plant N content was on average 17.81 mg N g−1 dry matter. Such N content at tillering can be considered as very low [42]. This indicates that the N availability in the soil was limiting plant growth, as observed by [43]. This was likely because of the intrinsic low soil N content and the previous cultivation of wheat, an N-depleting species.
Root AM infection of plots with natural AM inoculum (NAT) was on average slightly higher than 20%, which is similar to [44] but markedly lower than [45,46]. This suggests that, in our experiment, the actual soil conditions were unfavourable for the infection of roots by the natural AM consortium, probably because of several factors, such as the high intensity of the tillage technique adopted (mouldboard ploughing) and the high availability of phosphorus in the soil, both of which are detrimental to natural AM infection [45,47,48]. Soil inoculation with AM fungi, either alone or in combination with PGPR, markedly increased wheat root colonization by AM fungi, as in other studies [44,48].
Means across standardised data of identified compounds suggested that soil inoculation with AMF alone decreased concentrations of low molecular weight compounds in roots, particularly AA and saturated FA&E. Similar results were found by [49] for Lotus japonicus grown with or without AM fungi under different conditions. Information about the effects of AMF on the root metabolome is scarce. Other experiments showed that in the legume Medicago truncatula, AMF increased the concentration of AA in the roots [19]. In Lolium perenne, colonisation by Neotyphodium lolii, a fungal endophyte, reduced the content of nitrate and several amino acids in the host plant [50]. Hodge and Fitter [51] showed that AM fungi have a high N demand and retain most N taken as organic compounds for their own growth. In the present experiment, native organic matter and wheat roots of the previous crop were the only sources of N available for plants. Thus, it is possible that the greater development of AM hyphae in the soil in AMF than NAT resulted in a reduced N available to the plant. Thus, the reduced amination activity in AMF than NAT observed in the present study could have resulted either directly from increased root mycorrhization, as shown in L. japonicus [49], or indirectly from reduced N availability for the plant in AMF than NAT. This is consistent with the finding that GABA, which is synthesised in competition with many other AA, increased in AMF compared to NAT, as this molecule is implicated in a wide range of plant responses to external factors, including plant microbe interaction and biotic and abiotic stresses [52] and nutrient limitation [53]. This compound is an important constituent of root exudates that alters the growth and activity of some PGPR [54–56]. Accordingly, the higher amino acid content in AMF+PGPR than AMF may be due to the mineralisation activity by PGPR and to the further increase in N availability, especially ammonium and nitrate for plants. Indeed, the Bacillus species used in the present study are sturdy plant growth promoters that could have benefitted plants through their mineralisation activity or by reducing competition with other bacteria. This is corroborated by the reduced concentration of pipecolic acid in AMF+PGPR compared to AMF or NAT. Pipecolic acid is implied in systemic acquired resistance against bacteria and stresses [57–59] and accumulates into the plant at increasing N availability [60]. Bacillus volatiles, especially 2,3-butanediol and acetoin, promote plant growth [61]. However, in the present study, the only annotated compound implicated in the 2,3-butanediol/acetoin/GABA system (map 00650) was pyruvate, which is included in 25 different annotated metabolic pathways. This should curb any speculation about any effect of PGPR on wheat metabolic reprogramming. Fatty acids and their esters and carbohydrates, especially those that are unannotated, also contributed to separate AMF samples from NAT but not from AMF+PGPR. Simple sugars are the most important carbon sources exported from roots to the intra-radical mycelium (IRM) [62]. Just as lipids are synthesised into the IRM, these sugars consistently flow to the the extra-radical mycelium (ERM) [63]. AM symbiosis and N and P availability can affect the fatty acid content in roots [64]. However, lipids arising from AM fungi usually emerge in the roots in the late stage of symbiosis [65]. This could explain why we observed no variation in carnitines, which accumulate in mycorrhizal roots only in the late stage of AM symbiosis [66]. In early, active stages, high demand for lipids by ERM [67] can result in a reduction of lipids in mycorrhizal roots compared to non-mycorrhizal roots.
Conclusions
The present data, obtained through metabolomics analyses of field grown wheat, showed that inoculation with AMF negatively affected amination activity in the root and concentrations of most of amino acids. Inoculation with AMF+PGPR increased concentrations of amino acids compared to AMF. This support the hypothesis that N availability is crucial for the AM benefit to the plant and that AM fungi can compete with the plant for N coming from the organic matter. Because most N taken directly in organic form from AM fungi is retained in AM structures [51], this result is likely due to the increase in soil inorganic N in AMF+PGPR compared to AMF. Inoculation of the soil with exotic AMF also resulted in a reprogramming of primary metabolism, with a clear shift from the biosynthesis of common AA to GABA. The low percentage of compound annotation in plant metabolic pathways (ca. 23% of GC peaks) obscures the metabolic reprogramming at play in carbohydrate metabolism. Nonetheless, many compounds implicated in C fixation were increased in AM rather than non-AM roots, and this agrees with the findings of several authors reporting increased C fixation even in the absence of a significant growth response [19,28,64]. In addition, the increase in xilitol, which is crucial in the interaction between plants and AM fungi [19,49,68], suggests that plants were actively feeding the fungal symbionts.
Supporting Information
Above ground biomass, grain yield and yield components at maturity of durum wheat grown in the field (Table A). Enrichment metabolomic analysis for Pathways (Table B), Enzyme interactions (Table C), Biological role (Table D), Chemical groups (Table E), and Other interactions (Table F) as displayed in MBRole. Group means ± S.E. across standardised data for identified GC peaks grouped per biological group (Table G). HILIC-Q-TOF MS identified compounds (Table H).
(DOCX)
Acknowledgments
The authors are grateful to Mr. Vincenzo Cannella and Mr. Francesco Cannella for invaluable help during the field experiment.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The present research was funded by the project "Sviluppo tecnologico e innovazione per la sostenibilità e competitività della cerealicoltura meridionale (PON01_01145-ISCOCEM)" (person in charge: Dr. Giuseppe Di Miceli - Fondazione A. e S. Lima Mancuso) of the Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR), Italy. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Smith SE, Read DJ (2008) Mycorrhizal Symbiosis. Smith SE, Read DJ, editors Academic Press; 787 p. 10.1126/science.1208473 [DOI] [Google Scholar]
- 2. Saia S, Amato G, Frenda AS, Giambalvo D, Ruisi P (2014) Influence of arbuscular mycorrhizae on biomass production and nitrogen fixation of berseem clover plants subjected to water stress. PLoS One 9: e90738 10.1371/journal.pone.0090738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smith SE, Smith FA (2011) Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annu Rev Plant Biol 62: 227–250. 10.1146/annurev-arplant-042110-103846 [DOI] [PubMed] [Google Scholar]
- 4. Hodge A, Storer K (2014) Arbuscular mycorrhiza and nitrogen: implications for individual plants through to ecosystems. Plant Soil. 10.1007/s11104-014-2162-1 24744450 [DOI] [Google Scholar]
- 5. Fellbaum CR, Gachomo EW, Beesetty Y, Choudhari S, Strahan GD, Pfeffer PE, et al. (2012) Carbon availability triggers fungal nitrogen uptake and transport in arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci U S A 109: 2666–2671. 10.1073/pnas.1118650109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johnson NC, Wilson GWT, Wilson JA, Miller RM, Bowker MA (2014) Mycorrhizal phenotypes and the Law of the Minimum. New Phytol. 10.1111/nph.13172 [DOI] [PubMed] [Google Scholar]
- 7. Tanaka Y, Yano K (2005) Nitrogen delivery to maize via mycorrhizal hyphae depends on the form of N supplied. Plant, Cell Environ 28: 1247–1254. 10.1111/j.1365-3040.2005.01360.x [DOI] [Google Scholar]
- 8. Saia S, Benítez E, García-Garrido JM, Settanni L, Amato G, Giambalvo D (2014) The effect of arbuscular mycorrhizal fungi on total plant nitrogen uptake and nitrogen recovery from soil organic material. J Agric Sci 152: 370–378. 10.1017/S002185961300004X [DOI] [Google Scholar]
- 9. Leigh J, Fitter AH, Hodge A (2011) Growth and symbiotic effectiveness of an arbuscular mycorrhizal fungus in organic matter in competition with soil bacteria. Fems Micriobiology Ecol 76: 428–438. 10.1111/j.1574-6941.2011.01066.x [DOI] [PubMed] [Google Scholar]
- 10. Barea JM, Toro M, Orozco MO, Campos E, Azcón R (2002) The application of isotopic (32P and 15N) dilution techniques to evaluate the interactive effect of phosphate-solubilizing rhizobacteria, mycorrhizal fungi and Rhizobium to improve the agronomic efficiency of rock phosphate for legume crops. Nutr Cycl Agroecosystems 63: 35–42. 10.1023/A:1020589732436 [DOI] [Google Scholar]
- 11. Govindarajulu M, Pfeffer PE, Jin H, Abubaker J, Douds DD, Allen JW, et al. (2005) Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 435: 819–823. 10.1038/nature03610 [DOI] [PubMed] [Google Scholar]
- 12. Jin H, Pfeffer PE, Douds DD, Piotrowski E, Lammers PJ, Shachar-Hill Y (2005) The uptake, metabolism, transport and transfer of nitrogen in an arbuscular mycorrhizal symbiosis. New Phytol 168: 687–696. 10.1111/j.1469-8137.2005.01536.x [DOI] [PubMed] [Google Scholar]
- 13. Guether M, Neuhäuser B, Balestrini R, Dynowski M, Ludewig U, Bonfante P (2009) A mycorrhizal-specific ammonium transporter from Lotus japonicus acquires nitrogen released by arbuscular mycorrhizal fungi. Plant Physiol 150: 73–83. 10.1104/pp.109.136390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guether M, Balestrini R, Hannah M, He J, Udvardi MK, Bonfante P (2009) Genome-wide reprogramming of regulatory networks, transport, cell wall and membrane biogenesis during arbuscular mycorrhizal symbiosis in Lotus japonicus . New Phytol 182: 200–212. 10.1111/j.1469-8137.2008.02725.x [DOI] [PubMed] [Google Scholar]
- 15. Scheible W-R, Morcuende R, Czechowski T, Fritz C, Osuna D, Palacios-Rojas N, et al. (2004) Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiol 136: 2483–2499. 10.1104/pp.104.047019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morcuende R, Bari R, Gibon Y, Zheng W, Pant BD, Bläsing O, et al. (2007) Genome-wide reprogramming of metabolism and regulatory networks of Arabidopsis in response to phosphorus. Plant, Cell Environ 30: 85–112. 10.1111/j.1365-3040.2006.01608.x [DOI] [PubMed] [Google Scholar]
- 17. Martinelli F, Remorini D, Saia S, Massai R, Tonutti P (2013) Metabolic profiling of ripe olive fruit in response to moderate water stress. Sci Hortic (Amsterdam) 159: 52–58. 10.1016/j.scienta.2013.04.039 [DOI] [Google Scholar]
- 18. Martinelli F, Basile B, Morelli G, D’Andria R, Tonutti P (2012) Effects of irrigation on fruit ripening behavior and metabolic changes in olive. Sci Hortic (Amsterdam) 144: 201–207. 10.1016/j.scienta.2012.07.012 [DOI] [Google Scholar]
- 19. Schliemann W, Ammer C, Strack D (2008) Metabolite profiling of mycorrhizal roots of Medicago truncatula . Phytochemistry 69: 112–146. 10.1016/j.phytochem.2007.06.032 [DOI] [PubMed] [Google Scholar]
- 20. Larimer AL, Bever JD, Clay K (2010) The interactive effects of plant microbial symbionts: a review and meta-analysis. Symbiosis 51: 139–148. 10.1007/s13199-010-0083-1 [DOI] [Google Scholar]
- 21. Artursson V, Finlay RD, Jansson JK (2006) Interactions between arbuscular mycorrhizal fungi and bacteria and their potential for stimulating plant growth. Environ Microbiol 8: 1–10. 10.1111/j.1462-2920.2005.00942.x [DOI] [PubMed] [Google Scholar]
- 22. Draper J, Rasmussen S, Zubair H (2011) Metabolite Analysis and Metabolomics in the Study of Biotrophic Interactions between Plants and Microbes In: Hall RD, editor. Annual Plant Reviews Volume 43: Biology of Plant Metabolomics. Oxford, UK: Wiley-Blackwell; 10.1002/9781444339956.ch2 [DOI] [Google Scholar]
- 23. Pii Y, Mimmo T, Tomasi N, Terzano R, Cesco S, Crecchio C (2015) Microbial interactions in the rhizosphere: beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biol Fertil Soils. 10.1007/s00374-015-0996-1 [DOI] [Google Scholar]
- 24. Glick BR (2012) Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica (Cairo) 2012: 1–15. 10.6064/2012/963401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lekberg Y, Koide RT (2014) Integrating physiological, community, and evolutionary perspectives on the arbuscular mycorrhizal symbiosis 1. Botany 92: 241–251. 10.1139/cjb-2013-0182 [DOI] [Google Scholar]
- 26. Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annu Rev Microbiol 63: 541–556. 10.1146/annurev.micro.62.081307.162918 [DOI] [PubMed] [Google Scholar]
- 27. Ceccarelli N, Curadi M, Martelloni L, Sbrana C, Picciarelli P, Giovannetti M (2010) Mycorrhizal colonization impacts on phenolic content and antioxidant properties of artichoke leaves and flower heads two years after field transplant. Plant Soil 335: 311–323. 10.1007/s11104-010-0417-z [DOI] [Google Scholar]
- 28. Walker V, Couillerot O, Von Felten A, Bellvert F, Jansa J, Maurhofer M, et al. (2011) Variation of secondary metabolite levels in maize seedling roots induced by inoculation with Azospirillum, Pseudomonas and Glomus consortium under field conditions. Plant Soil 356: 151–163. 10.1007/s11104-011-0960-2 [DOI] [Google Scholar]
- 29. Lisec J, Schauer N, Kopka J, Willmitzer L, Fernie AR (2006) Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat Protoc 1: 387–396. 10.1038/nprot.2006.59 [DOI] [PubMed] [Google Scholar]
- 30. Kind T, Fiehn O (2006) Metabolomic database annotations via query of elemental compositions: mass accuracy is insufficient even at less than 1 ppm. BMC Bioinformatics 7: 234 10.1186/1471-2105-7-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gika HG, Wilson ID, Theodoridis GA (2014) LC-MS-based holistic metabolic profiling. Problems, limitations, advantages, and future perspectives. J Chromatogr B Analyt Technol Biomed Life Sci 966: 1–6. 10.1016/j.jchromb.2014.01.054 [DOI] [PubMed] [Google Scholar]
- 32. Strehmel N, Kopka J, Scheel D, Böttcher C (2013) Annotating unknown components from GC/EI-MS-based metabolite profiling experiments using GC/APCI(+)-QTOFMS. Metabolomics 10: 324–336. 10.1007/s11306-013-0569-y [DOI] [Google Scholar]
- 33. Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55: 158–IN18. 10.1016/S0007-1536(70)80110-3 [DOI] [Google Scholar]
- 34. Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84: 489–500. 10.1111/j.1469-8137.1980.tb04556.x [DOI] [Google Scholar]
- 35. Weckwerth W, Wenzel K, Fiehn O (2004) Process for the integrated extraction, identification and quantification of metabolites, proteins and RNA to reveal their co-regulation in biochemical networks. Proteomics 4: 78–83. 10.1002/pmic.200200500 [DOI] [PubMed] [Google Scholar]
- 36. Gullberg J, Jonsson P, Nordström A, Sjöström M, Moritz T (2004) Design of experiments: An efficient strategy to identify factors influencing extraction and derivatization of Arabidopsis thaliana samples in metabolomic studies with gas chromatography/mass spectrometry. Anal Biochem 331: 283–295. 10.1016/j.ab.2004.04.037 [DOI] [PubMed] [Google Scholar]
- 37. Lee DY, Fiehn O (2008) High quality metabolomic data for Chlamydomonas reinhardtii . Plant Methods 4: 7 10.1186/1746-4811-4-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. SAS (2008) SAS/STAT 9.2 User’s Guide. SAS Inst Inc. [Google Scholar]
- 39. Chagoyen M, Pazos F (2011) MBRole: enrichment analysis of metabolomic data. Bioinformatics 27: 730–731. 10.1093/bioinformatics/btr001 [DOI] [PubMed] [Google Scholar]
- 40. Leader DP, Burgess K, Creek D, Barrett MP (2011) Pathos: a web facility that uses metabolic maps to display experimental changes in metabolites identified by mass spectrometry. Rapid Commun Mass Spectrom 25: 3422–3426. 10.1002/rcm.5245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Benjamini Y, Hochberg Y (1995) Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser B 57: 289–300. 10.2307/2346101 [DOI] [Google Scholar]
- 42. Justes E, Mary B, Meynard J (1997) Evaluation of a nitrate test indicator to improve the nitrogen fertilisation of winter wheat crops. Diagnostic Proced Crop N Manag a Work Poitiers, Fr 22–23 November, 1995. [Google Scholar]
- 43. Longnecker N, Kirby EJM, Robson A (1993) Leaf Emergence, Tiller Growth, and Apical Development of Nitrogen-Dificient Spring Wheat. Crop Sci 33: 154 10.2135/cropsci1993.0011183X003300010028x [DOI] [Google Scholar]
- 44. Al-Karaki G, McMichael B, Zak J (2004) Field response of wheat to arbuscular mycorrhizal fungi and drought stress. Mycorrhiza 14: 263–269. 10.1007/s00572-003-0265-2 [DOI] [PubMed] [Google Scholar]
- 45. Gao X, Akhter F, Tenuta M, Flaten DN, Gawalko EJ, Grant CA (2010) Mycorrhizal colonization and grain Cd concentration of field-grown durum wheat in response to tillage, preceding crop and phosphorus fertilization. J Sci Food Agric 90: 750–758. 10.1002/jsfa.3878 [DOI] [PubMed] [Google Scholar]
- 46. Germida JJ, Walley FL (1996) Plant growth-promoting rhizobacteria alter rooting patterns and arbuscular mycorrhizal fungi colonization of field-grown spring wheat. Biol Fertil Soils 23: 113–120. 10.1007/BF00336050 [DOI] [Google Scholar]
- 47. Teng W, Deng Y, Chen X-P, Xu X-F, Chen R-Y, Lv Y, et al. (2013) Characterization of root response to phosphorus supply from morphology to gene analysis in field-grown wheat. J Exp Bot 64: 1403–1411. 10.1093/jxb/ert023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mohammad A, Mitra B, Khan AG (2004) Effects of sheared-root inoculum of Glomus intraradices on wheat grown at different phosphorus levels in the field. Agric Ecosyst Environ 103: 245–249. 10.1016/j.agee.2003.09.017 [DOI] [Google Scholar]
- 49. Fester T, Fetzer I, Buchert S, Lucas R, Rillig MC, Härtig C (2011) Towards a systemic metabolic signature of the arbuscular mycorrhizal interaction. Oecologia 167: 913–924. 10.1007/s00442-011-2037-6 [DOI] [PubMed] [Google Scholar]
- 50. Rasmussen S, Parsons AJ, Fraser K, Xue H, Newman JA (2008) Metabolic profiles of Lolium perenne are differentially affected by nitrogen supply, carbohydrate content, and fungal endophyte infection. Plant Physiol 146: 1440–1453. 10.1104/pp.107.111898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hodge A, Fitter AH (2010) Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proc Natl Acad Sci U S A 107: 13754–13759. 10.1073/pnas.1005874107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bouché N, Fromm H (2004) GABA in plants: just a metabolite? Trends Plant Sci 9: 110–115. 10.1016/j.tplants.2004.01.006 [DOI] [PubMed] [Google Scholar]
- 53. Carvalhais LC, Dennis PG, Fedoseyenko D, Hajirezaei M-R, Borriss R, von Wirén N (2011) Root exudation of sugars, amino acids, and organic acids by maize as affected by nitrogen, phosphorus, potassium, and iron deficiency. J Plant Nutr Soil Sci 174: 3–11. 10.1002/jpln.201000085 [DOI] [Google Scholar]
- 54. Somers E, Vanderleyden J, Srinivasan M (2004) Rhizosphere bacterial signalling: a love parade beneath our feet. Crit Rev Microbiol 30: 205–240. 10.1080/10408410490468786 [DOI] [PubMed] [Google Scholar]
- 55. Ramos-Gonzalez MI, Campos MJ, Ramos JL (2005) Analysis of Pseudomonas putida KT2440 Gene Expression in the Maize Rhizosphere: In Vivo Expression Technology Capture and Identification of Root-Activated Promoters. J Bacteriol 187: 5504–5504. 10.1128/JB.187.15.5504.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Carvalhais LC, Dennis PG, Fan B, Fedoseyenko D, Kierul K, Becker A, et al. (2013) Linking plant nutritional status to plant-microbe interactions. PLoS One 8: e68555 10.1371/journal.pone.0068555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Návarová H, Bernsdorff F, Döring A-C, Zeier J (2012) Pipecolic acid, an endogenous mediator of defense amplification and priming, is a critical regulator of inducible plant immunity. Plant Cell 24: 5123–5141. 10.1105/tpc.112.103564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vogel-Adghough D, Stahl E, Návarová H, Zeier J (2013) Pipecolic acid enhances resistance to bacterial infection and primes salicylic acid and nicotine accumulation in tobacco. Plant Signal Behav 8: e26366 10.4161/psb.26366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vranova V, Rejsek K, Skene KR, Formanek P (2011) Non-protein amino acids: Plant, soil and ecosystem interactions. Plant Soil 342: 31–48. 10.1007/s11104-010-0673-y [DOI] [Google Scholar]
- 60. Pálfi G, Dézsi L (1968) Pipecolic acid as an indicator of abnormal protein metabolism in diseased plants. Plant Soil 29: 285–291. 10.1007/BF01348946 [DOI] [Google Scholar]
- 61. Ryu C-M, Farag MA, Hu C-H, Reddy MS, Wei H-X, Paré PW, et al. (2003) Bacterial volatiles promote growth in Arabidopsis . Proc Natl Acad Sci U S A 100: 4927–4932. 10.1073/pnas.0730845100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pfeffer P, Douds DD Jr, Becard G, Shachar-Hill Y (1999) Carbon uptake and the metabolism and transport of lipids in an arbuscular mycorrhiza. Plant Physiol 120: 587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bago B, Pfeffer PE, Abubaker J, Jun J, Allen JW, Brouillette J, et al. (2003) Carbon export from arbuscular mycorrhizal roots involves the translocation of carbohydrate as well as lipid. Plant Physiol 131: 1496–1507. 10.1104/pp.102.007765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Moche M, Stremlau S, Hecht L, Göbel C, Feussner I, Stöhr C (2010) Effect of nitrate supply and mycorrhizal inoculation on characteristics of tobacco root plasma membrane vesicles. Planta 231: 425–436. 10.1007/s00425-009-1057-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kobae Y, Gutjahr C, Paszkowski U, Kojima T, Fujiwara T, Hata S (2014) Lipid droplets of arbuscular mycorrhizal fungi emerge in concert with arbuscule collapse. Plant Cell Physiol. 10.1093/pcp/pcu123 [DOI] [PubMed] [Google Scholar]
- 66. Laparre J, Malbreil M, Letisse F, Portais JC, Roux C, Bécard G, et al. (2014) Combining metabolomics and gene expression analysis reveals that propionyl- and butyryl-carnitines are involved in late stages of arbuscular mycorrhizal symbiosis. Mol Plant 7: 554–566. 10.1093/mp/sst136 [DOI] [PubMed] [Google Scholar]
- 67. Bago B, Zipfel W, Williams RM, Jun J, Arreola R, Lammers PJ, et al. (2002) Translocation and utilization of fungal storage lipid in the arbuscular mycorrhizal symbiosis. Plant Physiol 128: 108–124. 10.1104/pp.010466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Helber N, Wippel K, Sauer N, Schaarschmidt S, Hause B, Requena N (2011) A versatile monosaccharide transporter that operates in the arbuscular mycorrhizal fungus Glomus sp is crucial for the symbiotic relationship with plants. Plant Cell 23: 3812–3823. 10.1105/tpc.111.089813 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Above ground biomass, grain yield and yield components at maturity of durum wheat grown in the field (Table A). Enrichment metabolomic analysis for Pathways (Table B), Enzyme interactions (Table C), Biological role (Table D), Chemical groups (Table E), and Other interactions (Table F) as displayed in MBRole. Group means ± S.E. across standardised data for identified GC peaks grouped per biological group (Table G). HILIC-Q-TOF MS identified compounds (Table H).
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.