Abstract
Cellular senescence is a state of permanent cellular arrest that provides an initial barrier to cell transformation and tumorigenesis. In this study, we report that expression of NAD(P)H:quinone oxidoreductase 1 (NQO1), a cytoplasmic 2-electron reductase, is induced during oncogene-induced senescence (OIS). Depletion of NQO1 resulted in the delayed onset of senescence. In contrast, ectopic expression of NQO1 enhanced the senescence phenotype. Analysis of the mechanism underlying the up-regulation of NQO1 expression during senescence identified that NQO1 promotes p53 accumulation in an MDM2 and ubiquitin independent manner, which reinforces the cellular senescence phenotype. Specifically, we demonstrated that NRF2/KEAP1 signaling regulates NQO1 expression during OIS. More importantly, we confirmed that depletion of NQO1 facilitates cell transformation and tumorigenesis, which indicates that NQO1 takes part in the senescence barrier and has anti-oncogenic properties in cell transformation.
Keywords: NQO1, p53, senescence
Introduction
Cellular senescence is a state of permanent cellular arrest triggered by several stimuli, which includes DNA damage, oxidative stress, oncogene activation (premature senescence) and telomere erosion (replicative senescence) 1. Genes that have the potential to cause cancer (oncogene) can trigger oncogene-induced cellular senescence (OIS) 2. Numerous studies have shown that OIS has an initial anti-tumorigenic function 3-5. Senescent cells develop a permanent cellular arrest by a number of factors. Several lines of evidence have implicated that the Arf/p53/p21, p16/pRB and the DNA-damage response (DDR) pathway regulates cellular senescence 6-12. However, lesions on those important regulators of cellular senescence make cells more vulnerable against oncogenic stress.
NAD(P)H:quinone oxidoreductase 1 (NQO1) is a cytoplasmic 2-electron reductase that is involved in the cellular defense mechanism against oxidative stress 13, 14. NQO1 is induced in response to endogenous and exogenous stress. It was reported that NQO1 has a gatekeeping role in regulating the MDM2 and ubiquitin independent proteasomal degradation of certain proteins 15-17. In addition, the NQO1 polymorphism C609T has been associated with cancer susceptibility. However, high levels of NQO1 expression have been observed in a number of cancers, including liver, breast, colon, and lung cancers, as compared with normal tissues of the same origin 18-21. Furthermore, NQO1 induces cell cycle progression 22 and the proliferation of melanoma cells, and genetic deletion of NQO1 potentiates apoptosis 23. NQO1 silencing in prostate cancer cells leads to interactions between nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and p300 that reinforce survival signaling 24.
We report for the first time that NQO1 is induced during OIS. Together, our investigation advances the understanding about the mechanisms regulating OIS, and the role of NQO1 in senescence and cell transformation.
Materials and Methods
Cell culture
Human diploid fibroblasts (HDF) 2BS and BJ cells were obtained from the National Institute of Biological Products (Beijing, China). IMR90 was purchased from ATCC (Rockefeller, MD, USA). Cells were cultured as described previously 25.
SA-β-gal and BrdU incorporation assay
SA-β-gal activity assay were performed as described previously 25. BrdU incorporation assay was performed as described previously 26.
Plasmid constructs
To obtain constructs of NQO1 promoter plasmids, -891, 5'-cggGGTACCGGAGTGCAGTGGCACGATCT and +135, 5'-gaAGATCTCTGGCCGGAACTAGGCTCTC were used to amplify the NQO1 promoter. The underlined sequences are the KpnI (New England Biolabs, Beverly, MA, USA) and BglII (New England Biolabs, Beverly, MA, USA) recognition sites. The PCR products of promoter were subcloned into pGL3-basic plasmid (Promega, Madison, WI, USA).
pITA-NQO1 was created as follows: the cDNA for NQO1 was amplified by PCR and subcloned into the NotI and BsrGI sites of the pITA vector. The sequences of primers were as follows: 5'-ataagaatGCGGCCGCGAGCCATGGTCGGCAG and 5'-gggTGTACATCAGGGAAGCCTGGAAAG. The underlined sequences are the NotI (New England Biolabs, Beverly, MA, USA) and BsrGI (New England Biolabs, Beverly, MA, USA) recognition sites.
shNQO1 were created with the following sense shRNA sequences into LentiLox 3.7 (pLL3.7) 27. shNQO1#1 5'-GGTTTGAGCGAGTGTTCATAG; shNQO1#2 5'-GCAGCCTCTTTGACCTAAACT.
RasG12V lentiviral vector was kindly provided by Judith Campisi (Buck Institute for Age Research). NRF2 and p53 knockdown lentiviral vectors were kindly provided by Li Shen (Health Science Center, Peking University, China).
Site-directed Mutagenesis
The NQO1-ARE promoter mutagenic primer: 5'- CTTCCAAATCCGCAGTCACAagactTCAatAGAATCTGAGCCTAGGG and 5'- atTGAagtctTGTGACTGCGGATTTGGAAGGCTGAGAGTCCTG were used to create mutant construct. The underlined bases are the mutated bases.
Viral transduction
Infectious virus was produced as previously described 28.
Cell proliferation assay
2BS were infected with the indicated lentivirus, briefly selected for proviral integration, and subsequently infected with the RasG12V-encoding virus. Cells were fixed and stained with crystal violet10 days after exposure to RasG12V.
Quantitative RT-PCR
Quantitative RT-PCR (qRT-PCR) analyses were performed as previously described 25. Primer sets used were as follows: GAPDH 5'-ACGGATTTGGTCGTATTGGG and 5'-TGATTTTGGAGGGATCTCGC; NRF2 5'-GTCACATCGAGAGCCCAGTC and 5'-ACCATGGTAGTCTCAACCAGC; NQO1 5'-GTGATATTCCAGAGTAAGAAGGCAG and 5'-ATTCTCCAGGCGTTTCTTCCAT; p53 5'-CCCAAGCAATGGATGATTTGA and 5'-GGCATTCTGGGAGCTTCATCT.
Immunoblotting
Western blot and immunoprecipitations were carried out by standard method. Antibodies used for western blotting were against β-actin (Cell Signaling, Danvers, MA, USA; 4967), NQO1 (Abcam, Cambridge, MA, USA; ab28947), p53 (Santa Cruz Biotechnology, Santa Cruz, CA, USA; sc-6243), p21 (Santa Cruz Biotechnology; sc-28777), p16 (Santa Cruz Biotechnology; sc-28777), and NRF2 (Abcam; ab62352). Antibodies used for immunoprecipitations were against p53 (Abcam; ab28), c-MAF (Santa Cruz Biotechnology; sc-7866), KEAP1 (Santa Cruz Biotechnology; sc-33569).
The densitometry data were analyzed by ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Luciferase assay
For the NQO1 promoter activity assay, 2 days after exposure to RasG12V, 2BS cells were seeded and transfected with 0.45 μg of NQO1 reporter plasmid together with 0.05 μg of control. After 48 h, luciferase activity was measured with a luminometer (Centro LB 960; BERTHOLD TECHNOLOGIES, Germany), and was normalized to that of the renilla control.
Chromatin immunoprecipitation (ChIP)
ChIP assay was performed as previously described by Carey et al. 29. Antibodies used for ChIP were anti-NRF2 (Abcam; ab62352). Primers for amplification of ARE specific, proximal region (nucleotides -417 to -359) within the NQO1 promoter were (primerA): 5'-GGACTCTCAGCCTTCCAAAT and 5'-CCCTAGGCTCAGATTCTGCT. For a non-specific distal NQO1 promoter region (nucleotides -1487 to -1413) the primers were (primerB): 5'-CTGCTGGCCACATTTCCAGT and 5'- CCCTATCTGTGCTGCCCAAG.
Tumorigenic Assay
In the experiment, 5x106 cells were inoculated subcutaneously into the right legs of male BALB/c nude mice. All animal experiments were performed in accordance with the guidelines of Peking University Health Science Center Animal Care and Use Committee.
Results
NQO1 expression is induced during OIS
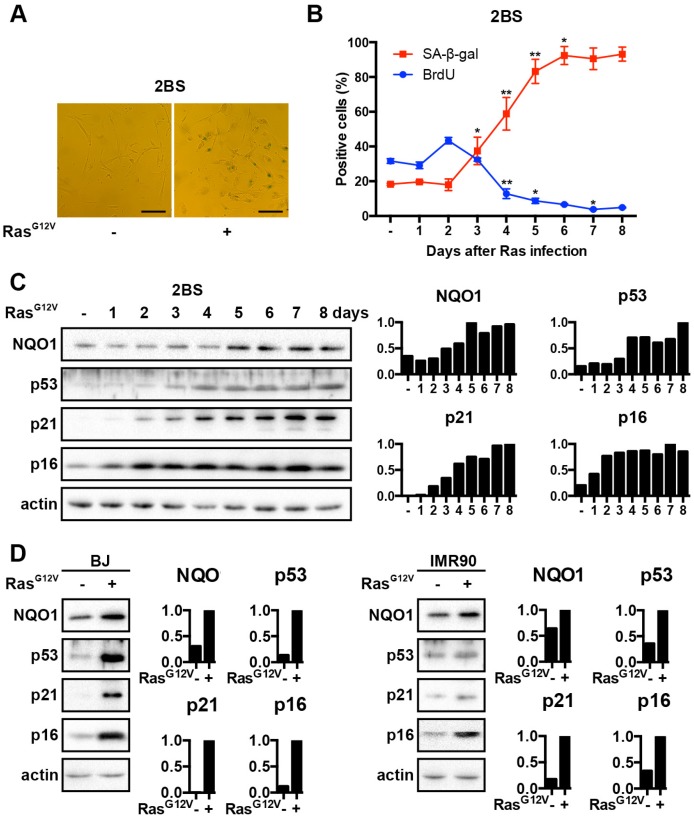
In has been previously demonstrated that upon introducing mutant RasG12V in HDF in the absence of cooperating lesions, cells enter premature senescence around day 5-6 26, 30. To investigate whether NQO1 is involved in OIS, we first performed senescence-associated beta-galactosidase (SA-β-gal) staining and analyzed BrdU incorporation to confirm cellular senescence. We studied cellular senescence resulting from oncogenic signaling by mutant RasG12V, allowing the cells to develop a senescence phenotype consisting of a morphology of flat cells with a vacuole-rich cytoplasm that stained positive for SA-β-gal (Fig. 1A). Consistent with previous reports, after introducing RasG12V into 2BS cells, the percentage of cells with SA-β-gal staining was increased and the percentage of cells with BrdU incorporated was decreased (Fig. 1B). Furthermore, we assessed the expression of the cell cycle regulators p53, p21 and p16 by western blot analysis after introducing RasG12V into 2BS cells. In agreement with previous reports that oncogenic stress could induce OIS through Arf/p53/p21- and p16/pRB-pathways, we observed a significant accumulation of p53 protein, as well as p21 protein, and marked changes in the level of p16 protein (Fig. 1C). Western blot showed that NQO1 expression was up regulated during the onset of OIS (Fig. 1C). Similar results were obtained in other types of HDF cells, BJ and IMR90 cells, suggesting that NQO1 up-regulation is not unique to 2BS cells (Fig. 1D).
Figure 1.
NQO1 expression is induced during OIS. (A) Representative microscopic view of 2BS cells upon exposure to RasG12V for 5d with SA-β-gal activity staining. Scale bars: 200 μm. (B) RasG12V-induced senescent 2BS cells at various time points were assessed for SA-β-gal activity and BrdU incorporation. Data are mean ± SD from 3 independent experiments, each performed in triplicate. (*p<0.05; **p < 0.01). (C) NQO1, p53, p21 and p16 protein levels at the various time points in RasG12V-induced senescent 2BS cells were determined by western blotting. β-actin served as a loading control. (D) NQO1, p53, p21 and p16 protein levels in RasG12V-induced senescent BJ cells and IMR90 cells were determined by western blotting. β-actin served as a loading control. The densitometry data were analyzed by ImageJ software and normalized to the highest signal in the corresponding row.
NQO1 mediates premature senescence
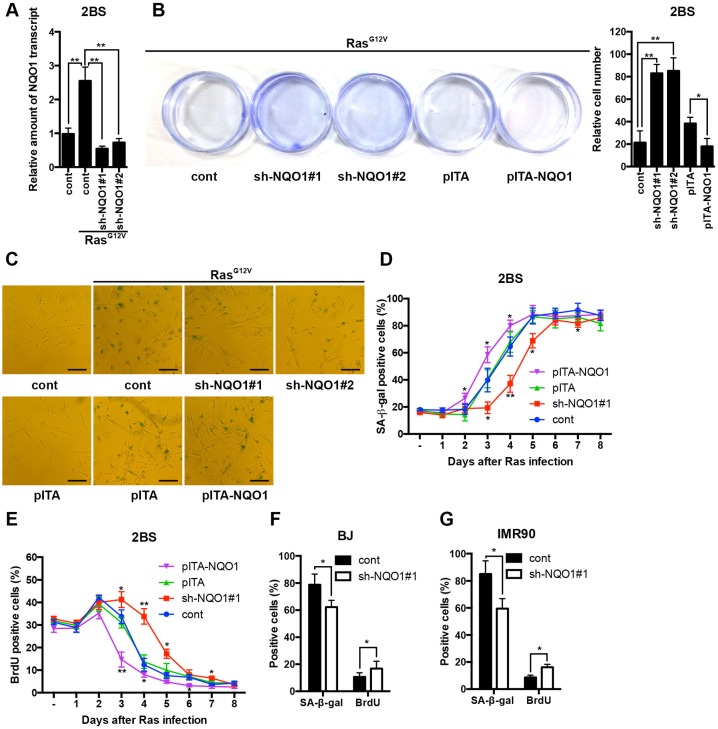
After finding that NQO1 levels correlated with senescence we wanted to understand if NQO1 could play a necessary role in senescence processing. To learn more about the contribution of NQO1 in senescence, short hairpin RNA (shRNA) was used to stably knock-down NQO1 in HDF cells, and separately NQO1 was over-expressed in HDF cells, thereby allowing us to acquire early passage 2BS cells with different levels of NQO1 expression. The amount of NQO1 mRNA in the HDF was reduced about 60% using two independent shRNAs (shNQO1#1 and shNQO1#2), as compared with the control (Fig. 2A). Subsequently, we introduced mutant RasG12V into these cells. We then performed a cell proliferation assay to test the relationship between NQO1 and OIS. NQO1 depletion resulted in continuous cell growth compared with corresponding control lentiviral vector (Cont) infected cells (Fig. 2B). In addition, the morphology changed during the OIS, with senescent cells exhibiting the typical enlarged and flattened shape. Five days after introducing RasG12V, NQO1 depleted 2BS cells showed a different morphology compared with control 2BS cells (Fig. 2C). Accordingly, we found that 2BS cells with NQO1 depletion reduced with SA-β-gal staining after expressing RasG12V (Fig. 2D). Lack of DNA replication is an obvious marker for senescent cells. As such, we preformed BrdU incorporation assay to further confirm our observation. We observed that knockdown of NQO1 resulted in an increased percentage of 2BS cells with BrdU incorporation after expressing RasG12V (Fig. 2E). This result suggests that NQO1 depletion delayed the onset of RasG12V-induced cellular senescence. Similar results were obtained in other types of HDF cells, BJ and IMR90 cells (Fig.2F and G). In contrast, ectopic expression of NQO1 clearly enhanced the senescence phenotypes induced by RasG12V, resulting in much stronger cell growth inhibition, elevated SA-β-gal activity and decreased levels of BrdU incorporation, compared with corresponding control lentiviral vector infected cells (Fig. 2B-E). These results can explain the finding of up-regulated NQO1 protein levels in premature senescence cells and highlight the functional relevance of NQO1 in senescence establishment.
Figure 2.
NQO1 mediates premature senescence. (A) 2BS stably expressing independent shRNAs against NQO1 upon exposure to RasG12V for 5d were analyzed for NQO1 transcript levels by qRT-PCR. Levels are represented relative to those found in control-infected cells as mean ± SD (n=3). (**p < 0.01). (B) Cells proliferation assay of polyclonal 2BS cells infected with the indicated vectors upon exposure to RasG12V for 12d. A representative experiment out of three independent experiments is shown. Crystal violet was extracted and quantified. (*p<0.05; **p < 0.01). (C) Representative microscopic view of 2BS cells infected with the indicated vectors upon exposure to RasG12V for 5d with SA-β-gal activity staining. Scale bars: 200 μm. (D) 2BS cells infected with the indicated vectors upon exposure to RasG12V for various times were assessed for SA-β-gal activity. Data are mean ± SD from 3 independent experiments, each performed in triplicate. (*p<0.05; **p < 0.01). (E) 2BS cells were assessed for BrdU incorporation. Data are mean ± SD from 3 independent experiments, each performed in triplicate. (*p<0.05; **p < 0.01). (F) BJ cells stably expressing shRNAs against NQO1 upon exposure to RasG12V for 5d were assessed for SA-β-gal and BrdU incorporation. Data are mean ± SD from 3 independent experiments, each performed in triplicate (*p < 0.05). (G) IMR90 cells stably expressing shRNAs against NQO1 upon exposure to RasG12V for 5d were assessed for SA-β-gal activity and BrdU incorporation. Data are mean ± SD from 3 independent experiments, each performed in triplicate (*p < 0.05).
NQO1 binds and stabilizes p53 in senescent cells
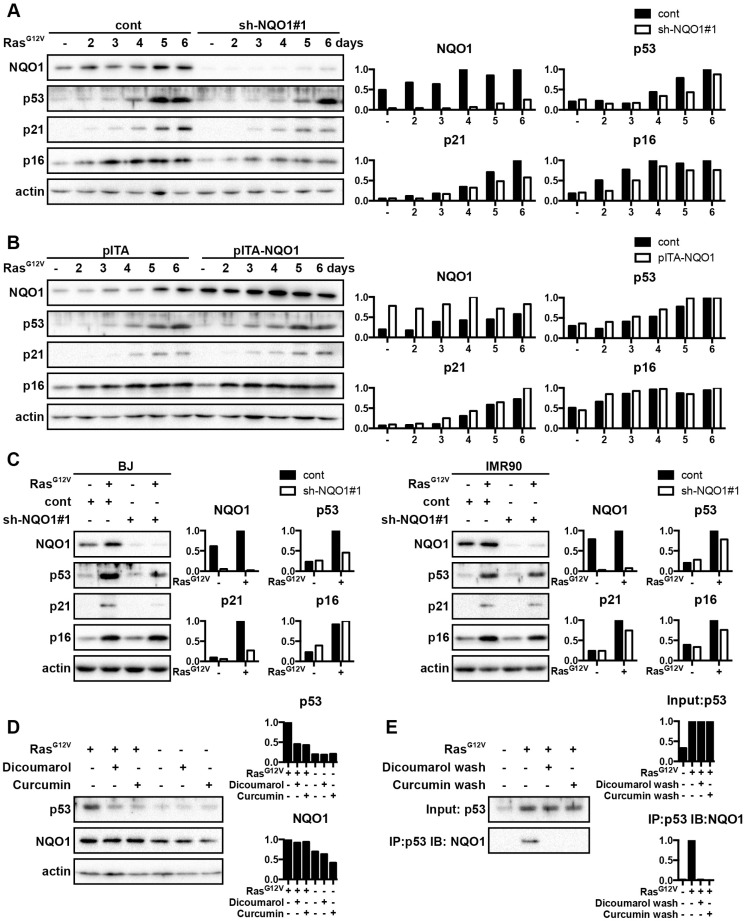
It has been shown that p53 increases in premature senescence, regulates transcription of genes involved in senescence, and plays an important role in cellular senescence. A previous study suggested that NQO1 regulates p53 stability by a mechanism that is independent of MDM2 and ubiquitination. Hence, we speculated that NQO1 also contributed to p53 accumulation during the onset of premature senescence. We found that NQO1 depletion clearly impeded p53 accumulation during OIS (Fig. 3A). Consistent with this result, NQO1 overexpression seems to contribute to p53 accumulation during the same stress (Fig. 3B). Therefore, we hypothesized that NQO1 contributed to p53 protein accumulation, thereby modulating its function. The senescent cell-derived inhibitor p21 is a major downstream target of p53 8. Consequently, in order to determine whether NQO1 activates p53 in OIS we analyzed p21 expression. As we postulated, western blot analysis verified that p21 expression was modulated by NQO1 expression (Fig. 3A and B). To define whether NQO1-mediated modulation of p53 has an impact on the onset of senescence, we used p16 as a hallmark of senescence. Depletion of NQO1 resulted in a noticeable up-regulation of p16 during OIS (Fig. 3A). In contrast, NQO1 overexpression modestly enhanced the p16 expression (Fig. 3B). Similar results were obtained in other types of HDF cells, BJ and IMR90 cells (Fig. 3C). These data suggest that NQO1 contributes to the establishment of senescence arrest.
Figure 3.
NQO1 binds and stabilizes p53 in senescent cells. (A) 2BS cells stably expressing shRNAs against NQO1 were exposed to RasG12V for various times. NQO1, p53, p21 and p16 protein levels were determined by western blotting. β-actin served as a loading control. (B) 2BS cells stably over-expressing NQO1 were exposed to RasG12V for various times. NQO1, p53, p21 and p16 protein levels were determined by western blotting. β-actin served as a loading control. (C) BJ and IMR cells stably expressing shRNAs against NQO1 were exposed to RasG12V for 5d. NQO1, p53, p21 and p16 protein levels were determined by western blotting. β-actin served as a loading control. (D) 2BS cells upon exposure to RasG12V for 5d were treated with vehicle control, dicoumarol (200 μM) or curcumin (60 μM) for 6 h. NQO1 and p53 protein levels were determined by western blotting. β-actin served as a loading control. (E) 2BS cells upon exposure to RasG12V for 5d were lysed for immunoprecipitation (IP) with p53 antibody. The beads were washed without dicoumarol and curcumin, with 300 μM dicoumarol (dicoumarol wash) or with 90 μM curcumin (curcumin wash) and followed by western blotting with NQO1 antibody. The densitometry data were analyzed by ImageJ software and normalized to the highest signal in the corresponding row.
We also investigated whether the NQO1-p53 interaction was affected by NQO1 activity during the onset of OIS. It has been reported that curcumin and dicoumarol are inhibitors of NQO1 activity 31. Analysis of the p53 level in 2BS cells showed that curcumin and dicoumarol only decreased the level of p53 in premature senescent 2BS cells (Fig. 3D), indicating the important role of NQO1 activity in p53 stabilization. Previous reports using various cancer cell lines have indicated that NQO1 binds to p53 15, 16. To test the relevance of NQO1-p53 binding in OIS, p53 was immunoprecipitated in 2BS cells. Western blot analysis verified that NQO1 specifically interacted with p53 in premature senescence (Fig. 3E). Moreover, we found that curcumin and dicoumarol efficiently diminished NQO1 interaction with p53 after OIS in 2BS cells (Fig. 3E).
NQO1 cooperates with p53 to promote senescence
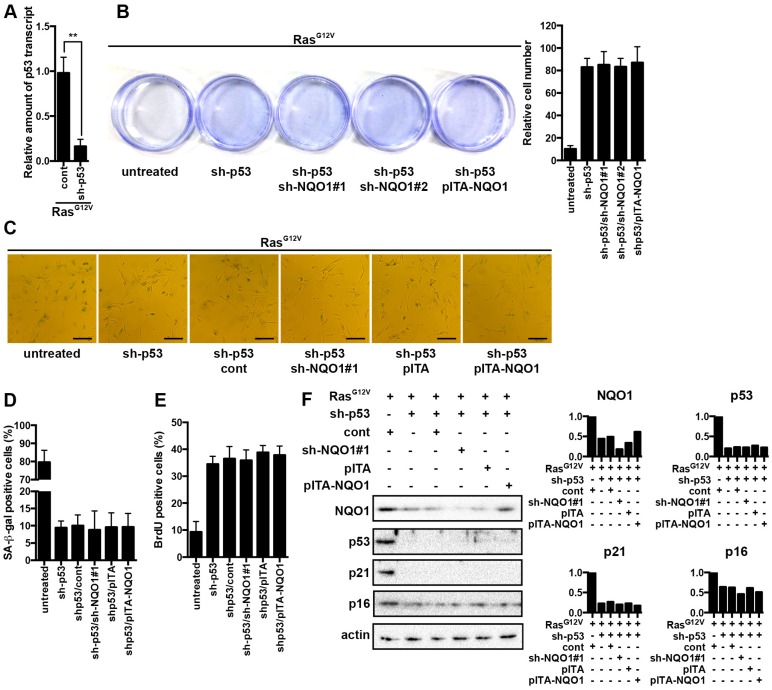
To further test whether NQO1 affects premature senescence mainly by stabilizing the downstream protein p53, we knocked down p53 in 2BS cells using shRNA (sh-p53) and in tandem either knocked down or over-expressed NQO1 in early passage 2BS cells to generate p53 depleted 2BS cells with different levels of NQO1 expression. Subsequently, we introduced RasG12V into these cells. The amount of p53 mRNA level in the HDF was reduced about 70% by shRNA, as compared with the control (Fig. 4A). We further confirmed the effect of the shRNA on p53 at the protein level (Fig. 4F). Considering p53 has an important role in cellular senescence, loss of p53 expression made a great impact on RasG12V induced senescence phenotypic features. We observed a bypass in the proliferation arrest, changes in senescent morphology, decreased SA-β-gal activity and loss in the inhibition of DNA synthesis (Fig. 4B-E). After introducing RasG12V to p53 depleted 2BS cells, overexpression or knockdown of NQO1 had less an impact on cell proliferation (Fig. 4B). These results lead us to evaluate other senescence hallmarks under the same condition. We observed that p53 depletion abolished the effect between NQO1 expression and SA-β-gal activity (Fig. 4D). In addition, the different levels of NQO1 expression did not change BrdU incorporation levels during the RasG12V induction. Furthermore, we assessed p16 protein level as a hallmark of senescence. In accordance with our previous result, we observed a significant decrease in the level of p16 protein in p53 depleted 2BS cells, but altering the levels of NQO1 in these cells did not regulate p16 expression (Fig. 1C). These results indicate that that shRNA-mediated depletion of p53 sufficient blocks the functional relevance between NQO1 and senescence (Fig. 4E) and NQO1 mediated senescence requires p53. Of note, the level of NQO1 protein in sh-p53/RasG12V cells was less than in cells expressing RasG12V alone, suggesting that p53 may take part in a positive feedback loop to up-regulate NQO1 during OIS (Fig. 4F).
Figure 4.
NQO1 cooperates with p53 to promote senescence. (A) 2BS cells stably expressing independent shRNAs against p53 upon exposure to RasG12V for 5d were analyzed for p53 transcript levels by qRT-PCR. Levels are represented relative to those found in control-infected cells as mean ± SD (n=3) (**p < 0.01). (B) Cells proliferation assay of polyclonal 2BS cells infected with the indicated vectors upon exposure to RasG12V for 12d. A representative experiment out of three independent experiments is shown. Crystal violet was extracted and quantified. (C) Representative microscopic view of 2BS cells infected with the indicated vectors upon exposure to RasG12V for 5d with SA-β-gal activity staining. Scale bars: 200 μm. (D) 2BS cells infected with the indicated vectors upon exposure to RasG12V for 5d were assessed for SA-β-gal activity. Data are mean ± SD from 3 independent experiments, each performed in triplicate. (E) 2BS cells were assessed for BrdU incorporation. Data are mean ± SD from 3 independent experiments, each performed in triplicate. (F) NQO1, p53, p21 and p16 protein levels in 2BS cells were determined by western blotting. β-actin served as a loading control. The densitometry data were analyzed by ImageJ software and normalized to the highest signal in the corresponding row.
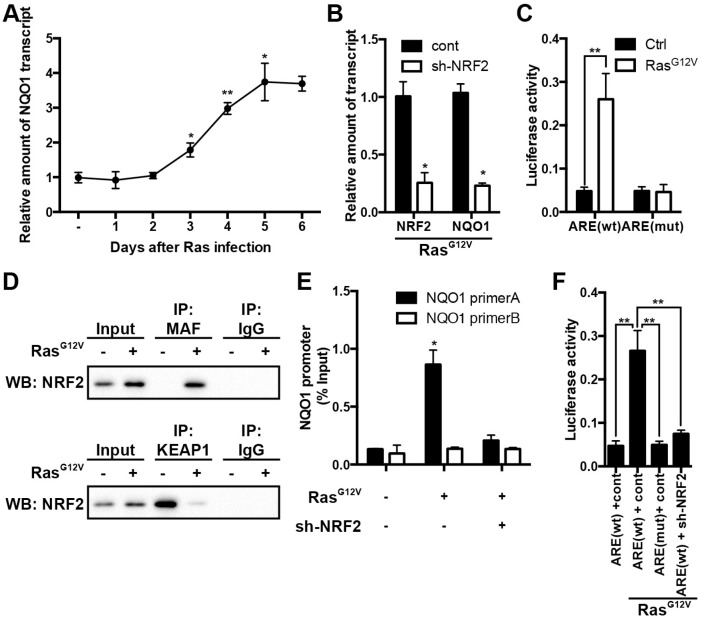
NRF2/KEAP1 signaling regulates NQO1 expression during OIS
Having identified the role of NQO1 in RasG12V induced OIS, we went on to resolve the mechanism by which NQO1 is up-regulated. To begin we measured NQO1 transcript levels by qPCR and we found that NQO1 is up-regulated at the mRNA level during OIS (Fig. 5A). Previous reports have shown that NQO1 gene expression is regulated by the transcription factor nuclear factor (erythroid-derived 2)-like 2 (NRF2) through an antioxidant-response element (ARE) in the gene's promoter. Higher levels of NQO1 may be due to an adaptive mechanism against oxidative stress in senescent cells. Thus, we wished to confirm whether the kelch-like ECH-associated protein 1 (KEAP1)/NRF2/ARE pathway modulates NQO1 in OIS. The amount of NRF2 mRNA level in the HDF was reduced about 60% by shRNA, as compared with the control (Fig. 5B). In support of our hypothesis, depletion of NRF2 sufficiently impaired NQO1 expression during OIS (Fig. 5B). Notably, the NQO1 promoter region contains an ARE element that when removed by mutation significantly attenuated the luciferase activity in premature senescent cells (Fig. 5C). To confirm whether NRF2 directly modulates NQO1 transcription we first analyzed whether OIS induced a shift in NRF2's interaction away from its repressor protein KEAP1 towards its transcriptional partner MAF. MAF and KEAP1 were immunoprecipitated from 2BS cells, and western blot analysis verified that during OIS NRF2 was dislocated from KEAP1 and bound with MAF, suggesting that OIS activates NRF2 and results in NRF2-mediated up-regulation of NQO1 (Fig. 5D). Next, we detected whether NRF2 bound to the endogenous NQO1 promoter by chromatin immunoprecipitation (ChIP) assay (Fig. 5E). Interaction between NRF2 and the NQO1 promoter region was detected by qPCR. As expected, interaction between NRF2 and NQO1 promoter only observed in senescent 2BS. To directly target NRF2, we stably infected 2BS cells with a sh-NRF2 lentiviral vector (Fig. 5B) and examined its binding to the NQO1 promoter. We found that knockdown of NRF2 diminished its interaction with the NQO1 promoter (Fig. 5E). In addition, we also determined whether NRF2 affected NQO1 promoter activity with ARE by the luciferase reporter assay (Fig. 5F). Taken together, these data strongly suggest that during OIS, NRF2/KEAP1 signaling regulates NQO1 expression.
Figure 5.
NRF2/KEAP1 signaling regulates NQO1 expression during OIS. (A) NQO1 transcript levels at the various time points in RasG12V-induced senescent 2BS cells were analyzed by qRT-PCR. Levels are represented relative to those found in control-infected cells as mean ± SD (n=3). (B) 2BS cells stably expressing shRNAs against NRF2 upon exposure to RasG12V for 5d were analyzed for NRF2 and NQO1 transcript levels by qRT-PCR. Levels are represented relative to those found in control-infected cells as mean ± SD (n=3) (*p < 0.05). (C) Effect of NRF2 binding site (ARE) mutations on the activity of the NQO1 promoter in 2BS cells upon exposure to RasG12V for 5d. Data were presented as means ± SD (n=3) (**p < 0.01). (D) 2BS cells upon exposure to RasG12V for 5d were lysed for immunoprecipitation (IP) with c-MAF, KEAP1 antibodies or control IgG, followed by western blotting with NQO1 antibody. (E) Chromatin immunoprecipitation (ChIP) was performed to examine the in vivo binding of NRF2 to the NQO1 promoter. We used NRF2 antibody on chromatin isolated from 2BS cells upon exposure to RasG12V for 5d. %Input NQO1 promoter binding was plotted as mean ± SD (n=3) (*p < 0.05). (F) Effect of NRF2 depletion on the activity of the NQO1 promoter in 2BS cells upon exposure to RasG12V for 5d. Data were presented as mean ± SD (n=3) (**p < 0.01)
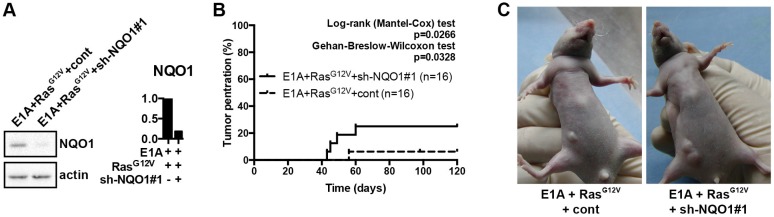
NQO1 deficiency promotes cell transformation and tumorigenesis
Although NQO1 is highly expressed in tumor cells, our results imply that it contributes to cellular senescence. We introduced E1A, RasG12V and shNQO1#1 or Cont in 2BS cells and injected subcutaneously into immunocompromised mice to investigate whether NQO1 has oncogenic properties in cell transformation (Fig. 6A). 2BS cells expressing E1A and RasG12V were weakly tumorigenic and NQO1 depletion clearly lead to a strongly tumorigenic cell (Fig. 6B). These results indicate that NQO1 has anti-oncogenic properties in cell transformation.
Figure 6.
NQO1 deficiency promotes cell transformation and tumorigenesis. (A) NQO1 protein levels in E1A/RasG12V/cont cells or E1A/RasG12V/shNQO1#1 containing cells were determined by western blotting. β-actin served as a loading control. The densitometry data were analyzed by ImageJ software and normalized to the highest signal in the corresponding row. (B) Tumorigenicity assay was performed using same cell as Fig. 6A injected subcutaneously into immunocompromised mice. (C) Two representative images of mice in Fig. 6B.
Discussion
In this study we assessed NQO1 expression during the onset of OIS. It was found that NQO1 expression increases during the onset of OIS, suggesting that NQO1 may be involved in senescence. It has been shown that depletion of NQO1 expression by shRNA delayed OIS. Consistently, an increase in NQO1 expression contributes to the onset of premature senescence. Collectively, we conclude that the increase of NQO1 expression contributes to cellular senescent phenotypes. However, the molecular mechanisms underlying NQO1 expression in OIS require further investigation.
Our results indicate that NQO1 modulates p53 protein level during OIS. Numerous studies have shown that p53 is a key component in the Arf/p53/p21 and DDR pathway, and that p53 can regulate growth arrest, apoptosis and senescence. Under physiological conditions p53 protein level is regulated by the E3 ligase MDM2, through an ubiquitin-dependent degradation that involves the 26S proteasome 32-34. During OIS, the activity of MDM2 is inhibited by DNA damage or oxidative stress, leading to p53 accumulation. Our results indicated that NQO1 specifically binds with p53 in premature senescence. It has been reported that NQO1 directly bound with p53 to protect it from 20S proteasomes degradation, act like a gatekeeper 16. However, NQO1 contributes to p53 regulation in a different mechanism that is ubiquitin-independent. Moreover, we identified that this increase in NQO1 expression was consistent with the NRF2 dislocation from KEAP1 and binding with MAF, which in turn, was found to bind and drive NQO1 promoter activity. It has been reported that the transcription factor NRF2 and its repressor protein KEAP1 respond to the rise in the levels of reactive oxygen species (ROS) 13. Therefore, considering oncogenes frequently increase the production of ROS, and the ROS can activate NRF2, which we have demonstrated to induce NQO1, it is plausible that OIS regulates NQO1 activity. In contrast to the Arf/p53/p21 and DDR pathway, during OIS NQO1 was induced in a different response element and accumulates p53 protein in a different mechanism that involves neither the E3-ubiquitin ligase MDM2 nor any E3 proteins 15. We propose that the ROS-NQO1-p53 signaling may provide an additional fail-safe pathway for cells to respond to oncogenic stress. Our current study suggests that NQO1 plays a distinctive role in p53 protein level regulation during the establishment of OIS.
It is widely accepted that premature senescence is an initial barrier to cell transformation and tumorigenesis 35, 36. In this study, it has been demonstrated that NQO1 itself is part of the barrier. In addition, NQO1 C609T polymorphism is significantly associated with colorectal cancer (CRC) susceptibility 37. In support of this notion, we observed an increase in tumorigenicity of NQO1 depleted cells. Combined expression of adenovirus E1A, Ha-RasG12V, and MDM2 is sufficient to convert a normal human cell into a cancer cell 38. Our results shows that HDF with E1A, RasG12V combined expression and NQO1 depletion can be transformed more efficiently than HDF with E1A and RasG12V expression, suggesting that NQO1 depletion has an overlapping function with MDM2 activity. This suggested to us that regulations on the MDM2 ubiquitin proteasomes degradation pathway and ubiquitin independent p53 proteasomal degradation are required for an intact senescence barrier.
Overexpressed NQO1 has been reported in a number of cancer cells, as well as solid tumors 18-21, which seems conflict with our findings. Actually, NQO1 has positive effects on malignant cells that are already in neoplastic state 22-24, but, as a part of senescence barrier, NQO1 also exhibits anti-tumorigenicity properties during the malignant progression by which normal cells evolve to cancer cells.
In conclusion, our results shed some light on the mechanisms surrounding NQO1's role in the senescence barrier. Specifically, we identified that NQO1 promotes p53 accumulation and thereby reinforces the cellular senescence phenotype. Moreover, we also confirmed that depletion of NQO1 facilitates cell transformation and tumorigenesis.
Acknowledgments
This work was supported by the National Basic Research Programs of China (Grant number 2013CB530801, 2011CB966203) and the Natural Science Foundation of China (Grant number 81471406).
References
- 1.Lee M, Lee JS. Exploiting tumor cell senescence in anticancer therapy. BMB reports. 2014;47:51–9. doi: 10.5483/BMBRep.2014.47.2.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 3.Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nature reviews Molecular cell biology. 2007;8:729–40. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 4.Collado M, Serrano M. Senescence in tumours: evidence from mice and humans. Nature reviews Cancer. 2010;10:51–7. doi: 10.1038/nrc2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes & development. 2010;24:2463–79. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alcorta DA, Xiong Y, Phelps D, Hannon G, Beach D, Barrett JC. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:13742–7. doi: 10.1073/pnas.93.24.13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atadja P, Wong H, Garkavtsev I, Veillette C, Riabowol K. Increased activity of p53 in senescing fibroblasts. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:8348–52. doi: 10.1073/pnas.92.18.8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown JP, Wei W, Sedivy JM. Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science. 1997;277:831–4. doi: 10.1126/science.277.5327.831. [DOI] [PubMed] [Google Scholar]
- 9.Kamijo T, Zindy F, Roussel MF, Quelle DE, Downing JR, Ashmun RA. et al. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell. 1997;91:649–59. doi: 10.1016/s0092-8674(00)80452-3. [DOI] [PubMed] [Google Scholar]
- 10.Lin AW, Barradas M, Stone JC, van Aelst L, Serrano M, Lowe SW. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes & development. 1998;12:3008–19. doi: 10.1101/gad.12.19.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaul Z, Cesare AJ, Huschtscha LI, Neumann AA, Reddel RR. Five dysfunctional telomeres predict onset of senescence in human cells. EMBO reports. 2012;13:52–9. doi: 10.1038/embor.2011.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–60. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 13.Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free radical biology & medicine. 2004;36:1199–207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 14.Talalay P, Fahey JW, Holtzclaw WD, Prestera T, Zhang Y. Chemoprotection against cancer by phase 2 enzyme induction. Toxicology letters. 1995;82-83:173–9. doi: 10.1016/0378-4274(95)03553-2. [DOI] [PubMed] [Google Scholar]
- 15.Asher G, Lotem J, Sachs L, Kahana C, Shaul Y. Mdm-2 and ubiquitin-independent p53 proteasomal degradation regulated by NQO1. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:13125–30. doi: 10.1073/pnas.202480499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asher G, Tsvetkov P, Kahana C, Shaul Y. A mechanism of ubiquitin-independent proteasomal degradation of the tumor suppressors p53 and p73. Genes & development. 2005;19:316–21. doi: 10.1101/gad.319905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garate M, Wong RP, Campos EI, Wang Y, Li G. NAD(P)H quinone oxidoreductase 1 inhibits the proteasomal degradation of the tumour suppressor p33(ING1b) EMBO reports. 2008;9:576–81. doi: 10.1038/embor.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marin A, Lopez de Cerain A, Hamilton E, Lewis AD, Martinez-Penuela JM, Idoate MA. et al. DT-diaphorase and cytochrome B5 reductase in human lung and breast tumours. British journal of cancer. 1997;76:923–9. doi: 10.1038/bjc.1997.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malkinson AM, Siegel D, Forrest GL, Gazdar AF, Oie HK, Chan DC. et al. Elevated DT-diaphorase activity and messenger RNA content in human non-small cell lung carcinoma: relationship to the response of lung tumor xenografts to mitomycin Cl. Cancer research. 1992;52:4752–7. [PubMed] [Google Scholar]
- 20.Belinsky M, Jaiswal AK. NAD(P)H:quinone oxidoreductase1 (DT-diaphorase) expression in normal and tumor tissues. Cancer metastasis reviews. 1993;12:103–17. doi: 10.1007/BF00689804. [DOI] [PubMed] [Google Scholar]
- 21.Joseph P, Xie T, Xu Y, Jaiswal AK. NAD(P)H:quinone oxidoreductase1 (DT-diaphorase): expression, regulation, and role in cancer. Oncology research. 1994;6:525–32. [PubMed] [Google Scholar]
- 22.Garate M, Wani AA, Li G. The NAD(P)H:Quinone Oxidoreductase 1 induces cell cycle progression and proliferation of melanoma cells. Free radical biology & medicine. 2010;48:1601–9. doi: 10.1016/j.freeradbiomed.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Ahn KS, Sethi G, Jain AK, Jaiswal AK, Aggarwal BB. Genetic deletion of NAD(P)H:quinone oxidoreductase 1 abrogates activation of nuclear factor-kappaB, IkappaBalpha kinase, c-Jun N-terminal kinase, Akt, p38, and p44/42 mitogen-activated protein kinases and potentiates apoptosis. The Journal of biological chemistry. 2006;281:19798–808. doi: 10.1074/jbc.M601162200. [DOI] [PubMed] [Google Scholar]
- 24.Thapa D, Meng P, Bedolla RG, Reddick RL, Kumar AP, Ghosh R. NQO1 suppresses NF-kappaB-p300 interaction to regulate inflammatory mediators associated with prostate tumorigenesis. Cancer research. 2014;74:5644–55. doi: 10.1158/0008-5472.CAN-14-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhuo de X, Niu XH, Chen YC, Xin DQ, Guo YL, Mao ZB. Vitamin D3 up-regulated protein 1(VDUP1) is regulated by FOXO3A and miR-17-5p at the transcriptional and post-transcriptional levels, respectively, in senescent fibroblasts. The Journal of biological chemistry. 2010;285:31491–501. doi: 10.1074/jbc.M109.068387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin B, Wang Y, Wu CL, Liu KY, Chen H, Mao ZB. PIM-1 modulates cellular senescence and links IL-6 signaling to heterochromatin formation. Aging cell. 2014;13:879–89. doi: 10.1111/acel.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubinson DA, Dillon CP, Kwiatkowski AV, Sievers C, Yang L, Kopinja J. et al. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nature genetics. 2003;33:401–6. doi: 10.1038/ng1117. [DOI] [PubMed] [Google Scholar]
- 28.Kutner RH, Zhang XY, Reiser J. Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nature protocols. 2009;4:495–505. doi: 10.1038/nprot.2009.22. [DOI] [PubMed] [Google Scholar]
- 29.Carey MF, Peterson CL, Smale ST. Chromatin immunoprecipitation (ChIP) Cold Spring Harbor protocols. 2009;2009:pdb. doi: 10.1101/pdb.prot5279. prot5279. [DOI] [PubMed] [Google Scholar]
- 30.Narita M, Narita M, Krizhanovsky V, Nunez S, Chicas A, Hearn SA. et al. A novel role for high-mobility group a proteins in cellular senescence and heterochromatin formation. Cell. 2006;126:503–14. doi: 10.1016/j.cell.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 31.Tsvetkov P, Asher G, Reiss V, Shaul Y, Sachs L, Lotem J. Inhibition of NAD(P)H:quinone oxidoreductase 1 activity and induction of p53 degradation by the natural phenolic compound curcumin. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:5535–40. doi: 10.1073/pnas.0501828102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–9. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 33.Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 34.Shmueli A, Oren M. Regulation of p53 by Mdm2: fate is in the numbers. Molecular cell. 2004;13:4–5. doi: 10.1016/s1097-2765(03)00529-x. [DOI] [PubMed] [Google Scholar]
- 35.Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–15. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- 36.Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–33. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Zheng B, Wang Z, Chai R. NQO1 C609T polymorphism and colorectal cancer susceptibility: a meta-analysis. Archives of medical science: AMS. 2014;10:651–60. doi: 10.5114/aoms.2014.44856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seger YR, Garcia-Cao M, Piccinin S, Cunsolo CL, Doglioni C, Blasco MA. et al. Transformation of normal human cells in the absence of telomerase activation. Cancer cell. 2002;2:401–13. doi: 10.1016/s1535-6108(02)00183-6. [DOI] [PubMed] [Google Scholar]