Abstract
Actinomyces meyeri is an uncommon cause of human actinomycosis. Here, we report a rare case of empyema caused by A. meyeri. A 49-year-old male presented with a history of 10 days of dyspnea and chest pain. A large amount of loculated pleural effusion was present on the right side and multiple lung nodules were documented on radiological studies. A chest tube was inserted and purulent pleural fluid was drained. A. meyeri was isolated in anaerobic cultures of the pleural fluid. The infection was alleviated in response to treatment with intravenous penicillin G (20 million IU daily) and oral amoxicillin (500 mg every 8 hours) for 4 months, demonstrating that short-term antibiotic treatment was effective.
1. Introduction
Actinomycosis is a chronic infection caused by bacteria belonging to the Actinomyces genus, with Actinomyces israelii most commonly implicated in human diseases, including cervicofacial disease. In contrast to other species of Actinomyces, Actinomyces meyeri often causes pulmonary infections and shows a tendency for hematogenous dissemination [1]. A. meyeri infection of the respiratory system presents with nonspecific symptoms and has no characteristic findings of diagnostic value in radiological tests. Therefore, histological and microbiological tests are important for diagnosis. We herein report a case of empyema due to A. meyeri that was treated with short-term antibiotic therapy and review the current literature.
2. Case Report
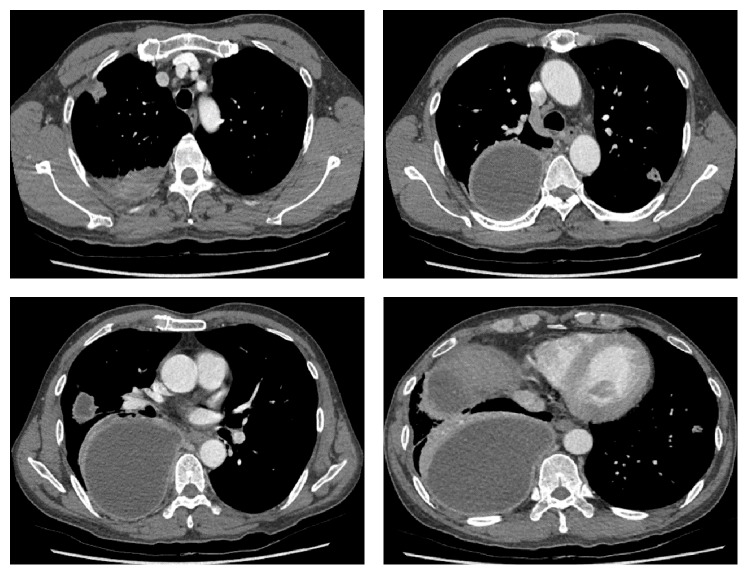
A 49-year-old male was hospitalized with a history of right chest pain occurring for 10 days and dyspnea. The patient had been in good health prior to this time and had no history of medical illness, except for smoking and alcohol abuse. The patient appeared acutely ill and a physical examination revealed that he was febrile (temperature 38.6°C). A chest examination revealed diminished breath sounds in the right side. Laboratory findings were significant for mild anemia (9.3 g/dL) and mild leukocytosis (12,260/mm3). Kidney and liver function tests were within normal limits. An arterial blood gas test without oxygen supply showed pH, partial pressure of oxygen, partial pressure of carbon dioxide, bicarbonate, and oxygen saturation readings of 7.472, 65.4 mmHg, 35.3 mmHg, 25.2 mmol/L, and 94%, respectively. A chest radiograph showed right side loculated pleural effusion (Figure 1). Computed tomography (CT) scans of the chest showed multiple lung nodules and right loculated pleural effusion (Figure 2). A chest tube was inserted and 1,380 mL of turbid yellow and pus-like pleural fluid was drained. A cell count of the pleural fluid revealed a white blood cell count of 56,000/mm3 with 89% polymorphonuclear leukocytes. The level of pleural lactate dehydrogenase was 20,530 IU/L and the levels of protein in the pleural fluid and serum were 3 g/dL and 5.9 g/dL, respectively. Gram stain of the pleural fluid specimen showed no organisms, and aerobic culture of the pleural fluid revealed no isolated pathogen. However, in anaerobic culture conditions, a 2 mm, elevated, gray colony was observed 48 hours after inoculation of the specimen onto phenylethyl alcohol agar. Gram stain of the colony growth showed a long, cribriform, non-spore-forming, Gram-positive Bacillus and this pathogen was ultimately identified as A. meyeri by the RAPID ID 32 A (bioMérieux, Marcy-l'Étoile, France) on the 10th day of hospitalization. The elapsed time from the collection of the pleural fluid specimen to incubation in an anaerobic environment was around 2 hours. From the day of admission, the patient was treated empirically with piperacillin/tazobactam (4.5 g IV every 8 hours) and the fever subsided after the third day of hospitalization. Antibiotics were changed to intravenous penicillin (20 million IU/day) after the pathogenic organism was identified as A. meyeri, and the course of penicillin treatment was 17 days. The symptoms were ameliorated and the lung nodules and pleural effusion were also improved on the follow-up chest radiograph and CT scan. The patient was discharged after hospitalization for 4 weeks and was prescribed oral amoxicillin (500 mg every 8 hours). After 8 weeks of oral amoxicillin therapy, a chest radiograph showed complete resolution of the empyema without pleural sequelae. The patient continued to receive amoxicillin therapy for approximately 5 more weeks to complete an almost 4-month course of antibiotic treatment and remained healthy during the 1-year follow-up period (Figure 3).
Figure 1.
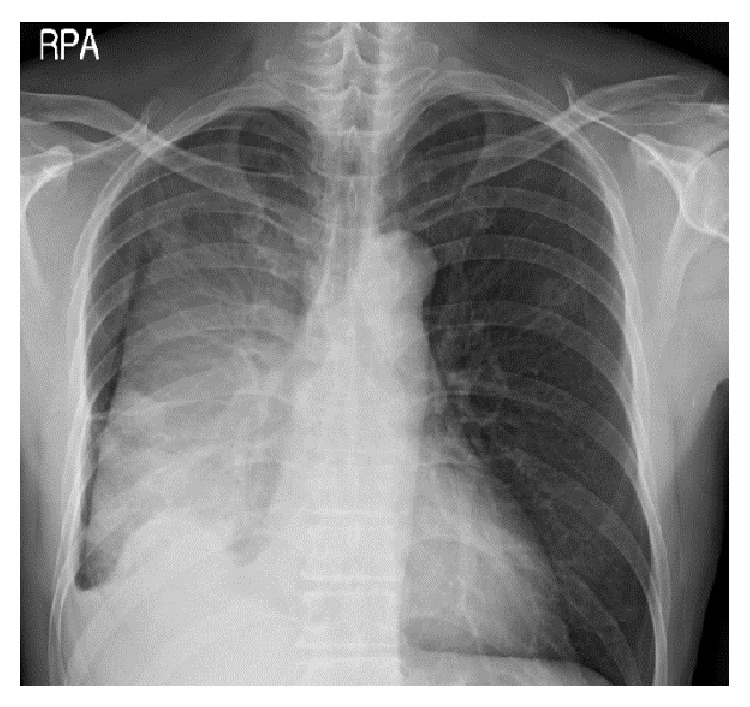
Large fissural loculated effusion in the right hemithorax.
Figure 2.
Multiple lung nodules and a large amount of multiloculated pleural effusion in the right hemithorax.
Figure 3.
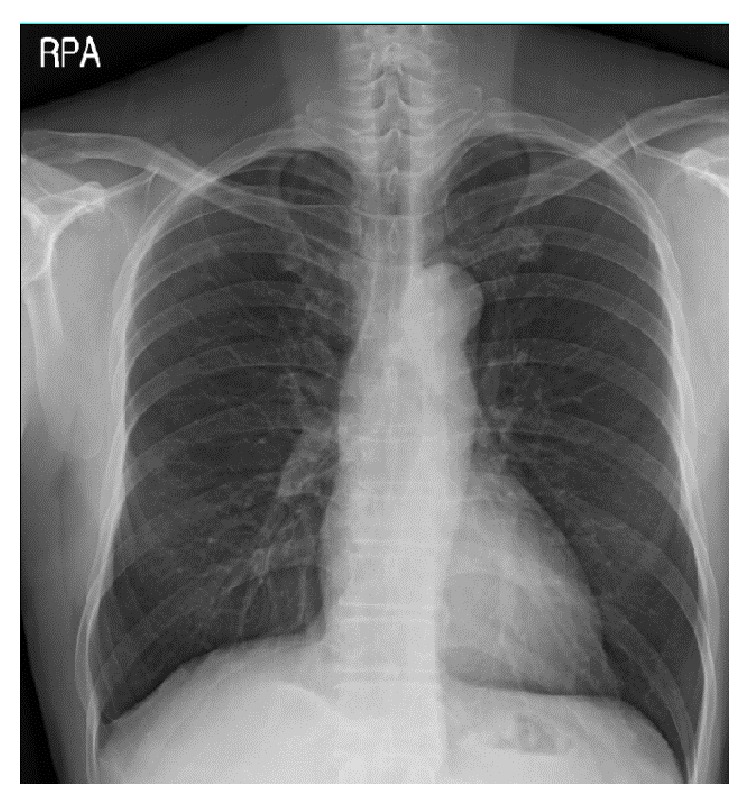
A chest radiograph showed the complete resolution of empyema without pleural sequelae 12 months after discontinuing treatment.
3. Discussion
Bacteria of the Actinomyces genus are anaerobic Gram-positive bacilli that typically reside in the mouth, gastrointestinal tract, and reproductive system. Disruption of the mucosal barrier is essential for the development of actinomycosis, which allows the organisms to invade. Cervicofacial and abdominal actinomycosis are associated with dental sepsis, appendicitis, diverticulitis, trauma, or surgery, while pelvic disease is related to intrauterine or intravaginal devices [1]. Pulmonary actinomycosis likely results from aspiration of oropharyngeal or gastrointestinal secretions into the respiratory tract and is initiated when saliva, or other material laden with Actinomyces species, is aspirated into a minor bronchus, causing atelectasis and pneumonitis [1].
Pulmonary actinomycosis frequently develops in middle- to old-aged males, especially those with a history of alcohol abuse and structural lung diseases [2]. Chest pain, productive cough, and dyspnea are the most common symptoms, and the most frequent radiologic feature is nonspecific consolidation. Although radiological tests may be helpful in diagnosing actinomycosis, differentiation from pneumonia or lung cancer is difficult. Direct isolation of the organism from a clinical specimen is necessary for a definitive diagnosis [3]. However, the failure rate of isolation is high (>50%) for various reasons, including previous antibiotic treatment, overgrowth of concomitant organisms, or inadequate methodology [3]. Most cases of actinomycosis reported to date had sulfur granules that were identified upon histological examination; however, sulfur granules may also be present in nocardiosis, coccidioidomycosis, and aspergillosis [4]. The difficulty of culturing and identifying Actinomyces has led to the use of new molecular genetic methods, such as 16s rRNA sequencing, which has facilitated more rapid and accurate identification of Actinomyces [3, 5, 6].
A. meyeri was first cultured from an empyema patient in 1911, but it is a very rare pathogen, with only 30 cases or so reported in the English-language literature to date [7, 8]. Unlike A. israelii, A. meyeri commonly infiltrates into the respiratory system, and it has the ability to spread to other organs such as the skin, long bones, liver, brain, and muscles. The propensity of A. meyeri to disseminate is difficult to explain because this organism does not differ from other Actinomyces species in its microbiological characteristics. It has been postulated that pulmonary infection is often the source of the hematogenous dissemination [9].
Actinomycosis in the respiratory system caused by A. meyeri may be effectively treated by antibiotics, similar to ordinary thoracic actinomycosis. Conventional therapy for actinomycosis is high-dose intravenous penicillin at a dosage of 18–24 million U/day for 2–6 weeks, followed by oral penicillin or amoxicillin for a period of 6–12 months [1]. However, the recommendations for the use of prolonged penicillin treatment date back to the early antibiotic era, and several studies have reported using shorter courses of antibiotics for actinomycosis [10]. A previous study described 19 patients exhibiting thoracic actinomycosis that were cured with a median duration of 6 weeks of antibiotic treatment (range, 1 week to 6 months) [11]. Another study including 16 patients reported an alleviation of symptoms in response to treatment consisting of a median duration of 2 weeks of intravenous penicillin and three months of oral penicillin [12]. These reports suggest that thoracic actinomycosis can be treated with relatively brief courses of antibiotic treatment.
A review of the English-language literature revealed five case reports of A. meyeri empyema [7, 8, 13–15] (Table 1). Four of five patients underwent a surgical procedure, and the duration of antibiotic therapy ranged from 6 to 12 months. In comparison to previous reports, the current case was diagnosed early and was effectively drained with a chest tube only. Additionally, there was no evidence of dissemination and symptoms and radiological findings were rapidly improved. On the basis of a clinical response, antibiotic treatment was maintained for a total of 4 months, and no evidence of recurrence was observed during the 1-year follow-up period. The duration of antibiotic treatment of actinomycosis depends on the initial burden of disease, the performance of resectional surgery, and the patient's response to treatment [3]. The traditional recommendation of 6–12 months of antibiotic therapy may not be necessary for all patients.
Table 1.
Summary of six cases of empyema due to A. meyeri.
| Case | Author (year) [reference] | Sex/age | Dissemination | Treatment | Antibiotic duration (months) |
|---|---|---|---|---|---|
| 1 | Rose et al. (1982) [13] | M/62 | Hip | Chest tube drainage | 13 |
| 2 | Lentino et al. (1985) [14] | M/16 | Bone marrow | Thoracotomy, resection of infected rib | 6 |
| 3 | Vallet et al. (2004) [15] | F/64 | None | Thoracotomy and decortication | 6.5 |
| 4 | Fazili et al. (2012) [7] | M/45 | None | Thoracotomy and decortication | 12 |
| 5 | Attaway and Flynn (2013) [8] | M/61 | None | Thoracotomy and decortication | 7.5 |
| 6 | Present | M/49 | None | Chest tube drainage | 4 |
In conclusion, empyema due to A. meyeri is uncommon, and anaerobic culture of pleural fluid plays a major role in the early diagnosis of actinomycosis involving pleura. Although the loculated pleural effusion was large, early diagnosis and successful drainage could shorten the duration of treatment. Short-term antibiotic therapeutic treatment of actinomycosis can be attempted when the clinical and radiological responses are rapid and there is no evidence of dissemination.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Mabeza G. F., Macfarlane J. Pulmonary actinomycosis. European Respiratory Journal. 2003;21(3):545–551. doi: 10.1183/09031936.03.00089103. [DOI] [PubMed] [Google Scholar]
- 2.Kim S. R., Jung L. Y., Oh I.-J., et al. Pulmonary actinomycosis during the first decade of 21st century: cases of 94 patients. BMC Infectious Diseases. 2013;13(1, article 216) doi: 10.1186/1471-2334-13-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong V. K., Turmezei T. D., Weston V. C. Actinomycosis. The British Medical Journal. 2011;343(7827) doi: 10.1136/bmj.d6099.d6099 [DOI] [PubMed] [Google Scholar]
- 4.Jung H. Y., Kim H. Y., Kim Y. K., et al. A rare case of pericardial actinomycosis. Infection and Chemotherapy. 2012;44(1):31–34. doi: 10.3947/ic.2012.44.1.31. [DOI] [Google Scholar]
- 5.Kakuta R., Hidaka H., Yano H., et al. Identification of Actinomyces meyeri actinomycosis in middle ear and mastoid by 16S rRNA analysis. Journal of Medical Microbiology. 2013;62(8):1245–1248. doi: 10.1099/jmm.0.051698-0. [DOI] [PubMed] [Google Scholar]
- 6.Song EH., Kwon HH., Jang EY. A case of brain abscess caused by Actinomyces meyeri and Haemophilus aphrophilus . The Korean Journal of Medicine. 2008;74:144–148. [Google Scholar]
- 7.Fazili T., Blair D., Riddell S., Kiska D., Nagra S. Actinomyces meyeri infection: case report and review of the literature. Journal of Infection. 2012;65(4):357–361. doi: 10.1016/j.jinf.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 8.Attaway A., Flynn T. Actinomyces meyeri: from ‘lumpy jaw’ to empyema. Infection. 2013;41(5):1025–1027. doi: 10.1007/s15010-013-0453-8. [DOI] [PubMed] [Google Scholar]
- 9.Apothéloz C., Regamey C. Disseminated infection due to Actinomyces meyeri: case report and review. Clinical Infectious Diseases. 1996;22(4):621–625. doi: 10.1093/clinids/22.4.621. [DOI] [PubMed] [Google Scholar]
- 10.Sudhakar S. S., Ross J. J. Short-term treatment of Actinomycosis: two cases and a review. Clinical Infectious Diseases. 2004;38(3):444–447. doi: 10.1086/381099. [DOI] [PubMed] [Google Scholar]
- 11.Kinnear W. J. M., MacFarlane J. T. A survey of thoracic actinomycosis. Respiratory Medicine. 1990;84(1):57–59. doi: 10.1016/S0954-6111(08)80095-9. [DOI] [PubMed] [Google Scholar]
- 12.Hsieh M.-J., Liu H.-P., Chang J.-P., Chang C.-H. Thoracic actinomycosis. Chest. 1993;104(2):366–370. doi: 10.1378/chest.104.2.366. [DOI] [PubMed] [Google Scholar]
- 13.Rose H. D., Varkey B., Kesavan Kutty C. P. Thoracic actinomycosis caused by Actinomyces meyeri . American Review of Respiratory Disease. 1982;125(2):251–254. doi: 10.1164/arrd.1982.125.2.251. [DOI] [PubMed] [Google Scholar]
- 14.Lentino J. R., Allen J. E., Stachowski M. Hematogenous dissemination of thoracic actinomycosis due to Actinomyces meyeri. Pediatric Infectious Disease. 1985;4(6):698–699. doi: 10.1097/00006454-198511000-00026. [DOI] [PubMed] [Google Scholar]
- 15.Vallet C., Pezzetta E., Nicolet-Chatelin G., El Lamaa Z., Martine O., Ris H.-B. Stage III empyema caused by Actinomyces meyeri: a plea for decortication. Journal of Thoracic and Cardiovascular Surgery. 2004;127(5):1511–1513. doi: 10.1016/j.jtcvs.2003.11.043. [DOI] [PubMed] [Google Scholar]