Abstract
Background
Skeletal muscle depletion (sarcopenia) is closely associated with limited physical ability and high mortality. This study evaluated the prognostic significance of skeletal muscle status before and after chemotherapy in patients with unresectable colorectal cancer (CRC).
Methods
We conducted a retrospective analysis of 215 consecutive patients with unresectable CRC who underwent systemic chemotherapy. Skeletal muscle cross-sectional area was measured by computed tomography. We evaluated the prognostic value of skeletal muscle mass before chemotherapy and the rate of skeletal muscle change in cross-sectional area after chemotherapy.
Results
One-hundred-eighty-two patients met our inclusion criteria. There were no significant differences in progression-free survival (PFS) or overall survival (OS) associated with skeletal muscle mass before chemotherapy. However, 22 patients with skeletal muscle loss (>5%) after chemotherapy showed significantly shorter PFS and OS compared with those without skeletal muscle loss (PFS, log-rank p = 0.029; OS, log-rank p = 0.009). Multivariate Cox regression analysis revealed that skeletal muscle loss after chemotherapy (hazard ratio, 2.079; 95% confidence interval, 1.194–3.619; p = 0.010) was independently associated with OS.
Conclusions
Skeletal muscle loss after chemotherapy was an independent, negative prognostic factor in unresectable CRC.
Introduction
Colorectal cancer (CRC) is the third leading cause of cancer deaths in Japan, with approximately 112,000 new cases and nearly 45,000 deaths in 2011 [1]. The past 20 years have seen remarkable progress in the treatment of CRC [2, 3]. However, its prognosis remains far from satisfactory.
Nutritional status and changes in body composition have been shown to affect perioperative surgical outcomes such as length of hospital stay and complication rates [4–6]. Skeletal muscle depletion (sarcopenia) is associated with increased toxicity from chemotherapy with 5-fluorouracil and its prodrug, capecitabine [7, 8]. Furthermore, sarcopenia is associated with poorer long-term outcomes in patients with gastrointestinal cancer, colorectal liver metastases, hepatocellular carcinoma, and melanoma [9–13]. In contrast, no consistent association between sarcopenia and survival has been demonstrated in pancreatic, oesophageal, or lung cancer [14–16].
We hypothesized that lower skeletal muscle mass before chemotherapy and skeletal muscle loss after chemotherapy might be associated with disease progression and higher mortality in patients with unresectable CRC. This study accordingly evaluated the association between skeletal muscle loss during chemotherapy and poor prognosis in patients with unresectable CRC.
Patients & Methods
Patients
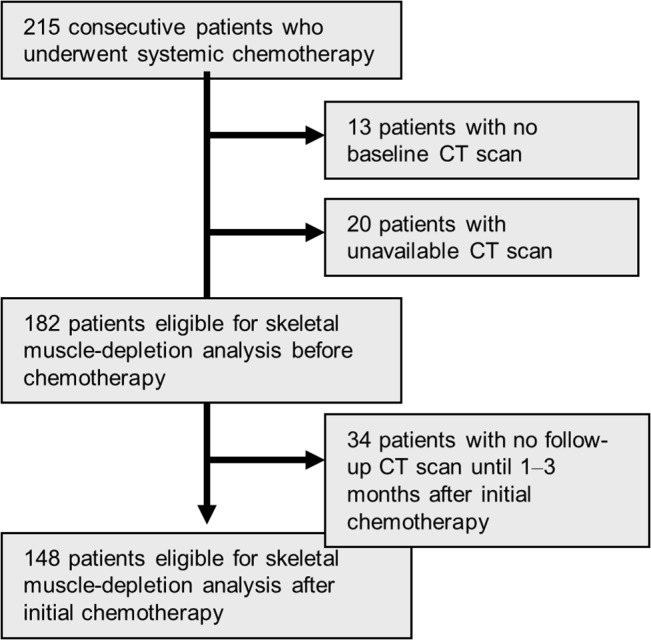
From January 2005 to December 2013, we performed a retrospective analysis of 215 consecutive patients with unresectable CRC who had undergone systemic first-line chemotherapy at Kumamoto University Hospital (Kumamoto, Japan). Patients with unresectable, histologically confirmed colorectal adenocarcinoma were eligible for the study. Patients were included if they had received a computed tomography (CT) scan within 30 days before their first chemotherapy. Patients with a CT scan performed within 30–90 days from their first chemotherapy were eligible for evaluation of skeletal muscle changes. This retrospective study was approved by the institutional review board of Kumamoto University Hospital and was conducted in accordance with the Declaration of Helsinki. Prior written comprehensive informed consent for routine CT scan studies and treatment had been obtained from all patients, and IRB waived the requirement for additional informed consent to participate in this study. In addition, all identifiers were removed from our records at the completion of our analyses to protect patient privacy.
Clinical data
We collected the following data from inpatient and outpatient records: relevant clinical data (age, sex, comorbidity and Eastern Cooperative Oncology Group performance status); tumour-specific data (location of primary tumour, number of organs with metastatic involvement and pre-treatment carcinoembryonic antigen [CEA] concentration); data concerning therapy (chemotherapy regimen); and overall response rate (ORR), progression-free survival (PFS), and overall survival (OS) data. We classified the primary tumour site as the right colon for tumours from the caecum through the transverse colon, and the left colon for tumours from the splenic flexure to the sigmoid colon and rectum.
Resectability was decided by a multidisciplinary team, including specialists in hepatic or colorectal surgery, during chemotherapy. There were no predefined criteria for resectability with regard to the number or size of the tumours, bilobarity, locoregional invasion, or presence of extrahepatic disease, though the resection needed to have the potential to be complete and macroscopically curative. The type of surgical resection was based on the results of preoperative and intraoperative diagnostic imaging. All detectable lesions were resected to achieve R0 resection.
Measurement of skeletal muscle area
Skeletal muscle area was measured retrospectively on CT scans performed before chemotherapy and at initial routine CT scan up to 3 months after chemotherapy, at the level of the third lumbar vertebra (L3) in the inferior direction, with the patient in the supine position. Briefly, we measured pixels using a window width of −30 to 150 HU to delineate the muscle compartments and to compute their cross-sectional areas in cm2 using the Volume Analyzer Synapse Vincent 3D image analysis system (Fujifilm Medical, Tokyo, Japan) (S1 Fig). The cross-sectional area of muscle (cm2) at the L3 level computed from each image was normalized by the square of the height (m2) to obtain the skeletal muscle index (cm2/m2). The rate of skeletal muscle change (%) between pre-treatment CT scan and first routine CT scan after chemotherapy was calculated. All measurements and calculations were performed independently by two trained examiners (Y.M. and Y. S.) who were blinded to the clinical outcomes at the time of quantification. Lin’s concordance correlation coefficient was 0.940 (95% confidence interval [CI], 0.922–0.954) (S1 Table).
Statistical analysis
Statistical analyses were performed using PASW Statistics 18 (SPSS Inc., Chicago, IL, USA), apart from classification and regression tree (CART) survival analysis, which was performed using the “party” package in R software ver.2.13.1 (http://cran.r-project.org/). All p values were two-sided. All data were expressed as mean ± standard deviation or median (range). Categorical variables were expressed as number (percentage). The p values for multiple hypothesis testing were adjusted by Bonferroni correction to p = 0.0042 (= 0.05/12). Mann–Whitney U and χ2 tests were used to compare groups and proportions between groups, respectively. Survival curves were estimated using the Kaplan–Meier method and analysed using log-rank tests. Univariate Cox proportional-hazards models of all potential baseline predictors were built to compute the hazard ratios (HRs) and their 95% CIs. We constructed a multivariate Cox proportional hazard model to compute the HR for skeletal muscle loss rate (<5% vs ≥5%), containing sex (male vs female), age at treatment (>70 vs ≤70 years), timing of metastases (simultaneous vs metachronous), primary tumour location (right colon vs left colon vs rectum), pre-treatment serum CEA level (>100 ng/ml vs ≤100 ng/ml), number of metastatic sites (1 vs ≥2), target agents (not combined vs combined), and R0 resection of metastatic lesions (no vs yes). A backward stepwise elimination with a threshold of p = 0.10 was used to select variables in the final model. The threshold p value for variable elimination was 0.05.
Results
From our database of 215 patients with unresectable CRC, 182 patients (84.7%) met our inclusion criteria for analysis (Fig 1). The median follow-up periods for assessing PFS and OS were 8.1 months (range, 1–92 months) and 23.2 months (range, 1–100 months), respectively. During the follow-up period, 128 patients (70%) developed progression and 111 (61%) died.
Fig 1. Flow chart representing the study selection process.
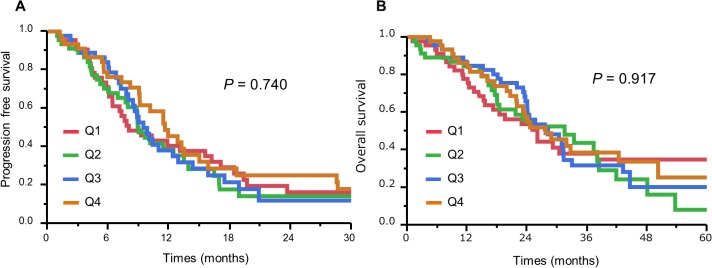
We examined the relationships between the skeletal muscle index in patients with unresectable CRC and various clinical and epidemiological variables. Female sex (p < 0.001) and BMI <25 (p < 0.001) were significantly associated with a lower skeletal muscle index (Table 1), though no other clinical CRC features were significantly correlated with skeletal muscle index. Cox regression analysis with skeletal muscle index before chemotherapy as a continuous variable showed no association between skeletal muscle index and increased mortality. Patients were divided into sex-specific quartiles according to pre-chemotherapy skeletal muscle index. Kaplan–Meier analysis found no significant difference in PFS (p = 0.740) or OS (p = 0.917) according to skeletal muscle index (Fig 2).
Table 1. Skeletal muscle index and clinical and tumour features in patients with unresectable colorectal cancer.
| n | (%) | Skeletal muscle index | p value | ||
|---|---|---|---|---|---|
| Age | 0.971 | ||||
| <70 | 128 | (70) | 50.03 | ±8.46 | |
| ≥70 | 54 | (54) | 50.04 | ±8.46 | |
| Sex | <0.001 | ||||
| Male | 112 | (62) | 53.37 | ±7.70 | |
| Female | 70 | (38) | 45.00 | ±7.02 | |
| PS | 0.438 | ||||
| 0–1 | 176 | (97) | 49.95 | ±8.37 | |
| 2 | 6 | (3) | 47.26 | ±6.92 | |
| BMI | <0.001 | ||||
| <25 | 144 | (79) | 48.65 | ±7.67 | |
| ≥25 | 38 | (21) | 55.83 | ±9.06 | |
| Location of primary | 0.872 | ||||
| Right | 48 | (26) | 50.46 | ±7.54 | |
| Left | 71 | (39) | 50.18 | ±9.05 | |
| Rectum | 63 | (35) | 49.89 | ±8.61 | |
| Timing to metastases | 0.108 | ||||
| Simultaneous | 132 | (73) | 49.54 | ±8.24 | |
| Metachronous | 50 | (27) | 51.76 | 8.97 | |
| Number of metastatic sites | 0.403 | ||||
| 1 | 97 | (53) | 50.66 | ±7.78 | |
| ≥2 | 85 | (47) | 49.57 | ±9.22 | |
| Pre-treatment CEA level | 0.266 | ||||
| ≤100 | 102 | (60) | 50.99 | ±8.70 | |
| >100 | 68 | (40) | 49.25 | ±7.60 | |
| 1st line chemotherapy | 0.080 | ||||
| Oxaliplatin base | 166 | (91) | 49.83 | ±8.53 | |
| Irinotecan base | 16 | (9) | 53.46 | ±7.41 | |
| Combined target therapy | 0.972 | ||||
| Not combined | 74 | (41) | 50.05 | ±8.31 | |
| Bevacizumab | 82 | (45) | 50.31 | ±8.93 | |
| Anti-EGFR antibody | 26 | (14) | 49.93 | ±7.73 | |
| Resection of metastases after chemotherapy | 0.108 | ||||
| No | 120 | (66) | 50.74 | ±8.49 | |
| Yes | 62 | (34) | 49.00 | ±8.39 | |
Abbreviations: CEA, carcinoembryonic antigen; EGFR, epidermal growth factor receptor.
Values given as mean ± standard deviation or number (%).
Fig 2. Kaplan–Meier curves of progression-free survival (A) and overall survival (B) in patients with unresectable colorectal cancer according to quartiles (Q1–Q4) based on skeletal muscle index before chemotherapy.
We also evaluated the association between changes in skeletal muscle mass after chemotherapy and survival. A total of 148 patients (68.8%) met our inclusion criteria for analysis (Fig 1). The median interval from first chemotherapy to initial CT scan after chemotherapy was 2.1 months (1.0–3.0). The median change in skeletal muscle was 4.2% (−30 to +39%) (S2 Fig). We found no correlation between the duration of chemotherapy (interval from first chemotherapy to initial CT scan after chemotherapy) and skeletal muscle change ratio (R2 = 0.012, p = 0.194) (S3 Fig).
We examined the relationship between the rate of skeletal muscle change and various clinical variables. None of the clinical CRC features was significantly correlated with the rate of skeletal muscle change (Table 2). We performed Cox regression analysis with the rate of skeletal muscle change as a continuous variable. Skeletal muscle change tended to be associated with increased overall mortality, but the effect was not significant (univariate analysis p = 0.083).
Table 2. Rate of skeletal muscle change after chemotherapy and clinical and tumour features in patients with unresectable colorectal cancer.
| n | (%) | Skeletal muscle change rate | p value | ||
|---|---|---|---|---|---|
| Age | 0.353 | ||||
| <70 | 108 | (73) | 3.33 | ±10.68 | |
| ≥70 | 40 | (27) | 5.44 | ±9.41 | |
| Sex | 0.057 | ||||
| Male | 85 | (57) | 2.57 | ±9.11 | |
| Female | 63 | (43) | 5.70 | ±11.68 | |
| PS | 0.799 | ||||
| 0–1 | 143 | (97) | 3.86 | ±10.41 | |
| 2 | 5 | (3) | 4.89 | ±10.04 | |
| BMI | 0.824 | ||||
| <25 | 120 | (81) | 3.87 | ±10.75 | |
| ≥25 | 28 | (19) | 4.00 | ±8.66 | |
| Location of primary | 0.826 | ||||
| Right | 40 | (27) | 3.55 | ±9.32 | |
| Left | 53 | (36) | 4.39 | ±13.03 | |
| Rectum | 55 | (37) | 3.68 | ±8.10 | |
| Timing to metastases | 0.092 | ||||
| Simultaneous | 108 | (73) | 4.42 | ±10.38 | |
| Metachronous | 40 | (27) | 2.49 | ±10.30 | |
| Number of metastatic sites | 0.122 | ||||
| 1 | 78 | (53) | 2.47 | ±8.14 | |
| ≥2 | 70 | (47) | 5.49 | ±12.24 | |
| Pre-treatment CEA level | 0.274 | ||||
| ≤100 | 84 | (59) | 4.76 | ±9.66 | |
| >100 | 59 | (41) | 2.72 | ±11.54 | |
| 1st line chemotherapy | 0.550 | ||||
| Oxaliplatin base | 166 | (91) | 3.89 | ±10.02 | |
| Irinotecan base | 16 | (9) | 4.02 | ±14.19 | |
| Combined target therapy | 0.238 | ||||
| Not combined | 57 | (39) | 5.12 | ±10.16 | |
| Bevacizumab | 67 | (45) | 4.16 | ±9.61 | |
| anti-EGFR antibody | 24 | (16) | 0.28 | ±12.35 | |
| Resection of metastases after chemotherapy | 0.435 | ||||
| No | 103 | (70) | 3.69 | ±11.24 | |
| Yes | 45 | (30) | 4.38 | ±8.09 | |
Abbreviations: CEA, carcinoembryonic antigen; EGFR, epidermal growth factor receptor.
Values given as mean ± standard deviation or number (%).
We then divided the 148 patients into four categories according to the rate of skeletal muscle change after chemotherapy: Q1 (>5%, n = 64), Q2 (0–5%, n = 35), Q3 (−5–0%, n = 27), and Q4 (<−5%, n = 22) and performed Cox regression analysis using categorical variables. According to univariate Cox regression analysis, Q4 patients had a significantly higher mortality than Q1 patients (p = 0.013; HR, 2.127; 95% CI, 1.179–3.703), whereas mortality in Q2 patients was similar to that in Q1 patients (p = 0.165 for Q2) (Table 3). Based on this analysis, we established a dichotomous skeletal muscle index variable, defining Q4 as the skeletal-muscle-loss group and combining Q1, Q2, and Q3 into the non-skeletal-muscle-loss group. Patient and tumour characteristics for both groups are summarized in Table 4. There was no significant difference between the two groups in any of the variables. CART survival analysis was used to validate the cut-off value for skeletal muscle change [17], and the first split point to partition the mortality risk for patients was a skeletal muscle change score of −3.6%. This result supports the validity of our cut-off value of ≥ −5.0%.
Table 3. Association between rate of skeletal muscle loss and mortality in patients with colorectal cancer.
| Univariate analysis | ||||
|---|---|---|---|---|
| Skeletal muscle change rate | Total n | HR | 95% CI | p value |
| Q1 (>5%) | 64 | 1.000 | ||
| Q2 (0–5%) | 35 | 1.042 | (0.590–1.786) | 0.165 |
| Q3 (−5–0%) | 27 | 1.335 | (0.741–2.320) | 0.326 |
| Q4 (<−5%) | 22 | 2.127 | (1.179–3.703) | 0.013 |
| Q1–3 | 126 | 1 | ||
| Q4 | 22 | 1.972 | (1.140–3.23) | 0.017 |
Abbreviations: HR, hazard ratio; CI, confidence interval.
Table 4. Characteristics of patients with initially unresectable colorectal cancer with or without skeletal muscle loss (>5%) during chemotherapy.
| Non-skeletal muscle-loss | Skeletal muscle-loss | p value | |||
|---|---|---|---|---|---|
| n | (126) | n | (22) | ||
| Age | 64 | (34–81) | 60 | (34–82) | 0.242 |
| Sex | 0.269 | ||||
| Male | 70 | (56) | 15 | (68) | |
| Female | 56 | (44) | 7 | (32) | |
| Co-morbidity | 0.896 | ||||
| Overall | 68 | (54) | 11 | (50) | 0.522 |
| Cardiovascular disorder | 7 | (6) | 2 | (9) | 0.08 |
| Respiratory disorder | 2 | (2) | 1 | (5) | 0.364 |
| Diabetes mellitus | 21 | (16) | 1 | (5) | 0.140 |
| BMI | 22.2 | (16.7–33.2) | 21.7 | (17.4–28.3) | 0.996 |
| Location of primary | 0.310 | ||||
| Right | 33 | (26) | 7 | (32) | |
| Left | 43 | (34) | 10 | (45) | |
| Rectum | 50 | (40) | 5 | (23) | |
| Timing to metastases | 0.583 | ||||
| Simultaneous | 93 | (74) | 15 | (68) | |
| Metachronous | 33 | (26) | 7 | (32) | |
| Number of metastatic sites | 0.230 | ||||
| 1 | 69 | (55) | 9 | (41) | |
| ≥2 | 57 | (45) | 13 | (59) | |
| Pre-treatment CEA level | 0.169 | ||||
| ≥100 | 74 | (61) | 10 | (45) | |
| <100 | 47 | (39) | 12 | (55) | |
| 1st line chemotherapy | 0.855 | ||||
| Oxaliplatin base | 116 | (92) | 20 | (91) | |
| Irinotecan base | 10 | (8) | 2 | (9) | |
| Combined target therapy | 0.015 | ||||
| Not combined | 49 | (39) | 8 | (36) | |
| Bevacizumab | 61 | (48) | 8 | (36) | |
| anti-EGFR antibody | 16 | (13) | 6 | (27) | |
| 2nd line chemotherapy* | 0.628 | ||||
| Done | 92 | (86) | 18 | (90) | |
| Undone | 15 | (14) | 2 | (10) | |
| ORR, % | 20.5 | 16.7 | 0.713 | ||
| PFS, months | 6.8 | 4.7 | 0.355 | ||
| 3rd line chemotherapy † | 0.074 | ||||
| Done | 61 | (62) | 8 | (40) | |
| Undone | 38 | (38) | 12 | (60) | |
| Resection of metastases after chemotherapy | 0.064 | ||||
| Yes | 42 | (33) | 3 | (14) | |
| No | 84 | (67) | 19 | (86) | |
| Skeletal muscle status before chemotherapy | 0.071 | ||||
| Non-sarcopenia | 92 | (73) | 20 | (91) | |
| Sarcopenia | 34 | (27) | 2 | (9) | |
Abbreviations: CEA, carcinoembryonic antigen; EGFR, epidermal growth factor receptor.
*16 patients did not require 2nd line chemotherapy and 5 patients had missing information.
†22 patients did not require 3rd line chemotherapy and 7 patients had missing information.
Values given as median value (range) for age and BMI, or number (%).
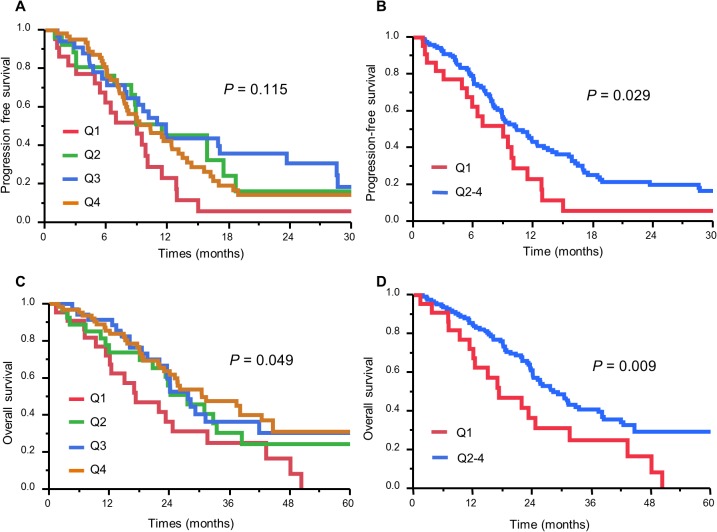
The ORRs were 41.9% in the skeletal-muscle-loss group and 51.5% in the non-skeletal-muscle-loss group. ORR was not significantly associated with skeletal muscle loss (p = 0.338). According to Kaplan–Meier analysis, patients in the skeletal-muscle-loss group (i.e., Q4 patients) experienced significantly shorter PFS (median PFS, 9.0 vs 10.3 months; log-rank p = 0.029) and OS (MST, 17.2 vs 28.2 months; log-rank p = 0.009) than those in the non-skeletal-muscle-loss group (Fig 3).
Fig 3. Analysis of survival in relation to change in skeletal muscle after chemotherapy in patients with unresectable colorectal cancer.
Kaplan–Meier survival curves of progression-free survival (A) and overall survival (C) according to quartiles (Q1–Q4) based on change in skeletal muscle after chemotherapy in patients with unresectable colorectal cancer. (B) and (D) show differences in PFS and OS, respectively, between Q1 and Q2–4. Q1 represents the skeletal-muscle-loss group and Q2, Q3 and Q4 collectively represent the non-skeletal-muscle-loss group.
Cox multivariate modelling was used to determine whether skeletal muscle loss was independently associated with OS when known clinical prognostic markers were included. Univariate analysis initially identified sex, timing of metastases, pre-treatment CEA level, number of metastatic sites, R0 resection of metastatic lesions, and skeletal muscle loss as being associated with OS. Subsequent multivariate Cox analysis identified skeletal muscle loss as an independent risk factor for OS (HR, 2.079; 95% CI, 1.194–3.619; p = 0.010) (Table 5).
Table 5. Univariate and multivariate analyses of factors associated with overall survival in patients with skeletal muscle analysis.
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Skeletal muscle loss rate | ||||||
| ≤5% | 1 | 1 | ||||
| >5% | 1.972 | 1.140–3.233 | 0.017 | 2.079 | 1.194–3.619 | 0.010 |
| Age (years) | ||||||
| <70 | 1 | |||||
| ≥70 | 1.274 | 0.800–1.974 | 0.299 | |||
| Sex | ||||||
| Male | 1 | |||||
| Female | 0.683 | 0.441–1.039 | 0.076 | |||
| Timing of metastases | ||||||
| Simultaneous | 1 | 1 | ||||
| Metachronous | 0.528 | 0.316–0.846 | 0.007 | 0.349 | 0.203–0.599 | <0.001 |
| Primary tumour location | ||||||
| Right | 1 | 1 | ||||
| Left | 0.544 | 0.328–0.904 | 0.019 | 0.406 | 0.242–0.681 | 0.001 |
| Rectum | 0.567 | 0.328–0.904 | 0.029 | 0.466 | 0.275–0.789 | 0.005 |
| Pre-treatment CEA level, ng/ml | ||||||
| ≤100 | 1 | |||||
| >100 | 1.838 | 1.213–2.779 | 0.004 | |||
| Number of metastatic sites | ||||||
| 1 | 1 | |||||
| ≥2 | 2.110 | 1.391–3.225 | <0.001 | |||
| Target agents | ||||||
| Not combined | 1 | |||||
| Combined | 0.810 | 0.525–1.233 | 0.328 | |||
| Resection of metastases after chemotherapy | ||||||
| No | 1 | 1 | ||||
| Yes | 0.290 | 0.167–0.478 | <0.001 | 0.276 | 0.161–0.474 | <0.001 |
Abbreviations: CEA: carcinoembryonic antigen; HR, hazard ratio; CI, confidence interval.
Discussion
In this study, we evaluated the association between skeletal muscle loss during chemotherapy and poor prognosis in patients with unresectable CRC. Pre-treatment skeletal muscle depletion was not a risk factor for survival in unresectable CRC. However, skeletal muscle loss (>5%) after chemotherapy was significantly associated with poorer PFS and OS.
Several studies have confirmed a close relationship between muscle wasting and poor prognosis in patients with various malignancies [11–13, 18]. In addition, accumulating evidence suggests that sarcopenia is linked to treatment toxicity in patients with various cancers [7, 19]. For example, Prado et al. [9] reported that sarcopenia was a significant predictor of toxicity and time to tumour progression in metastatic breast cancer patients treated with oral fluoropyrimidine [20]. In this study, however, there was no relationship between pre-treatment sarcopenia and tumour progression/survival in unresectable CRC patients. Sarcopenia may reflect the increased metabolic activity of a more aggressive tumour biology leading to systemic inflammation, causing muscle loss [21]. Achieving tumour control with effective chemotherapy therefore has the potential to reverse the catabolic processes causing cachexia.
The results of this study demonstrated that PFS in first-line chemotherapy and OS were significantly better in patients without skeletal muscle loss, compared with those with skeletal muscle loss. However, although the ORR was slightly lower in patients with skeletal muscle loss (42%) than in those without (52%), ORR was not significantly associated with skeletal muscle change (p = 0.338). The reason for this apparent discrepancy is unclear. However, the mechanisms underlying the negative impact of skeletal muscle loss on chemotherapeutic response and chemoresistance are likely to be multifactorial. In addition, the continuity of third-line or salvage chemotherapy was lower in patients with skeletal muscle loss (40%) compared with those without (62%) (p = 0.074). These data suggest that skeletal muscle loss may be associated with difficulties in continuing chemotherapy, and subsequent poor compliance might be a risk factor for a poor prognosis. Further studies are needed to verify the effects of skeletal muscle loss on the efficacy of chemotherapy.
There is currently limited knowledge of changes in muscle mass during chemotherapy. The present study found a median rate of change in skeletal muscle mass of 4.2% from pre- to post-chemotherapy, with almost two-thirds of patients remaining stable or gaining skeletal muscle mass. We confirmed that skeletal muscle mass began to change during the initial stage of chemotherapy. To the best of our knowledge, this study provides the first evidence for an association between skeletal muscle loss after chemotherapy and poor prognosis in patients with unresectable CRC. Stene et al. reported that skeletal muscle loss, but not sarcopenia at baseline, was a significant negative prognostic factor in patients with advanced non-small-cell lung cancer receiving palliative chemotherapy [22]. These results may confirm the value of skeletal muscle loss after chemotherapy as a prognostic factor in various cancers.
The most common definition of sarcopenia is currently an appendicular skeletal muscle index of more than two standard deviations below that of healthy adults (5.45 kg/m2 for women and 7.26 kg/m2 for men) [23]. However, these values are related to dual-energy X-ray scanning and we did not use this technique to obtain our primary measurements. Unlike dual-energy X-ray scanning, diagnostic CT is performed in all patients with unresectable CRC as part of routine examinations, and body-composition analysis using CT images is therefore readily available with no additional patient burden or cost, thus demonstrating wider clinical use. In this study, CART survival analysis calculated an optimal discriminatory cut-off value of 3.9% for skeletal muscle decrease, though we established 5% as a simple and reasonable cut-off value.
This study had some limitations. First, it was a single-institution, retrospective study with a small number of patients and a relatively short follow-up duration. Second, the definitions of both sarcopenia and skeletal muscle loss remain controversial. Nonetheless, our results provide important information relevant to the management of patients with unresectable CRC.
In conclusion, skeletal muscle loss after chemotherapy was an independent negative prognostic factor in unresectable CRC. Further prospective studies are needed to clarify the potential clinical benefits of these differences in patients with CRC.
Supporting Information
a) We identified at the level of the third lumbar vertebra (L3) in the inferior direction, b) The skeletal muscle thresholds (-30 to +150HU) are applied, (c) the abdominal contents are cropped and the skeltal muscle cross sectional area calculated in cm2.
(TIF)
(TIF)
(TIF)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research (C), grant number 26462020.
References
- 1. Matsuda A, Matsuda T, Shibata A, Katanoda K, Sobue T, Nishimoto H. Cancer incidence and incidence rates in Japan in 2008: a study of 25 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol 2014; 44:388–396. 10.1093/jjco/hyu003 [DOI] [PubMed] [Google Scholar]
- 2. Meulenbeld HJ, van Steenbergen LN, Janssen-Heijnen ML, Lemmens VE, Creemers GJ. Significant improvement in survival of patients presenting with metastatic colon cancer in the south of The Netherlands from 1990 to 2004. Ann Oncol 2008; 19:1600–1604. 10.1093/annonc/mdn176 [DOI] [PubMed] [Google Scholar]
- 3. Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 2009; 27:3677–3683. 10.1200/JCO.2008.20.5278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer 2012; 107:931–936. 10.1038/bjc.2012.350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peng PD, van Vledder MG, Tsai S, de Jong MC, Makary M, Ng J, et al. Sarcopenia negatively impacts short-term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB (Oxford) 2011; 13:439–446. 10.1111/j.1477-2574.2011.00301.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hasselager R, Gogenur I. Core muscle size assessed by perioperative abdominal CT scan is related to mortality, postoperative complications, and hospitalization after major abdominal surgery: a systematic review. Langenbecks Arch Surg 2014; 399:287–295. 10.1007/s00423-014-1174-x [DOI] [PubMed] [Google Scholar]
- 7. Barret M, Antoun S, Dalban C, Malka D, Mansourbakht T, Zaanan A, et al. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr Cancer 2014; 66:583–589. 10.1080/01635581.2014.894103 [DOI] [PubMed] [Google Scholar]
- 8. Prado CM, Baracos VE, McCargar LJ, Mourtzakis M, Mulder KE, Reiman T, et al. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res 2007; 13:3264–3268. [DOI] [PubMed] [Google Scholar]
- 9. Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 2009; 15:2920–2926. 10.1158/1078-0432.CCR-08-2242 [DOI] [PubMed] [Google Scholar]
- 10. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 2008; 9:629–635. 10.1016/S1470-2045(08)70153-0 [DOI] [PubMed] [Google Scholar]
- 11. van Vledder MG, Levolger S, Ayez N, Verhoef C, Tran TC, Ijzermans JN. Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg 2012; 99:550–557. 10.1002/bjs.7823 [DOI] [PubMed] [Google Scholar]
- 12.Iritani S, Imai K, Takai K, Hanai T, Ideta T, Miyazaki T, et. al. Skeletal muscle depletion is an independent prognostic factor for hepatocellular carcinoma. J Gastroenterol 10.1007/s00535-014-0964-92014 [Online May 10, 2014]. [DOI] [PubMed]
- 13.Voron T, Tselikas L, Pietrasz D, Pigneur F, Laurent A, Compagnon P, et. al. Sarcopenia Impacts on Short- and Long-term Results of Hepatectomy for Hepatocellular Carcinoma. Ann Surg 10.1097/SLA.0000000000000743 [Online June 19, 2014]. [DOI] [PubMed]
- 14. Sabel MS, Lee J, Cai S, Englesbe MJ, Holcombe S, Wang S. Sarcopenia as a prognostic factor among patients with stage III melanoma. Ann Surg Oncol 2011; 18:3579–3585. 10.1245/s10434-011-1976-9 [DOI] [PubMed] [Google Scholar]
- 15. Collins J, Noble S, Chester J, Coles B, Byrne A. The assessment and impact of sarcopenia in lung cancer: a systematic literature review. BMJ Open 2014; 4:e003697 10.1136/bmjopen-2013-003697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yip C, Goh V, Davies A, Gossage J, Mitchell-Hay R, Hynes O, et al. Assessment of sarcopenia and changes in body composition after neoadjuvant chemotherapy and associations with clinical outcomes in oesophageal cancer. Eur Radiol 2014; 24:998–1005. 10.1007/s00330-014-3110-4 [DOI] [PubMed] [Google Scholar]
- 17. Kovarik M, Hronek M, Zadak Z. Clinically relevant determinants of body composition, function and nutritional status as mortality predictors in lung cancer patients. Lung Cancer 2014; 84:1–6. 10.1016/j.lungcan.2014.01.020 [DOI] [PubMed] [Google Scholar]
- 18. Segal MR. Regression trees for censored data. Biometrics 1988; 44:35–4835e48. [Google Scholar]
- 19. Tan BH, Birdsell LA, Martin L, Baracos VE, Fearon KC. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res 2009; 15:6973–6979. 10.1158/1078-0432.CCR-09-1525 [DOI] [PubMed] [Google Scholar]
- 20. Antoun S, Borget I, Lanoy E. Impact of sarcopenia on the prognosis and treatment toxicities in patients diagnosed with cancer. Curr Opin Support Palliat Care 2013; 7:383–389. 10.1097/SPC.0000000000000011 [DOI] [PubMed] [Google Scholar]
- 21. Dodson S, Baracos VE, Jatoi A, Evans WJ, Cella D, Dalton JT, et al. Muscle wasting in cancer cachexia: clinical implications, diagnosis, and emerging treatment strategies. Annu Rev Med 2011; 62:265–279. 10.1146/annurev-med-061509-131248 [DOI] [PubMed] [Google Scholar]
- 22.Stene GB, Helbostad JL, Amundsen T, Sorhaug S, Hjelde H, Kaasa S, et al. Changes in skeletal muscle mass during palliative chemotherapy in patients with advanced lung cancer. Acta Oncol 1–9 2014;:. [DOI] [PubMed] [Google Scholar]
- 23. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998; 147:755–763. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
a) We identified at the level of the third lumbar vertebra (L3) in the inferior direction, b) The skeletal muscle thresholds (-30 to +150HU) are applied, (c) the abdominal contents are cropped and the skeltal muscle cross sectional area calculated in cm2.
(TIF)
(TIF)
(TIF)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.