Abstract
The worldwide prevalence of metabolic diseases, including obesity and diabetes, is increasing. Mitochondrial dysfunction is recognized as a core feature of these diseases. Emerging evidence also suggests that defects in mitochondrial biogenesis, number, morphology, fusion, and fission, contribute to the development and progression of metabolic diseases. Our previous studies revealed that balanced deep-sea water (BDSW) has potential as a treatment for diabetes and obesity. In this study, we aimed to investigate the mechanism by which BDSW regulates diabetes and obesity by studying its effects on mitochondrial metabolism. To determine whether BDSW regulates mitochondrial biogenesis and function, we investigated its effects on mitochondrial DNA (mtDNA) content, mitochondrial enzyme activity, and the expression of transcription factors and mitochondria specific genes, as well as on the phosphorylation of signaling molecules associated with mitochondria biogenesis and its function in C2C12 myotubes. BDSW increased mitochondrial biogenesis in a time and dose-dependent manner. Quantitative real-time PCR revealed that BDSW enhances gene expression of PGC-1α, NRF1, and TFAM for mitochondrial transcription; MFN1/2 and DRP1 for mitochondrial fusion; OPA1 for mitochondrial fission; TOMM40 and TIMM44 for mitochondrial protein import; CPT-1α and MCAD for fatty acid oxidation; CYTC for oxidative phosphorylation. Upregulation of these genes was validated by increased mitochondria staining, CS activity, CytC oxidase activity, NAD+ to NADH ratio, and the phosphorylation of signaling molecules such as AMPK and SIRT1. Moreover, drinking BDSW remarkably improved mtDNA content in the muscles of HFD-induced obese mice. Taken together, these results suggest that the stimulatory effect of BDSW on mitochondrial biogenesis and function may provide further insights into the regulatory mechanism of BDSW-induced anti-diabetic and anti-obesity action.
Introduction
The decrease of mitochondrial DNA (mtDNA) and mitochondrial dysfunction play an important role in diverse age-associated disorders and metabolic diseases such as type 2 diabetes and obesity [1]. Emerging evidence suggests that impaired mitochondrial metabolism is accompanied by reduced transcription factor activity regulating mitochondrial biogenesis in patients with insulin resistance, type 2 diabetes and obesity [2, 3].
Mitochondrial biogenesis is regulated by a complex network of transcription factors such as peroxisome proliferator-activated receptor co-activator 1 alpha (PGC-1α), the nuclear respiratory factors (NRFs), and mitochondrial transcription factor A (mtTFA) [4]. Furthermore, reduced mtDNA copy number has also been shown in muscle and adipose tissue from diabetic patients [5]. Treatment with thiazolidinedione (TZD), an insulin-sensitizing drug currently used to treat type 2 diabetes, restored the decreased mtDNA content as well as the expression of genes involved in mitochondrial biogenesis and fatty acid oxidation [6, 7]. Therefore, the promotion of mitochondrial biogenesis and function could be a strategy for preventing and treating metabolic diseases, including insulin resistance, obesity, and diabetes.
Deep-sea water (DSW), that is, sea water found at a depth of more than 200 m, is a stable natural resource and is available in infinite supply as compared to other natural products. It contains high levels of essential minerals such as magnesium (Mg), calcium (Ca), and potassium (K), as well as beneficial trace minerals for human health, such as chromium (Cr), selenium (Se), zinc (Zn), and vanadium (V). Several studies investigating the physiological effects of DSW at various degrees of hardness found that DSW might aid in preventing hypertension [8], atopic eczema/dermatitis syndrome [9], and arteriosclerosis [10].
Previously, we demonstrated that balanced DSW (BDSW), which is a mixture of DSW mineral extracts and desalinated water, has anti-diabetic potential via the inhibition of hyperglycemia and improvement in glucose intolerance by increasing glucose uptake in type 1 [11] and 2 diabetic mice [12]. In addition, BDSW has antiobesity potential because it can inhibit adipocyte hypertrophy and reduce liver steatosis in high-fat diet (HFD)–induced obese mice [13]. Reduced mitochondrial content has also been observed in hypertrophic epididymal fat and fatty livers of HFD-induced obese mice, suggesting a potential role for the disruption of mitochondrial content in adipose and liver tissue during development of obesity and type 2 diabetes. Interestingly, drinking BDSW remarkably increased mitochondrial biogenesis by inducing the expression of the main genes involved in its regulation such as PGC-1α, NRF1, and mtTFA in the adipose and liver of HFD-induced obese mice [13]. A decrease in mitochondrial function or mtDNA content has been correlated with the degree of insulin resistance and dysregulated lipid metabolism [14].
Based on the above findings, we hypothesized that the increase in mitochondrial content and expression of transcription factor genes by BDSW could stimulate mitochondrial function and prevent diabetes and obesity-related mitochondrial dysfunction. Therefore, in the present study, we first determined whether BDSW could stimulate mitochondrial function by stimulating mitochondrial biogenesis and its regulatory mechanism in C2C12 myotubes. To expand on the information obtained in a previous study, we investigated the effect of drinking BDSW on mitochondrial biogenesis in the muscles of HFD-induced obese mice.
Materials and Methods
Ethics statement
No permission was required for the areas studied involving collection of water. Animal experiments were performed by the guidelines established by the Animal Ethics Committee of Kyungpook National University, and the protocols were approved by this committee (Approval No. KNU 2012–88).
Materials
The C2C12 cell line was purchased from the American Type Culture Collection (ATCC No.CRL-1772, Manassas, VA, USA). The following items were purchased from the stated commercial sources: 5-amino-1-β-d-ribofuranosyl-imidazole-4-carboxamide (AICAR), nicotinamide, ethidium bromide (EtBr), 4′,6-Diamidino-2-phenylindole (DAPI) from Sigma-Aldrich Co. LLC. (St. Louis, MO, USA); anti-phospho AMPKα (Thr172), anti-AMPKα and anti-phospho SIRT1 (Ser47) from Cell Signaling Technology (Danvers, MA, USA); citrate synthase (CS) assay kit, cytochrome oxidase (COX) activity assay kit, and NAD+/NADH quantification kit from BioVision, Inc. (Milpitas, CA, USA); SRT1720, protein A/G agarose, anti-SIRT1, anti-PGC-1α, anti-acetylated-lysine (Ac-Lys), anti-β-actin, horseradish peroxidase (HRP)-conjugated anti-mouse IgG, and anti-rabbit IgG-HRP antibodies from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA); ECL Plus Western Blotting Substrate from Pierce Biotechnology (Rockford, IL, USA); TRIzol and MitoTracker Red CMXRos from Invitrogen Life Technologies (Carlsbad, CA, USA); PrimeScript 1st strand cDNA Synthesis Kit from Takara Bio Inc. (Shiga, Japan); FastStart Universal SYBR Green Master from Roche Applied Science (Basel, Switzerland); Phosphatase Inhibitor Cocktail and Protease Inhibitor Cocktail solutions from GenDEPOT (Barker, TX, USA).
BDSW preparation
As described in detail previously [11], original DSW that had been pumped up from a depth of 0.5 km and a distance of 6.7 km off Oho-Ri, Goseong (38°20´ N and 128°34´ E, Gangwon-Do, Korea) was filtered using a microfilter system (Synopex INC, Pohang, Korea) to remove phytoplankton and microorganisms. Next, the filtered DSW was passed through a reverse osmosis membrane (Vontron Technology Co. Ltd., Beijing, China) to obtain DSW mineral extracts and desalinated water. The BDSW was serially diluted using desalinated DSW with regard to hardness. In this study, we defined the hardness of BDSW by using the concentrations of Mg and Ca. The hardness was calculated according to the following equation: Hardness = Mg (mg/L) × 4.1 + Ca (mg/L) × 2.5 [15]. The mineral content of samples were measured by Dionex ICS-1100 basic integrated ion chromatography system (Thermo Scientific Inc., Sunnyvale, CA, USA) for cation analysis such as Ca and Mg, and ELAN 9000/6X00/DRC-e ICP-MS (PerkinElmer Inc., Waltham, MA, USA) for trace minerals analysis such as Se, V, and Zn. S1 Table indicates the mineral content of the original DSW and BDSW samples with a hardness of 4680 ppm.
Cell culture and myotube differentiation
As described in detail previously [11], murine C2C12 myoblast was maintained in Dulbecco’s modified Eagle medium (DMEM) containing 10% FBS (HyClone Laboratories, South Logan, UT, USA) and 1% antibiotics (penicillin-streptomycin) in 5% CO2 at 37°C. To induce myotubes differentiation, C2C12 myoblasts were incubated in differentiation medium containing DMEM with 2% horse serum (Life Technologies Inc., Grand Island, NY, USA) for 2 days after reaching confluency. The media was changed daily, and C2C12 myotubes were prepared for experiments 4–5 days after plating.
Animal experiment
To determine the effects of BDSW on mitochondrial biogenesis in muscles of HFD-induced obese mice, we used muscles isolated from a previous animal study [13]. Briefly, the C57BL/6J mice (6 weeks of age) in each of the 5 groups (eight mice per group) were given either the normal diet (D12450B, 10% kcal% fat; Research diet, New Brunswick, NJ, USA), HFD (D12451, 45% kcal% fat; Research diet, New Brunswick, NJ, USA), or HFD with BDSW of differing hardness (500, 1000, and 2000 ppm) for 20 weeks. At the end of the feeding period, muscles was collected under anesthesia with zoletil (Virbac S.A, Carros, France). All samples were stored at −70°C until assayed.
Mitochondrial biogenesis analysis
To assess mitochondrial biogenesis, C2C12 myotubes were incubated in DMEM containing desalinated water, BDSW at different levels of hardness (0, 100, 500, 1000, 1500, 2000), or 1 mM AICAR (AC) for the indicated time (0, 1, 2, 3 days). To investigate the effect of inhibitors on mitochondrial biogenesis, C2C12 myotubes were incubated with BDSW at hardness 2000 only and BDSW 2000 ppm with inhibitors such as 2 mM nicotinamide (NA), 10 μM compound C (CC), and 20 ng/ml EtBr, and 1 mM AICAR (AC) for 1 day. As described in detail previously [13], mitochondrial biogenesis was determined by the ratio of mitochondrial DNA (mtDNA) to nuclear DNA (nDNA), quantified by real-time qPCR, assuming that nDNA levels remain constant. DNA was extracted from C2C12 myotubes and muscles of HFD-induced obese mice, using a genomic DNA purification kit (Promega Corp., Madison, WI, USA), according to the manufacturer’s instructions. For each DNA extract, mRNA levels of the nuclear gene ribosomal protein large p0 (RPLP0) and the mitochondrial gene cytochrome c oxidase subunit I (COX I) were quantified by quantitative real-time polymerase chain reaction (qRT-PCR). All primers are listed in S2 Table. SYBR Green was used to measure expression levels and data were normalized against the expression of RPLP0 (ΔΔCT analysis).
Mitochondrial staining and quantification
C2C12 myotubes were differentiated in 8-well chamber slides (Thermo Scientific Inc., Rochester, NY, USA) and then treated with DMEM containing BDSW 2000, 1 mM AICAR, or 10 μM SRT1720 for 24 h. Next, myotubes were incubated with DMEM containing 20 nM Mito Tracker Red CMXRos at 37°C for 30 min. After fixing with 4% paraformaldehyde, cells were stained with 1 μg/mL DAPI for 10 min. Image acquisition was performed using a digital microscope (Nikon eclipse 80i; Tokyo, Japan) at × 400 magnification. As described in detail previously [11], to quantify the fluorescent signal, cells were washed with PBS and incubated with lysis buffer (0.1 M potassium phosphate, 1% Triton X-100, pH 10) for 10 min with shaking. Subsequently, DMSO was added and the cells were allowed to shake for 10 min. The intensity of the fluorescent signal was measured using a microplate reader (FLUOstar OPTIMA, BMG LABTECH, Germany) at excitation and emission wavelengths of 466 nm and 540 nm, respectively.
Citrate synthase activity, cytochrome c oxidase activity, and NAD+/NADH ratio analysis
After C2C12 myotubes were incubated with DMEM containing BDSW at different hardness (0, 100, 500, 1000, 1500, 2000 ppm), or 1 mM AICAR for 6 h, enzyme activity was measured using citrate synthase activity kit and cytochrome c oxidase activity kit (BioVision, Inc. Milpitas, CA, USA), and the NAD+ and NADH content was determined using NAD+ /NADH quantification kit (BioVision, Inc. Milpitas, CA, USA) in cell lysates (10 μg), respectively, according to the manufacturer's instructions. Using a microplate reader (Model AD200; Beckman Coulter, Brea, CA, USA), citrate synthase (CS) and cytochrome c oxidase (COX) activity were determined using a kinetic program at OD 412 nm and OD 550 nm, respectively. The amount of total NAD+ or NADH in the lysates was determined at OD 450 nm. The ratio of NAD+ to NADH was calculated by dividing the total NAD+ or NADH concentration by the total protein concentration.
Nuclear extraction and immunoprecipitation to assess PGC-1α activity
To assess PGC-1α activity, C2C12 myotubes were incubated with DMEM containing BDSW at different hardness levels, 1 mM AICAR, or 10 μM SRT1720 for 6 h. As described in detail previously [16], immunoprecipitation of PGC-1α was performed using the Nuclear Complex Co-IP Kit (Active Motif Inc. Carlsbad, CA, USA), according to manufacturer’s instructions. Briefly, PGC-1α was immunoprecipitated from nuclear extracts (200 μg) using a rabbit anti-PGC-1α polyclonal antibody (Santa Cruz, CA, USA) with gentle rocking overnight at 4°C and was then incubated with protein A/G plus agarose beads (40 μl of 50% bead slurry; Santa Cruz, CA, USA) for 2 h at 4°C. Subsequently, immunoprecipitates were immunoblotted with either an anti-acetylated lysine antibody to determine the extent of PGC-1α acetylation (Ac-Lys) or a rabbit anti-PGC-1α polyclonal antibody to determine the total amount of PGC-1α.
Western blot analysis
For determination of AMPK and SIRT1 activity, C2C12 myotubes were incubated with DMEM containing BDSW with differing hardness (0, 100, 500, 1000, 1500, 2000 ppm), 1 mM AICAR, or 10 μM SRT1720 for 1 h, and then the levels of AMPK and SIRT1 phosphorylation were analyzed. To investigate the effect of several inhibitors on AMPK and SIRT1 phosphorylation, the cells were preincubated in DMEM in the presence or absence of 2 mM nicotinamide (NA), 10 μM compound C (CC), and 20 ng/ml EtBr for 1 h, and then the cells were incubated in DMEM containing BDSW (hardness, 2000 ppm) only, with or without inhibitors for 1 h. After incubation was completed, the cells were washed with PBS, and lysed in RIPA buffer (50 mM NaCl, 10 mM Tris, 0.1% SDS, 1% Triton X-100, 0.1% sodium deoxycholate, 5 mM EDTA, 1 mM Na3VO4, pH 7.4). Equal amounts of cell lysate protein (40 μg/lane) was subjected to SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane (Whatman, Dassel, Germany). The membrane was blocked with 5% non-fat dried milk (BD Bioscience, San Jose, CA) for 1 h and incubated with anti-PGC-1α, anti-phospho AMPKα (Thr172), anti-AMPK, anti-phospho SIRT1 (Ser47), anti-SIRT1, anti-acetylated-lysine (Ac-Lys), and anti-β-actin (diluted 1:1000) overnight at 4°C. After washing with TBS containing 0.1% Tween-20, the membrane was incubated with HRP-conjugated anti-mouse IgG or anti-rabbit IgG antibodies (diluted 1:3000) for 1 h at room temperature. Antibody binding on the nitrocellulose membrane was detected with an ECL plus solution and radiography. The images were detected with a lumino image analyzer (Model LAS-4000 Mini; Fujifilm, Tokyo, Japan) and coupled with image analysis software (Multi Gauge Ver. 3.0; Fujifilm).
Quantitative real time- polymerase chain reaction (qRT-PCR) analysis
As described in detail previously [11], total RNA was isolated from C2C12 myotubes and muscles of HFD-induced obese mice using TRIzol, and cDNA was synthesized using PrimeScript1st strand cDNA synthesis kit (Takara Bio Inc., Shiga, Japan), according to the manufacturer’s instructions. qRT-PCR was performed in triplicate using a FastStart SYBR Green Master Mix in an ABI Prism 7300 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The expression levels of target genes relative to that of the endogenous reference gene actin were calculated using the delta cycle threshold method. The primer sequences are listed in S2 Table.
Statistical analysis
All results were compared by one-way analysis of variance (ANOVA) using Statistical Package for the Social Sciences (SPSS, ver. 11.0), and are expressed as means ± SE in three independent experiments. Group means were considered as significantly different at p < 0.05, as determined by the technique of protective least significant difference when ANOVA indicated an overall significant treatment effect of p < 0.05.
Results
BDSW enhances mitochondrial biogenesis in C2C12 myotubes
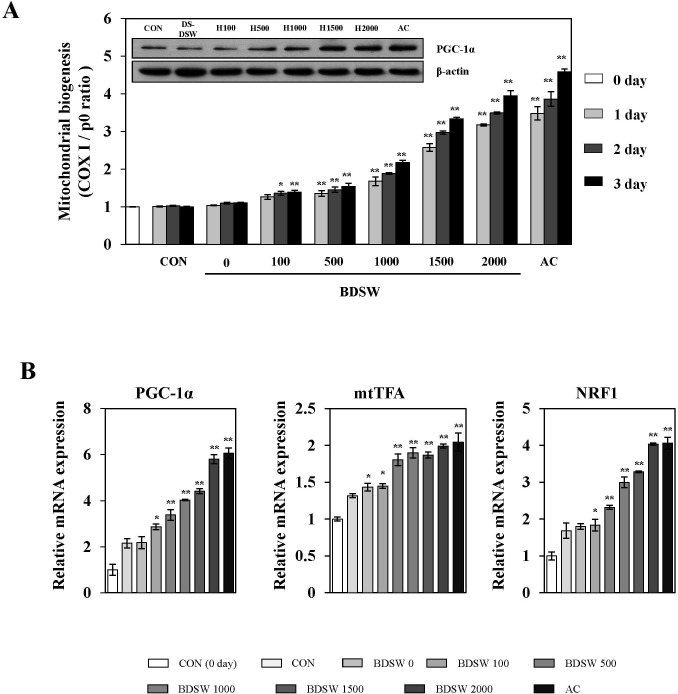
To determine whether BDSW regulates mitochondrial biogenesis, we investigated the gene expression ratio of COX Ι and RPLP0 representing mitochondrial DNA (mtDNA) and nuclear DNA (nDNA), respectively in C2C12 myotubes. BDSW increased mitochondrial biogenesis in a dose- and time-dependent manner. The expression of PGC-1α, a primary regulator for mitochondrial biogenesis, was increased in a dose-dependent manner after 3 days of BDSW treatment (Fig 1A). To determine how BDSW stimulates the increase in mitochondrial biogenesis, we next investigated the mRNA expression of various genes related to mitochondrial transcription, fatty acid oxidation, and oxidative phosphorylation. As shown in Fig 1B, BDSW increased the gene expression of transcription co-activators such as PGC-1α, mtTFA, and NRF1 and in a dose-dependent manner. To further investigate the regulatory mechanism related to mitochondrial biogenesis, we investigated gene expression related to fatty acid oxidation, oxidative phosphorylation, and mitochondrial dynamics. BDSW increased the gene expression of carnitine palmitoyltransferase 1-α (CPT1-α) and medium-chain acyl-CoA dehydrogenase (MCAD) for fatty acid oxidation, cytochrome c (CytC) for oxidative phosphorylation (Fig 2A), translocase of outer mitochondrial membrane 40 (TOMM40) and translocase of inner mitochondrial membrane 44 (TIMM44) for protein import, dynamin-related protein 1 (DRP1) for fission (Fig 2B), optic atrophy 1 (OPA1), mitofusin-1 (MFN1), and mitofusin-2 (MFN2) for fusion (Fig 2C) in a dose-dependent manner. These results suggest that BDSW is an effective stimulator of mitochondrial biogenesis.
Fig 1. Effect of BDSW on mtDNA, expression of PGC-1α (A), and PGC-1α, mtTFA, and NRF1 gene expression (B) in C2C12 myotubes.
Myotubes were incubated in DMEM containing desalinated water, BDSW at different levels of hardness (0, 100, 500, 1000, 1500, 2000), or 1 mM AICAR (AC) for the indicated time (0, 1, 2, 3 days). All media were changed daily. Expression of PGC-1α was determined by western blot analysis (3 days). β–actin served as the standard. B. After C2C12 myotubes were incubated with BDSW at different levels of hardness for 24 h, gene expression of markers of mitochondrial biogenesis were determined by quantitative real-time PCR. Each value represents the mean ± SE of three independent experiments. *P < 0.05, **P < 0.01: significant difference vs. 0 day (A) or CON group (B). CON group: DMEM media treated control group; DS-W, desalinated water.
Fig 2. Effect of BDSW on expression of genes involved in fatty acid oxidation, oxidative phosphorylation, and mitochondrial dynamic in C2C12 myotubes.
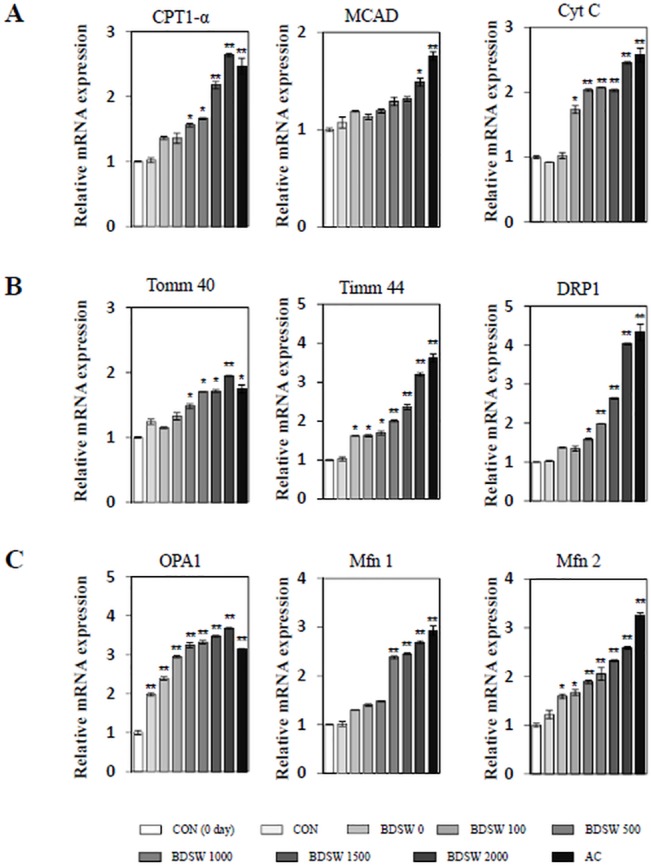
After C2C12 myotubes were incubated in DMEM containing desalinated water, BDSW at different levels of hardness or 1 mM AICAR (AC) for 24 h, expression of CPT1-α and MCAD for fatty acid oxidation and CytC for oxidative phosphorylation (A), TOMM 40 and TIMM 44 for mitochondrial protein import and DRP1 for mitochondrial fission (B), and OPA1, MFN1, and MFN2 for mitochondrial fusion (C) were determined by quantitative real-time PCR. Each value represents the mean ± SE of three independent experiments. *P < 0.05, **P < 0.01: significant difference vs. CON group. CON group: DMEM media treated control group; DS-W, desalinated water.
BDSW enhances mitochondrial enzyme activity by increasing AMPK and SIRT1 phosphorylation and PGC-1α deacetylation
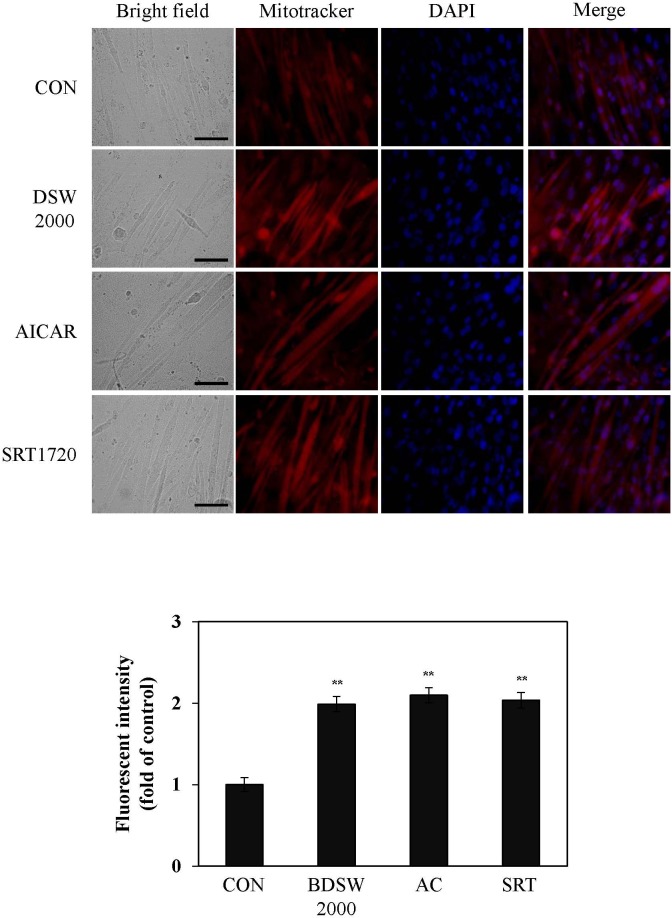
To examine whether BDSW-induced mitochondrial gene expression was associated with mitochondrial function, we next stained mitochondria in C2C12 cells using MitoTracker Red CMXRos. After BDSW treatment for 24 h, there was increased mitochondrial staining compared with untreated controls. As compared with AICAR, an activator of AMPK, and SRT1720, an activator of SIRT1, treated cells, the stimulatory effect of BDSW was similar to that of activators for mitochondrial biogenesis (Fig 3). To confirm that the increased mitochondrial membrane potential was associated with increased mitochondrial function, we investigated the effects of BDSW on CS activity, COX activity, and the ratio of NAD+ to NADH. BDSW increased CS, COX activity as well as the ratio of NAD+ to NADH in a dose- dependent manner (Fig 4A). Moreover, BDSW increased the phosphorylation of AMPK and SIRT1, and the deacetylation of PGC-1α, which are key signaling molecules in mitochondrial biogenesis and function (Fig 4B). These results suggest that BDSW may increase mitochondrial function via the induction of gene expression and the activation of signaling molecules.
Fig 3. Effect of BDSW on mitochondrial mass in C2C12 myotubes.
A. Representative MitoTracker Red CMXRos and DAPI staining of the C2C12 myotubes are shown at × 400 magnification. Scale bar, 50 μm. B. Quantification of MitoTracker Red CMXRos staining (n = 10 per group). Each value represents the mean ± SE of three independent experiments. **P < 0.01: significant difference vs. CON group. CON group: non-treated control group.
Fig 4. Effect of BDSW on the activity of citrate synthase and cytochrome c oxidase, the ratio of NAD+/NADH, and the activity of AMPK, SIRT1, and PGC-1α.
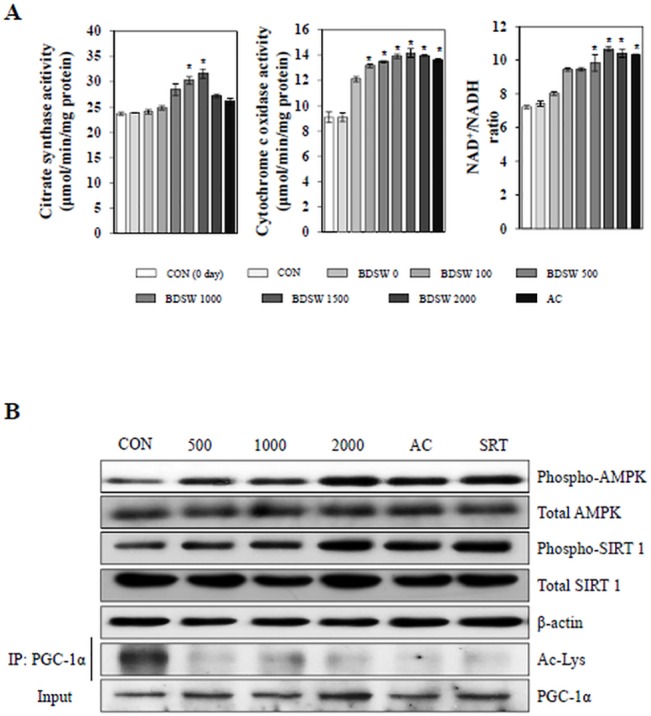
A. The activity of citrate synthase and cytochrome c oxidase, and the ratio of NAD+/NADH were determined in cell lysates (10 μg). B. Phospho-AMPK, total AMPK, phospho-SIRT1, and total SIRT1 expression were determined in lysates (20 μg) of C2C12 myotubes after BDSW treatment for 1 h. β-actin was used as a loading control. For the activity of PGC-1α, after BDSW treatment for 6 h, PGC-1α was immunoprecipitated from nuclear extracts and then immunoblotted with either an antiacetylated lysine antibody to determine the extent of PGC-1α acetylation (Ac-Lys) or PGC-1α antibody to determine the total amount of PGC-1α. Each value represents the mean ± SE of three independent experiments. *P < 0.05: significant difference vs. CON group. CON group: DMEM media treated control group.
BDSW stimulates mitochondrial biogenesis dependent on AMPK and SIRT1 signaling pathways
To confirm the mechanism by which BDSW affects mitochondrial biogenesis and function, we analyzed the phosphorylation of AMPK and SIRT1. BDSW increased the phosphorylation of AMPK and SIRT1 (Fig 5A). To further investigate the more detailed mechanisms of BDSW-mediated mitochondrial biogenesis, we next examined the effects of several inhibitors, namely, nicotinamide (NA, an SIRT1 inhibitor), compound C (CC, an ATP-competitive inhibitor of AMPK), and EtBr (an inhibitor of mtDNA synthesis). The promotion in protein phosphorylation (Fig 5B) and mitochondrial biogenesis (Fig 5C) by BDSW was inhibited by treatment with NA, CC, or EtBr. Therefore, these results suggest that the BDSW-mediated increase in mitochondrial biogenesis and function is dependent on the AMPK and SIRT1 signaling pathways.
Fig 5. Effect of BDSW on the phosphorylation of AMPK and SIRT1.
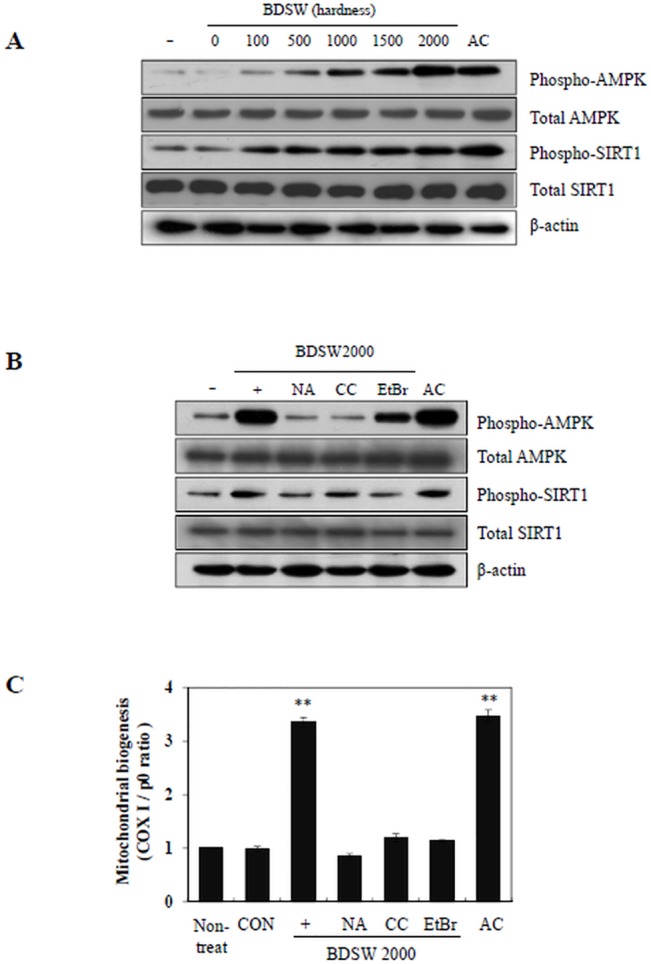
A. Western blot analyses of phospho-AMPK, total AMPK, phospho-SIRT1, and total SIRT1 in C2C12 myotubes treated with BDSW at different levels of hardness for 1 h. β-actin was used as a loading control. B. Western blot analysis following treatment with 2 mM nicotinamide (NA), 10 μM compound C (CC), and 20 ng/ml EtBr, for 1 h, followed by incubation with BDSW 2000 ppm hardness for 1 h C. For mitochondrial biogenesis using several inhibitors, after C2C12 myotubes were incubated with BDSW at hardness 2000 only, BDSW 2000 ppm with inhibitors and 1 mM AICAR (AC) for 1 day, mitochondria biogenesis were determined by the ratio of relative amount of mitochondrial DNA (COX I) and nuclear DNA (p0) quantified by real-time PCR. Non-treated group was used as the 0 day group. Each value represents the mean ± SE of three independent experiments. **P < 0.01: significant difference vs. non-treated group. CON: 1 day control group.
Drinking BDSW enhances mitochondrial biogenesis in muscles of HFD-induced obese mice
To determine whether BDSW regulates mitochondrial biogenesis in vivo, we investigated mtDNA content and the expression of genes related to mitochondrial biogenesis such as PGC-1α, NRF1, and mtTFA in muscles isolated during a previous study using HFD-induced obese mice. The mitochondrial biogenesis in HFD-induced obese mice was very low; however, BDSW significantly increased mtDNA content in the muscles of HFD-induced obese mice (Fig 6A). Moreover, BDSW enhanced the expression of PGC-1α, NRF1, and mtTFA (Fig 6B) as well as estrogen-related receptor α (ERRα), peroxisome proliferator-activated receptor α (PPARα), and PPARδ, which are co-transcription factors involved in the regulation of muscle mitochondrial oxidative phosphorylation activity (Fig 6C). These results suggest that BDSW is a powerful enhancer of mitochondrial biogenesis and function in vivo.
Fig 6. Effect of BDSW on mtDNA (A) and gene expression of PGC-1α, NRF1, and mtTFA (B), and ERRα, PPARα, and PPARδ (C) in muscles of mice fed the ND, HFD, and HFD with BDSW for 20 weeks.
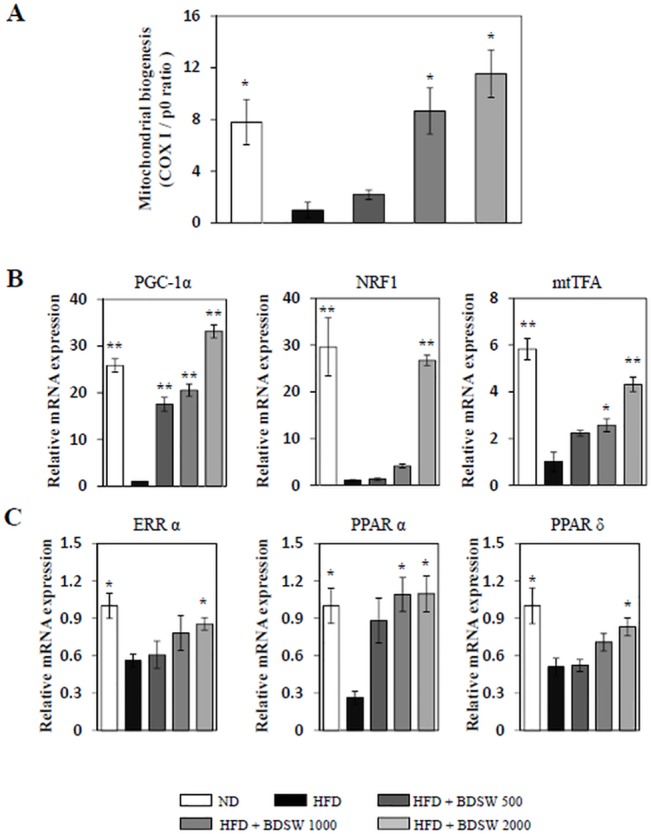
Each value represents the mean ± SE (n = 8 per group). *P < 0.05, **P < 0.01: significant difference vs. HFD group. ND, normal diet; HFD, high-fat diet.
Discussion
Identifying the mechanisms of mitochondrial dysfunction, and developing drugs or nutrients that target the mitochondria, are well known strategies for preventing and treating mitochondria-related disease including type 2 diabetes and obesity [17]. In the present study, we demonstrated that BDSW enhances mitochondrial biogenesis and function by inducing gene expression of PGC-1α, NRF1, and mtTFA, increasing the activity of mitochondrial enzymes such as CS, CytC oxidase, and the ratio of NAD+ to NADH, and regulating the activity of signaling molecules such as PGC-1α, AMPK, and SIRT1 in C2C12 myotubes. Moreover, BDSW improved mitochondrial biogenesis by inducing gene expression of main transcription factors in muscles of HFD-induced obese mice. Although most of our conclusions are based on in vivo models and in vitro pharmacological interventions, the novel association between mitochondria metabolism and the anti-obesity and anti-diabetic effects of BDSW, led us to propose that the regulation of mitochondrial metabolism by BDSW is important in our understanding of obesity and diabetes. Therefore, these findings suggest that there is an association between mitochondrial metabolism and BDSW-induced inhibitory effects on obesity and diabetes.
AMP-activated protein kinase (AMPK) and the silent information regulator T1 (SIRT1), an NAD+-dependent deacetylase, are energy sensing molecules regulated by the AMP/ATP or NAD+/NADH ratio, respectively. Recent studies showed that they have common effects on diverse physiological processes such as cellular energy metabolism and inflammation as well as mitochondrial biogenesis and function [18, 19]. These similarities occur because AMPK and SIRT1 have a common mechanism that regulates the functional activity of targets such as PGC-1α, endothelial nitric oxide synthase (eNOS), and forkhead box O (FOXO). In particular, both AMPK and SIRT1 act in cooperation with PGC-1α, a key regulator of mitochondrial biogenesis, to regulate energy homeostasis in response to environmental, nutritional, and hormonal stimuli [20]. The activation of AMPK, SIRT1, and PGC-1α by BDSW may converge to enhance mitochondrial function, as demonstrated by significant increases in CS and COX activity, and induction of key metabolic genes including CPT1-α, MCAD, and CytC. Therefore, enhanced mitochondrial biogenesis and function due to BDSW, with increased activity of energy sensing molecules further support our hypothesis that BDSW improves HFD-induced obesity and type 2 diabetes by regulating energy metabolism.
Mitochondrial alterations, including reduced biogenesis and function as well as impaired morphology and dynamics, are closely associated with the development of metabolic diseases such as diabetes and obesity [21]. Mitochondrial biogenesis is the process of formation of new mitochondria, which is defined as the growth and division of pre-existing mitochondria influenced by environmental stimuli such as exercise, caloric restriction, low temperature, oxidative stress, cell division, renewal, and differentiation. It is well known that mitochondrial biogenesis is accompanied by changes in the number, size, and mass of mitochondria [22]. Mitochondrial function can have diverse effects on whole body metabolism. In particular, muscles [23] and adipose [24]tissues, which are metabolically flexible tissues, are most affected. As predicted, our findings show that BDSW enhances mitochondrial biogenesis and function with increased expression of mitochondrial genes and enzyme activity. Moreover, BDSW increased gene expression related to mitochondrial morphology and dynamics, which form an essential axis of mitochondrial quality control. Therefore, BDSW may regulate bioenergetics efficiency and energy expenditure by enhancing mitochondrial biogenesis and function.
Metabolic flexibility is the capacity of a system to adapt the oxidation of fuels, such as glucose and fatty acids, according to nutrient and oxidative availability. Conversely, metabolic inflexibility is impaired metabolic flexibility observed in peripheral tissue, such as muscle and adipose tissue, that is closelyassociated with diabetes and obesity [25]. The relationship between metabolic inflexibility and insulin resistance was observed in obese adults [26] and obese adolescents [27]. Several mitochondrial parameters including number, structure, and function, are impaired in insulin-resistant and insulin sensitive subjects [28]. These suggest that mitochondrial dysfunction might contribute to metabolic inflexibility and insulin resistance [29]. Mitochondrial deficiency in skeletal muscle is also associated with insulin resistance [30]. Taken together, our studies suggest that BDSW might enhance metabolic flexibility by enhancing mitochondrial biogenesis and function.
Optimal metabolic function of mitochondria requires the availability of essential vitamins, minerals, and other metabolites. These micronutrients are the primary cofactors that regulate basic mitochondrial function, including ATP synthesis, heme synthesis, assembly of electron transport complexes, and oxygen detoxification [31]. Deficiency of micronutrients leads to a decrease in several enzymatic activities of the electron transport complexes, while increasing the production of reactive oxidants and the functional decay of the mitochondria [32]. Recent studies showed that deficiency of essential minerals including Mg [33], Ca [34], and K [35], as well as trace minerals including Se [36], Zn [37], and V [38], was closely associated with development, prevention, and treatment of several chronic diseases including Alzheimer’s disease, stroke, hypertension, cardiovascular disease, and type 2 diabetes [39]. Although the exact roles of these minerals in metabolic disease are unknown, their deficiency results in reproductive problems, skeletal abnormalities, and diabetic cardiovascular complications. Therefore, further studies are needed to investigate the use of DSW mineral extracts in the treatment of obese and type 2 diabetes.
Mg is present as a complex with ATP in the mitochondria and is essential for the structural function of several mitochondrial proteins, including mitochondrial electron transport chain complex subunits, methylenetetrahydrofolate dehydrogenase 2, and pyruvate dehydrogenase phosphatase [40]. Studies on Mg-deficient cultured human cells [41] and animals [42] showed that mitochondrial dysfunction may induce a decrease in antioxidant defenses. However, the underlying mechanism remains unclear. Ca is a key regulator of mitochondrial function and acts as a cofactor in several mitochondrial enzymes. It plays a critical role in allosteric activation of mitochondrial enzymes such as pyruvate dehydrogenase, isocitrate dehydrogenase, and α-ketoglutarate dehydrogenase, as well as stimulation of the ATP synthase (complex V), α-glycerophosphate dehydrogenase, and adenine nucleotide translocase (ANT) [43]. The increase in cytosolic Ca level leads to increase in the levels of mitochondria-encoded COX I and mtTFA in human granulosa cells [44], as well as PGC-1α expression and mitochondrial biogenesis in rat epitrochlearis muscle [45]. On the other hand, Mendelev et al. showed that supplementation of Se increases the phosphorylation of Akt, an upstream regulator of PGC-1α, and cAMP response element-binding (CREB). In addition, Se improves mitochondrial function, including mitochondrial respiration and the activities of mitochondrial respiratory complexes I, II+III, and IV in murine hippocampal neuronal HT22 cells [46]. Moreover, it is known that low-dose supplementation of Se increases the level of glutathione (GSH) and the activities of glutathione peroxidases (GPXs), thioredoxin reductases (TRXRs), and iodothyronine deiodinases [47], as well as regulating diverse signaling pathways, including the mitogen-activated protein kinase (MAPK) [48], phosphatidylinositol 3-kinase (PI3K)-AKT [49], and NF-kB [50] pathways. Consequentially, these results support that the stimulatory effects of BDSW on mitochondrial biogenesis and function may be due to the physiological activities of BDSW components, including Mg, Ca, and Se.
For now, we speculate that the stimulatory effect of BDSW on mitochondria-associated gene expression and enzyme activity affects the synthesis of ATP or NAD+, which are known to be substrates of AMPK and SIRT1, respectively, contributing to an increase in AMPK and SIRT1 activity. As shown by Nathan et al. [51], SIRT1 plays an essential role in resveratrol-induced stimulation of AMPK and improvement of mitochondrial function, both in vitro and in vivo. We also speculate that SIRT1 activity is required for AMPK activation as well as the stimulatory effects of BDSW on mitochondrial biogenesis and function, although our present findings indicate only some indirect evidence for the association between SIRT1 and AMPK in the regulatory mechanism of BDSW on mitochondrial function. As shown in Fig 5B, NA completely inhibited the phosphorylation of both AMPK and Sirt1. In contrast, CC completely inhibited the phosphorylation of AMPK but not Sirt1. Therefore, further studies are needed to investigate what is essential for the stimulatory effect of BDSW on AMPK and SIRT1 activity as well as mitochondrial biogenesis and function, and clarify the regulatory mechanism by BDSW on AMPK and SIRT1 activity. In conclusion, our findings suggest that BDSW effectively stimulates mitochondrial biogenesis and function, and may provide a possible therapeutic approach for preventing or treating diabetes and obesity.
Supporting Information
(PDF)
(PDF)
Data Availability
All relevant data are within the paper and its supporting information files.
Funding Statement
This research was supported by a grant (technical development for supporting the DSW industry) from the Ministry of Oceans and Fisheries of the Republic of Korea.
References
- 1. Aguer C, Harper ME. Skeletal muscle mitochondrial energetics in obesity and type 2 diabetes mellitus: endocrine aspects (2012) Best Pract Res Clin Endocrinol Metab 26: 805–819. 10.1016/j.beem.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 2. Vijgen GH, Bouvy ND, Hoeks J, Wijers S, Schrauwen P, van Marken Lichtenbelt WD. (2013) Impaired skeletal muscle mitochondrial function in morbidly obese patients is normalized one year after bariatric surgery. Surg Obes Relat Dis 9: 936–941. 10.1016/j.soard.2013.03.009 [DOI] [PubMed] [Google Scholar]
- 3. van de Weijer T, Sparks LM, Phielix E, Meex RC, van Herpen NA, Hesselink MK, et al. (2013) Relationships between mitochondrial function and metabolic flexibility in type 2 diabetes mellitus. PLoS One 8: e51648 10.1371/journal.pone.0051648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scarpulla RC, Vega RB, Kelly DP (2012) Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol Metab 23: 459–466. 10.1016/j.tem.2012.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hsieh CJ, Weng SW, Liou CW, Lin TK, Chen JB, Tiao MM, et al. (2011) Tissue-specific differences in mitochondrial DNA content in type 2 diabetes. Diabetes Res Clin Pract 92(1):106–110. 10.1016/j.diabres.2011.01.010 [DOI] [PubMed] [Google Scholar]
- 6. Bogacka I, Xie H, Bray GA, Smith SR (2005) Pioglitazone induces mitochondrial biogenesis in human subcutaneous adipose tissue in vivo. Diabetes 54: 1392–1399. [DOI] [PubMed] [Google Scholar]
- 7. Fujisawa K, Nishikawa T, Kukidome D, Imoto K, Yamashiro T, Motoshima H, et al. (2009) TZDs reduce mitochondrial ROS production and enhance mitochondrial biogenesis. Biochem Biophys Res Commun 379: 43–48. 10.1016/j.bbrc.2008.11.141 [DOI] [PubMed] [Google Scholar]
- 8. Sheu MJ, Chou PY, Lin WH, Pan CH, Chien YC, Chung YL, et al. (2013) Deep sea water modulates blood pressure and exhibits hypolipidemic effects via the AMPK-ACC pathway: an in vivo study. Mar Drugs 11: 2183–2202. 10.3390/md11062183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bak JP, Kim YM, Son J, Kim CJ, Kim EH (2012) Application of concentrated deep sea water inhibits the development of atopic dermatitis-like skin lesions in NC/Nga mice. BMC Complement Altern Med 12: 108 10.1186/1472-6882-12-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Katsuda S, Yasukawa T, Nakagawa K, Miyake M, Yamasaki M, Katahira K, et al. (2008) Deep-sea water improves cardiovascular hemodynamics in Kurosawa and Kusanagi-Hypercholesterolemic (KHC) rabbits. Biol Pharm Bull 31: 38–44. [DOI] [PubMed] [Google Scholar]
- 11. Ha BG, Park JE, Shin EJ, Shon YH (2014) Modulation of glucose metabolism by balanced deep-sea water ameliorates hyperglycemia and pancreatic function in streptozotocin-induced diabetic mice. PLoS One 9: e102095 10.1371/journal.pone.0102095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ha BG, Shin EJ, Park JE, Shon YH (2013) Anti-diabetic effect of balanced deep-sea water and its mode of action in high-fat diet induced diabetic mice. Mar Drugs 11: 4193–4212. 10.3390/md11114193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ha BG, Park JE, Shin EJ, Shon YH (2014) Effects of balanced deep sea water on adipocyte hypertrophy and liver steatosis in high-fat diet-induced obese mice. Obesity (Silver Spring) 22: 1669–1678. 10.1002/oby.20740 [DOI] [PubMed] [Google Scholar]
- 14. Cheng Z, Almeida FA (2014) Mitochondrial alteration in type 2 diabetes and obesity. Cell Cycle 13: 890–897. 10.4161/cc.28189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Standard methods for the examination of water and wastewater 2–37 (1998) #2340 hardness. 20th ed. American Public Health Association/American Water Works Association/Water Environment Federation, Washington, DC, USA
- 16. Hiraoka Y, Matsuoka T, Ohno M, Nakamura K, Saijo S, Matsumura S, et al. (2014) Critical roles of nardilysin in the maintenance of body temperature homoeostasis. Nat Commun 5:3224 10.1038/ncomms4224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andreux PA, Houtkooper RH, Auwerx J (2013) Pharmacological approaches to restore mitochondrial function. Nat Rev Drug Discov 12: 465–483. 10.1038/nrd4023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, et al. (2012) SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab 15: 675–690. 10.1016/j.cmet.2012.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higashida K, Kim SH, Jung SR, Asaka M, Holloszy JO, Han DH. (2013) Effects of resveratrol and SIRT1 on PGC-1alpha activity and mitochondrial biogenesis: a reevaluation. PLoS Biol 11: e1001603 10.1371/journal.pbio.1001603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ruderman NB, Xu XJ, Nelson L, Cacicedo JM, Saha AK, Lan F, et al. (2010) AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab 298: E751–760. 10.1152/ajpendo.00745.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liesa M, Shirihai OS (2013) Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab 17: 491–506. 10.1016/j.cmet.2013.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wenz T (2013) Regulation of mitochondrial biogenesis and PGC-1alpha under cellular stress. Mitochondrion 13: 134–142. 10.1016/j.mito.2013.01.006 [DOI] [PubMed] [Google Scholar]
- 23. Chomentowski P, Coen PM, Radiková Z, Goodpaster BH, Toledo FG (2011) Skeletal muscle mitochondria in insulin resistance: differences in intermyofibrillar versus subsarcolemmal subpopulations and relationship to metabolic flexibility. J Clin Endocrinol Metab 96(2):494–503. 10.1210/jc.2010-0822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sparks LM, Ukropcova B, Smith J, Pasarica M, Hymel D, Xie H, et al. (2009) Relation of adipose tissue to metabolic flexibility. Diabetes Res Clin Pract 83(1):32–43. 10.1016/j.diabres.2008.09.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gao AW, Canto C, Houtkooper RH (2014) Mitochondrial response to nutrient availability and its role in metabolic disease. EMBO Mol Med 6: 580–589. 10.1002/emmm.201303782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prior SJ, Ryan AS, Stevenson TG, Goldberg AP (2014) Metabolic inflexibility during submaximal aerobic exercise is associated with glucose intolerance in obese older adults. Obesity (Silver Spring) 22: 451–457. 10.1002/oby.20609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee S, Rivera-Vega M, Alsayed HM, Boesch C, Libman I (2014) Metabolic inflexibility and insulin resistance in obese adolescents with non-alcoholic fatty liver disease. Pediatr Diabetes Apr 23. 10.1111/pedi.12141 [DOI] [PMC free article] [PubMed]
- 28. Martin SD, McGee SL (2014) The role of mitochondria in the aetiology of insulin resistance and type 2 diabetes. Biochim Biophys Acta 1840: 1303–1312. 10.1016/j.bbagen.2013.09.019 [DOI] [PubMed] [Google Scholar]
- 29. Galgani JE, Moro C, Ravussin E (2008) Metabolic flexibility and insulin resistance. Am J Physiol Endocrinol Metab 295: E1009–1017. 10.1152/ajpendo.90558.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yuzefovych LV, Musiyenko SI, Wilson GL, Rachek LI (2013) Mitochondrial DNA damage and dysfunction, and oxidative stress are associated with endoplasmic reticulum stress, protein degradation and apoptosis in high fat diet-induced insulin resistance mice. PLoS One 8: e54059 10.1371/journal.pone.0054059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huskisson E, Maggini S, Ruf M (2007) The role of vitamins and minerals in energy metabolism and well-being. J Int Med Res 35: 277–289. [DOI] [PubMed] [Google Scholar]
- 32. Ames BN, Atamna H, Killilea DW. (2005) Mineral and vitamin deficiencies can accelerate the mitochondrial decay of aging. Mol Aspects Med 26(4–5):363–78. [DOI] [PubMed] [Google Scholar]
- 33. Volpe SL (2013) Magnesium in disease prevention and overall health. Adv Nutr 4: 378S–383S. 10.3945/an.112.003483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mostafavi E, Nakhjavani M, Ghazizadeh Z, Barakati H, Mirmiranpour H, Safari R. (2013) Protective role of calcium ion against stress-induced osmotic fragility of red blood cells in patients with type 2 diabetes mellitus. Clin Hemorheol Microcirc 53: 239–245. 10.3233/CH-2012-1546 [DOI] [PubMed] [Google Scholar]
- 35. Lee H, Lee J, Hwang SS, Kim S, Chin HJ, Han JS, et al. (2013) Potassium intake and the prevalence of metabolic syndrome: the Korean National Health and Nutrition Examination Survey 2008–2010. PLoS One 8: e55106 10.1371/journal.pone.0055106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rees K, Hartley L, Day C, Flowers N, Clarke A, Stranges S. (2013) Selenium supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 1: CD009671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kumar SD, Vijaya M, Samy RP, Dheen ST, Ren M, Watt F, et al. (2012) Zinc supplementation prevents cardiomyocyte apoptosis and congenital heart defects in embryos of diabetic mice. Free Radic Biol Med 53: 1595–1606. 10.1016/j.freeradbiomed.2012.07.008 [DOI] [PubMed] [Google Scholar]
- 38. Clark TA, Deniset JF, Heyliger CE, Pierce GN (2014) Alternative therapies for diabetes and its cardiac complications: role of vanadium. Heart Fail Rev 19: 123–132. 10.1007/s10741-013-9380-0 [DOI] [PubMed] [Google Scholar]
- 39. Huang HY, Caballero B, Chang S, Alberg A, Semba R, Schneyer C, et al. (2006) Multivitamin/mineral supplements and prevention of chronic disease. Evid Rep Technol Assess (Full Rep) 139: 1–117. [PMC free article] [PubMed] [Google Scholar]
- 40. Wolf FI, Torsello A, Fasanella S, Cittadini A (2003) Cell physiology of magnesium. Mol Aspects Med 24(1–3):11–26. [DOI] [PubMed] [Google Scholar]
- 41. Killilea DW, Ames BN (2008) Magnesium deficiency accelerates cellular senescence in cultured human fibroblasts. Proc Natl Acad Sci U S A 105(15):5768–5773. 10.1073/pnas.0712401105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bussière FI, Gueux E, Rock E, Girardeau JP, Tridon A, Mazur A, et al. (2002) Increased phagocytosis and production of reactive oxygen species by neutrophils during magnesium deficiency in rats and inhibition by high magnesium concentration. Br J Nutr 87(2):107–113. [DOI] [PubMed] [Google Scholar]
- 43. Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS (2004) Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol 287(4):C817–833. [DOI] [PubMed] [Google Scholar]
- 44. Yeh TS, Ho JD, Yang VW, Tzeng CR, Hsieh RH (2005) Calcium stimulates mitochondrial biogenesis in human granulosa cells. Ann N Y Acad Sci 1042:157–162. [DOI] [PubMed] [Google Scholar]
- 45. Wright DC, Geiger PC, Han DH, Jones TE, Holloszy JO (2007) Calcium induces increases in peroxisome proliferator-activated receptor gamma coactivator-1alpha and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J Biol Chem 282(26):18793–18799. [DOI] [PubMed] [Google Scholar]
- 46. Mendelev N, Mehta SL, Idris H, Kumari S, Li PA (2012) Selenite stimulates mitochondrial biogenesis signaling and enhances mitochondrial functional performance in murine hippocampal neuronal cells. PLoS One 7(10):e47910 10.1371/journal.pone.0047910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Panee J, Stoytcheva ZR, Liu W, Berry MJ (2007) Selenoprotein H is a redox-sensing high mobility group family DNA-binding protein that up-regulates genes involved in glutathione synthesis and phase II detoxification. J Biol Chem 282(33):23759–23765. [DOI] [PubMed] [Google Scholar]
- 48. Hui K, Yang Y, Shi K, Luo H, Duan J, An J, et al. , (2014) The p38 MAPK-regulated PKD1/CREB/Bcl-2 pathway contributes to selenite-induced colorectal cancer cell apoptosis in vitro and in vivo. Cancer Lett 354(1):189–199. 10.1016/j.canlet.2014.08.009 [DOI] [PubMed] [Google Scholar]
- 49. Yoon SO, Kim MM, Park SJ, Kim D, Chung J, Chung AS (2002) Selenite suppresses hydrogen peroxide-induced cell apoptosis through inhibition of ASK1/JNK and activation of PI3-K/Akt pathways. FASEB J 16(1):111–3. [DOI] [PubMed] [Google Scholar]
- 50. Zhang W, Zhang R, Wang T, Jiang H, Guo M, Zhou E, et al. , (2014) Selenium inhibits LPS-induced pro-inflammatory gene expression by modulating MAPK and NF-κB signaling pathways in mouse mammary epithelial cells in primary culture. Inflammation 37(2):478–485. 10.1007/s10753-013-9761-5 [DOI] [PubMed] [Google Scholar]
- 51. Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, et al. , (2012) SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab 15(5):675–90 10.1016/j.cmet.2012.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its supporting information files.