Abstract
Orchid bees compose an exclusive Neotropical pollinators group, with bright body coloration. Several of those species build their own nests, while others are reported as nest cleptoparasites. Here, the objective was to evaluate whether the inclusion of a strong biotic interaction, such as the presence of a host species, improved the ability of species distribution models (SDMs) to predict the geographic range of the cleptoparasite species. The target species were Aglae caerulea and its host species Eulaema nigrita. Additionally, since A. caerulea is more frequently found in the Amazon rather than the Cerrado areas, a secondary objective was to evaluate whether this species is increasing or decreasing its distribution given South American past and current climatic conditions. SDMs methods (Maxent and Bioclim), in addition with current and past South American climatic conditions, as well as the occurrences for A. caerulea and E. nigrita were used to generate the distribution models. The distribution of A. caerulea was generated with and without the inclusion of the distribution of E. nigrita as a predictor variable. The results indicate A. caerulea was barely affected by past climatic conditions and the populations from the Cerrado savanna could be at least 21,000 years old (the last glacial maximum), as well as the Amazonian ones. On the other hand, in this study, the inclusion of the host-cleptoparasite interaction complex did not statistically improve the quality of the produced models, which means that the geographic range of this cleptoparasite species is mainly constrained by climate and not by the presence of the host species. Nonetheless, this could also be caused by unknown complexes of other Euglossini hosts with A. caerulea, which still are still needed to be described by science.
Introduction
Associations between host and parasitic species among Neotropical orchid bees (Hymenoptera: Apidae: Euglossini) have been mostly established based on casual and fortuitous reports (revised by [1,2]). Two genera among orchid bees comprise exclusively cleptoparasitic species: the monotypic Aglae Lepeletier & Serville, and Exaerete Hoffmannsegg, comprising eight species [3]. Besides field observations of cleptoparasitism, such behavior can also be inferred from particular morphological features of females of the parasitic species, such as the lack of corbiculae, a synapomorphic character shared by females of all non-parasitic Apini, Bombini, Euglossini and Meliponini, used in harvesting pollen and returning it to the nest or hive [4]. Species belonging to these two genera, Aglae and Exaerete, are known to be cleptoparasites on the nests of Eufriesea Cockerell and Eulaema Lepeletier species (revised by [2]).
Usually considered as an Amazonian orchid bee, Aglae caerulea Lepeletier & Servile (Apidae: Euglossini) has been recently reported within several savannic areas [5–7]. Although not widely distributed, individuals of this species are not particularly rare where the species occur (e.g. [8]). At least 15 species of Eulaema and 20 species of Eufriesea occur sympatrically with A. caerulea, but it has been only reported as an obligatory cleptoparasite on the nests of Eulaema nigrita Lepeletier, based on a single anedoctal record by J. G. Myers, in 1935 [9]. Eulaema nigrita is a large-bodied orchid bee which pollinates several different plant species [10–12] presenting a wide distribution in the Neotropics, ranging from Central America to southern Brazil and Argentina, being usually associated with fragmented and disturbed areas [13–16]. The aforementioned new occurrences for A. caerulea in savannic areas in Central Brazil raised doubts whether its populations are expanding their ranges towards central South America or are retreating their distribution backwards to the Amazon after several expansion and contraction events of this forest formation in previous moments of South America climatic history [17–23].
Here, species distribution models (SDMs hereon) were used to predict the potential distribution of a cleptoparasite orchid bee considering the distribution of its host as a predictor variable on both current and past scenarios of climatic conditions. Climatic conditions are one of the main factors determining species distributions, and thus, their inter-specific relationships [24–27]. Although South America was not covered by ice in any of the known glacial events that occurred in the planet, the climatic conditions of the Last Glacial Maximum (LGM hereon) were different from the current climate, and with an expected impact on the ranges of species [28,29]. Despite previous warmer moments, where the Amazonian was interconnected to the Atlantic forest, the palynological and vegetation evidences [18,30–33] show such connection was not reestablished with the temperature rise after the LGM, around five thousand years ago. The same period when the dry South American diagonal last developed with the interconnection of the current biomes of Caatinga, Cerrado, and Chaco [34,35].
Paleodistribution modelling has been successfully employed to analyze Quaternary megafaunal extinction events [36,37] and these methods are usually considered interesting tools to predict past species ranges [38,39]. These models correlate known occurrences of the focus species with environmental variables to create a multidimensional environmental space. Then, the species potential distribution is projected onto areas in the geographic space with similar environmental conditions to those found in the known occurrences [40].
Despite the advantages involved with the use of SDMs, they usually do not include biotic interactions to determine species ranges [41,42], an important determinant of species occurrences elsewhere [43]. Biotic interactions could vary from extremely positive interactions (e.g. mutualism) to extremely negative interactions (e.g. predation). Positive interactions could allow species to inhabit areas outside their own physiologic limits, while negative interactions (e.g. competition, parasitism, predation) could prevent species from inhabiting environmental favorable areas [44,45]. Many studies have already been developed on the attempt to integrate biotic interactions and species potential distributions, with results on the predictions of the potential distributions of the modeled species varying from significant improvement [46–49], mild or no improvement [50], and even decreases in the predictions of the modeled species [51].
Here, two different SDMs, Bioclim and Maxent, were used to predict the potential distribution of A. caerulea and E. nigrita. Two different models for A. caerulea were constructed, one based on the climatic conditions, and a second one in which the strong biotic interaction between the species was included by using the presence of E. nigrita as a predictor in the model of A. caerulea. Specifically, the study’s aims were: 1) to understand the role of Quaternary climatic changes on the distribution of both species; 2) to determine whether the recently reported occurrences of A. caerulea from savannic areas in Central Brazil are recent expansions from the Amazonian populations, or on the contrary, whether they are ancient populations of the species; 3) to test whether the map predictions for A. caerulea change after including a strong biotic interaction like host-parasite dependence in the model. As far as we are concerned, this is the first attempt to integrate host-parasite interactions across time using hindcast predictions with paleodistribution modeling.
Methods
Species occurrences
The casual sampling of new occurrences for A. caerulea used here, that generated the question whether this species was increasing or decreasing its current distribution when compared to the its past one, were the same used in Silva et al. [7]. However, additional occurrences obtained from Abrahamczyk et al. [52] and Freitas and Nemésio [6] were included here. The occurrences of E. nigrita used here were obtained from CRIA’s Species Link (www.splink.org.br) and GBIF (www.gbif.org), with the same methods employed by Silva et al. [7] to georeference occurrences. For those occurrences with reliable geographic information, but lacking specific latitude/longitude information, Google Earth [53] was employed to acquire municipality geographic information, later used in the modeling procedures. Additional occurrences were obtained in the published literature, as shown in the Supplementary Material (S1 Text). The samplings of new occurrecens for A. caeruea in Silva et al. [7] occurred in particular areas with the consent of the property’s owner. The occurrence data for both host and cleptoparasite bees are available in the supplementary material (S1 File and S2 File).
Climatic variables
Worldclim’s current variables and CCSM and MIROC General Circulation Models Worldclim variables (www.worldclim.org) at 2.5 minutes resolution were used to produce the different models. Five variables out of the 19 variables available from WorldClim [54] were selected to produce the distribution models: BIO 4 (Temperature Seasonality (standard deviation *100)), BIO8 (Mean Temperature of Wettest Quarter), BIO9 (Mean Temperature of Driest Quarter), BIO16 (Precipitation of Wettest Quarter) and BIO17 (Precipitation of Driest Quarter). Temperature seasonality was used because this variable has been related to the distribution of euglossine species [52]. Temperature and precipitation of the driest and the wettest quarter were also considered. In tropical and subtropical South America, the dry and the wet season clearly influence the biotic cycles of these orchid bee species [52,55].
Ecological niche models
Two different algorithms, Maxent [56,57] and Bioclim [58] were selected to produce the potential distributions. Those two models are different approaches for predicting the distribution of species when the data sample has no real absences (necessary if logistic regressions or classification techniques are required; [59]). Maxent model fits an algorithm to separate the occurrence records from the background conditions [60], while Bioclim map the percentile distribution of the occurrences in the studied extent [61].
Maxent and Bioclim model predictions could be sensitive to bias and errors in the data [62]. For that reason, species occurrences have been filtered to select a balanced calibration data set [63,64]. The selected calibration data sets should be balanced in the environmental space, but not necessarily in the geographic space (geographically close occurrences could have different and thus, important information for the model, while geographically separated occurrences could have similar environmental information). The envSamp R-function (https://github.com/SaraVarela/envSample) was used to filter the data. Three environmental filters were set: BIO4 (Temperature seasonality: standard deviation *100) = 200, BIO16 (Precipitation of Wettest Quarter) = 200 mm, BIO17 (Precipitation of Driest Quarter) = 200 mm. By doing so, 25 out of the 44 presumably biased raw occurrences for A. caerulea were used (S1 Fig). The 19 discarded points were used to test the model predictions. In addition, the same filter was also applied to the 290 raw occurrences from E. nigrita, and 66 balanced occurrences were selected to calibrate the models (S2 Fig). The 224 discarded occurrences were used to test the model predictions. The Area Under the receiver-operator Curve (AUC hereon) was calculated as a measure of the discriminative power of the Maxent results. The AUC values for A. caerulea could be compared between models because the same training and testing occurrence records were used, plus the same geographic extent to train the Maxent models [65].
For constructing the models, the functions Bioclim and Maxent from the dismo R-package [66] were used. The model using the filtered occurrences (see above) was calibrated to overcome the potential biases of the raw occurrence dataset. For Maxent, the model was calibrated using the filtered occurrences, plus 10,000 background points from the study extent (South America). Model overfitting was avoided by setting the argument betamultiplier = 2, not allowing the algorithm to construct a model using ad-hoc and complex equations [67]. To evaluate the models, the occurrences discarded from the training dataset were used, plus the same number of absences selected randomly from the study extent. Thus, since the number of occurrences and pseudoabsences were the same, the default value of prevalence 0.5 was used to evaluate the model predictions [65]. Other parameters not mentioned here were set as default.
Finally, the threshold that maximizes both sensitivity and specificity was used [62,68], for constructing the binary predictions for Bioclim and Maxent. The LGM maps are the result of averaging the predictions from the two general circulation models (CCSM and MIROC) and, subsequently, using the threshold to obtain the binary prediction. Omission errors (percentage of errors in the prediction of presences) were calculated. Neither commission errors nor True Skilled Statistics were calculated because there were no real georeferenced absences for either of the species considered. Consequently, the Akaike Criterion (AIC) and the relative likelihood (exp((AICminO-OAICj/2)) were used in order to compare the models for A. caerulea, after including and excluding the models of E. nigrita, its host, as a predictor variable. AIC is a measure of the quality of a model given a set of data [69] and these methods were done using ENMeval [70]. All R-scripts are available in https://github.com/SaraVarela/host_parasite_ENM.
Results
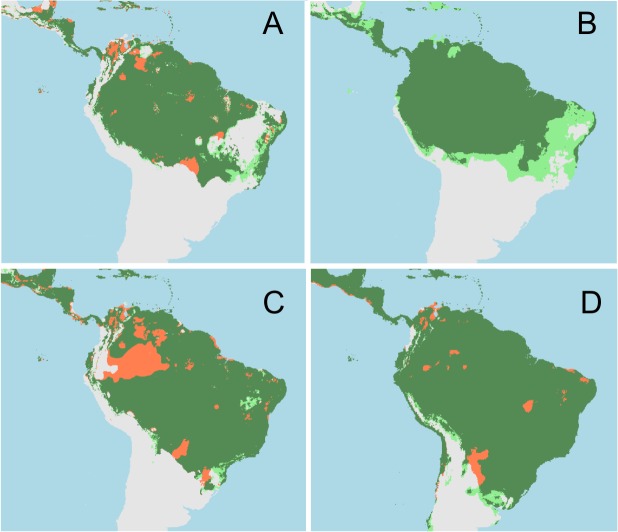
Precipitation of the wettest quarter (BIO16), Temperature seasonality (BIO4), and Precipitation of the driest quarter (BIO17) determined the distribution of A. caerulea, based in the Maxent results (Table 1). Both training and testing AUC values for the models considering the distribution of A. caerulea were high, reaching 0.79. Omission rate (percentage of errors in the occurrences) was equal to 0, what means that the occurrences of A. caerulea are being perfectly predicted. Thus, the model was able to predict both training and testing datasets. Maxent and Bioclim models predicted that the distribution of A. caerulea was not strongly affected by the LGM (Fig 1A and 1B). Following the results, current populations of A. caerulea from the Brazilian Cerrado could be as old as Amazonian populations.
Table 1. Maxent model results for A. caerulea, using only climatic variables to calibrate the model.
| Model training | |
| Training occurrences | 25 |
| Training background data | 10025 |
| Beta multiplier | 2 |
| Iterations | 260 |
| Training AUC | 0.790 |
| Individual contribution of the variables | |
| bio16 | 45.819 |
| bio17 | 12.626 |
| bio4 | 41.554 |
| bio8 | 0 |
| bio9 | 0 |
| Permutation importance of the variables | |
| bio16 | 60.076 |
| bio17 | 11.607 |
| bio4 | 28.316 |
| bio8 | 0 |
| bio9 | 0 |
| Model testing | |
| Testing occurrences | 19 |
| Testing absence data | 19 |
| Prevalence | 0.500 |
| Testing AUC | 0.790 |
Fig 1. Potential range shift of the species as a consequence of the climatic changes of the last 21,000 years.
Stable areas for both species are in dark green, areas predicted suitable during the Last Glacial Maximum are in red, and new expansions (younger than 21,000 years) in pale green. A) Aglae caerulea distribution according to Bioclim. B) Aglae caerulea distribution according to Maxent; C) Eulaema nigrita distribution according to Bioclim, D) Eulaema nigrita distribution according to Maxent. Climatic changes of the last 21,000 years did not strongly affect any of these species.
Temperature seasonality, Mean Temperature of Wettest Quarter, Precipitation of the driest quarter, and Mean Temperature of driest Quarter were the four variables that determined the distribution of E. nigrita according to Maxent results (Table 2). AUC reached 0.76 for the training data set and 0.75 for the testing data set. Thus, the model had a good discriminative power for the training and the testing datasets. In this case, omission rate was 0.02, what means that the presences of E. nigrita are also being perfectly predicted by the models. Bioclim and Maxent models predicted that E. nigrita have had a large potential suitable area in South America during both, glacial and interglacial periods (Fig 1C and 1D). The overlap of A. caerulea with its host, E. nigrita, was very similar from the LGM to the present, showing only 0.02% of change between each climatic scenario.
Table 2. Maxent model results for E. nigrita, using only climatic variables to calibrate the model.
| Model training | |
| Training occurrences | 66 |
| Training background data | 10066 |
| Beta multiplier | 2 |
| Iterations | 500 |
| Training AUC | 0.760 |
| Individual contribution of the variables | |
| bio16 | 0.914 |
| bio17 | 14.927 |
| bio4 | 46.306 |
| bio8 | 23.601 |
| bio9 | 14.250 |
| Permutation importance of the variables | |
| bio16 | 4.622 |
| bio17 | 14.320 |
| bio4 | 55.012 |
| bio8 | 26.044 |
| bio9 | 0 |
| Model testing | |
| Testing occurrences | 224 |
| Testing absence data | 224 |
| Prevalence | 0.500 |
| Testing AUC | 0.750 |
The Maxent results showed that climatic variables are still the most important factors determining the distribution of A. caerulea, after the distribution of E. nigrita was considered as an environmental predictor (Table 3). Precipitation of the wettest quarter (BIO16), Temperature seasonality (BIO4), and Precipitation of the driest quarter (BIO17) determined the distribution of A. caerulea based in the Maxent results (Table 3). The presence of E. nigrita had a small contribution to the final model of A. caerulea (Table 3). Overall, this model had an omission rate of 0.03, what means that the presences are being perfectly predicted) and a lower discriminative power than the climatic models, with a training AUC equal to 0.70. However, it has a high predictive power in the test sample, with AUC equal to 0.85. These results indicate that the relative likelihood of both models is similar (the relative likelihood of the complete model with the host species is < 0.0001), and statistically, no distinction of the best model solution can be made. When the presence of E. nigrita was included as one predictor variable for modeling the distribution of A. caerulea, the map predictions decreased. Specifically, the Bioclim predictions excluded western portions of the Amazon as potential distribution areas for A. caerulea (Fig 2A). Conversely, in the predictions generated by Maxent, eastern areas for A. caerulea became unstable (Fig 2B). Nonetheless, as E. nigrita has a broad suitable range, the potential distribution of A. caerulea potential distribution was not strongly affected by its host species in any of the periods considered.
Table 3. Maxent model results for A. caerulea, using climatic variables plus the presence of E. nigrita, its host species, as a predictor variable.
| Training the model | |
| Training occurrences | 25 |
| Training background data | 10025 |
| Beta multiplier | 2 |
| Iterations | 420 |
| Training AUC | 0.700 |
| Contribution of the variables | |
| bio16 | 45.800 |
| bio17 | 18.235 |
| bio4 | 35.031 |
| bio8 | 0 |
| bio9 | 0.250 |
| Presence of E. nigrita | 0.681 |
| Permutation importance of the variables | |
| bio16 | 22.285 |
| bio17 | 14.788 |
| bio4 | 61.446 |
| bio8 | 0 |
| bio9 | 0.529 |
| Presence of E. nigrita | 0.950 |
| Testing the model | |
| Testing occurrences | 19 |
| Testing absence data | 19 |
| Prevalence | 0.500 |
| Testing AUC | 0.850 |
Fig 2. Predictions regarding the current distribution of Aglae caerulea according to A) Bioclim and B) Maxent.
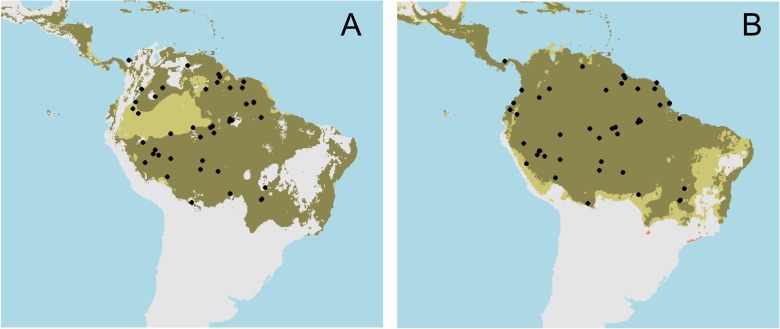
The areas in pale brown become unsuitable for the species after including the host species, Eulaema nigrita, as a predictor in the models. Points are the observed occurrences for A. caerulea. The main difference between the models is that Bioclim predictions with E. nigrita exclude the Western areas of the Amazonas and thus, when including this variable in the model for predicting A. caerulea, the resulting map shows this pattern.
Discussion
The predictions of the potential distribution of a host-parasite complex of South American orchid bees were assessed when the host species distribution was included as a predictor variable of the parasitic bee, in the current and past climatic conditions for the LGM event. Additionally, climatically stable areas for the potential distribution of A. caerulea in both periods were estimated, on an attempt to discover whether the newer distributional occurrences for this species in the Brazilian Cerrado savanna [5–7] are related to range increases or decreases of the species. Although the map predictions for the potential distribution of A. caerulea did not increase when the distribution of E. nigrita was considered as a predictor, suitable areas for A. caerulea in the Cerrado seemed to be as stable as the Amazonian ones. Additionally, some areas in the eastern Brazilian coast became climatically suitable for this species after the LGM. Finally, some areas became unsuitable for A. caerulea distribution in western Amazon and northeastern Brazil after the inclusion of the distribution of E. nigrita as a predictor variable determining the distribution of A. caerulea, depending on the algorithm considered.
Although ice sheets did not cover South America, past climatic changes had severe effects on South American forested areas (e.g. Amazon and Atlantic Forests) and their related biota [19–23]. Nonetheless, the distribution of A. caerulea, in both past and current climatic conditions, covers a similar area. Despite its usual xeric-like environment, the Brazilian Cerrado, the second largest biome in South America [33], has many evergreen gallery forests surrounding its rivers and streamlets. These habitats provide both environmental and biotic conditions for the maintenance of Amazonian fauna which disperse through the Cerrado biome, especially for Euglossini bees [71]. Consequently, this vegetation serve as an important dispersal route for A. caerulea [7] and may have exerted the same role in past contraction events of the Amazonian forest, also supporting the high climatic stability of A. caerulea distribution across time.
Nonetheless, host-parasite interactions may also explain the climatic stability observed for A. caerulea in this study. While A. caerulea is mainly distributed in Amazon with scattered occurrences in Cerrado, E. nigrita is a wide-ranged species, which benefits from and have higher abundances in opened areas or in anthropic areas [16,72,73]. The anecdotal report of this host-parasite complex occurred more than 80 years ago [9] and since then, A. caerulea has not been reported to parasitize nests of other orchid bees.
On the other hand, E. nigrita has been confirmed to be host to at least another widespread cleptoparasitic orchid bee, Exaerete smaragdina Guérin-Menéville, which is suggested to exert strong control on the populations of E. nigtrita [2]. This latter association may limit the availability of nests to be parasitized by A. caerulea in the region where the three species are sympatric, i.e., the Amazon and the Brazilian Cerrado. However, it should be stressed that A. caerulea might parasitize orchid bee species other than E. nigrita. Based on the relative frequencies of known host-parasite associations, Nemésio and Silveira (2) showed that some of these associations could not be exclusive, since the known parasitic species occurred in sites where the host had never been recorded or, alternatively, the relative frequency of the parasitic species was higher than that observed for the host species. The only explanation found by those authors to deal with such a discrepancy, considering the database is reliable, is that many parasitic species may have more than one host species. Almost 30 species of Eulaema and more than 60 species of Eufriesea are described from the Neotropical region [3]. Most of them have a similar size or are larger when compared to A. caerulea and many of them occur in sympatry with A. caerulea. Therefore, especially for those areas depicted as climatically unstable with the inclusion of E. nigrita as a predictor determining the distribution of A. caerulea, other orchid bees may serve as hosts for A. caerulea. Since host-parasite relationships in orchid bees are generally based on fortuitous observations [2], Eulaema nests other than E. nigrita, besides those of Eufriesea species, are plausible to be recorded as hosts for A. caerulea in future field studies aiming to establish such associations.
The use of biotic related variables in species distribution modeling are becoming increasingly frequent in macroecology, with variable results ranging from positive [46–49], neutral [50] to negative [51]. Even so, in this study, the inclusion of E. nigrita as a predictor of the distribution of A. caerulea did not increase the prediction of its potential distribution. Despite these results, the attempts to make distribution models more realistic, and consequently, encompass the biotic component of the Biotic-Abiotic-Migration (BAM) diagram [41,74] into the distribution models are interesting perspectives, especially if ongoing climate changes are considered. Many insect species rely on specific inter-specific biotic interactions (e.g. mutualism, parasitism) to survive and reproduce. Nonetheless, during historical and current scenarios of climate change, such interactions may uncouple or fail given spatial or phenological mismatches between the interacting species [75–79]. Therefore, further investigation on how interacting species will behave upon future climate changes are needed especially given concerning scenarios of biodiversity loss [80–82] for a better assessment on how will species interactions maintain themselves in the future. Similar approaches to those employed here, considering the potential range of interactions in different climatic scenarios, are already being done elsewhere and considering different biological groups and areas (e.g. [50,79,83,84]). These methods should be replicated either to obtain distribution models that are biologically more meaningful than those only using bioclimatic variables as well as to better understand the behavior of the SDMs when these biotic interactions are used as determinants of species distributions.
Supporting Information
Occurrences selected for calibrating the model are depicted in red, while those selected for testing the model are in grey. Selection was based on envSample R-function (https://github.com/SaraVarela/envSample).
(DOC)
The 66 occurrences selected for calibrating the model are depicted in red, while the 264 selected for testing the model are in grey. Selection was based on envSample R-function (https://github.com/SaraVarela/envSample).
(DOC)
(XLS)
(XLS)
(DOC)
Acknowledgments
The authors are grateful to S. Lodi, who revised a previous version of the manuscript. Two anonymous reviewers are also acknowledged for the important contributions to the first submitted version of the manuscript. PDMJ was continuously supported by several CNPq grants. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
PDMJ was continuously supported by several CNPq grants. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kimsey LS. Systematics of bees of the genus Eufriesea . Univ Calif Publ Entomol. 1982;95: 1–125. [Google Scholar]
- 2. Nemésio A, Silveira FA. Deriving ecological relationships from geographical correlations between host and parasitic species: An example with orchid bees. J Biogeogr. 2006;33: 91–97. [Google Scholar]
- 3. Nemésio A, Rasmussen C. Nomenclatural issues in the orchid bees (Hymenoptera: Apidae: Euglossina) and an updated catalogue. Zootaxa. 2011;42: 1–42. 10.1093/jncimonographs/lgr006 [DOI] [PubMed] [Google Scholar]
- 4. Michener CD. The Bees of the World 2nd ed. Baltimore: The Johns Hopkins University Press; 2007. [Google Scholar]
- 5. Anjos-Silva EJ, Camillo E, Garófalo CA. Occurrence of Aglae caerulea Lepeletier & Serville (Hymenoptera: Apidae: Euglossini) in the Parque Nacional da Chapada dos Guimarães, Mato Grosso State, Brazil. Neotrop Entomol. 2006;35: 868–870. [DOI] [PubMed] [Google Scholar]
- 6. Freitas F V, Nemésio A. Registro de fêmea de Aglae caerulea Lepeletier & Serville, 1825 (Hymenoptera: Apidae: Euglossina) em flor de Swartzia sp. (Fabaceae) em área de transição de Cerrado e Floresta Amazônica no Norte do Estado do Mato grosso, Brasil In: Simões ZLP, Antônio DSM, Bitondi MMG, editors. Anais do IX Encontro Sobre Abelhas. Ribeirão Preto, SP—Brasil: FUNPEC Editora; 2010. p. 425. [Google Scholar]
- 7. Silva DP, Aguiar AJC, Melo GAR, Anjos-Silva EJ, De Marco P Jr. Amazonian species within the Cerrado savanna: new records and potential distribution for Aglae caerulea (Apidae: Euglossini). Apidologie. 2013;44: 673–683. [Google Scholar]
- 8. Nemésio A, Rasmussen C. Sampling a biodiversity hotspot: the orchid-bee fauna (Hymenoptera: Apidae) of Tarapoto, northeastern Peru, the richest and most diverse site of the Neotropics. Rev Bras Biol. 2014;74: S033–S044. [DOI] [PubMed] [Google Scholar]
- 9. Myers JG. Ethological observations on the citrus bee, Trigona silvestriana Vachal, and other neotropical bees (Hym. Apoidea). Trans R Entomol Soc London. 1935;83: 131–142. [Google Scholar]
- 10. Dressler RL. Biology of the orchid bees (Euglossini). Annu Rev Ecol Syst. 1982;13: 373–394. [Google Scholar]
- 11. Williams H, Mark W. Orchid floral fragrances and male euglossine bees: methods and advances in the last sesquidecade. Biol Bull. 1983;164: 355–395. [Google Scholar]
- 12. Zucchi R, Sakagami SF, Camargo JMF. Biological observations on a neotropical parasocial bee, Eulaema nigrita, with a review on the biology of Euglossinae (Hymenoptera, Apidae). J Fac Sci Hokkaido Univ Ser VI Zool. 1969;17: 271–380. [Google Scholar]
- 13.Morato EF. Estudos sobre comunidades de abelhas Euglossini. Anais do III Encontro sobre Abelhas de Ribeirão Preto, São Paulo. Ribeirão Preto, SP—Brasil; 1998. pp. 135–143.
- 14. Nemésio A, Silveira FA. Edge effects on the orchid-bee fauna (Hymenoptera: Apidae) at a large remnant of Atlantic Rain Forest in southeastern Brazil. Neotrop Entomol. 2006;35: 313–323. [DOI] [PubMed] [Google Scholar]
- 15. Peruquetti RC, Campos LAO, Coelho CDP, Abrantes CVM, Lisboa LCO. Abelhas Euglossini (Apidae) de áreas de Mata Atlântica: abundância, riqueza e aspectos biológicos. Rev Bras Zool. 1999;16: 101–118. [Google Scholar]
- 16. Tonhasca A Jr, Blackmer JL, Albuquerque GS. Abundance and diversity of Euglossine bees in the Fragmented lndscape of the Brazilian Atlantic forest. Biotropica. 2002;34: 416–422. [Google Scholar]
- 17. Rizzini CT. Nota prévia sobre a divisão fitogeográfica do Brasil. Rev Bras Geogr. 1963;25: 1–64. [Google Scholar]
- 18. Bigarella JJ, Andrade-Lima D, Riehs PJ. Considerações a respeito das mudanças paleoambientais na distribuição de algumas espécies vegetais e animais no Brasil. An Acad Bras Cienc. 1975;47: 411–464. [Google Scholar]
- 19. Ledru MP. Late quaternary environmental and climatic changes in central Brazil. Quat Res. 1993;39: 90–98. [Google Scholar]
- 20. De Vivo M. Mammalian evidence of historical ecological change in the Caatinga semiarid vegetation of northeastern Brazil. J Comp Biol. 1997;2: 65–73. [Google Scholar]
- 21. Costa LP. The historical bridge between the Amazon and the Atlantic Forest of Brazil: a study of molecular phylogeography with small mammals. J Biogeogr. 2003;30: 71–86. [Google Scholar]
- 22. De Vivo M, Carmignotto AP. Holocene vegetation change and the mammal faunas of South America and Africa. J Biogeogr. 2004;31: 943–957. [Google Scholar]
- 23. Batalha-Filho H, Fjeldså J, Fabre PH, Miyaki CY. Connections between the Atlantic and the Amazonian forest avifaunas represent distinct historical events. J Ornithol. 2012;154: 41–50. [Google Scholar]
- 24. Araújo MB, Pearson RG. Equilibrium of species’ distributions with climate. Ecography. 2005;28: 693–695. [Google Scholar]
- 25. Barry JP, Baxter CH, Sagarin RD, Gilman SE. Climate-related, long-term faunal changes in a California rocky intertidal community. Science. 1995;267: 672–675. [DOI] [PubMed] [Google Scholar]
- 26. Lima-Ribeiro MS, Diniz-Filho JAF, Barberi M. Climate stability and the current patterns of terrestrial vertebrate species richness on the Brazilian Cerrado. Quat Int. 2010;222: 230–236. [Google Scholar]
- 27. Lenoir J, Svenning J-C. Climate-related range shifts—a global multidimensional synthesis and new research directions. Ecography. 2014;37: 1–14. [Google Scholar]
- 28. Lima-Ribeiro MS, Varela S, Nógues-Bravo D, Diniz-Filho JAF. Potential suitable areas of Giant Ground Sloths dropped before its extinction in South America: the evidences from bioclimatic envelope modeling. Nat Conserv. 2012;10: 145–151. [Google Scholar]
- 29. Terribile LC, Lima-Ribeiro MS, Araújo MB, Bizão N, Collevatti RG, Dobrovolski R, et al. Areas of climate stability of species ranges in the Brazilian Cerrado: Disentangling uncertainties through time. Nat Conserv. 2012;10: 152–159. [Google Scholar]
- 30. De Oliveira PE, Magno A, Suguio K. Late Pleistocene/Holocene climatic and vegetational history of the Brazilian Caatinga: the fossil dunes of the middle São Francisco River. Palaeogeogr Palaeoclimatol Palaeoecol. 1999;152: 319–337. [Google Scholar]
- 31. Méio BB, Freitas C V, Jatobá L, Silva MEF, Ribeiro RPB. Influência da flora das florestas Amazônica e Atlântica na vegetação do cerrado sensu stricto . Rev Bras Botânica. 2003;26: 437–444. 10.1590/S1984-29612013000400026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Coimbra-Filho AF, Câmara IG. Os limites originais do Bioma Mata Atlântica na região Nordeste do Brasil 1st ed. Rio de Janeiro: FBCN; 1996. [Google Scholar]
- 33. Ab’Saber AN. Os domínios morfoclimáticos da América do Sul. Primeira Aproximação. Geomorfologia. 1977;52: 1–21. [Google Scholar]
- 34. Werneck FP. The diversification of eastern South American open vegetation biomes: Historical biogeography and perspectives. Quat Sci Rev. Elsevier Ltd; 2011;30: 1630–1648. [Google Scholar]
- 35. Werneck FP, Costa GC, Colli GR, Prado DE, Sites JW Jr. Revisiting the historical distribution of Seasonally Dry Tropical Forests: New insights based on palaeodistribution modelling and palynological evidence. Glob Ecol Biogeogr. 2011;20: 272–288. [Google Scholar]
- 36. Nogués-Bravo D, Rodríguez J, Hortal J, Batra P, Araújo MB. Climate Change, Humans, and the Extinction of the Woolly Mammoth. PLoS Biol. 2008;6: 685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Varela S, Lobo JM, Rodríguez J, Batra P. Were the Late Pleistocene climatic changes responsible for the disappearance of the European spotted hyena populations? Hindcasting a species geographic distribution across time. Quat Sci Rev. Elsevier Ltd; 2010;29: 2027–2035. [Google Scholar]
- 38. Araújo MB, Peterson AT. Uses and misuses of bioclimatic envelope modeling. Ecology. 2012;93: 1527–1539. [DOI] [PubMed] [Google Scholar]
- 39. Guisan A, Zimmermann NE. Predictive habitat distribution models in ecology. Ecol Modell. 2000;135: 147–186. [Google Scholar]
- 40. Araújo MB, Guisan A. Five (or so) challenges for species distribution modelling. J Biogeogr. 2006;33: 1677–1688. [Google Scholar]
- 41. Soberón J. Grinnellian and Eltonian niches and geographic distributions of species. Ecol Lett. 2007;10: 1115–23. [DOI] [PubMed] [Google Scholar]
- 42.Svenning J-C, Gravel D, Holt RD, Schurr FM, Thuiller W, Münkemüller T, et al. The influence of interspecific interactions on species range expansion rates. Ecography. 2014; no–no. [DOI] [PMC free article] [PubMed]
- 43. Hutchinson GE. Concluding remarks. Cold Spring Harb Symp Quant Biol. 1957;22: 415–427. [Google Scholar]
- 44. Lentz DL, Bye R, Sanchez-Cordero V. Ecological niche modelling and distribution of wild sunflower (Helianthus annuus L.) in Mexico. Int J Plant Sci. 2008;167: 541–549. [Google Scholar]
- 45. Gutiérrez EE, Boria RA, Anderson RP. Can biotic interactions cause allopatry? Niche models, competition, and distributions of South American mouse opossums. Ecography. 2014;37: 741–753. [Google Scholar]
- 46. Heikkinen RK, Luoto M, Virkkala R, Pearson RG, Körber JH. Biotic interactions improve prediction of boreal bird distributions at macro-scales. Glob Ecol Biogeogr. 2007;16: 754–763. [Google Scholar]
- 47. Araújo MB, Luoto M. The importance of biotic interactions for modelling species distributions under climate change. Glob Ecol Biogeogr. 2007;16: 743–753. [Google Scholar]
- 48. Meier ES, Kienast F, Pearman PB, Svenning J-C, Thuiller W, Araújo MB, et al. Biotic and abiotic variables show little redundancy in explaining tree species distributions. Ecography. 2010;33: 1038–1048. [Google Scholar]
- 49. Giannini TC, Chapman DS, Saraiva AM, Alves-dos-Santos I, Biesmeijer JC. Improving species distribution models using biotic interactions: A case study of parasites, pollinators and plants. Ecography. 2013;36: 649–656. [Google Scholar]
- 50. Silva DP, Gonzalez VH, Melo GAR, Lucia M, Alvarez LJ, De Marco P Jr. Seeking the flowers for the bees: integrating biotic interactions into niche models to assess the distribution of the exotic bee species Lithurgus huberi in South America. Ecol Modell. 2014;273: 200–209. [Google Scholar]
- 51. Pellissier L, Bråthen KA, Pottier J, Randin CF, Vittoz P, Dubuis A, et al. Species distribution models reveal apparent competitive and facilitative effects of a dominant species on the distribution of tundra plants. Ecography. 2010;33: 1004–1014. [Google Scholar]
- 52. Abrahamczyk S, Gottleuber P, Matauschek C, Kessler M. Diversity and community composition of euglossine bee assemblages (Hymenoptera: Apidae) in western Amazonia. Biodivers Conserv. 2011;20: 2981–3001. [Google Scholar]
- 53. Google Inc. Google Earth, version 7.0.3.8542 Google Incorporated; 2015. [Google Scholar]
- 54. Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25: 1965–1978. [Google Scholar]
- 55. Nemésio A, Vasconcelos HL. Beta diversity of orchid bees in a tropical biodiversity hotspot. Biodivers Conserv. 2013;22: 1647–1661. [Google Scholar]
- 56. Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol Modell. 2006;190: 231–259. [Google Scholar]
- 57. Phillips SJ, Dudík M. Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography. 2008;31: 161–175. [Google Scholar]
- 58. Nix HA. A biogeographic analysis of Australian elapid snakes In: Longmore R, editor. Atlas of Elapid Snapkes of Australia—Australian Flora and Fauna series Number 7 1st ed. Canberra: Australian Government Publishing Service; 1986. pp. 4–15. [Google Scholar]
- 59. Elith J, Graham CH, Anderson RP, Dudik M, Ferrier S, Guisan A, et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography. 2006;29: 129–151. 16622301 [Google Scholar]
- 60. Elith J, Phillips SJ, Hastie T, Dudík M, Chee YE, Yates CJ. A statistical explanation of MaxEnt for ecologists. Divers Distrib. 2011;17: 43–57. [Google Scholar]
- 61. Hijmans RJ, Graham CH. The ability of climate envelope models to predict the effect of climate change on species distributions. Glob Chang Biol. 2006;12: 2272–2281. [Google Scholar]
- 62. Varela S, Mateo RG, Garcia-Valdés, Fernandéz-Gonzaléz F. Macroecología y ecoinformática: sesgos, errores y predicciones en el modelado de distribuciones. Ecosistemas. 2014;23: 46–53. [Google Scholar]
- 63. De Oliveira G, Rangel TF, Lima-Ribeiro MS, Terribile LC, Diniz-Filho JAF. Evaluating, partitioning, and mapping the spatial autocorrelation component in ecological niche modeling: A new approach based on environmentally equidistant records. Ecography. 2014;37: 637–647. [Google Scholar]
- 64. Varela S, Anderson RP, García-Valdés R, Fernández-González F. Environmental filters reduce the effects of sampling bias and improve predictions of ecological niche models. Ecography. 2014;37: 1084–1091. [Google Scholar]
- 65. Lobo JM, Jiménez-Valverde A, Real R. AUC: A misleading measure of the performance of predictive distribution models. Glob Ecol Biogeogr. 2008;17: 145–151. [Google Scholar]
- 66.Hijmans RJ, Phillips SJ, Leathwick JR, Elith J. Package “dismo”. Species distribution modeling. R project; 2012.
- 67. Anderson RP, Gonzalez I Jr. Species-specific tuning increases robustness to sampling bias in models of species distributions: An implementation with Maxent. Ecol Modell. 2011;222: 2796–2811. [Google Scholar]
- 68. Liu CR, Berry PM, Dawson TP, Pearson RG. Selecting thresholds of occurrence in the prediction of species distributions. Ecography. 2005;28: 385–393. [Google Scholar]
- 69. Burnham KP, Andersen DR. Model selection and multimodel inference: A practical information-theoretic approach 2nd ed. New Yor: Springer-Verlag; 2003. [Google Scholar]
- 70. Muscarella R, Galante PJ, Soley-Guardia M, Boria RA, Kass JM, Uriarte M, et al. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol Evol. 2014;5: 1198–1205. [Google Scholar]
- 71. Moura DC, Schlindwein C. Mata ciliar do Rio São Francisco como biocorredor para Euglossini (Hymenoptera: Apidae) de florestas tropicais úmidas. Neotrop Entomol. 2009;38: 281–284. [DOI] [PubMed] [Google Scholar]
- 72. Nemésio A, Faria-Junior LRR. First assessment of the orchid-bee fauna (Hymenoptera: Apidae) at Parque Estadual do Rio Preto, a cerrado area in southeastern. Lundiana. 2004;5: 113–117. 15287965 [Google Scholar]
- 73. Silva DP, De Marco P Jr. No evidence of habitat loss affecting the orchid bees Eulaema nigrita Lepeletier and Eufriesea auriceps Friese (Apidae: Euglossini) in the Brazilian Cerrado Savanna. Neotrop Entomol. 2014;43: 509–518. [DOI] [PubMed] [Google Scholar]
- 74. Peterson AT, Soberón J, Pearson RG, Anderson RP, Martínez-Meyer E, Nakamura M, et al. Ecological niches and geographic distributions 1st ed. Princeton: Princeton University Press; 2011. [Google Scholar]
- 75. Warren RJ, Bradford MA. Mutualism fails when climate response differs between interacting species. Glob Chang Biol. 2014;20: 466–74. 10.1111/gcb.12407 [DOI] [PubMed] [Google Scholar]
- 76. Hegland SJ, Nielsen A, Lázaro A, Bjerknes AL, Totland Ø. How does climate warming affect plant-pollinator interactions? Ecol Lett. 2009;12: 184–95. 10.1111/j.1461-0248.2008.01269.x [DOI] [PubMed] [Google Scholar]
- 77. Tylianakis JM, Didham RK, Bascompte J, Wardle DA. Global change and species interactions in terrestrial ecosystems. Ecol Lett. 2008;11: 1351–1363. [DOI] [PubMed] [Google Scholar]
- 78. Memmott J, Craze PG, Waser NM, Price M V. Global warming and the disruption of plant-pollinator interactions. Ecol Lett. 2007;10: 710–7. [DOI] [PubMed] [Google Scholar]
- 79. Schweiger O, Heikkinen RK, Harpke A, Hickler T, Klotz S, Kudrna O, et al. Increasing range mismatching of interacting species under global change is related to their ecological characteristics. Glob Ecol Biogeogr. 2012;21: 88–99. [Google Scholar]
- 80. Thomas CD, Cameron A, Green RE, Bakkenes M, Beaumont LJ, Collingham YC, et al. Extinction risk from climate change. Nature. 2004;427: 145–8. [DOI] [PubMed] [Google Scholar]
- 81. Loyola RD, Lemes P, Faleiro F V, Trindade-Filho J, Machado RB. Severe loss of suitable climatic conditions for marsupial species in Brazil: challenges and opportunities for conservation. PLoS One. 2012;7: e46257 10.1371/journal.pone.0046257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ferro VG, Lemes P, Melo AS, Loyola R. The Reduced Effectiveness of Protected Areas under Climate Change Threatens Atlantic Forest Tiger Moths. PLoS One. 2014;9: e107792 10.1371/journal.pone.0107792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Parida M, Hoffmann AA, Hill MP. Climate change expected to drive habitat loss for two key herbivore species in an alpine environment. J Biogeogr. 2015; 10.1111/jbi.12490 [DOI]
- 84.Filz KJ, Schmitt T. Niche overlap and host specificity in parasitic Maculinea butterflies (Lepidoptera: Lycaenidae) as a measure for potential extinction risks under climate change. Org Divers Evol. 2015; 10.1007/s13127-015-0210-1 [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Occurrences selected for calibrating the model are depicted in red, while those selected for testing the model are in grey. Selection was based on envSample R-function (https://github.com/SaraVarela/envSample).
(DOC)
The 66 occurrences selected for calibrating the model are depicted in red, while the 264 selected for testing the model are in grey. Selection was based on envSample R-function (https://github.com/SaraVarela/envSample).
(DOC)
(XLS)
(XLS)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.