Abstract
We analyzed the Hg concentration, and δ¹³C and δ15N values in the scalp hair of residents from seven countries; Vietnam, New Zealand, Spain, the USA, South Korea, Brazil and Japan. Relationships among the data in each country and among the seven countries were then examined. The highest Hg concentration as well as the highest or higher δ15N value in each country was found in the hair of a heavy marine fish-eater, whereas the lowest Hg concentration and δ15N value were found in the hair of a vegetarian or non (marginal)-fish eater. Hg concentrations were positively correlated with the δ15N values in each country, and increased markedly in samples with δ15N values exceeding 9.0 ‰, probably due to fish consumption. The highest Hg concentration could be found in sample, with a δ¹³C value between -19 and -18‰, probably reflecting the δ¹³C value of the marine food web.
Introduction
Mercury (Hg) is distributed throughout the environment via both natural and anthropogenic processes, and Hg toxicity has resulted in widespread public health concerns. The main source of human Hg intake is the consumption of fish and other marine animals contaminated with methyl mercury [1–5]. As Hg accumulation in marine animals increases with increased trophic level, predators accumulate higher levels of Hg [6]-[8].
Hg concentration in scalp hair is the preferred marker for evaluating Hg exposure over a period of several weeks or months, as the hair to blood ratio in humans has been estimated to be about 250:1 [9]. The World Health Organization (WHO) has reported that an Hg level of 50μg/g in human hair corresponds to the “no observed adversary effect level” (NOAEL) for methyl mercury in adults, as determined from neurotoxicological data [9]. In 2003, the Food and Agriculture Organization (FAO)/WHO Joint Expert Committee on Food Additives (JECFA) lowered its guideline value for the provisional tolerable weekly intake (PTWI) of methyl mercury from 3.3 μg/kg-bw/week to 1.6 μg/kg-bw/week [10]. The previous and revised PTWIs correspond to hair Hg levels of 5.0 and 2.2 μg/g, respectively [11]. Most heavy eaters of predatory fish and marine mammals exceed this revised PTWI for methyl mercury [11–13].
Stable isotope analysis has been used as a tool to obtain information on feeding ecology. The stable isotope ratio of nitrogen (δ15N) is used to estimate the trophic level of an organism, while the stable isotope ratio of carbon (δ13C) is used to estimate the relative contribution to the diet of potential primary sources [14]: a 3 ~ 4 ‰ enrichment in δ15N has been shown to occur between the muscle of predators and their prey in wild animals, whereas only a small enrichment is generally found in the δ13C value [15], [16]. This enrichment in δ15N is the mean value of a significant spread of δ15N values between particular consumer-diet pairs. The trophic discrimination values of δ13C and δ15N were reviewed by McCutchan et al. [17]. Many researchers have reported high levels of δ15N in the muscle of marine mammals and predatory fish, reflecting the high trophic positions of the species [7], [18], [19].
Terrestrial plants following the C3 photosynthesis cycle show significantly depleted 13C values (about -26 ‰) compared to C4 plants (about -13 ‰) [14]. A large amount of basic human food as well as feed for domestic animals is derived from C3 plants (wheat, barley, soy, potatoes, rice, beans, sugar beets, grass, etc.), although a few species of C4 plants (maize, sugar cane, millet, etc.) are dominant in large regions. In contrast, the δ13C values in marine phytoplankton and sea grasses are about -22 ‰ and between -15 and -3 ‰, respectively, and the δ13C values in coastal fauna are generally higher than those in pelagic fauna [14]. The shift from coastal or benthic feeding to pelagic feeding, due to the growth of fish, leads to decreased δ13C and δ15N values [14], [20–22]. To our knowledge, the δ13C values in the marine fish reported to date have ranged from -20 to -14 ‰: the δ13C values of pelagic fish, such as tuna, swordfish, and sharks, were in the range of -20 to -16 ‰, and those of coastal fish were slightly higher in range, mainly reflecting the trophic positions and the inhabiting areas [7], [19], [23].
Scalp hair and nails are convenient human matrices, as they are stable and easy to collect, transport and store compared to other matrices (e.g., blood and urine). According to previous reports [24–29], δ13C in scalp hair samples collected from Europe, Asia, North and South America, and Oceania ranges from -22 to -15 ‰, values which fall between the levels found in C3 plants (about -26 ‰) and C4 plants (about -13 ‰) and similar in range to the levels observed in predatory fish (between -20 and -16 ‰). On the other hand, the level of δ15N in scalp hair has been reported to be between 6 and 13 ‰ [24–29], which fall between the levels found in plants (usually between 0 and 5 ‰) [3] and those in marine mammals and predatory fish (generally higher than 12 ‰). As the δ13C and δ15N values in scalp hair are similar to those in the fingernails (enriched in δ13C by ~ 0.2 ‰ and depleted in δ15N by ~ 0.6 ‰ compared with fingernail values [30]), the determination of the δ13C and δ15N values in scalp hair and fingernail samples provides information on the dietary habits and dietary changes from a C3 plant-based diet to a C4 plant-enriched diet as well as from the consumption of animal products to marine products [31].
The consumption of marine products and C4 plant-enriched diets has been found to increase the δ15N and δ13C values in scalp hair and fingernails, respectively [3], [32]. The enrichment of δ13C and δ15N values in the scalp hair from diet has been reported to be 2.4 ‰ and 4.5 ‰, respectively [33], which is in contrast with the small δ13C enrichment observed in the muscle of wild animals. Macko et al. [33] estimated the dietary habit of ancient humans based on the δ13C and δ15N values of C3 plant-vegetarians, C4 plant-vegetarians and marine products, as well as the enrichments of δ13C and δ15N values in scalp hair that result from the diet. Furthermore, the determination of δ13C and δ15N provides information on geographical location and origin, eating disorders and forensic parameters [3], [30], [32], [34].
The bioaccumulation of methyl mercury in marine animals mainly depends on their trophic level, and positive correlations between δ15N values and Hg concentrations in animal muscle have been reported [6–8]. However, the relationships between δ13C values and Hg concentrations in wild animals are unclear, probably as δ13C is not greatly enriched and the δ13C values in plants and marine animals are distributed across a wide range. We previously analyzed the δ15N and δ13C values as well as the Hg concentration in the scalp hair of whale meat-eaters and non-eaters in Japan, and reported positive correlations between the δ15N value and the Hg concentration, and between the δ13C and δ15N values in the hair of whale meat-eaters [29]. To our knowledge, in spite of many reports concerning δ15N and δ13C values in scalp hair and many reports concerning Hg concentrations in hair, our previous report was the first to analyze the Hg concentration and δ15N and δ13C values in the same hair samples. Further study is still, however, necessary to identify the δ15N and δ13C values and Hg concentration in the scalp hair of heavy fish-eaters.
We collected scalp hair samples from fish-eaters and non-eaters from Vietnam, New Zealand, Spain, South Korea, the USA, Brazil and Japan, and analyzed the Hg concentrations, and δ13C and δ15N values in the hair samples. We compared the data among the seven countries as well as with the data for whale-meat eaters reported previously [29], and investigated the relationships among the Hg concentration, δ15N and δ13C values, as well as the frequency of fish consumption.
Materials and Methods
Ethic statement
This research project with consenting procedures was approved by the Human Research Ethics Committee of the Graduate School of Pharmaceutical Sciences, Health Sciences University of Hokkaido. Hair donors provided their written informed consent to participate in this study. The principles of Declaration of Helsinki were considered in each part of this study.
Sampling of scalp hair
Scalp hair samples (about 5 ~ 10 cm in length and 0.5 ~ 1.0 g) from healthy donors were once collected in seven countries, Vietnam, New Zealand, Spain, South Korea, the USA, Brazil and Japan, with the assistance of local collaborators after obtaining written informed consent (Table 1). At the time of hair collection, a simple questionnaire for the collection of data in relation to age, frequency of fish and marine product consumption (per week or day), species of favorite fish, and the daily staple food (rice, wheat, potato, corn, etc.). Eighteen hair samples were collected in Hai Phon and Ha Long, Vietnam, in December 2009. Twenty-one hair samples were collected in Christchurch and Auckland, New Zealand, in February 2010. Twenty-eight and thirty-three samples were collected from Spain (Barcelona) and South Korea (Seoul and its environs) in July-October and September-October 2010, respectively. Twenty-two hair samples were collected in Honolulu, Hawaii (USA), in December 2012, and 31 samples were collected in Salvador, Brazil, in February 2011. The hair donors from each country included heavy fish-eaters who worked as fishmongers, in seafood (Japanese) restaurants or as fishermen. We looked at the fish markets near the hair sampling area in each country to confirm and compare the responses to the questionnaire in relation to the species of fish consumed and consumption levels of fish products.
Table 1. Number of hair samples from each of the seven countries and the analytical results.
| No. of samples | Age | Hg (μg/g) | δ15N (‰) | δ13C (‰) | Frequency | |
|---|---|---|---|---|---|---|
| Vietnam | 18 | 39±14 | 1.6±1.8 | 9.5±0.7 | -20.7±0.7 | 3.6±2.0 |
| (m = 11, f = 7) | 21–61 | 0.4–6.9 | 8.2–10.9 | -22.2 to -19.4 | 0–7 | |
| New Zealand | 21 | 27±7.5 | 2.0±2.5 | 9.3±0.7 | -20.2±0.5 | 2.2±2.4 |
| (m = 7, f = 14) | 15–45 | 0.2–10.1 | 7.6–10.7 | -21.3 to- 19.6 | 0–10 | |
| Spain | 28 | 37±14 | 7.9±12.6 | 9.7±0.6 | -19.8±0.5 | 3.7±2.2 |
| (m = 9, f = 19) | 20–72 | 0.1–42.1 | 8.7–10.8 | -20.3 to -18.8 | 1–8 | |
| USA | 22 | 37±20 | 3.2±5.0 | 9.3±0.6 | -18.3±0.9 | 7.8±6.8 |
| (m = 12, f = 10) | 6–65 | 0.2–24.1 | 7.9–10.5 | -20.1 to -16.6 | 0–21 | |
| South Korea | 33 | 42±14 | 0.8±1.7 | 9.9±0.9 | -18.3±0.8 | 1.4±2.0 |
| (m = 17, f = 16) | 20–77 | 0.1–7.2 | 9.0–12.1 | -19.6 to -16.3 | 0–7 | |
| Brazil | 31 | 33±18 | 2.2±3.1 | 10.3±0.8 | -16.8±0.7 | 3.2±2.9 |
| (m = 14, f = 17) | 1–70 | 0.4–15.3 | 8.7–12.0 | -18.3 to -15.8 | 0–14 | |
| Japan | 90 | 37±22 | 2.6±2.9 | 9.3±0.5 | -18.9±0.6 | N.D |
| (m = 38, f = 52) | 7–88 | 0.4–18.3 | 8.6–11.4 | -20.2 to -17.6 | ||
| Whale meat-eaters a | 45 | 60±18 | 23.415.5 | 10.2±0.6 | -18.4 ±0.5 | N.D |
| (m = 30, f = 15) | 15–87 | 3.8–67.2 | 8.9–11.4 | -19.5 to -17.2 |
The sample numbers of males (m) and females (f) are shown in parentheses.The analytical data are shown as the mean ± SD with range.
aQuoted from Endo et al. (23).
Scalp hair samples from 90 donors living in Hokkaido, Aomori, Miyagi and Iwate Prefectures and the Tokyo Metropolitan area, Japan, were collected during November 2009 and September 2013. Most of these Japanese donors consumed fish products, but none consumed products from either toothed whales or dolphins.
All hair samples were packed in polyethylene bags and stored at room temperature until analysis. Prior to analysis, all hair samples were washed twice in a 2:1 chloroform/methanol mixture to remove lipids and other surface contaminants. Washed samples were air-dried and minced by scissors as finely as possible. Table 1 lists the analytical data for the seven countries as well as the data for donors in Japan who ate whale products from toothed whales and dolphins, as reported previously [29].
Chemical analyses
Total mercury (Hg) concentration in the minced hair samples was determined using a flameless atomic absorption spectrophotometer (Hiranuma Sangyo Co. Ltd., HG-310, Ibaraki, Japan) after digestion by a mixture of HNO3, HCO4 and H2SO4 [35]. Human hair No. 13, a certified reference material from NIES (Japan), was used as an analytical quality control for Hg (4.43 ± 0.20 μg/g), and the recovery of Hg was 96 ± 3% (n = 3).
The stable isotope ratios of carbon and nitrogen (δ13C and δ15N) in the minced hair samples were analyzed using a mass spectrometer (Delta S, Finnigan MAT, Bremen, Germany) coupled with an elemental analyzer (EA1108, Fisons, Roano, Milan, Italy) held in the Center for Ecological Research, Kyoto University, as described previously [36]. δ13C and δ15N were calculated based on the two-point anchoring method using CERKU-1, -2 and -5, certified by the Center for Ecology Research, Kyoto University [37].
Statistical analyses
Data were expressed as the mean ± SD. Data were analyzed by Pearson’s coefficient test and multiple linear regression analysis, using the Statcel 2 program, with a value of p<0.05 considered to be significant.
Results
Analytical results for hair samples from the seven countries
Table 1 summarizes the age, frequency of fish consumption (per week), and analytical data for Hg concentration, and δ13C and δ15N values in the scalp hair of residents of the seven countries. The average Hg concentration as well as δ13C and δ15N values in the male hair from each country were higher, although not significantly, than those in female hair. Table 1 shows the total data for males and females. The data for whale meat-eaters are taken from a previous report [29].
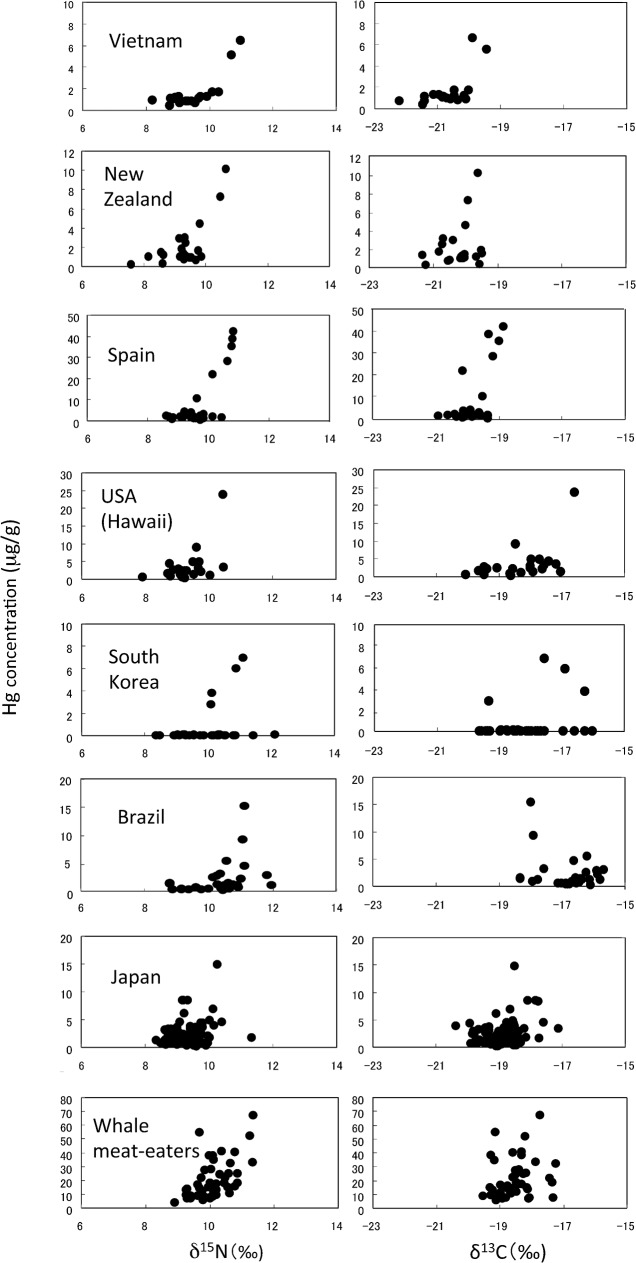
Fig 1 shows the relationships between Hg concentration and δ15N or δ13C value in the donors from each country and in the whale meat-eaters. The Hg concentration in the donors from each country as well as in the whale-meat eaters increased markedly with δ15N values exceeding 9.0 ‰. The seven countries were classified in three groups according to the average of δ13C and the distribution pattern of δ13C: the first group included Vietnam, New Zealand and Spain (below -19 ‰), the second group included the USA, Japan and the whale meat-eaters (-21 to -17 ‰), and the third group included South Korea and Brazil (-20 to -15 ‰).
Fig 1. The relationship between Hg concentration and the δ13C or δ15N value in hair samples from seven countries and Japanese whale meat-eaters.
Samples from Vietnam
The highest and second highest concentrations of Hg (6.9 and 5.7 μg/g) were found in the hair samples from young brothers who worked as fisherman (21 and 22 years). The δ13C and δ15N values found in these brothers were -19.9 and 10.9 ‰, and -19.4 and 10.6 ‰, respectively, and these values were the highest and second highest found among the samples from Vietnam. The lowest concentration of Hg (0.4 μg/g) as well as the lowest value of δ13C (-22.2 ‰) and a relatively low value of δ15N (9.1 ‰) were found in a young woman (25 years) who seldom ate fish products. According to the responses to questionnaires, most of the donors sometimes ate small fish, but not predatory fish such as tuna, swordfish or shark. These predatory fish were not found in the fish markets where the donors lived.
Samples from New Zealand
The highest and second highest concentrations of Hg (10.1 and 7.2 μg/g) were found in the hair samples of a manager and cook of Japanese (seafood) restaurants (both male, aged 37 and 45 years), respectively. They also accounted for the highest and second highest values of δ13C and δ15N (-19.6 and 10.7 ‰, and -19.9 and 10.5 ‰, respectively). Both donors ate predatory fish almost every day. The lowest Hg concentration as well as δ13C and δ15N values were found in a 43-year-old vegetarian female (0.2 μg/g, -21.3 ‰ and 7.6 ‰, respectively). The average age of the New Zealand donors was the youngest among the seven counties investigated, and the range of ages was the narrowest (Table 1).
Samples from Spain
The three highest concentrations of Hg (42.1, 38.6 and 35.2 μg/g) among the samples from Spain were found in the hair from a male fishermen and two male heavy tuna-eaters, all aged in their 50s. The three highest values of δ13C and δ15N were also found in these donors (-18.8 and 10.8 ‰, -19.3 and 10.8 ‰, and -19.0 and 10.8 ‰). The lowest concentration of Hg (0.1 μg/g) was found in the hair of a female (70 years) who did not eat fish products. This donor also showed a relatively low value of δ15N (9.8 ‰) and an intermediate value of δ13C (-19.4 ‰).
Samples from the USA (Hawaii)
The highest concentration of Hg (24.1 μg/g) among the samples from the USA was found in the sample from a male fishmonger (40 years) who ate marine products at every meal and predatory fish almost every day. This donor also had the highest values of δ13C and δ15N (-16.6 and 10.5 ‰). More than half of the donors ate predatory fish at least once a month and showed Hg concentrations above 2.0 μg/g. The lowest concentration of Hg (0.2 μg/g) was found in the hair of a male donor (47 years) who scarcely ate fish. This donor also showed intermediate values of δ13C and δ15N (-18.61 ‰ and 9.26 ‰, respectively) in comparison with the other donors. The lowest δ13C and δ15N values (-20.1 and 7.9 ‰) were found in the hair from a female donor (56 years) who did not eat fish products, and the Hg concentration in this donor was 0.4 μg/g.
Samples from South Korea
The highest and second highest concentration of Hg (7.2 and 6.3 μg/g) among the South Korean samples were found in the hair of two male managers of Japanese (seafood) restaurants (60 and 57 years) who ate fish products almost everyday. These donors also showed relatively high values of δ15N (11.1 and 10.9 ‰) and δ13C (-17.6 and -16.9 ‰). The average Hg and frequency of fish consumption were the lowest among the seven countries investigated. The Hg concentrations in most of the South Korean samples were below 0.5 μg/g. The δ15N and δ13C values in some donors exceeded 10 ‰ and -17 ‰, but the Hg concentrations in these donors were trace, which is in contrast with the Hg distribution pattern of the other countries (Fig 1). According to the responses to the questionnaires, most of the donors sometimes ate small fish, but not predatory fish such as tuna, swordfish or shark. We seldom found predatory fish in the markets where the donors shopped.
Samples from Brazil
The highest concentration of Hg (15.3 μg/g) was found in the hair of a female fishmonger (60 years) who frequently ate fish products. This donor also showed a relatively high value of δ15N (11.1 ‰) and a relatively low value of δ13C (-18.0 ‰) compared to the other samples from Brazil. Some of the donors occasionally ate predatory fish, particularly shark, and high concentrations of Hg were found in their hair. The lowest concentration of Hg (0.4 μg/g) was found in the hair sample from a female donor (20 years) who seldom ate fish products. This donor also showed the lowest value of δ15N (8.7 ‰) and an intermediate value of δ13C (-16.9 ‰). The average δ13C (-16.8 ‰) and δ15N (10.3 ‰) values from the Brazilian samples were the highest among the seven counties investigated, although the average Hg concentration was at an intermediate value (Table 1).
We analyzed the δ13C and δ15N values and Hg concentration in shark muscle randomly purchased from supermarkets. The δ13C values from five shark samples were in the range of -17.5 to -15.2 ‰, the δ15N values were in the range of 9.8 to 13.4 ‰, and the Hg concentrations were in the range of 0.4 to 3.02 μg/g. The δ13C values found in the shark muscle were negatively correlated with the Hg concentrations (γ = 0.941, p<0.05).
Samples from Japan
The highest concentration of Hg (18.3 μg/g) among the Japanese samples was found in the hair from a male fishmonger (80 years) who frequently ate predatory fish. This donor also showed relatively high values of δ13C (-18.1 ‰) and δ15N (10.4 ‰). The lowest concentration of Hg (0.4 μg/g) was found in the hair from a female donor, aged 35 years, who seldom ate fish products. She also showed intermediate values of δ13C (-19.0 ‰) and δ15N (9.6 ‰) compared with the other Japanese samples.
According to our previous report [29], the average value of δ15N in whale meat-eaters was significantly higher than those in non-eaters (shown in Table 1). The highest Hg concentration among the whale meat-eaters was 67.2 μg/g, with the second highest value of δ15N being 11.3 ‰. The δ15N and δ13C values of the cetaceans and predatory fish consumed were between 9 and13 ‰ and between -16 and -18 ‰, respectively. The average concentration of Hg in the hair of whale meat-eaters (23.4 ± 15.5 μg/g, n = 45) was about nine times higher than that of non-eaters (2.6 ± 2.9 μg/g, n = 90). The average age of whale meat-eaters (60 ± 18 yr, n = 45) was significantly older than that of non-eaters (37 ± 22 yr, n = 90) and of the hair sample donors from other countries.
Correlations among δ13C and δ15N values and Hg concentration, and between Hg concentration and age or frequency of fish consumption in the samples from each country
Table 2 lists the correlation coefficients (γ) among Hg concentration, δ15N and δ13C values, and between Hg concentration and age or frequency of fish consumption per week.
Table 2. Correlation coefficients among Hg concentration, andδ13C andδ15N values, and between age and Hg concentration shown in Table 1.
| Number of samples | Hg vs δ15N | Hg vs δ13C | δ13C vs δ15N | Age vs Hg | Hg vs frequency | |
|---|---|---|---|---|---|---|
| Vietnam | 18 | 0.748* | 0.607* | 0.409 | 0.410 a | 0.592* |
| New Zealand | 21 | 0.677* | 0.261 | 0.311 | 0.396 | 0.636* |
| Spain | 28 | 0.763* | 0.584* | 0.692* | 0.432* | 0.746* |
| USA | 22 | 0.518* | 0.493* | 0.620* | 0.122 b | 0.437* |
| South Korea | 33 | 0.321 | 0.313 | 0.638* | 0.699* | 0.785* |
| Brazil | 31 | 0.362* | - 0.448* | 0.284 | 0.326 | 0.279 |
| Japan | 90 | 0.372* | 0.248* | 0.305* | 0.406* | N.D |
| Whale meat-eaters c | 45 | 0.549* | 0.202 | 0.422* | 0.227 | N.D |
*Significant correlation (p<0.05)
aTwo samples with Hg concentrations of 6.9 and 5.7 μg/g were deleted (n = 16).
bOne sample with an Hg concentration of 24.1 μg/g was deleted (n = 21).
cQuoted from Endo et al. (23)
The δ13C values in the hair samples from Spain, South Korea, the USA and Japan were positively correlated with their δ15N values (p<0.05), but no significant positive correlation was found in samples from Vietnam, New Zealand and Brazil.
The Hg concentrations in the hair samples from Vietnam, New Zealand, Spain, the USA, Brazil and Japan were positively correlated with their δ15N values (p<0.05), but this correlation was not significant in samples from South Korea (p<0.10). Similar or slightly higher correlations (γ) were found between the logarithmic concentrations of Hg and the δ15N values.
It is worthy of note that the δ13C value in the Brazilian samples was negatively correlated with the Hg concentration (p<0.05), which was in contrast with the tendency towards positive correlations found in the other countries (Table 2).
Significant positive correlations (p<0.05) were found between Hg concentration and the age of donors from Vietnam, Spain, South Korea and Japan, and positive tendencies were observed in the donors from New Zealand, the USA and Brazil (p>0.05). However, no correlation was found between the age and δ13C value or δ15N value in the combined donors from the seven countries (data not shown in Table 2).
Significant positive correlations (p<0.05) were found between the Hg concentration and frequency of fish consumption (per week) of donors from all countries except for Brazil (Table 2). Although the frequency data was not available for all Japanese donors, the Hg concentrations in nineteen donors increased significantly with increased frequency of consumption (p<0.05, data not shown in Table 2).
The Hg concentrations in the donors from the seven countries and whale meat-eaters were analyzed by multiple linear regression analysis (Table 3). This analysis showed that the Hg concentrations of the donors from all countries, except for the USA and Brazil, had significant positive correlations with at least one of parameters examined; δ15N value, δ13C value, age and frequency of fish consumption. In contrast, the Hg concentration in the donors from the USA tended to show a positive relationship with the δ13C value, whereas that from Brazil tended to show a negative relationship with the δ13C value.
Table 3. Standardized partial coefficients for the seven countries and whale meat-eaters from the multiple linear regression analysis of Hg concentration.
| Vietnam | New Zealand | Spain | USA | South Korea | Brazil | Japan | Whale meat-eaters | |
|---|---|---|---|---|---|---|---|---|
| δ15N | 0.498* | 0.385* | 0.406* | 0.286 | 0.027 | 0.232 | 0.243* | 0.527* |
| δ13C | 0.298 | 0.202 | 0.120 | 0.355 | 0.039 | -0.343 | 0.169 | -0.005 |
| Age | 0.012 | 0.453* | 0.049 | 0.168 | 0.227 | 0.268 | 0.350* | 0.128 |
| Frequency | 0.205 | 0.446* | 0.455* | 0.002 | 0.773* | 0.138 | —- | —— |
* p<0.05
Correlations among δ13C and δ15N values and Hg concentration in the combined samples from the seven countries
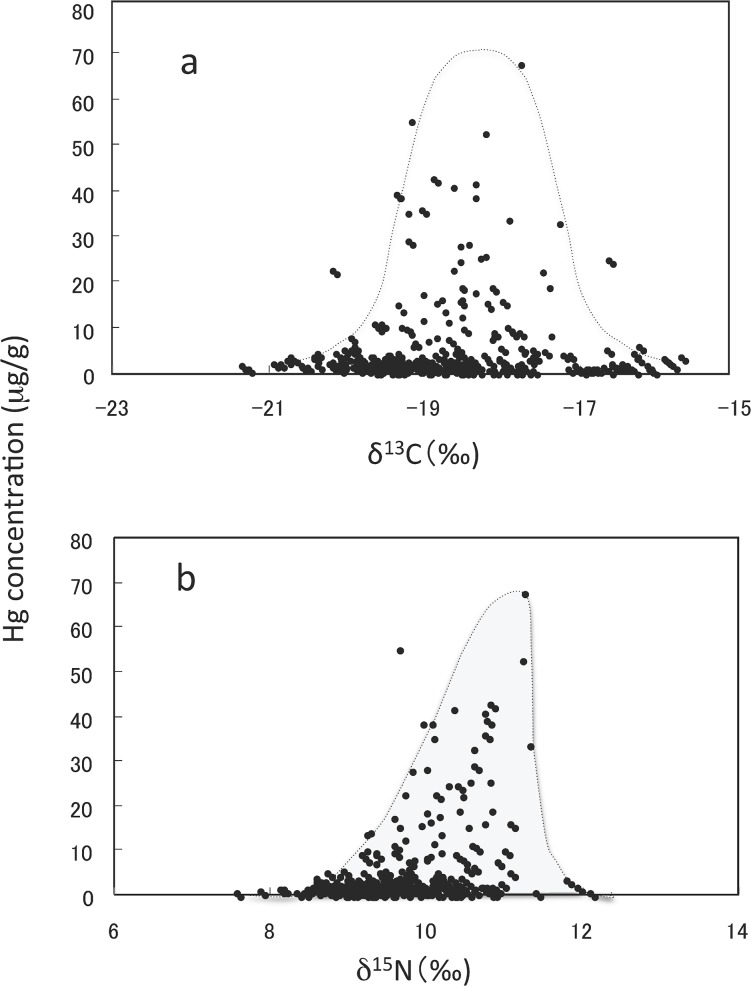
Fig 2A shows the relationship between the δ13C value and Hg concentration in the combined samples from the seven countries and the whale meat-eaters (n = 288). The peak of the curve for δ13C appears to be between -19 and -18 ‰.
Fig 2. The relationship between Hg concentration and the δ13C or δ15N value in the combined hair samples from seven countries and Japanese whale meat-eaters.
Fig 2B similarly shows the relationship between the δ15N value and Hg concentration in the combined samples from the seven countries and the whale-meat eaters (n = 288). The Hg concentration increased with δ15N values up to about 11.5 ‰, but further increases in Hg could not be confirmed as only five samples showed δ15N values exceeding 11.5 ‰ (the highest value was 12.1 ‰).
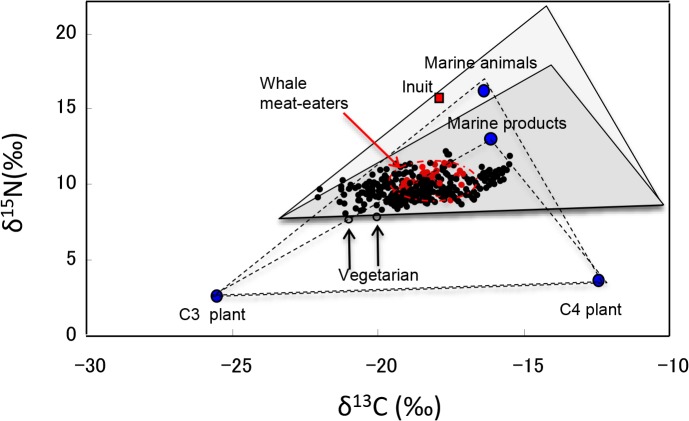
Fig 3 shows the relationship between the δ13C and δ15N values in the combined samples from the seven countries and the whale meat-eaters (n = 288). Triangles in the figure link the average values for C3 plants, C4 plants and marine products (unbroken lines) or marine animals (dashed lines). The average δ13C and δ15N values in the C3 plants, C4 plants and marine products used were -25.7 and 2.5 ‰, -12.3 and 3.5 ‰, and -16.3 and 12.5 ‰, respectively [31], and those of marine animals were -16.5 and 16.0 ‰, respectively [3]. To estimate dietary habits, these triangles were shifted toward the top right corner to correct for δ13C and δ15N enrichments [34]. The enrichment values of δ13C and δ15N from the diet to the hair used were +2.4 ‰ and +4.5 ‰, respectively [33]. The shifted triangles are shown in gray.
Fig 3. Isotope analyses of carbon and nitrogen in the combined hair samples from donors from seven countries, Japanese whale meat-eaters and the Inuit.
Most of the hair samples from the seven countries and from the whale meat-eaters were distributed over the central region from the left edge of gray triangle shown by the unbroken lines. The δ13C and δ15N values of the two vegetarians from New Zealand and the USA were located almost on the unbroken line connecting the C3 plants and C4 plants, and most of the samples from Brazil and South Korea were distributed across the central region.
Furthermore, we also estimated the dietary habits of Inuit using the δ13C and δ15N values of marine animals eaten this ethnic group. The average values of δ13C and δ15N from the hair of Inuit (-18.0 and 15.4 ‰, respectively), estimated from the fingernail data provided by Buchardt et al. [3], were distributed along the dashed line connecting the C3 plants and marine animals, which was just above those of the whale meat-eaters.
Discussion
To our knowledge, the δ13C and δ15N values in scalp hair reported in most countries are distributed between -22 ‰ and -15 ‰ and between 6 ‰ and 13 ‰, respectively [24–28]. The δ13C and δ15N values in the combined samples from the seven countries (n = 243) and in the whale meat-eaters (n = 45) shown in Table 1 were distributed widely across the reported ranges of δ13C and δ15N. The Hg concentrations in the hair samples from donors from the seven countries and from the whale meat-eaters ranged widely between 0.1 to 67.2 μg/g (n = 288). Among the donors from the seven countries (n = 243), the Hg concentration exceeded 2.2 μg/g (PTWI of methyl mercury) in 58 donors, and exceeded 5 μg/g (the previous PTWI) in 26 donors, but no donor showed an Hg concentration exceeding 50 μg/g (NOAEL). Many studies have reported similar Hg concentrations in the hair of fish-eaters [2], [3], [11], [13], [38].
The donor with the highest Hg concentration and the highest or relatively high value of δ15N in each country was a heavy fish-eater. The Hg concentrations in the hair of non-fish eaters in the present study were about 0.4 μg/g or lower (Table 1), and increased with the frequency of fish consumption (Table 2). In agreement, Petzke et al. [31] reported a positive correlation between δ15N value in hair and fish consumption, and Elhamri et al. [2] reported that the Hg concentration in the hair of non-fish eaters and marginal fish-eaters seldom exceeded 0.4 μg/g. As shown in Table 2, the Hg concentration in the hair increased with increased δ15N value. One notable result was that the Hg concentrations in the hair of donors, probably of fish-eaters, increased markedly when δ15N values exceeded 9.0 ‰, but further increases in Hg concentration when the δ15N value exceeded 11.5 ‰ could not be confirmed due to the limited data available for the higher δ15N values (Fig 2B). On the other hand, the Hg concentration in the hair could be the highest when the δ13C values ranged between -19 and -18 ‰ (Fig 2A). The δ13C values reported in marine predators ranged from -20 ‰ to -16 ‰ [6], [18], [29], while those in marine animals captured in the Brazilian coastal waters were slightly higher than this range [7]. The peak δ13C value shown in Fig 2A may reflect the δ13C values of predatory fish as well as the intermediate values of C3 plants and C4 plants (-26 ‰ and -13 ‰). The present report is the first study to clarify the relationship among Hg concentration, δ15N and δ13C values in scalp hair. One exception to the above conclusion was that the δ15N values in five donors from Brazil (two adults), South Korea (two adults) and Japan (an infant) exceeded 11.0 ‰, although the Hg concentrations in those donors were trace. The high δ15N values in breast-fed infants [24], [39] may explain the high δ15N value found in the Japanese infant. Possible reasons for the high δ15N values found in the adults are that grey hair (lacking pigment) contains undetectable amounts of Hg [40], artificial waving decreases the Hg concentration [41], or that dyeing and bleaching of scalp hair affects the analysis of δ15N and δ13C values whereas the lack of pigment does not [39]. Furthermore, the potential influence of urban pollution of δ15N [42] cannot be ruled out for the cause of high δ15N values in the donors.
The present survey included two vegetarians. The δ13C and δ15N values of the samples from vegetarian from New Zealand were -21.3 ‰ and 7.6 ‰, respectively, and from the vegetarian from the USA were -20.1 ‰ and 7.9 ‰, respectively. The Hg concentrations in those vegetarians (0.2 and 0.4 μg/g) were the lowest among the New Zealand samples and the second lowest among the USA samples. In agreement with the present data, most of the previously reported δ15N values in vegans were distributed between 6 and 8 ‰ [24], [34], [39], the Hg concentrations in the scalp hair of vegetarians were markedly lower those in omnivores [43], and the Hg concentrations in the hair of non-fish eaters seldom exceeded 0.4 μg/g [2].
The average values of δ13C and δ15N in the samples from Brazil were the highest among the seven countries. The four highest δ13C values in the Brazil samples were -15.7, -15.8, -15.8 and -15.9 ‰, but the Hg concentrations in those four samples (1.2 to 2.9 μg/g) were close to the average value for the Brazil samples. It is worthy of note that the δ13C values in the Brazil samples were negatively correlated with their Hg concentrations (p<0.05), while the δ13C values in the other countries were positively correlated (Table 2). According to the multiple linear regression analysis (Table 3), the Hg concentration more closely associated with the δ13C value, although negatively, than with the δ15N value, age or frequency of fish consumption (positively). The scatter plot of δ13C versus Hg concentration for Brazilians (Fig 1) may be typical of donors consuming a C4-plant-enriched diet. High levels of δ13C were reported in the scalp hair and fingernails of Brazilians [25], [26], although the Hg concentrations in those tissues were not determined. Interestingly, the δ13C values found in the shark muscle sold in the Brazilian markets were negatively correlated with the Hg concentrations (p<0.05). Bisi et al. [7] investigated the relationships between δ15N value and Hg concentrations and between δ13C and δ15N values in marine animals inhabiting the Brazilian coastal waters, and reported that the δ15N value was positively correlated with the logarithmic concentration of Hg. However, they did not analyze the relationship between the δ13C value and Hg concentration.
Fig 3 shows triangles generated from the average δ13C and δ15N values of C3 plants, C4 plants and marine products. The samples from Brazil and South Korea were distributed near the center of the shifted triangle (gray), and other samples were mainly distributed in the corner of the shifted triangle. These results suggest that the donors from Brazil and South Korea consume C4 plant-enriched diets whereas the donors from the other countries mainly consume C3 plant-based diets. Interestingly, the δ13C and δ15N values of the two vegetarians were located almost on the solid line connecting the C3 plants and C4 plants, which is in agreement with their dietary habits.
We estimated the dietary habits of Inuit in the same manner as described above using the δ13C and δ15N values of marine animals eaten by this ethnic group. The estimated values of δ13C and δ15N in the Inuit hair were distributed along the dashed line of the gray-shaded triangle (connecting C3 plants and marine animals). This result is in good agreement with the fact that the Inuit diet consists of marine animals and C3 plants in a 1:1 ratio. To estimate dietary habits, we used the values of δ13C and δ15N in the C3 and C4 plants and marine products reported by Huelsemann et al. [31], and the enrichments in δ13C and δ15N values in scalp hair reported by Tokui et al. [33]. Similar δ13C and δ15N values in foods were reported by Nardoto et al. [25] and similar values for the enrichments from the diet to the hair were reported by Yoshinaga et al. [44].
The estimated δ15N value in the hair of the Inuit (15.4 ‰) was markedly higher than the reported values of δ15N in the heavy fish-eaters and whale meat-eaters reported by many investigators (lower than 13 ‰), whereas the estimated δ13C value in the Inuit corresponded to the average value of the whale meat-eaters (Table 1 and Fig 3). The Hg concentrations in the hair of Inuit could not be estimated from Fig 2A because no hair samples in this survey showed a δ15N value exceeding 12.1 ‰. According to Weihe et al. [12], the average Hg concentration in the maternal hair of the Inuit is 15.5 μg/g (n = 31) and that in the hair of children is 5.5 μg/g (n = 43). The δ15N values in the marine mammals consumed by the Inuit may be higher than those by the whale-meat eaters (9 ∼ 13 ‰) [29]. Analyses of Hg concentration, δ13C and δ15N values in the same hair samples from Inuit donors in addition to their food are necessary to clarify the relationships among these parameters.
As we did not select donors at random from the general population and the sample numbers were limited, the present data may not be representative of the population of each country, except for Japan (n = 90). The average Hg concentration in the scalp hair from Spain previously reported was about 1.0 μg/g [5], which is in contrast to the present data (7.9 ± 12.6 μg/g). On the other hand, the Hg concentrations in scalp hair samples from South Korea previously reported [38], [45], [46] were similar to the present data. Unfortunately, previous studies on δ13C and δ15N in scalp hair from Spain and South Korea are lacking. The average δ13C and δ15N values in the hair from the central USA were reported to be -16.8 ± 0.8 ‰ and 8.8 ± 0.4 ‰ (n = 206), respectively [28], and the average Hg concentration was reported to be below 1.0 μg/g [1]. The present data for δ13C values in the USA (Hawaii) were slightly lower, whereas the present data forδ15N values and Hg concentrations were higher than those previously reported for the USA. The δ13C and δ15N values in the Brazilian samples were the highest among the seven countries (Table 1). In agreement with our data, a high level of δ13C was reported in the scalp hair [26] and fingernails [25] from Brazilian donors. Not only their C4 plant-enriched diet but also geographical factors may contribute to these high δ13C and δ15N values. Many research groups have reported high concentrations of Hg in scalp hair due to the consumption of Hg-contaminated freshwater fish caught off the gold mining areas in the Amazon, Brazil [47]. However, little is known about the relationship among the Hg concentration, and δ13C and δ15N values in hair samples from donors who ate freshwater fish contaminated with Hg from anthropogenic origins. Further, no information is available on the Hg concentration, and δ13C and δ15N values in the scalp hair from Vietnam and New Zealand. The present data for Hg concentrations in hair samples from Japan (n = 90) are in good agreement with those of large scale surveys in Japan (n = 8665) reported by Yasutake et al. [11]. Unfortunately, there is an absence of large scale surveys of δ13C and δ15N in hair samples taken from Japanese. The Hg concentration in the hair of Japanese donors (n = 90) was found to increase with age (Table 2), and a similar tendency was observed in the donors from the other countries. We speculate that the Hg concentration in the hair of fish-eaters tends to increase with age.
In conclusion, we analyzed hair samples from donors from seven countries. The highest Hg concentration in each county was found in the hair of heavy fish-eaters. These donors also showed the highest or relatively high values of δ15N. The lowest Hg concentrations and the lowest δ15N values were found in the hair of vegetarians or non (marginal)-fish eaters. The average δ13C and δ15N values in hair from Brazilian donors were the highest, probably reflecting not only their dietary diversity but also their geographic diversity. The highest Hg concentration in hair samples was found in the samples with a δ13C value ranging from between -19 and -18 ‰, probably reflecting the δ13C value of the predatory fish consumed. The Hg concentrations in hair samples tended to increase markedly with δ15N values exceeding 9.0 ‰, which could reflect high levels of fish consumption. Not only dietary and geographical diversities, but also the nutritional and metabolic status of the donor affect the δ13C and δ15N values in hair [32], and increased age and frequency of fish consumption increase the Hg concentration. Further well-designed studies are necessary to confirm and develop the present findings.
Acknowledgments
Determinations of δ13C and δ15N were conducted using the Joint Usage/Research Grant from the Center for Ecological Research, Kyoto University.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was partially supported by a Grant-in-Aid for Scientific Research (C) (No. 24614012 O. K.) and a Grant-in-Aid for Scientific Research (B) (No. 24310049 K. H.) from Japan Society for the Promotion of Science. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Park D, Johnson MA (2006) Awareness of fish advisories and mercury exposure in women of childbearing age. Nutrition Reveiw 64: 250–256. [DOI] [PubMed] [Google Scholar]
- 2. Elhamri H, Idrissi L, Coquery M, Azemard S, El-Abidi A, Benlemlit M, et al. (2007) Hair mercury levels in relation to fish consumption in a community of the Moroccan Mediterranean coast. Food Additives & Contaminations 24: 1236–1246. [DOI] [PubMed] [Google Scholar]
- 3. Buchardt B, Bunch V, Helin P (2007) Fingernails and diet: Stable isotope signatures of a marine hunting community from modern Uummannaq, north Greenland. Chemical Geology 244: 316–329. [Google Scholar]
- 4. Díez S, Montuori P, Pagano A, Sanacchiaro P, Bayona JM, Triassi M (2008) Hair mercury levels in an urban population from southern Italy: fish consumption as a determinant of exposure. Environment International 34: 162–167. [DOI] [PubMed] [Google Scholar]
- 5. Freire G, Ramos R, Lopez-Espinosa MJ, Díetz S, Vioqúe J, Ballester F, et al. (2010) Hair mercury levels, fish consumption, and cognitive development in preschool children from Granada, Spain. Environmental Research 110: 96–104. 10.1016/j.envres.2009.10.005 [DOI] [PubMed] [Google Scholar]
- 6. Campbell LM, Norstrom RJ, Hobson KA, Muir DCG, Backus S, Fisk AT (2005) Mercury and other trace elements in a pelagic Arctic marine food web (Northwater Polynya, Baffin Bay). Science of the Total Environment 351–352: 247–263. [DOI] [PubMed] [Google Scholar]
- 7. Bisi TL, Lepont G, Azevedo ADF, Dorneles PR, Flach L, Das K, et al. (2012) Trophic relationships and mercury biomagnification in Brazilian tropical coastal food webs. Ecological Indicators 18: 291–302. [Google Scholar]
- 8. Harmelin-Vivien M, Bodiguel X, Charmasson S, Loizeau V, Mellon-Duval G, Troncyñski J, et al. (2012) Differential biomagnification of PCB, PBDE, Hg and radiocesium in the food web of European hake from the NW Mediterranean. Marine Pollution Bulletin 64: 974–983. 10.1016/j.marpolbul.2012.02.014 [DOI] [PubMed] [Google Scholar]
- 9.WHO. World Health Organization. International program on chemical safety Environmental health criteria. 101, methylmercury 1990.
- 10.JECFA. Summary and Conclusions of the 61st Meeting of the Joint FAO/WHO Expert Committee of Food Additives (JECFA). JECFA/61/SC. 2003. Rome, Italy.
- 11. Yasutake A, Matsumoto M, Yamaguchi M, Hachiya N (2004) Current hair mercury levels in Japanese for estimation of methylmercury exposure. Journal Health Science 50: 120–125. [Google Scholar]
- 12. Weihe P, Hansen JC, Murata K, Debes F, Jɸrgensen PJ, Steuerwald U, et al. (2002). Neurobehavioral performance of Inuit children with increased prenatal exposure to methylmercury. International Journal of Circumpolar Health 61: 41–49. [DOI] [PubMed] [Google Scholar]
- 13. Endo T, Haraguchi K (2010) High mercury levels in hair samples from residents of Taiji, a Japanese whaling town. Marine Pollution Bulletin 60: 743–747. 10.1016/j.marpolbul.2009.11.020 [DOI] [PubMed] [Google Scholar]
- 14. Kelly JF (2000) Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Canadian Journal of Zoology 78: 1–27. [Google Scholar]
- 15. DeNiro MJ, Epstein S (1981) Influence of diet on the distribution of nitrogen isotopes in animals. Geochimica Cosmochimica Acta 45: 341–351. [Google Scholar]
- 16. Minagawa M, Wada E (1984) Stepwise enrichment of 15N along food chains further evidence and relation between 15N and animal age. Geochimica Cosmochimica Acta 48: 1135–1140. [Google Scholar]
- 17. McCutchan JHJ, Lewis WMJ, Kendall C, McGrath C (2003) Variation in trophic sift for stable isotope ratios of carbon, nitrogen, and sulfur. OIKOS 102:378–390. [Google Scholar]
- 18. Hobson KA, Welch HE (1992) Determination of trophic relationships within a high Arctic marine food web using δ13C and δ15N analysis. Marine Ecology Progress Series 84: 9–18. [Google Scholar]
- 19. Endo T, Hisamichi Y, Kimura O, Haraguchi K, Lavery S, Dalebout ML, et al. (2010) Stable isotope ratios of carbon and nitrogen and mercury concentration in 13 toothed whale species from the western Pacific Ocean off Japan. Environmental Science & Technology 44: 2675–268. [DOI] [PubMed] [Google Scholar]
- 20. Walker JL, Potter CW, Macko SA (1999) The diet of modern and historic bottlenose dolphin populations reflected through stable isotopes. Marine Mammal Science 15:335–350. [Google Scholar]
- 21. Chen CY, Dionne M, Mayes BM, Ward DM, Sturup S, Jackson BP (2009) Mercury bioavailability and bioaccumulation in estuarine food webs in Gulf of Maine. Environmental Science & Technology 43: 1804–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Karimi R, Frisk M, Fisher NS (2013) Contrasting food web and body size relationships with Hg and Se concentrations in marine biota. PLOS ONE 8: e74695 10.1371/journal.pone.0074695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hisamichi Y, Haraguchi K, Endo T (2010) Contamination levels of mercury and organochlorine compounds, and stable isotope ratios in three tuna species taken from different regions of Japan. Environmental Science & Technology 44: 5971–5978. 10.1021/es1008856 [DOI] [PubMed] [Google Scholar]
- 24. Bol R, Pflieger C (2002) Stable isotope (13C, 15N and 34S) analysis of the hair of modern humans and their domestic animals. Rapid Communications in Mass Spectrometry 16: 2195–2200. [DOI] [PubMed] [Google Scholar]
- 25. Nardoto GB, Silva S, Kendall C, Ehleringer JR, Chesson LA, Ferraz ESB, et al. (2006) Geographical patterns of human diet derived from stable-isotope analysis of fingernails. American Journal of Physical Anthropology 131: 137–146. [DOI] [PubMed] [Google Scholar]
- 26. Mützel E, Lehn C, Peschel O, Hölzl S, Rossman A (2009) Assignment of unknown persons to their geographical origin by determination of stable isotopes in hair samples. International Journal of Legal Medicine 123: 35–40. 10.1007/s00414-008-0286-7 [DOI] [PubMed] [Google Scholar]
- 27. Thompson AH, Chesson LA, Podlesak DW, Bowen G J, Cerling TE, Ehleringer JR (2010) Stable isotope analysis of modern human hair collected from Asia (China, India, Mongolia, and Pakistan). American Journal of Physical Anthropology 141: 440–451. 10.1002/ajpa.21162 [DOI] [PubMed] [Google Scholar]
- 28. Valenzuela LO, Chesson LA, O’Grady SO, Cerling TE. Ehleringer JR (2011) Spatial distributions of carbon, nitrogen and sulfur isotope ratios in human hair across the central United States. Rapid Communications in Mass Spectrometry 25: 861–868. 10.1002/rcm.4934 [DOI] [PubMed] [Google Scholar]
- 29. Endo T, Hayasaka M, Hisamichi Y, Kimura O, Haraguchi K (2013) Carbon and nitrogen stable isotope ratios and mercury concentration in the scalp hair of residents from Taiji, a whaling town. Marine Pollution Bulletin 69: 116–121. 10.1016/j.marpolbul.2013.01.018 [DOI] [PubMed] [Google Scholar]
- 30. O’Connell TC, Hedges REM, Healey MA, Simpson AHRW (2001) Isotopic comparison of hair, nail and bone: modern analyses. Journal of Archaeological Science 28: 1247–1255. [Google Scholar]
- 31. Huelsemann F, Flenker U, Koehler K, Schaenzer W (2009) Effect of a controlled dietary change on carbon and nitrogen stable isotope ratios of human hair. Rapid Communications in Mass Spectrometry 23: 2448–2454. 10.1002/rcm.4039 [DOI] [PubMed] [Google Scholar]
- 32. Petzke KJ, Fuller BT, Metges CC (2010) Advance in natural stable isotope ratio analysis of human hair to determine nutritional and metabolic status. Current Opinion in Clinical Nutrition and Metabolic Care 13: 532–540. 10.1097/MCO.0b013e32833c3c84 [DOI] [PubMed] [Google Scholar]
- 33. Tokui N, Minari Y, Lusunoki K, Yoshimura T, Yamamoto T, Minagawa M (2000) Evaluation of dietary intake using carbon and nitrogen isotope analysis of human hair of Chinese living in southern part of China. Journal of UOEH 22: 219–228. [DOI] [PubMed] [Google Scholar]
- 34. Macko SA, Engel MH, Andrusevich V, Lubec G, O’Connell TC, Hedges REM (1999) Documenting the diet in ancient human populations through stable isotope analysis of hair. Philosophical Transactions of the Royal Society of London Series B 354: 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Endo T, Haraguchi K, Sakata M (2002) Mercury and selenium concentrations in the internal organs of toothed whales and dolphins marketed for human consumption in Japan. Science of the Total Environment 300: 15–22. [DOI] [PubMed] [Google Scholar]
- 36. Endo T, Hotta Y, Hisamichi Y, Kimura O, Sato R, Haraguchi K, et al. (2012) Stable isotope ratios and mercury levels in red meat products from six baleen whales sold in Japanese markets. Ecotoxicology and Environmental Safety 79: 35–41. 10.1016/j.ecoenv.2012.01.020 [DOI] [PubMed] [Google Scholar]
- 37. Tayasu I, Hirasawa R, Ogawa NO, Ohkouchi N, Yamada K (2011) New organic reference materials for carbon- and nitrogen-stable isotope ratio measurements provided by Center for Ecological Research, Kyoto University, and Institute of Biogeosciences, Japan Agency for Marine-Earth Science and Technology. Limnology 12: 261–266. [Google Scholar]
- 38. Kim SA, Jeon CK, Paek DM (2008) Hair mercury concentrations of children and mothers in Korea: Implication fro exposure and evaluation. Science of the Total Environment 402: 36–42. 10.1016/j.scitotenv.2008.04.010 [DOI] [PubMed] [Google Scholar]
- 39. O’Connell TC, Hedges REM (1999) Investigations into the effect of diet on modern human hair isotopic values. American Journal of Physical Anthropology 108: 409–425. [DOI] [PubMed] [Google Scholar]
- 40. Al-Majed NB, Preston MR (2000) Factors influencing the total mercury and methyl mercury in the hair of the fisherman of Kuwait. Environmental Pollution 109: 239–250. [DOI] [PubMed] [Google Scholar]
- 41. Yasutake A, Matsumoto M, Yamaguchi M, Hachiya N (2003) Current hair mercury levels in Japanese: Survey in five districts. The Tohoku Journal of Experimental Medicine 199: 161–169. [DOI] [PubMed] [Google Scholar]
- 42. Xiao HY, Tang CG, Xiao HW, Wang YL, Lie XY, Liu CQ (2010) Tissue S/N ratios and stable isotopes (δ34S and δ15N) of epilithic mosses (Haplocladium microphyllum) for showing air pollution in urban cities in South China. Environmental Pollution 158, 1726–1732. 10.1016/j.envpol.2009.11.016 [DOI] [PubMed] [Google Scholar]
- 43. Yamaguchi S, Matsumoto H, Kaku S, Tateishi M, Shiramizu M (1975) Factors affecting the amount of mercury in human scalp hair. American Journal of Public Health May 65: 484–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yoshinaga J, Minagawa M, Suzuki T, Ohtsuka R, Kawabe T, Inaoka T, et al. (1996) Stable carbon and nitrogen isotopic composition of diet and hair of Gidra-speaking Papuans. American Journal of Physical Anthropology 100: 23–34. [DOI] [PubMed] [Google Scholar]
- 45. Lee WC, Lee MJ, Lee SM, Kim JS, Bae CS, Park TK (2000) An observation on the mercury contents of scalp hair in the urban residents of South Korea. Environmental Toxicology and Pharmacology 8: 275–278. [DOI] [PubMed] [Google Scholar]
- 46. Hong SR, Lee SM, Lim NR, Chung HW, Ahn HS (2009) Association between hair mineral and age, BMI and nutrient intakes among Korean female adults. Nutrition Research and Practice 3: 212–219. 10.4162/nrp.2009.3.3.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Barbosa AC, Jardim W, Dórea JG, Fosberg B, Souza J (2001) Hair mercury speciation as function of gender, age, and body mass index in inhabitants of the Negro River basin, Amazon, Brazil. Archives of Environmental Contamination and Toxicology 40: 439–444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.