Abstract
Parapneumonic effusions complicating pneumonia are associated with increased morbidity and mortality. Along with increased mortality, complicated parapneumonic effusion and empyema often necessitate prolonged treatment, longer hospital stay and interventions. Parapneumonic effusions arise from inflammation in the lungs and pleural space from direct invasion of bacteria, cascade of inflammatory events and bacteriologic virulence features. Patient factors and comorbidities also contribute to the pathophysiology of parapneumonic effusion development. The evolution of parapneumonic effusion can be divided into three progressive stages: (I) exudative stage; (II) fibrinopurulent stage; and (III) organizing stage with pleural peel formation. These stages can help categorize effusions into groups in order to evaluate the risk of a complicated course requiring intervention. We recommend that clinical data be evaluated and a stepwise approach be taken in management of these patients. This review article discusses current understanding of the development and relationship of parapneumonic effusions with pneumonia.
Keywords: Parapneumonic effusion, pleural infection, empyema, pneumonia
Community acquired pneumonia results in significant morbidity and mortality, and is the 9th leading cause of death in the United States when combined with influenza (1). About 6 million cases are reported annually in the United States population, resulting in 500,000 to 1.1 million hospitalizations annually (2,3). Among pneumonia patients admitted to the hospital, 20% to 40% have pleural effusion, and 10% of these develop complicated parapneumonic effusion or empyema (4,5). There is considerable variation in the course and aggressiveness of parapneumonic effusions; therefore an understanding of its progression is important. Along with increased mortality, complicated parapneumonic effusion and empyema often necessitate prolonged treatment, longer hospital stay and interventions. Thus, identification of these patients and prompt management is critical.
This review article discusses current understanding of the development and relationship of parapneumonic effusions with pneumonia.
Historical information
An Egyptian physician, Imhotep was most likely the first to describe pleural infections around 3000 BC, however Hippocrates is more often cited for its recognition in 500 BC. It was not until the 19th century that open lung drainage was recommended for treatment, however mortality was as high as 70%, likely related to complications of surgery. Closed chest tube drainage was first described in 1876 and widely used during the influenza epidemic of 1917-1919, resulting in improved survival (6,7). The introduction of antibiotics has not only reduced the incidence of empyema, but also changed its bacteriology. In the pre-antibiotic era, 60-70% of empyemas were due to Streptococcus pneumoniae (S. pneumoniae), which now only accounts for about 10% (8-10). Staphylococcus aureus empyema has become more common, along with anaerobic and Gram-negative bacteria infections (7,8,10-14). With the advent of antibiotics, the incidence of empyema was dramatically reduced; however more recent studies indicate the incidence of pleural infection is increasing (15,16).
Parapneumonic effusion is defined as any pleural effusion secondary to viral or bacterial pneumonia or lung abscess. “Complicated” parapneumonic effusion is a paraneumonic effusion that requires an invasive procedure, such as tube thoracostomy, to resolve, often with positive pleural fluid cultures (5). Empyema is defined by the presence of bacteria or pus in the pleural space. Pus is thick, viscous fluid that appears purulent. About 60% of empyemas are related to a primary pneumonic process, therefore risk factors for pleural infection are similar to those for pneumonia (3,17). However, up to 40% of empyema may be secondary to a non-pneumonic process, such as systemic infection with hematogenous spread or abdominal etiology. Independent risk factors for the development of empyema include diabetes, immunosuppression, gastro-esophageal reflux disease, alcohol and intravenous drug abuse, aspiration, and poor oral hygiene (18). Other causes of empyema include complications after thoracic surgical procedures, trauma, esophageal perforation, thoracentesis and subdiaphragmatic infection (3,17).
Pathophysiologic features
In homeostatic conditions, pleural fluid arises from the systemic pleural vessels, traverses across leaky pleural membranes into the pleural space and exits via the parietal pleural lymphatics in the dependent part of the cavity (19,20). In healthy adults, the pleural space contains a small volume (1-20 mL) of low protein fluid that forms a lubricating film about 10 µm thick between the visceral and parietal pleural surfaces (6,19). A pressure gradient facilitates movement into, but not out of the pleural space, as intrapleural pressure is lower than interstitial pressure, and pleural membranes are leaky, offering little resistance to liquid or protein movement. The majority of pleural fluid exits the space by bulk flow, rather than diffusion or active transport, through the parietal lymphatics (20). Pleural fluid turnover is estimated to be ~0.15 mL/kg·h (19).
Pleural fluid accumulates when the rate of formation exceeds the rate of absorption. The flow of pleural lymphatics can efficiently increase in response to an increase in pleural fluid filtration, acting as a negative feedback mechanism. The lymphatic flow is about 15 mL/day, as this is the typical amount of pleural fluid formed per day. However, the capacity of the lymphatics is about 300-700 mL/day. Due to the large lymphatic capacity, unless the lymphatic drainage is severely impaired, another factor must be present for pleural fluid to accumulate (3,19).
The most common cause of increased pleural fluid formation is increased interstitial edema. This can occur as a result of several processes and is the predominant mechanism for the formation of parapneumonic effusions along with pleural effusions related to congestive heart failure (CHF), pulmonary embolism and acute respiratory distress syndrome. Decreased pleural pressures can also contribute to pleural fluid accumulation, as in advanced empyema when the visceral pleura becomes coated with a collagenous peel and traps the lung. Increased capillary permeability, particularly when the pleura becomes inflamed, also contributes to pleural effusion formation. Lymphatic obstruction is a common mechanism contributing to malignant effusions (3,19).
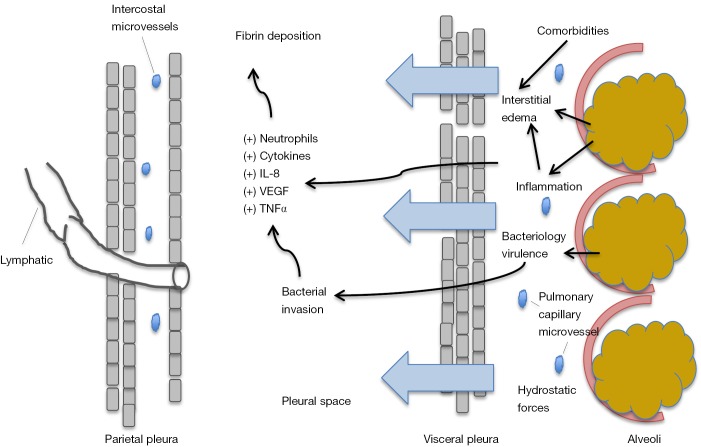
The evolution of parapneumonic effusion is divided into three progressive stages: (I) exudative stage; (II) fibrinopurulent stage; and (III) organizing stage with pleural peel formation (21). In the early exudative stage there is a rapid outpouring of fluid and inflammatory cells into the pleural space due to increased capillary microvascular permeability. This directly results from proinflammatory cytokines, such as interleukin 8 (IL-8) and tumor necrosis factor α (TNF-α) (22,23). The inflammatory process of the pulmonary parenchyma extends to the visceral pleura causing changes to the mesothelial cells lining the pleura, allowing increased fluid movement. This causes a local pleuritic reaction, and the characteristic pleuritic chest pain described by patients (5,21). Researchers studying rabbits infected with intrapulmonary Pseudomonas found a dose dependent relationship between bacterial levels and extent of alveolar epithelial injury, which further facilitated entry of alveolar protein and bacteria into the pleural space. This occurred within hours of inoculation (24). See Figure 1.
Figure 1.
Schema shows mechanism of pleural effusion development in pneumonia. Initial bacterial infection causes local inflammatory reaction resulting in increased capillary microvascular permeability and a rapid outpouring of fluid containing inflammatory cells into the pleural space. Comorbidities such as heart failure also further contribute to interstitial edema. IL-8, interleukin 8; TNF-α, tumor necrosis factor α; VEGF, vascular endothelial growth factor.
The pleural fluid in this early exudative stage is usually clear free-flowing exudative fluid with predominance of neutrophils, and characterized by negative bacterial cultures, glucose level greater than 60 mg/dL, pH above 7.20, lactic acid dehydrogenase (LDH) less than three times the upper limit of normal for serum (often <1,000 units/L) and low white cell count (4,5,25-31). Pleural fluid that develops during this stage is usually considered a “simple” parapneumonic effusion and treatment with antibiotics is often adequate, without the need for tube drainage (4,27,28).
Patients can progress to stage 2, the fibrinopurulent stage within hours if effective treatment is not provided. This next stage is characterized by deposition of fibrin clots and fibrin membranes in the pleural space, leading to loculations and isolated collections of fluid. Invasion of bacteria from the pulmonary parenchyma occurs across the damaged endothelium. This invasion accelerates the immune response and directly contributes to fluid loculation (25) by promoting further migration of neutrophils and activation of the coagulation cascade. This leads to increased pro-coagulant and decreased fibrinolytic activity, which encourages fibrin deposition and promotes formation of septations within the fluid. The inflammatory reaction is further fueled by neutrophil phagocytosis and bacterial death, which results in release of more bacteria cell wall derived fragments and proteases (22).
This process has been demonstrated in mice with empyema after being infected intranasally with S. pneumoniae. Researchers found rapid bacterial invasion and increased inflammatory markers in the pleural space, such as IL-8, vascular endothelial growth factor (VEGF), monocyte chemotactic protein 1 (MCP-1) and TNF-α, which caused significant neutrophilia and development of fibrinous pleural adhesions. The pleural cavity offered a protected compartment for the bacteria, as bacterial clearance from the pleural space was poor in this animal model (31).
The pleural fluid in the fibrinopurulent stage is often turbid and characterized by positive bacteria on Gram stain and culture. Cytology shows neutrophils and degenerated cells. The combination of bacterial invasion and increased inflammatory response leads to increased lactic acid and carbon dioxide production, resulting in a fall in pleural fluid pH, increased glucose metabolism and a rise in LDH, consistent with “complicated” parapneumonic effusion. Typical pleural fluid studies in this stage have a glucose level less than 60 mg/dL, pH below 7.20, and pleural LDH more than three times the upper limit normal for serum (often >1,000 units/L) (5,25).
If stage 2 pleural fluid is not drained in conjunction with effective antibiotic therapy, the effusion may progress to stage 3, the organizing stage. This final stage is characterized by fibroblasts that proliferate and invade the pleural fluid from both the visceral and parietal pleura, forming a thick pleural peel. Fibrin membranes are transformed by fibroblast into a web of thick nonelastic pleura. This can occasionally encase the lung, preventing re-expansion and resulting in “trapped lung”. This can functionally result in impaired gas exchange and produce a persistent pleural space, increasing risk for continued infection (6,25). Clinical course varies considerably, from spontaneous healing with persistent defects of lung function, to chronic forms of empyema with high risk of complications, such as bronchopleural fistula, trapped restricted lung, fibro-thorax or spontaneous perforation through the chest wall (25).
Classification
Classifying the prognosis of a patient with a parapneumonic effusion is a critical first step in management. In 2000, the American College of Chest Physicians developed a classification system based on the anatomic features (A), bacteriology (B), and chemistry of the pleural fluid (C) (32). The anatomy (A) of the pleural fluid is based on three features; size, whether it is free flowing and whether the parietal pleura are thickened. The bacteriology (B) of the effusion is based on whether pleural cultures or smears are positive. The chemistry (C) of the pleural fluid is based on pH measured with a blood gas machine. Pleural fluid glucose can be used as an alternative to pH with a cutoff level of 60 mg/dL. Based on the A, B, and C classification, the effusion is categorized. Risk of poor outcome is based on the category of the effusion, as are recommendations to drain the effusion (Table 1) (5,32). Similarly, the British Thoracic Society has published a diagnostic algorithm for management of these patients (6).
Table 1. Parapneumonic effusion classification.
| Anatomy | Bacteriology | Chemistry | Category | Drainage intervention |
|---|---|---|---|---|
| Very small to small free flowing effusion* | Unknown | Unknown | 1 | No |
| Small to moderate free flowing effusion* | Negative culture and Gram stain | Normal pH and glucose | 2 | No |
| Large effusion or loculation* | Positive culture or Gram stain | Low pH or glucose | 3 | Yes |
| Any size | Pus | 4 | Yes |
*, small <10 mm on lateral decubitus; moderate less than half hemithorax; large greater than or equal to half hemithorax. Risk of poor outcome is very low to low for category 1 and 2; however category 3 and 4 have moderate to high risk.
Bacteriology
The infectious organisms of community-acquired pneumonia (CAP) vary according to patient population, host immunity and geographic region, with the most common pathogens including S. pneumoniae, Haemophilus influenzae and Staphylococcus aureus (25). However, despite the relationship with pneumonia, studies suggest the bacteriology of pleural infections differ from that of pneumonia and have been altered significantly with the institution of antibiotic treatment. In a study of 434 patients with pleural infections, approximately 50% of parapneumonic infections were due to Streptococcal species, with the most common being S. intermedius [S. anginosus (milleri) group], followed by S. pneumoniae. Staphylococcus species were also common and accounted for about 14% of the parapneumonic infections. In this series, another, 20% of parapneumonic effusions were due to anaerobic bacteria (24). Most other series report similar rates of anaerobes (12-24%). However when DNA amplification and research laboratories are used to identify organisms, anaerobes may be present in up to 76% of the cases (14,33-35).
The difference in the bacteriology between pneumonia and pleural infections may be related to the acidic and hypoxic environment of the infected pleural space and bacterial virulence factors favoring certain pathogens (18,24). Furthermore, the difference in bacterial species between pleural infections and pneumonia, along with lack of chest imaging evidence of pneumonia in some patients, have led some experts to question the conventional belief that empyema and pneumonia are inherently related. Hematogenous spread of bacteria from systemic infection or an abdominal process with rapid growth of bacterial in the pleural space, as observed in animal models, offers a plausible explanation (24,31).
Predictive factors
Aside from inflammation in the lungs and pleural space from direct invasion of bacteria and bacteriologic virulence features contributing to parapneumonic effusion, patient factors and comorbidities also contribute to the pathophysiology of parapneumonic effusion development. A recent study (11), analyzed 4,715 patients with CAP and 882 (19%) had pleural effusions, of which 261 (30%) had empyema or complicated parapneumonic effusion. In a multivariable analysis, no single baseline patient characteristic distinguished patients without pleural effusion from those with uncomplicated parapneumonic effusion. However, five independent baseline characteristics could predict the development of empyema or complicated parapneumonic effusion in patients with pneumonia: age <60 years old, alcoholism, pleuritic pain, tachycardia and leukocytosis. These investigators, and others have found a reduced prevalence of clinical manifestations in older patients, suggesting possible age-related change in the immune response (11,13,36,37). In this cohort, researchers also found patients with a history of tobacco abuse had increased risk of developing a complicated parapneumonic effusion or empyema, whereas, chronic obstructive pulmonary disease and heart failure decreased risk. Diabetes, chronic renal disease and liver disease were not associated with risk of pleural infection in the cohort (11). Similar results have been found in other prospective observational studies of patients diagnosed with CAP (12,13).
Pneumonia is a leading cause of death and pleural infections complicating pneumonia has been established to have considerable morbidity and mortality, with mortality approximately 20% for patients with empyema (16,38,39). This may be related to something inherent about the parapneumonic effusion and/or a more robust inflammatory response. Underlying comorbidities or patient factors may not only contribute to the development of a parapneumonic effusion, but might be the cause of increased mortality.
In a prospective cohort study of 1,906 patients with CAP, Hasley et al. (36) found an overall 30-day mortality of 4.9%. Patients with associated pleural effusion had 30-day mortality of 14.7% and those with bilateral effusions had an even higher mortality of 26.0%. In multivariate analysis of radiographic features and clinical characteristics, the presences of bilateral pleural effusions were independently associated with mortality. Other radiographic characteristics including infiltrates involving two or more lobes, the presence of bronchopneumonia, bilateral infiltrates, air bronchograms, postobstructive pneumonia, or an aspiration pattern, had univariate associations with mortality. However, none of these factors were independently associated with death after controlling for confounding variables know to be associated with mortality. The most common comorbidities in this pneumonia cohort were coronary artery disease, chronic obstructive pulmonary disease, and CHF. Mortality rates were greatest for patients with both CHF and bilateral pleural effusions (25.6%).
Why bilateral pleural effusions are associated with mortality in pneumonia is not clear nor is the observation that patients with bilateral effusions and CHF are at greatest risk of mortality. Bilateral effusions in pneumonia patients may be a marker of severe pneumonia or may be attributable to underlying comorbidities. The increased mortality in patients with comorbidities such as CHF may reflect overall health status and those who are more likely to die from co-existing heart disease rather than directly from the pneumonia. Further research is needed (5,12,36).
Management
Management of parapneumonic effusions involves a stepwise approach in addition to appropriate and timely antibiotic treatment. The treatment options include: observation, therapeutic thoracentesis, tube thoracostomy, intrapleural instillation of fibrinolytics, thoracoscopy with breakdown of adhesions and/or decortication, and open drainage procedures (5,6,32). The details of these procedures go beyond the scope of this article.
Summary
Pneumonia can be complicated by the development of a parapneumonic effusion, which has increased morbidity and mortality. Complicated parapneumonic effusion and empyema often necessitate prolonged treatment, longer hospital stay and interventions. Parapneumonic effusions arise from inflammation in the lungs and pleural space from a cascade of inflammatory events including, direct invasion of bacteria and bacteriology virulence features. Patient factors and comorbid illnesses such as heart failure also contribute to the pathophysiology of parapneumonic effusion development.
The evolution of parapneumonic effusions can be divided into three progressive stages: (I) exudative; (II) fibrinopurulent; and (III) organizing stages. These stages can help categorize effusions into groups in order to evaluate risk of an uncomplicated or complicated course requiring intervention. Clinical data should be collected to classify patients and a stepwise approach be taken in the management.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep 2013;61:1-117. [PubMed] [Google Scholar]
- 2.Marston BJ, Plouffe JF, File TM, Jr, et al. Incidence of community-acquired pneumonia requiring hospitalization. Results of a population-based active surveillance Study in Ohio. The Community-Based Pneumonia Incidence Study Group. Arch Intern Med 1997;157:1709-18. [PubMed] [Google Scholar]
- 3.Light RW. editor. Pleural disease. 6th ed. Philadelphia: Williams & Wilkins, 2013. [Google Scholar]
- 4.Light RW, Girard WM, Jenkinson SG, et al. Parapneumonic effusions. Am J Med 1980;69:507-12. [DOI] [PubMed] [Google Scholar]
- 5.Light RW. Parapneumonic effusions and empyema. Proc Am Thorac Soc 2006;3:75-80. [DOI] [PubMed] [Google Scholar]
- 6.Davies HE, Davies RJ, Davies CW. Management of pleural infection in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii41-53. [DOI] [PubMed] [Google Scholar]
- 7.Wallenhaupt SL. Surgical management of thoracic empyema. J Thorac Imaging 1991;6:80-8. [DOI] [PubMed] [Google Scholar]
- 8.Maskell NA, Davies CW, Nunn AJ, et al. U.K. Controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 2005;352:865-74. [DOI] [PubMed] [Google Scholar]
- 9.Heffner JE. Diagnosis and management of thoracic empyemas. Curr Opin Pulm Med 1996;2:198-205. [DOI] [PubMed] [Google Scholar]
- 10.Alfageme I, Muñoz F, Peña N, et al. Empyema of the thorax in adults. Etiology, microbiologic findings, and management. Chest 1993;103:839-43. [DOI] [PubMed] [Google Scholar]
- 11.Falguera M, Carratalà J, Bielsa S, et al. Predictive factors, microbiology and outcome of patients with parapneumonic effusion. Eur Respir J 2011;38:1173-9. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed RA, Marrie TJ, Huang JQ. Thoracic empyema in patients with community-acquired pneumonia. Am J Med 2006;119:877-83. [DOI] [PubMed] [Google Scholar]
- 13.Chalmers JD, Singanayagam A, Murray MP, et al. Risk factors for complicated parapneumonic effusion and empyema on presentation to hospital with community-acquired pneumonia. Thorax 2009;64:592-7. [DOI] [PubMed] [Google Scholar]
- 14.Bartlett JG. Anaerobic bacterial infections of the lung and pleural space. Clin Infect Dis 1993;16 Suppl 4:S248-55. [DOI] [PubMed] [Google Scholar]
- 15.Finley C, Clifton J, Fitzgerald JM, et al. Empyema: an increasing concern in Canada. Can Respir J 2008;15:85-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farjah F, Symons RG, Krishnadasan B, et al. Management of pleural space infections: a population-based analysis. J Thorac Cardiovasc Surg 2007;133:346-51. [DOI] [PubMed] [Google Scholar]
- 17.Smith JA, Mullerworth MH, Westlake GW, et al. Empyema thoracis: 14-year experience in a teaching center. Ann Thorac Surg 1991;51:39-42. [DOI] [PubMed] [Google Scholar]
- 18.Maskell NA, Batt S, Hedley EL, et al. The bacteriology of pleural infection by genetic and standard methods and its mortality significance. Am J Respir Crit Care Med 2006;174:817-23. [DOI] [PubMed] [Google Scholar]
- 19.Miserocchi G. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J 1997;10:219-25. [DOI] [PubMed] [Google Scholar]
- 20.Mason R, Broaddus VC, Martin T, et al. editors. Murray and Nadel’s textbook of respiratory medicine. 5th ed. Philadelphia: Saunders Elsevier, 2010. [Google Scholar]
- 21.Andrews NC, Parker EF, Shaw RR, et al. Management of non-tuberculous empyema: a statement of the subcommittee on surgery: from American Thoracic Society. Am Rev Respir Dis 1962;85:935-6. [Google Scholar]
- 22.Kroegel C, Antony VB. Immunobiology of pleural inflammation: potential implications for pathogenesis, diagnosis and therapy. Eur Respir J 1997;10:2411-8. [DOI] [PubMed] [Google Scholar]
- 23.Alemán C, Alegre J, Monasterio J, et al. Association between inflammatory mediators and the fibrinolysis system in infectious pleural effusions. Clin Sci (Lond) 2003;105:601-7. [DOI] [PubMed] [Google Scholar]
- 24.Wiener-Kronish JP, Sakuma T, Kudoh I, et al. Alveolar epithelial injury and pleural empyema in acute P. aeruginosa pneumonia in anesthetized rabbits. J Appl Physiol (1985) 1993;75:1661-9. [DOI] [PubMed] [Google Scholar]
- 25.Hamm H, Light RW. Parapneumonic effusion and empyema. Eur Respir J 1997;10:1150-6. [DOI] [PubMed] [Google Scholar]
- 26.Good JT, Jr, Taryle DA, Maulitz RM, et al. The diagnostic value of pleural fluid pH. Chest 1980;78:55-9. [DOI] [PubMed] [Google Scholar]
- 27.Light RW, MacGregor MI, Ball WC, Jr, et al. Diagnostic significance of pleural fluid pH and PCO2. Chest 1973;64:591-6. [DOI] [PubMed] [Google Scholar]
- 28.Potts DE, Levin DC, Sahn SA. Pleural fluid pH in parapneumonic effusions. Chest 1976;70:328-31. [DOI] [PubMed] [Google Scholar]
- 29.Potts DE, Taryle DA, Sahn SA. The glucose-pH relationship in parapneumonic effusions. Arch Intern Med 1978;138:1378-80. [PubMed] [Google Scholar]
- 30.Sasse SA, Causing LA, Mulligan ME, et al. Serial pleural fluid analysis in a new experimental model of empyema. Chest 1996;109:1043-8. [DOI] [PubMed] [Google Scholar]
- 31.Wilkosz S, Edwards LA, Bielsa S, et al. Characterization of a new mouse model of empyema and the mechanisms of pleural invasion by Streptococcus pneumoniae. Am J Respir Cell Mol Biol 2012;46:180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colice GL, Curtis A, Deslauriers J, et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest 2000;118:1158-71. [DOI] [PubMed] [Google Scholar]
- 33.Bartlett JG, Gorbach SL, Thadepalli H, Bacteriology of empyema. Lancet 1974;1:338-40. [DOI] [PubMed] [Google Scholar]
- 34.Brook I, Frazier EH. Aerobic and anaerobic microbiology of empyema. A retrospective review in two military hospitals. Chest 1993;103:1502-7. [DOI] [PubMed] [Google Scholar]
- 35.Civen R, Jousimies-Somer H, Marina M, et al. A retrospective review of cases of anaerobic empyema and update of bacteriology. Clin Infect Dis 1995;20 Suppl 2:S224-9. [DOI] [PubMed] [Google Scholar]
- 36.Hasley PB, Albaum MN, Li YH, et al. Do pulmonary radiographic findings at presentation predict mortality in patients with community-acquired pneumonia? Arch Intern Med 1996;156:2206-12. [PubMed] [Google Scholar]
- 37.Metlay JP, Schulz R, Li YH, et al. Influence of age on symptoms at presentation in patients with community-acquired pneumonia. Arch Intern Med 1997;157:1453-9. [PubMed] [Google Scholar]
- 38.Ferguson AD, Prescott RJ, Selkon JB, et al. The clinical course and management of thoracic empyema. QJM 1996;89:285-9. [DOI] [PubMed] [Google Scholar]
- 39.Davies CW, Kearney SE, Gleeson FV, et al. Predictors of outcome and long-term survival in patients with pleural infection. Am J Respir Crit Care Med 1999;160:1682-7. [DOI] [PubMed] [Google Scholar]