Abstract
Duchenne muscular dystrophy (DMD) is an X-linked recessive disorder characterized by progressive muscle weakness, with eventual loss of ambulation and premature death. The approved therapy with corticosteroids improves muscle strength, prolongs ambulation, and maintains pulmonary function. However, the osteoporotic impact of chronic corticosteroid use further impairs the underlying reduced bone mass seen in DMD, leading to increased fragility fractures of long bones and vertebrae. These serious sequelae adversely affect quality of life and can impact survival. The current clinical issues relating to bone health and bone health screening methods in DMD are presented in this review. Diagnostic studies, including biochemical markers of bone turnover and bone mineral density by dual energy X-ray absorptiometry (DXA), as well as spinal imaging using densitometric lateral spinal imaging, and treatment to optimize bone health in patients with DMD are discussed. Treatment with bisphosphonates offers a method to increase bone mass in these children; oral and intravenous bisphosphonates have been used successfully although treatment is typically reserved for children with fractures and/or bone pain with low bone mass by DXA.
1. Introduction
Duchenne muscular dystrophy (DMD) is an X-linked recessive disorder (one of the dystrophinopathies) that is due to mutations in the dystrophin gene, encoding the dystrophin protein. This mutation leads to a deficiency of dystrophin, a protein that stabilizes the extracellular matrix and the cytoskeleton. Cell membrane destabilization, via loss of dystrophin, results in necrosis of myofibers and progressive muscle weakness [1]. Affecting approximately 1 in 3,500–5,000 males, it is the most common and severe form of muscular dystrophy [2]. DMD is usually marked by onset of symptoms between the ages of 3 and 5 years. The early symptoms are typically related to gait, including delayed onset of walking, toe walking, and/or a waddling gait. Serum creatine kinase (CK) is typically elevated 50 to 100 times above normal. Historically, loss of ambulation occurred between the ages of 7 and 12 years and death by the end of the second decade. In nearly 90% of cases, DMD is due to mutations that truncate the reading frame, leading to an absence of dystrophin expression.
In patients with DMD, prior to corticosteroid treatment, muscle weakness would inevitably progress and early in the second decade of life these boys would become nonambulatory and eventually require respiratory support. Two corticosteroids commonly used to treat DMD are prednisone and deflazacort, an oxazolone derivative of prednisolone [3–5]. Five recently published, long-term controlled nonrandomized trials (now extending beyond 3 years) with prednisone or deflazacort [6] demonstrated that, with either drug, patients retain muscle function longer, ambulate 2 to 5 years longer [4, 5], require less spinal stabilization surgery [7], and have delayed need for noninvasive ventilation [8–10] and less cardiac dysfunction than boys not receiving corticosteroid therapy [10–12].
Corticosteroid therapy along with better supportive care for cardiopulmonary status has improved survival in DMD from an average of 14.4 years in the 1960s to an average of 24.7 years according to a report published in 2010 [6]. The beneficial effects of corticosteroids in DMD are thought to be due to their potent anti-inflammatory activity that reduces the inflammatory response in dystrophin-deficient muscle, which in turn delays the loss of muscle strength and preserves the ability to ambulate longer than in boys without corticosteroid therapy. Unfortunately, this therapy comes with many adverse effects including negative effects on bone health such as low bone mass and bone fragility fractures [13–19]. For a more detailed discussion of corticosteroid therapy concerning timing of initiation, treatment after loss of ambulation, as well as the mechanism of corticosteroids, and other treatments in DMD, the reader is referred to recent reviews [20, 21]. In this review, we present the current risks of poor bone health, screening methods, diagnostic studies, and treatments to optimize bone health in patients with DMD.
2. Risks to Bone Health
Several pathophysiological mechanisms can contribute to the deterioration of bone health seen in DMD patients. Mechanisms that can alter bone health in children with DMD are as follows:
Progressive muscle weakness.
Effects of cytokines released due to the inflammatory response in dystrophin-deficient muscles.
Activation of osteoclastogenesis by altered muscle metabolism.
-
Corticosteroid side effects:
- delayed puberty with decreased sex steroids,
- impaired osteoblast bone formation/mineralization,
- poor calcium absorption from the intestine.
Immobilization effects on bone calcium homeostasis.
-
Insufficient vitamin D stores:
- poor vitamin D absorption from corticosteroids,
- reduced sun exposure from lack of outdoor physical activity.
Many patients experience multiple factors and corticosteroid therapy is associated with additional risk factors for poor bone health. Genetic factors that may contribute to poor bone mineralization or exaggerated bone mineral loss have not been explored.
3. Bone Fragility in DMD
King et al. found that long bone fractures were 2.6 times more frequent in DMD patients treated with steroids than steroid naïve patients [16]. The steroid naïve group also had no vertebral fractures while 32% of the steroid treated group had vertebral fractures [16]. About 20–25% of DMD patients have a long bone fracture [22], and those of the lower extremities often result in the onset of permanent loss of ambulation [23]. Immobilization, such as full-time wheelchair use, can further alter bone health and the risk for fracture by causing additional demineralization of bone [24, 25].
Tian et al., in a recent retrospective study in a large cohort of 408 glucocorticoid-treated DMD patients aged 3–19 years, demonstrated that fracture prevalence increased progressively with age, along with worsening motor function [26]. Prevalence of total fractures was 16.5%, 37.4%, and 83.3% at ages 5, 10, and 18 years, respectively. Prevalence of vertebral compression fractures was 4.4%, 19.1%, and 58.3% at the same ages. This high prevalence of fractures with increasing age heightens the importance of monitoring bone health in DMD patients and providing interventions to improve bone health in this susceptible population [26].
4. Bone Mineral Density (BMD) in DMD
In 2000, Larson and Henderson [23] described bone density findings in 36 corticosteroid naïve DMD boys. They showed that BMD was decreased in boys with DMD compared to normal controls but also that the mean lumbar spine Z-score of 17 ambulatory DMD boys (−0.8 ± 0.2) decreased further with the loss of ambulation (mean lumbar spine Z-score −1.7 ± 0.2) (P = 0.004). In a group of 32 DMD boys (22 on prednisone and 10 corticosteroid naïve), Bianchi et al. [17] found that cumulative corticosteroid dose correlated to decreased BMD (P < 0.05) and better lower limb muscle strength correlated with better BMD (P < 0.01). They also showed that BMD was lower in DMD boys than in normal control patients at the spine and total body, but no significant difference was seen in the spine Z-scores once adjusted for body surface area or for vertebral volume. Mayo et al. [27] followed 39 DMD boys on deflazacort therapy for about 8 years and demonstrated that there was a decrease in the age-adjusted BMD Z-score within the first 2 years of starting deflazacort (P < 0.05), with a consistent decline thereafter; in those who lost ambulation, the decline was greater. Compared to age-based Z-scores, height-adjusted BMD Z-scores were less impaired in this population. This may reflect better bone health than what is anticipated in these DMD boys on corticosteroids; since children on corticosteroids tend to be short, when their Z-scores were corrected for their smaller size, their BMD was not as poor as it would be when compared to normal heighted control boys.
5. Bone Health Screening
Leung et al. reported screening recommendations derived from a 2010 DMD conference [28]. Suggested screening included (1) nutritional and fracture history, (2) reviewing radiographic evidence of past fractures, (3) obtaining a back pain assessment on initial and each subsequent visit, and (4) checking the serum 25-hydroxyvitamin D level every 1-2 years prior to steroid therapy and maintaining a consistent level ≥30 ng/mL (≥75 nmol/L). Upon starting steroids, a dual energy X-ray absorptiometry (DXA) scan of the lumbar spine and a lateral spinal radiograph is recommended to screen for low BMD and vertebral fractures, respectively. Once a DMD patient has symptoms/signs of vertebral fracture, starting bisphosphonate therapy is recommended; in regard to bisphosphonate treatment before vertebral fractures, this should be decided on a case by case basis.
Similar screening suggestions were derived from a 2009 DMD conference as summarized by Quinlivan et al. [29]. Major additional recommendations included (1) encouraging physical activity and standing and questions related to sun exposure (as a source of vitamin D) at initial and subsequent visits, (2) performing a baseline DXA, and (3) repeating the DXA every 12–24 months if the body size corrected lumbar spine Z-score is >−2 and no back pain is present. If the lumbar spine Z-score is <−2 or there is back pain, then lateral imaging is required for vertebral fracture screening. Once a vertebral fracture is present, bisphosphonate treatment should be considered. Table 1 outlines a summary of the bone health screening and course of action recommendations for boys with DMD [28, 29].
Table 1.
| Screening | Timing | Course of action |
|---|---|---|
| Back pain assessment | Each visit | If present, obtain vertebral imaging |
|
| ||
| Calcium intake and vitamin D intake (diet and amount of sun exposure) | Initial and subsequent visits | Calcium and vitamin D supplementation as needed; see Table 2. |
|
| ||
| Serum 25-hydroxyvitamin D | Every 1-2 years | Vitamin D insufficiency/deficiency treatment without clinical signs of rickets. Ergocalciferol or cholecalciferol dose based on vitamin D level: 20–30 ng/mL: 1000 IU PO daily, <20 ng/mL: 2000 IU PO daily, <10 ng/mL: 4000 IU PO daily. (i) Dose may need to be higher in patients with malabsorption, chronic glucocorticoids use, dark skin pigmentation, or obesity. (ii) Serum 25-OH vitamin D level should be repeated in 3 months after giving pharmacologic doses of vitamin D. (iii) When the level is optimal, vitamin D dose should be reduced to a supplementation dose at 400–800 IU/day (or higher in chronic glucocorticoid use). |
|
| ||
| Bone turnover markers | Not formally recommended at this time | Further research is needed and may be useful in monitoring bisphosphonate therapy. |
|
| ||
| DXA scan | Obtain baseline prior to glucocorticoid use every 1-2 years thereafter | If height-adjusted lumbar BMD Z score <−1, should repeat DXA in 1 year. Worsening BMD and/or BMD Z score or the gain in BMD is less than expected, consider vertebral imaging. |
|
| ||
| Vertebral imaging (X-rays or densitometric lateral spinal imaging) | Obtain if back pain present or lumbar height-adjusted Z-score < −2 | If vertebral fracture is present, start bisphosphonate therapy. |
As studies have suggested, mobility influences bone density [30] and fracture risk [24]. There are several different mobility scales used in DMD to measure muscle function. Some scales are based on timed functional tests, such as the 6-minute walk test, 2-minute walk test, and time to rise from the floor (lying supine on the floor to full standing position). Others are based on clinical evaluation, such as the Vignos lower extremity functional grade and Brooke upper extremity functional grade [31–33]. To our knowledge, there are no reported studies that correlate mobility scales to BMD and fracture risk.
5.1. Biochemical Screening
5.1.1. Calcium and Vitamin D
Screening for calcium intake can be completed by asking patients about the amounts, types, and frequency of dairy products they consume. Vitamin D intake can also be assessed from the diet history or 72-hour dietary record. It is more difficult to quantify and qualify the amount of sun light a child obtains. However, a simple blood test (serum 25-hydroxyvitamin D level) can screen for vitamin D deficiency. The Endocrine Society defines 25-hydroxyvitamin D levels of <20 ng/mL as deficient, 20–30 ng/mL as insufficient, and ≥30 ng/mL as sufficient [34].
5.1.2. Bone Formation and Turnover Markers
Markers for bone formation and turnover have been examined in a limited number of DMD patients. These include bone formation markers, such as osteocalcin (OC) and bone-specific alkaline phosphatase (BALP), and bone breakdown products, such as pyridinoline, deoxypyridinoline, type 1 procollagen intact amino-terminal propeptide (PINP), carboxyterminal telopeptide of type 1 collagen (ICTP), N-terminal cross-linked telopeptide (NTx), and osteoclast-derived, tartrate-resistant, acid phosphatase isoform 5b (TRACP-5b) [17, 18]. Research shows that the values for these markers change with age, but currently there are no well-established normal ranges for different ages for some of the newer markers [35]. Bisphosphonates have been shown to decrease bone turnover markers (BALP, OC, NTx, and pyridinoline) in other patient populations [36]. One study compared OC and NTx in DMD patients treated with and without corticosteroids to a normal control group [17]. They found that while the OC was in the upper limit of normal, the NTx was nearly 4 times higher than the control group. An additional study showed that, for DMD patients on corticosteroid treatment, BALP, PINP, OC, CTX, and TRACP-5b were decreased compared to controls [18]. Clearly, further research is needed to evaluate the clinical utility of bone turnover markers in assessing bone fragility, predicting fractures, and monitoring treatment in this population. Until bone turnover markers are better understood, they cannot be recommended for bone health screening, at least at this time [29].
5.2. Imaging Screening
5.2.1. Spinal Assessment
Chronic glucocorticoid use has a significant impact on trabecular bone, particularly vertebra, which can result in vertebral compression fractures. The prevalence of symptomatic vertebral fractures in corticosteroid-treated DMD ranges from 32 to 40% [14, 16]. The frequency of asymptomatic vertebral fractures DMD is unknown. It has been shown that 10% of patients with juvenile idiopathic arthritis on glucocorticoids had asymptomatic vertebral fractures [37]. More generally, vertebral fractures are often asymptomatic in children with low bone mass with only 18%–56% of diagnosed vertebral fractures in this population presenting with clinical signs and symptoms in children [38, 39].
Screening for vertebral fractures in corticosteroid-treated patients with DMD is essential, as prior vertebral fractures are a powerful predictor of subsequent fractures and, when present, are an indication for treatment with bisphosphonates. Spinal radiographs should be obtained when patients complain of back pain or height-adjusted lumbar spine Z-score is <−2 [29]. Separate films of the thoracic and lumbar spine are required to visualize the entire vertebral column at risk for fractures. Anterior wedging or biconcave deformity is indicative of a vertebral compression fracture. Currently, there is no consensus for when to perform spinal screening in asymptomatic patients. In our institution, we obtain a densitometric lateral spinal imaging (Figure 1) at the time of the DXA bone density test. This technique has been proven to be a low-cost method of vertebral fracture screening, which can be done efficiently and quickly at the time of DXA, with much less radiation exposure compared to a traditional spinal radiograph (10–20 μSv versus 800 μSv) [40]. This technique allows for vertebral screening in asymptomatic patients with DMD who undergo routine DXA BMD testing.
Figure 1.
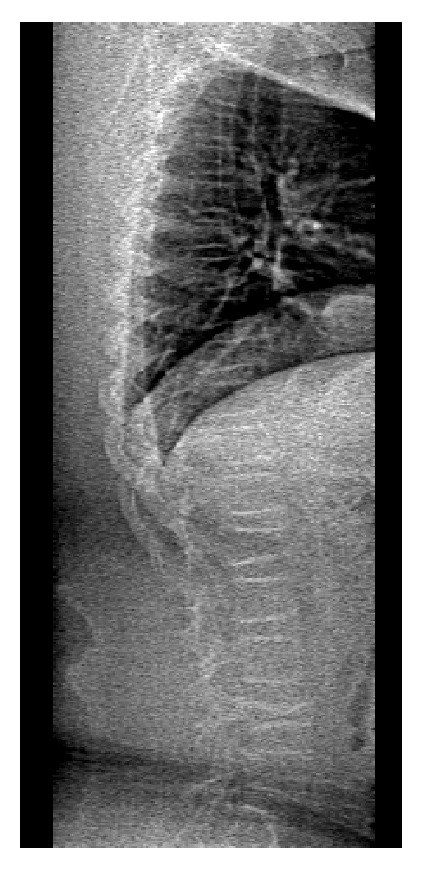
Densitometric lateral spinal imaging obtained at the time of bone density test by DXA shows L1 and L4 compression fracture with a mild anterior wedging of L2 in a 14-year-old boy with DMD. (Film courtesy of Dr. Sasigarn Bowden.)
5.2.2. BMD Measurement
Imaging methods for bone densitometry include dual energy X-ray absorptiometry (DXA) scan, quantitative computed tomography (QCT), and qualitative ultrasound; the DXA scan is the most widely used method due to its relatively low radiation exposure, precision, and opportunities to follow the skeleton sequentially over time. The current recommendation is to use (1) total body less head BMD and (2) lumbar spine BMD spine as the preferred sites of measurements because of the speed and precision of measurements and well-established pediatric normative data. The rationale for excluding the head in the total body bone density measurement is that the skull constitutes a large percentage of the total body bone mass but does not respond to growth, physical activity, or metabolic changes. Inclusion of the skull potentially under- or overestimates BMD gains or losses at other skeletal sites. Total body less head analysis of total body BMD has been shown to improve BMD assessment in boys with DMD [41]. Currently, there is not enough data to support routine BMD measurements of other skeletal sites, such as the forearm and proximal or distal femur; reference data for these sites are only available for certain machines and scan software as well as additional specialized personnel training would be required [41–43]. In children, DXA measurements of the hip region (total hip or femoral neck) are not as reliable due to difficulties in identifying the bony landmarks for this region of interest [43].
Baseline DXA scans should be obtained prior to starting glucocorticoids, every 1-2 years while on glucocorticoids and yearly if on bisphosphonate therapy [29]. DXA scans in children must be interpreted correctly; T-scores should not be used before 20 years of age, as this compares the patient's BMD with that of a healthy young adult. Age-adjusted Z-score is used in pediatric patients but this might not be appropriate in DMD patients with short stature, which would result in lower Z-score when compared to healthy children of normal height. More useful is the height-adjusted Z-score [43, 44] which represents the number of standard deviations; the BMD of a subject is away from the height-adjusted predicted mean BMD of that subject. This method corrects for the tendency for smaller children to have lower bone density than taller children of the same age. Additional factors that may affect BMD measurements include scoliosis, which may require placement of corrective hardware, and vertebral fractures. Both phenomena can lead to inaccurate spinal bone density measurement and an erroneous Z-score [44].
6. Treatments to Optimize Bone Health
6.1. Calcium and Vitamin D
Upon optimizing the calcium and vitamin D intake in patients with DMD, Bianchi et al. [45] reported that over 65% of 33 DMD patients on corticosteroid treatment displayed increased BMD after 12 months. Additional studies are needed to define the optimal vitamin D level for DMD patients; thus, current treatment goals are to maintain sufficient 25-hydroxyvitamin D levels [29]. Recommended daily calcium and vitamin D intake varies by age and is detailed in Table 2. Recommended adult vitamin D3 daily intake is 800–1,000 IU while the pediatric recommended daily intake is based on age [28]. Treatment recommendations for vitamin D insufficiency and deficiency are outlined in Table 1. Higher dose of vitamin D may be required in individuals with high adiposity, malabsorption, and dark skin pigmentation or those taking chronic glucocorticoids. Thus, it is important to monitor vitamin D levels periodically and increase the dosage if levels remain low.
Table 2.
Recommended daily allowance for calcium and vitamin D [67].
| Age | Calcium RDA∗ (mg/d) | Vitamin D RDA∗ (IU/D) |
|---|---|---|
| 0–6 months old | 200∗∗ | 400∗∗ |
| 6–12 months old | 260∗∗ | 400∗∗ |
| 1–3 years old | 700 | 600 |
| 4–8 years old | 1000 | 600 |
| 9–13 years old | 1300 | 600 |
| 14–18 years old | 1300 | 600 |
∗RDA = recommended daily allowance.
∗∗RDAs not established, and thus values are adequate intake reference.
6.2. Vibration Therapy
Whole body vibration has also been evaluated as a means to preserve or improve muscle and bone function in DMD patients. Patients tolerate whole body vibration well, but studies, even after 1 year of treatment, have not shown improvement in muscle function [46] or bone density [47]. A Cochrane review for whole body vibration in adults with Parkinson's disease and multiple sclerosis showed insufficient evidence to support beneficial effects [48]. However, in children with cerebral palsy, there have been modest improvements in bone mass with high-frequency and low-intensity vibrations seen at 6 months [49, 50] and 12 months of therapy [51]. More investigation is needed to determine whether longer duration of treatment or earlier intervention with vibration therapy would be beneficial for DMD patients.
6.3. Bisphosphonate Treatment
Bisphosphonate treatment has become increasingly used in pediatric patients with clinical bone fragility in the last 2 decades. Reports of bisphosphonate use in pediatric patients with DMD have been limited in the literature, typically with oral alendronate, intravenous pamidronate, or zoledronic acid (Table 3) [52–56]. The benefits of bisphosphonates include improvement in BMD and Z-scores [52, 55, 56] and amelioration of back pain after vertebral fracture [53, 55]. Interestingly, one study from Canada showed that bisphosphonate treatment improved survival [54]. Additional studies will be required to validate this finding. Overall, bisphosphonates were well tolerated in these studies [52–56]. Oral bisphosphonates carry the risk of esophagitis in those unable to sit up right for 30 minutes after taking the medication [57]; IV bisphosphonates may be a better option for DMD patients lacking truncal stability or inability to sit up reliably.
Table 3.
Summary of bisphosphonate use in DMD patients.
| Study | Year | Study type | Patient number | Steroids | Bisphosphonate | Mean Age at Bisphosphonate Initiation | Results | Comments |
|---|---|---|---|---|---|---|---|---|
| Houston et al. [52] | 2014 | Retrospective cohort | 39 | 29 were on prednisone or deflazacort | Alendronate PO | 12 years old (no range given) |
Z-score trended up at the hip with alendronate, but it is not statistically significant | 10 did not receive alendronate, varying dosages of alendronate used |
|
| ||||||||
| Sbrocchi et al. [53] | 2012 | Retrospective observational | 7 | All but 1 were reported as prednisone equivalents | Pamidronate IV 9 mg/kg/year or zoledronic acid IV 0.1 mg/kg/year | 11.6 years old (range: 8.5–14.3 years old) | Improved back pain and stabilization to improvement in vertebral height ratios for the previously fractured vertebrae | Only patients with vertebral fractures were included in the study |
|
| ||||||||
| Gordon et al. [54] | 2011 | Retrospective observational | 44 | 5 prednisone only; 13 changed from prednisone to deflazacort; 26 deflazacort only | 11 used pamidronate only; 1 changed from pamidronate to alendronate; 3 alendronate only; 1 clodronate only | 12.5 years old (range: 7–23 years old) | Survival curve showed improvement in survival rate (P = 0.005, log-rank test); also, possible therapy duration effect could be present (P = 0.007, log-rank test) | Pamidronate was IV; alendronate was PO; clodronate was PO |
|
| ||||||||
| Atance et al. [55] | 2011 | Case reports | 3 | 2 on deflazacort | Alendronate 10 mg daily PO | 11.4 years old (range: 8.1–15.8 years old) | Reduced back pain and improved BMD | Only 3 patients were reported |
|
| ||||||||
| Hawker et al. [56] | 2005 | Before-after trial | 23 | All on deflazacort | Alendronate 0.08 mg/kg/day PO | 10.8 years old (range: 6.9–15.6 years old) | Positive effect on BMD and Z-scores, better BMD outcome when given early in the course of disease | Also received 750 mg daily calcium and 1000 IU vitamin D |
Many issues remain to be defined regarding the use of bisphosphonates in patients with DMD who exhibit clinical bone fragility and/or low bone density, as to the best dose, best preparation, ideal length of therapy, or whether patients should receive a drug holiday (time-off bisphosphonate therapy). Moreover, it is unclear whether low bone mass without fractures should justify prophylactic therapy with bisphosphonates, similar to what has been recommended in adults for prophylactic treatment of steroid-induced osteoporosis (prior to first fracture) [58]. Sarkozy et al. presented 43 DMD boys who received prophylactic oral risedronate for an average of 24 months whose median lumbar spine adjusted BMD Z-scores remained stable during treatment (0.11 at baseline and 0.21 at 3-year followup); however, 7 boys had a long bone fracture and 3 had vertebral fractures on therapy (the majority having been on glucocorticoids for >36 months by the time of fracture) [59]. More research is needed to address these important questions.
6.4. Prospective Treatments
Two possible treatments for low bone density that have not been evaluated in DMD patients are denosumab and recombinant parathyroid hormone (PTH). Denosumab is a human monoclonal antibody against receptor activator of nuclear factor-κB-ligand (RANKL). Inhibiting RANKL, which leads to decreased osteoclast number and activity, results in less bone resorption [60]. Denosumab has been used in a pediatric patient with Paget's disease [60] and also a few children with osteogenesis imperfecta type VI [61]. Another possible treatment would be recombinant PTH (teriparatide). In a 19-year-old patient with osteoporosis pseudoglioma, teriparatide was used to increase BMD [62].
A new treatment (for DMD in general) under clinical study is ataluren, a molecule that binds with ribosomes and may allow the insertion of an amino acid in the premature termination codon, and exon-skipping, which binds with ribonucleic acid (RNA) and excludes specific sites of RNA splicing, producing a dystrophin that is a smaller but more functional molecule. There are also studies underway that attempt to modulate other muscular proteins, such as myostatin and utrophin, to reduce DMD symptoms [21].
Melatonin may also have a role in treating both muscle function and bone metabolism in DMD. Intraperitoneal injections and subcutaneous implants of melatonin demonstrated improved muscle function in the dystrophic mdx5Cv mouse model for DMD [63]. This improved function may be due to melatonin's ability to normalize proinflammatory cytokines (such as nitrites (NOx), interleukin- (IL-) 1β, IL-2, IL-6, tumor necrosis factor-α, and interferon-γ) and reduce oxidative stress [64]. When cultured with melatonin, mouse osteoblastic MC3T3-E1 cells showed increased differentiation and mineralization by Alizarin red S staining [65]. In ddy mice, pharmacological doses of melatonin demonstrated a 36% increase in BMD (P < 0.005) possibly by decreasing the RANKL (and thus reducing bone resorption) [66]. The muscle function and BMD benefits need to be investigated specifically in DMD patients prior to widespread use.
7. Conclusions
Poor bone health is a significant concern in DMD patients as it can contribute to loss of ambulation and adversely affect quality of life and possibly impact survival. There is a great need for evidence based practice guidelines of management of bone health in patients with DMD. Screening for vitamin D deficiency/insufficiency and low bone mass should be routinely pursued in all patients with DMD, particularly those treated with corticosteroids, while ensuring adequate vitamin D and calcium intake. More studies are needed to determine the timing of first bone density test and the clinical usefulness of biochemical bone turnover markers and other newer imaging techniques such as vertebral morphometry by DXA, peripheral quantitative computed tomography, and micromagnetic resonance imaging to accurately assess bone quality and predict fracture risk. Early intervention to improve bone health by vibration therapy requires more study to validate the benefits as seen in other neuromuscular diseases. Bisphosphonates have generally been recommended to treat bone fragility, but prospective randomized controlled studies with long-term followup are needed to confirm their antifracture efficacy and safety. New investigations as to the benefit of teriparatide, melatonin, or denosumab on BMD and fracture risk in this patient population should be explored.
Abbreviations
- DMD:
Duchenne muscular dystrophy
- CK:
Creatine kinase
- BMD:
Bone mineral density
- DXA:
Dual energy X-ray bsorptiometry
- OC:
Osteocalcin
- BALP:
Bone-specific alkaline phosphatase
- PINP:
Procollagen intact amino-terminal propeptide
- ICTP:
Carboxyterminal telopeptide
- NTx:
N-terminal cross-linked telopeptide
- TRACP-5b:
Osteoclast-derived, tartrate-resistant, acid phosphatase isoform 5b
- QCT:
Quantitative computed tomography
- PTH:
Parathyroid hormone
- RANKL:
Receptor activator of nuclear factor κB-ligand
- RNA:
Ribonucleic acid
- RDA:
Recommended daily allowance
- IL:
Interleukin.
Conflict of Interests
The authors declare that they have no conflict of interests or financial disclosures.
References
- 1.Deconinck N., Dan B. Pathophysiology of duchenne muscular dystrophy: current hypotheses. Pediatric Neurology. 2007;36(1):1–7. doi: 10.1016/j.pediatrneurol.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 2.Flanigan K. M. The muscular dystrophies. Seminars in Neurology. 2012;32(3):255–263. doi: 10.1055/s-0032-1329199. [DOI] [PubMed] [Google Scholar]
- 3.Biggar W. D., Bachrach L. K., Henderson R. C., Kalkwarf H., Plotkin H., Wong B. L. Bone health in Duchenne muscular dystrophy: a workshop report from the meeting in Cincinnati, Ohio, July 8, 2004. Neuromuscular Disorders. 2005;15(1):80–85. doi: 10.1016/j.nmd.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Matthews D. J., James K. A., Miller L. A., et al. Use of corticosteroids in a population-based cohort of boys with duchenne and becker muscular dystrophy. Journal of Child Neurology. 2010;25(11):1319–1324. doi: 10.1177/0883073810362762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonifati M. D., Ruzza G., Bonometto P., et al. A multicenter, double-blind, randomized trial of deflazacort versus prednisone in duchenne muscular dystrophy. Muscle and Nerve. 2000;23(9):1344–1347. doi: 10.1002/1097-4598(200009)23:9&lt;1344::AID-MUS4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 6.Moxley R. T., III, Pandya S., Ciafaloni E., Fox D. J., Campbell K. Change in natural history of Duchenne muscular dystrophy with long-term corticosteroid treatment: implications for management. Journal of Child Neurology. 2010;25(9):1116–1129. doi: 10.1177/0883073810371004. [DOI] [PubMed] [Google Scholar]
- 7.Lebel D. E., Corston J. A., McAdam L. C., Biggar W. D., Alman B. A. Glucocorticoid treatment for the prevention of scoliosis in children with Duchenne muscular dystrophy: long-term follow-up. The Journal of Bone & Joint Surgery: American Volume. 2013;95(12):1057–1061. doi: 10.2106/jbjs.l.01577. [DOI] [PubMed] [Google Scholar]
- 8.Daftary A. S., Crisanti M., Kalra M., Wong B., Amin R. Effect of long-term steroids on cough efficiency and respiratory muscle strength in patients with Duchenne muscular dystrophy. Pediatrics. 2007;119(2):e320–e324. doi: 10.1542/peds.2006-1400. [DOI] [PubMed] [Google Scholar]
- 9.Balaban B., Matthews D. J., Clayton G. H., Carry T. Corticosteroid treatment and functional improvement in Duchenne muscular dystrophy long-term effect. American Journal of Physical Medicine and Rehabilitation. 2005;84(11):843–850. doi: 10.1097/01.phm.0000184156.98671.d0. [DOI] [PubMed] [Google Scholar]
- 10.Biggar W. D., Harris V. A., Eliasoph L., Alman B. Long-term benefits of deflazacort treatment for boys with Duchenne muscular dystrophy in their second decade. Neuromuscular Disorders. 2006;16(4):249–255. doi: 10.1016/j.nmd.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Schram G., Fournier A., Leduc H., et al. All-cause mortality and cardiovascular outcomes with prophylactic steroid therapy in Duchenne muscular dystrophy. Journal of the American College of Cardiology. 2013;61(9):948–954. doi: 10.1016/j.jacc.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Hussein G., Mansour L., Ghafar H. A., Mostafa F. A., Fawaz L. Short-term effects of corticosteroid therapy on cardiac and skeletal muscles in muscular dystrophies. Journal of Investigative Medicine. 2014;62(6):875–879. doi: 10.1097/01.JIM.0000446835.98223.ce. [DOI] [PubMed] [Google Scholar]
- 13.Talim B., Malaguti C., Gnudi S., Politano L., Merlini L. Vertebral compression in Duchenne muscular dystrophy following deflazacort. Neuromuscular Disorders. 2002;12(3):294–295. doi: 10.1016/S0960-8966(01)00307-8. [DOI] [PubMed] [Google Scholar]
- 14.Bothwell J. E., Gordon K. E., Dooley J. M., MacSween J., Cummings E. A., Salisbury S. Vertebral fractures in boys with Duchenne muscular dystrophy. Clinical Pediatrics. 2003;42(4):353–356. doi: 10.1177/000992280304200408. [DOI] [PubMed] [Google Scholar]
- 15.Van Staa T. P., Cooper C., Leufkens H. G. M., Bishop N. Children and the Risk of Fractures Caused by Oral Corticosteroids. Journal of Bone and Mineral Research. 2003;18(5):913–918. doi: 10.1359/jbmr.2003.18.5.913. [DOI] [PubMed] [Google Scholar]
- 16.King W. M., Ruttencutter R., Nagaraja H. N., et al. Orthopedic outcomes of long-term daily corticosteroid treatment in Duchenne muscular dystrophy. Neurology. 2007;68(19):1607–1613. doi: 10.1212/01.wnl.0000260974.41514.83. [DOI] [PubMed] [Google Scholar]
- 17.Bianchi M. L., Mazzanti A., Galbiati E., et al. Bone mineral density and bone metabolism in Duchenne muscular dystrophy. Osteoporosis International. 2003;14(9):761–767. doi: 10.1007/s00198-003-1443-y. [DOI] [PubMed] [Google Scholar]
- 18.Söderpalm A.-C., Magnusson P., Åhlander A.-C., et al. Low bone mineral density and decreased bone turnover in Duchenne muscular dystrophy. Neuromuscular Disorders. 2007;17(11-12):919–928. doi: 10.1016/j.nmd.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Bachrach L. K. Taking steps towards reducing osteoporosis in Duchenne muscular dystrophy. Neuromuscular Disorders. 2005;15(1):86–87. doi: 10.1016/j.nmd.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Bushby K., Finkel R., Birnkrant D. J., et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. The Lancet Neurology. 2010;9(1):77–93. doi: 10.1016/s1474-4422(09)70271-6. [DOI] [PubMed] [Google Scholar]
- 21.de Los Angeles Beytía M., Vry J., Kirschner J. Drug treatment of Duchenne muscular dystrophy: available evidence and perspectives. Acta Myologica. 2012;31(1):4–8. [PMC free article] [PubMed] [Google Scholar]
- 22.McDonald D. G. M., Kinali M., Gallagher A. C., et al. Fracture prevalence in Duchenne muscular dystrophy. Developmental Medicine & Child Neurology. 2002;44(10):695–698. doi: 10.1017/s0012162201002778. [DOI] [PubMed] [Google Scholar]
- 23.Larson C. M., Henderson R. C. Bone mineral density and fractures in boys with Duchenne muscular dystrophy. Journal of Pediatric Orthopaedics. 2000;20(1):71–74. doi: 10.1097/00004694-200001000-00016. [DOI] [PubMed] [Google Scholar]
- 24.James K. A., Cunniff C., Apkon S. D., et al. Risk factors for first fractures among males with duchenne or becker muscular dystrophy. Journal of Pediatric Orthopaedics. 2014 doi: 10.1097/bpo.0000000000000348. [DOI] [PubMed] [Google Scholar]
- 25.Vestergaard P., Glerup H., Steffensen B. F., Rejnmark L., Rahbek J., Mosekilde L. Fracture risk in patients with muscular dystrophy and spinal muscular atrophy. Journal of Rehabilitation Medicine. 2001;33(4):150–155. doi: 10.1080/16501970119066. [DOI] [PubMed] [Google Scholar]
- 26.Tian C., Wong B., Hornung L., et al. MON-0162: Age-Specific Prevalence of Osteoporosis and Frequency of Poor Bone Health Indices in Duchenne Muscular Dystrophy. 2014. [Google Scholar]
- 27.Mayo A. L., Craven B. C., McAdam L. C., Biggar W. D. Bone health in boys with Duchenne Muscular Dystrophy on long-term daily deflazacort therapy. Neuromuscular Disorders. 2012;22(12):1040–1045. doi: 10.1016/j.nmd.2012.06.354. [DOI] [PubMed] [Google Scholar]
- 28.Leung D. G., Germain-Lee E. L., Denger B. E., Wagner K. R. Report on the second endocrine aspects of Duchenne Muscular Dystrophy Conference December 1-2, 2010, Baltimore, Maryland, USA. Neuromuscular Disorders. 2011;21(8):594–601. doi: 10.1016/j.nmd.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Quinlivan R., Shaw N., Bushby K. 170th ENMC International Workshop: Bone protection for corticosteroid treated Duchenne muscular dystrophy. 27–29 November 2009, Naarden, The Netherlands. Neuromuscular Disorders. 2010;20(11):761–769. doi: 10.1016/j.nmd.2010.07.272. [DOI] [PubMed] [Google Scholar]
- 30.Iuliano-Burns S., Saxon L., Naughton G., Gibbons K., Bass S. L. Regional specificity of exercise and calcium during skeletal growth in girls: a randomized controlled trial. Journal of Bone and Mineral Research. 2003;18(1):156–162. doi: 10.1359/jbmr.2003.18.1.156. [DOI] [PubMed] [Google Scholar]
- 31.McDonald C. M., Fowler W. M., Jr. The role of the neuromuscular medicine and physiatry specialists in the multidisciplinary management of neuromuscular disease. Physical Medicine and Rehabilitation Clinics of North America. 2012;23(3):475–493. doi: 10.1016/j.pmr.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henricson E., Abresch R., Han J. J., et al. The 6-minute walk test and person-reported outcomes in boys with duchenne muscular dystrophy and typically developing controls: longitudinal comparisons and clinically-meaningful changes over one year. PLoS Currents. 2013;5 doi: 10.1371/currents.md.9e17658b007eb79fcd6f723089f79e06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lue Y.-J., Lin R.-F., Chen S.-S., Lu Y.-M. Measurement of the functional status of patients with different types of muscular dystrophy. The Kaohsiung Journal of Medical Sciences. 2009;25(6):325–333. doi: 10.1016/s1607-551x(09)70523-6. [DOI] [PubMed] [Google Scholar]
- 34.Holick M. F., Binkley N. C., Bischoff-Ferrari H. A., et al. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. The Journal of Clinical Endocrinology & Metabolism. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 35.Jürimäe J. Interpretation and application of bone turnover markers in children and adolescents. Current Opinion in Pediatrics. 2010;22(4):494–500. doi: 10.1097/MOP.0b013e32833b0b9e. [DOI] [PubMed] [Google Scholar]
- 36.Cimaz R., Gattorno M., Sormani M. P., et al. Changes in markers of bone turnover and inflammatory variables during alendronate therapy in pediatric patients with rheumatic diseases. The Journal of Rheumatology. 2002;29(8):1786–1792. [PubMed] [Google Scholar]
- 37.Valta H., Lahdenne P., Jalanko H., Aalto K., Mäkitie O. Bone health and growth in glucocorticoid-treated patients with juvenile idiopathic arthritis. The Journal of Rheumatology. 2007;34(4):831–836. [PubMed] [Google Scholar]
- 38.Mäkitie O., Doria A. S., Henriques F., et al. Radiographic vertebral morphology: a diagnostic tool in pediatric osteoporosis. The Journal of Pediatrics. 2005;146(3):395–401. doi: 10.1016/j.jpeds.2004.10.052. [DOI] [PubMed] [Google Scholar]
- 39.Nakhla M., Scuccimarri R., Duffy K. N. W., et al. Prevalence of vertebral fractures in children with chronic rheumatic diseases at risk for osteopenia. The Journal of Pediatrics. 2009;154(3):438–443. doi: 10.1016/j.jpeds.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 40.Blake G. M., Rea J. A., Fogelman I. Vertebral morphometry studies using dual-energy X-ray absorptiometry. Seminars in Nuclear Medicine. 1997;27(3):276–290. doi: 10.1016/s0001-2998(97)80029-3. [DOI] [PubMed] [Google Scholar]
- 41.King W. M., Kissel J. T., Visy D., Goel P. K., Matkovic V. Skeletal health in Duchenne dystrophy: bone-size and subcranial dual-energy X-ray absorptiometry analyses. Muscle & Nerve. 2014;49(4):512–519. doi: 10.1002/mus.23963. [DOI] [PubMed] [Google Scholar]
- 42.Kalkwarf H. J., Abrams S. A., DiMeglio L. A., Koo W. W. K., Specker B. L., Weiler H. Bone densitometry in infants and young children: the 2013 ISCD pediatric official positions. Journal of Clinical Densitometry. 2014;17(2):243–257. doi: 10.1016/j.jocd.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Crabtree N. J., Arabi A., Bachrach L. K., et al. Dual-energy x-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD pediatric official positions. Journal of Clinical Densitometry. 2014;17(2):225–242. doi: 10.1016/j.jocd.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Fewtrell M. S. Bone densitometry in children assessed by dual X ray absorptiometry: uses and pitfalls. Archives of Disease in Childhood. 2003;88(9):795–798. doi: 10.1136/adc.88.9.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bianchi M. L., Morandi L., Andreucci E., Vai S., Frasunkiewicz J., Cottafava R. Low bone density and bone metabolism alterations in Duchenne muscular dystrophy: response to calcium and vitamin D treatment. Osteoporosis International. 2011;22(2):529–539. doi: 10.1007/s00198-010-1275-5. [DOI] [PubMed] [Google Scholar]
- 46.Myers K. A., Ramage B., Khan A., Mah J. K. Vibration therapy tolerated in children with duchenne muscular dystrophy: a pilot study. Pediatric Neurology. 2014;51(1):126–129. doi: 10.1016/j.pediatrneurol.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 47.Söderpalm A.-C., Kroksmark A.-K., Magnusson P., Karlsson J., Tulinius M., Swolin-Eide D. Whole body vibration therapy in patients with Duchenne muscular dystrophy—a prospective observational study. Journal of Musculoskeletal Neuronal Interactions. 2013;13(1):13–18. [PubMed] [Google Scholar]
- 48.Sitjà Rabert M., Rigau Comas D., Fort Vanmeerhaeghe A., et al. Whole-body vibration training for patients with neurodegenerative disease. The Cochrane Database of Systematic Reviews. 2012;2 doi: 10.1002/14651858.CD009097.pub2.CD009097 [DOI] [PubMed] [Google Scholar]
- 49.Reyes M. L., Hernández M., Holmgren L. J., Sanhueza E., Escobar R. G. High-frequency, low-intensity vibrations increase bone mass and muscle strength in upper limbs, improving autonomy in disabled children. Journal of Bone and Mineral Research. 2011;26(8):1759–1766. doi: 10.1002/jbmr.402. [DOI] [PubMed] [Google Scholar]
- 50.Ruck J., Chabot G., Rauch F. Vibration treatment in cerebral palsy: a randomized controlled pilot study. Journal of Musculoskeletal Neuronal Interactions. 2010;10(1):77–83. [PubMed] [Google Scholar]
- 51.Wren T. A. L., Lee D. C., Hara R., et al. Effect of high-frequency, low-magnitude vibration on bone and muscle in children with cerebral palsy. Journal of Pediatric Orthopaedics. 2010;30(7):732–738. doi: 10.1097/BPO.0b013e3181efbabc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Houston C., Mathews K., Shibli-Rahhal A. Bone density and alendronate effects in Duchenne muscular dystrophy patients. Muscle & Nerve. 2014;49(4):506–511. doi: 10.1002/mus.23948. [DOI] [PubMed] [Google Scholar]
- 53.Sbrocchi A. M., Rauch F., Jacob P., et al. The use of intravenous bisphosphonate therapy to treat vertebral fractures due to osteoporosis among boys with Duchenne muscular dystrophy. Osteoporosis International. 2012;23(11):2703–2711. doi: 10.1007/s00198-012-1911-3. [DOI] [PubMed] [Google Scholar]
- 54.Gordon K. E., Dooley J. M., Sheppard K. M., MacSween J., Esser M. J. Impact of bisphosphonates on survival for patients with Duchenne muscular dystrophy. Pediatrics. 2011;127(2):e353–e358. doi: 10.1542/peds.2010-1666. [DOI] [PubMed] [Google Scholar]
- 55.Atance E. P., Herrera M. B., de la Plata M. A. M., Cano E. M., Vilchez R. C. Alendronato en el tratamiento de la osteoporosis secundaria a la distrofia muscular de Duchenne. Anales de Pediatría. 2011;74(2):122–125. doi: 10.1016/j.anpedi.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 56.Hawker G. A., Ridout R., Harris V. A., Chase C. C., Fielding L. J., Biggar W. D. Alendronate in the treatment of low bone mass in steroid-treated boys with Duchenne's muscular dystrophy. Archives of Physical Medicine and Rehabilitation. 2005;86(2):284–288. doi: 10.1016/j.apmr.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 57.de Groen P. C., Lubbe D. F., Hirsch L. J., et al. Esophagitis associated with the use of alendronate. The New England Journal of Medicine. 1996;335(14):1016–1021. doi: 10.1056/nejm199610033351403. [DOI] [PubMed] [Google Scholar]
- 58.Grossman J. M., Gordon R., Ranganath V. K., et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care & Research. 2010;62(11):1515–1526. doi: 10.1002/acr.20295. [DOI] [PubMed] [Google Scholar]
- 59.Sarkozy A., Srinivasan R., Rawlings D., et al. G.P.97: prophylactic oral bisphosphonate therapy in Duchenne muscular dystrophy: the Newcastle upon Tyne experience. Neuromuscular Disorders. 2014;24(9-10):p. 824. doi: 10.1016/j.nmd.2014.06.111. [DOI] [Google Scholar]
- 60.Grasemann C., Schündeln M. M., Hövel M., et al. Effects of RANK-ligand antibody (denosumab) treatment on bone turnover markers in a girl with juvenile paget's disease. The Journal of Clinical Endocrinology & Metabolism. 2013;98(8):3121–3126. doi: 10.1210/jc.2013-1143. [DOI] [PubMed] [Google Scholar]
- 61.Hoyer-Kuhn H., Netzer C., Koerber F., Schoenau E., Semler O. Two years' experience with denosumab for children with osteogenesis imperfecta type VI. Orphanet Journal of Rare Diseases. 2014;9, article 145 doi: 10.1186/s13023-014-0145-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arantes H. P., Barros E. R., Kunii I., Bilezikian J. P., Lazaretti-Castro M. Teriparatide increases bone mineral density in a man with osteoporosis pseudoglioma. Journal of Bone and Mineral Research. 2011;26(12):2823–2826. doi: 10.1002/jbmr.530. [DOI] [PubMed] [Google Scholar]
- 63.Hibaoui Y., Reutenauer-Patte J., Patthey-Vuadens O., Ruegg U. T., Dorchies O. M. Melatonin improves muscle function of the dystrophic mdx5Cv mouse, a model for Duchenne muscular dystrophy. Journal of Pineal Research. 2011;51(2):163–171. doi: 10.1111/j.1600-079X.2011.00871.x. [DOI] [PubMed] [Google Scholar]
- 64.Chahbouni M., Escames G., Venegas C., et al. Melatonin treatment normalizes plasma pro-inflammatory cytokines and nitrosative/oxidative stress in patients suffering from Duchenne muscular dystrophy. Journal of Pineal Research. 2010;48(3):282–289. doi: 10.1111/j.1600-079x.2010.00752.x. [DOI] [PubMed] [Google Scholar]
- 65.Park K.-H., Kang J. W., Lee E.-M., et al. Melatonin promotes osteoblastic differentiation through the BMP/ERK/Wnt signaling pathways. Journal of Pineal Research. 2011;51(2):187–194. doi: 10.1111/j.1600-079x.2011.00875.x. [DOI] [PubMed] [Google Scholar]
- 66.Koyama H., Nakade O., Takada Y., Kaku T., Lau K.-H. W. Melatonin at pharmacologic doses increases bone mass by suppressing resorption through down-regulation of the RANKL-mediated osteoclast formation and activation. Journal of Bone and Mineral Research. 2002;17(7):1219–1229. doi: 10.1359/jbmr.2002.17.7.1219. [DOI] [PubMed] [Google Scholar]
- 67.Golden N. H., Abrams S. A., Daniels S. R., et al. Optimizing bone health in children and adolescents. Pediatrics. 2014;134(4):e1229–e1243. doi: 10.1542/peds.2014-2173. [DOI] [PubMed] [Google Scholar]


