Significance
Refractory organics are the main hosts of carbon, nitrogen, and other biogenic elements in primitive solar system material. We have synthesized refractory organics by ionizing a gas mixture reminiscent of the composition of the protosolar nebula, at temperatures up to 1,000 K in a plasma. Synthesized compounds share chemical and structural features with chondritic organics, and trapped noble gases reproduce well the elemental and isotopic characteristics of meteoritic noble gases. Our study suggests that organosynthesis took place in the solar system, including in its warm regions, and was ubiquitous anywhere the nebular gas was subject to ionization.
Keywords: organics, meteorites, noble gases, accretion disk, ionization
Abstract
In the nascent solar system, primitive organic matter was a major contributor of volatile elements to planetary bodies, and could have played a key role in the development of the biosphere. However, the origin of primitive organics is poorly understood. Most scenarios advocate cold synthesis in the interstellar medium or in the outer solar system. Here, we report the synthesis of solid organics under ionizing conditions in a plasma setup from gas mixtures (H2(O)−CO−N2−noble gases) reminiscent of the protosolar nebula composition. Ionization of the gas phase was achieved at temperatures up to 1,000 K. Synthesized solid compounds share chemical and structural features with chondritic organics, and noble gases trapped during the experiments reproduce the elemental and isotopic fractionations observed in primitive organics. These results strongly suggest that both the formation of chondritic refractory organics and the trapping of noble gases took place simultaneously in the ionized areas of the protoplanetary disk, via photon- and/or electron-driven reactions and processing. Thus, synthesis of primitive organics might not have required a cold environment and could have occurred anywhere the disk is ionized, including in its warm regions. This scenario also supports N2 photodissociation as the cause of the large nitrogen isotopic range in the solar system.
The solar system formed from the gravitational collapse of a dense core within a molecular cloud that led to the ignition of a central star surrounded by a protoplanetary disk. Protoplanetary disks are evolving and dynamic systems, in which complex chemistry results in the formation and aggregation of solids. Among them, organic molecules are of great astrobiological interest because they may represent the first building blocks of prebiotic molecules. Moreover, they are the main carriers of volatile elements (i.e., H, C, N, and noble gases) that formed the atmospheres of the inner planets. Organic matter is ubiquitous in primitive solar system bodies [e.g., chondrites, especially the carbonaceous ones, interplanetary dust particles (IDPs), and cometary dust—Comet 81P/Wild 2 and ultracarbonaceous Antarctic micrometeorites (UCAMMs) (1–3)]. Most meteoritic organics (up to 99%) are in the form of a refractory and insoluble macromolecular solid, commonly referred to as insoluble organic matter (IOM) (e.g., ref. 4). Notably, IOM displays bulk D and 15N isotope excesses relative to solar composition, which can reach extreme values at a microscopic scale (5, 6). These compositional and isotopic characteristics bear a unique record of the processes and environmental conditions of IOM synthesis, but the message is still cryptic.
IOM is also the main carrier of the heavy noble gases (Ar, Kr, and Xe) as well as of a small fraction of He and Ne trapped in chondrites. The exact host phase of these elements—nicknamed Phase Q (7)—is not well characterized and appears to be a part of, or at least chemically associated with, refractory organic compounds (7–9). This Q component is ubiquitous in primitive chondrites (10, 11), in IDPs, and in Antarctic micrometeorites (12). Thus, Q gases may represent the most important noble gas reservoir outside the Sun at the time of accretion in the protosolar solar nebula (PSN). Q gases are elementally and isotopically fractionated relative to the solar composition [as inferred by the analysis of solar wind composition (13–16)], in favor of heavy elements and isotopes, by about 1%/atomic mass unit (amu) for Xe isotopes (11). So far, only plasma experiments involving noble gas ionization were able to fractionate noble gases elementally and isotopically to extents comparable to those of Q (17–22). Both Q gases and IOM are intimately related and ubiquitous in primitive solar system bodies, likely pointing to a common and preaccretion origin.
Several processes for IOM synthesis have been proposed and/or studied experimentally, reflecting the variety of astrophysical regions where organosynthesis may occur. Most of them involve cold scenarios (e.g., below 40 K) via UV-induced grain surface polymerization of organic molecules in the icy mantles of dust grains, either in the interstellar medium (ISM) or in the outer solar nebula (e.g., refs. 23 and 24). It has been also proposed that organosynthesis could have occurred onto the parent body of chondrites via polymerization of interstellar formaldehyde (25, 26), or via Fischer−Tropsch-like reactions onto metal grains at warm temperature (e.g., 300 K or above) (27, 28). Laboratory works reproduced partially the compositional features of chondritic/cometary refractory organics, but generally failed to reproduce the large D and 15N excesses (relative to the PSN composition) observed in the inner planets, meteorites, and comets. These experiments did not address either the elemental or isotopic fractionations of noble gases.
In this work, we present a plasma experiment designed to produce refractory solid organics from mixtures of C- and N-bearing gases representative of the composition of the solar nebula (i.e., CO, N2 with addition of noble gases). This is the first integrative study, to our knowledge, that investigates organosynthesis and noble gas issues simultaneously.
Materials and Methods
Production of carbonaceous solids was undertaken in a microwave (2.45 GHz) plasma reactor nicknamed Nebulotron (ref. 29, Fig. S1, and Tables S1 and S2) from gas mixtures composed of CO ± N2 ± noble gases (plus traces of H2/H2O). The Ar/C, O/C, and N/C ratios of the starting gas mixtures were close to those inferred for the solar gas composition. However, the H/C (hydrogen mostly coming from H2/H2O impurities from the gas tanks), Kr/C, and Xe/C ratios were orders of magnitude different from an expected solar composition, due to technical and/or analytical limitations (e.g., detection limit for analysis). Typical experiments were run with a total gas flow of 6–10 standard cubic centimeters per minute (sccm) at ∼1 mbar for 2−6 h. Carbonaceous solid particles synthesis followed ionization and dissociation of CO/CO−N2 by electronic and heavy particle excitation in the gas phase at ∼800–1,000 K, a temperature typical of microwave discharges (29, 30). This process produces radicals that react to form small organic precursors such as HCO+, HCN, NH3, and hydrocarbons. These precursors copolymerize into larger organic molecules that eventually coagulate to form solid particles. The synthesized carbonaceous solids from CO ± noble gases and CO−N2 ± noble gases experiments were recovered and analyzed by a combination of analytical techniques to address their nature and structure and their noble gas content (see SI Materials and Methods for experimental and analytical details). Our laboratory conditions are by essence far from the ones expected in the PSN, notably in terms of pressure and duration. This issue is discussed in Discussion.
Results
Complex CHON Compounds.
The elemental analyses and FTIR data show that the N-poor Nebulotron samples (produced from CO + traces of H2/H2O ± noble gases) display a chemical composition very close to that of IOM from unheated chondrites (Table S1 and Fig. 1A). Solids produced from CO and CO–noble gas mixtures are N-poor samples (i.e., N/C < 0.1), whereas those prepared from a CO–N2 ± noble gas mixture contain significant amounts of nitrogen (i.e., N/C > 0.5), demonstrating the very efficient incorporation of nitrogen into the organic solids when N2 is present in the flowing gas mixture (Tables S1 and S2). Interestingly, hydrogen is also efficiently incorporated into molecular precursors of solid compounds although low in abundance in the gas flow.
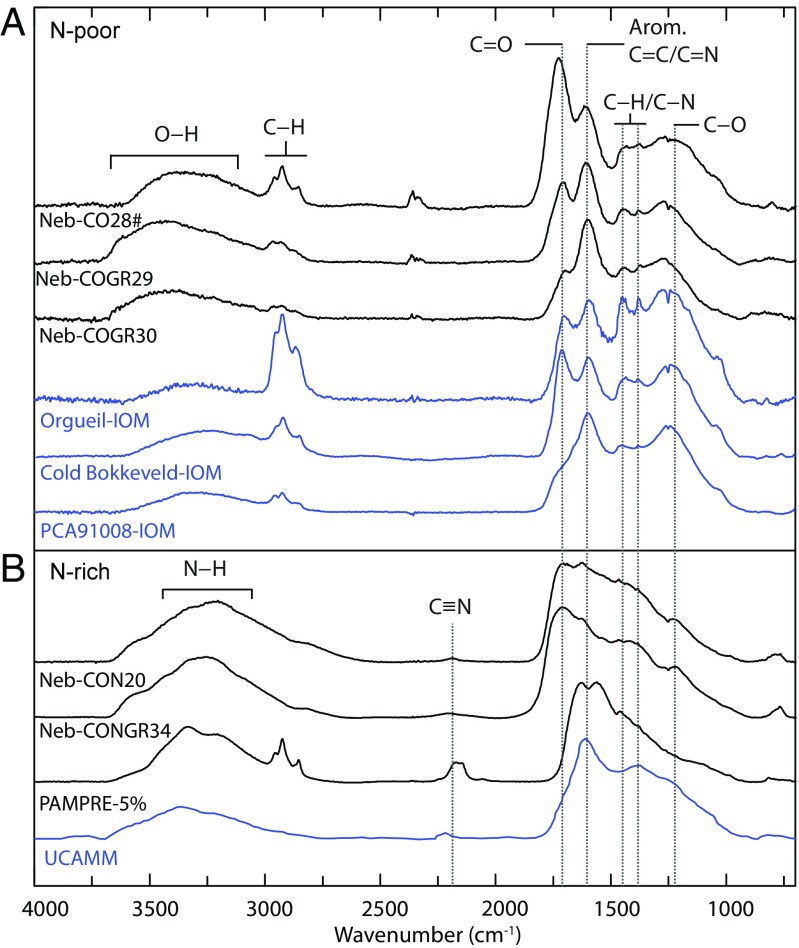
Fig. 1.
FTIR baseline-corrected spectra of the carbonaceous solids synthesized from CO ± noble gas (A) or from CO + N2 ± noble gas (B) mixtures in the Nebulotron. Shown for comparison: IOMs isolated from Orgueil, Cold Bokkeveld, and PCA91008 (62) (A), tholins from the PAMPRE setup [plasma setup designed to simulate Titan's atmospheric chemistry - sample PAMPRE-5%, produced from a mixture composed of 5% CH4 in N2 (63) (B)], and organics from the UCAMM particle DC65 (64) (B). The main absorption bands are highlighted. All spectra are normalized to the intensity at 1,600 cm−1 (C = C), to compare the relative intensities of the other bands. Sample Neb-CO28 was heated at 80 °C under vacuum [Neb-CO28#, (A)] in order to remove water adsorbed onto the sample surfaces. Qualitatively, no significant difference is observed between IR spectra of this particular sample and the other ones.
The organic and complex nature of the Nebulotron-synthesized compounds is apparent in their FTIR spectra (Fig. 1), which display characteristic bands of aliphatic and aromatic/oleifinic groups, and O-rich functional groups (hydroxyl, carbonyl, and ether groups). The N-rich Nebulotron samples synthesized from CO–N2 experiments present additional bands identifying N-rich functional groups (amine/amide, nitrile, and imine groups). The C = O and C = C bands display the largest relative intensities, suggesting an unsaturated molecular structure for the Nebulotron-synthetized organics. The absence of the aromatic C–H stretching band (∼3,050 cm−1) is consistent with high cross-linking and substitution of aromatics in those solids. The N-poor Nebulotron samples display elemental ratios in the range of those of IOM from primitive chondrites (31), and their FTIR spectra are comparable to those of IOM (Fig. 1A). The Nebulotron N-rich samples are more comparable to data obtained for ultracarbonaceous micrometeorites (32), which are thought to be cometary in origin (Fig. 1B).
A Low Degree of Carbon Structural Organization: High-Resolution Transmission Electron Microscopy and Raman.
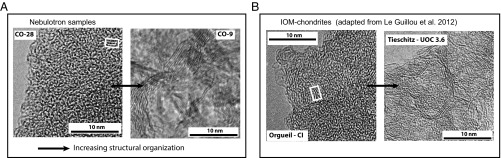
High-resolution transmission electron microscopy (HRTEM) images (Fig. 2) and Raman spectra (Fig. 3) indicate comparable structural features between the Nebulotron-synthesized organics and IOM (see SI Materials and Methods for further details). In the Nebulotron samples, the HRTEM images (Fig. 2) show a gradual increase in the structural organization of the carbon structure with the temperature of the discharge. The degree of organization ranges from highly disordered or “fluffy” (33), with very short fringes staked by two or three, to lamellar and porous nanostructures with fringes staked by more than five and longer than 10 nm.
Fig. 2.
High-resolution transmission electron microscopy. (A) Two Nebulotron solids (CO-28 and CO-9) presenting different degrees of structural organization. (B) Chondritic IOMs. Adapted from ref. 33. Nebulotron samples CO-28 and CO-9 were produced at discharge power of 30 W and 60 W, respectively.
Fig. 3.
Quantitative extraction of spectral parameters from 244 nm (A and B) and 514 nm (C) Raman spectra. (A) D band and ID/IG ratio extracted from 244-nm Raman spectra. Chondritic IOM and UCAMM data are from refs. 65 and 32, respectively. (B) G-band Raman spectral parameters extracted from 244-nm Raman spectra. Chondritic IOM data are from ref. 65. The N-rich synthetic samples (produced in the Nebulotron and in the PAMPRE plasma setups) present a much larger G band as pointed out in Fig. S3. N-poor Nebulotron solids present G-band parameters that are in the area covered by chondritic IOMs within errors. (C) G-band spectral parameters extracted from the 514-nm Raman spectra for N-poor Nebulotron samples (empty circles). Data for UCAMMs (32), IDP and Stardust grains (66–68), carbonaceous chondrites (34, 35, 65, 69), and ion-irradiated organics (70) are shown for comparison. Evolution trends (arrows) are from ref. 70.
The Raman spectra show that the Nebulotron samples and IOM display comparable D and G carbon band variations (Figs. S2 and S3) that confirm a low degree of carbon structural organization (34, 35). Quantitative extraction of 244-nm Raman spectral parameters demonstrates limited heterogeneity among Nebulotron samples (Fig. 3), except for the ID/IG ratio (Fig. 3A), and for the FWHM G, which is larger for N-rich Nebulotron samples (Fig. 3B). The Nebulotron CO-9 sample falls out of the trend, reflecting a higher degree of structural organization consistent with HRTEM imaging (Fig. 2). This particular sample was produced with a higher electric discharge power compared with other samples, implying a higher gas temperature [>1,000 K (30)] in the plasma setup.
Altogether, these observations show that organosynthesis from very simple gaseous molecules at temperatures of >500 K up to 1,000 K can account for the production of complex and unsaturated organic compounds with carbon structural disorder comparable to that of chondritic IOMs.
Trapping and Elemental and Isotopic Fractionation of Heavy Noble Gases.
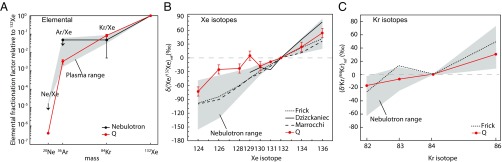
The present data show that ionization of noble gases leads to their efficient trapping and generates strong elemental and isotopic fractionations, in agreement with previous plasma studies (17–21). The elemental and isotopic fractionations of trapped Xe-Q and Kr-Q in meteorites relative to the solar composition (11) are well reproduced in the Nebulotron plasma experiment (Fig. 4).
Fig. 4.
Noble gases. (A) Elemental fractionation of noble gases in the Nebulotron solids (black dots), in synthetic solids produced in plasma setups (gray area) (17–19), and in chondritic IOM for Q gases (red dots) (11), relative to 132Xe and to the gas reference (air composition for laboratory compounds and solar composition for Q gases). Arrows show upper limits, in particular for Ar, as the blank contribution was larger than the trapped Ar content (see SI Materials and Methods for details). (B) Isotopic composition of Xe in the Nebulotron solids (gray range corresponding to 15 samples), in other plasma-synthesized solids (17, 18, 20), and in Q (average of chondrites measured by ref. 11). The delta notation is used, and the ratios are normalized to 132Xe and to air composition for plasma experiments and to solar composition for Q. Typical errors (1σ) are shown for Q only for visibility. Xe-Q isotopes do not follow a linear trend with the mass, contrary to Xe trapped in plasma-synthesized organics. This has been interpreted as the result of the addition of other noble gas components found in meteorites such as Xe-HL, carried by nanodiamonds, s-process Xe, and radiogenic 129Xe to account for the 129Xe excess displayed by Xe-Q (16). (C) Isotopic composition of Kr in the Nebulotron solids (gray range corresponding to seven samples), in plasma-synthesized solids by ref. 17, and in Q (average of chondrites measured by ref. 11). The delta notation is used, and the ratios are normalized to 84Kr and to air composition for plasma experiments and to solar composition for Q. Errors (1σ) are smaller than the symbol size for Kr-Q and are ±5–40‰ for synthetic samples, depending on the isotopic ratio.
All of the Nebulotron samples produced from CO–noble gases ± N2 mixtures present large Kr and Xe mass-dependent isotopic fractionations, where heavy isotopes are enriched relative to the lighter ones by 1.3 ± 0.7%/amu and 1.0 ± 0.4%/amu, respectively (Fig. 4 B and C). Xe atoms are efficiently trapped in the Nebulotron-synthesized solids as attested by (i) the abundance of xenon (2.0 × 10−12 mol/g to 2.4 × 10−9 mol/g) and (ii) the trapping yields (e.g., the amounts of atoms trapped in solids relative to those flowing in the reaction zone; Table S2). It is worth noting that without ionization, the trapping efficiency of Xe atoms in organics is very low and does not induce detectable isotopic fractionation (20, 22). Both Ar and Kr trapped in the synthesized solids are depleted by at least one order of magnitude relative to Xe and to the starting gas mixture composition, similarly to Q noble gases (Fig. 4A). Ionization has been advocated for accounting for the extremely high noble gas content of Phase Q (20) and for the depletion of light noble gases relative to Xe (36).
This characterization points to the synthesized compounds being similar to chondritic organics in respect to chemical, structural, and noble gas features. A qualitative and quantitative comparison between the Nebulotron solids and chondritic refractory organics is discussed in SI Materials and Methods.
Discussion
Noble Gases and Nitrogen Isotopes: Tracers of Organic Synthesis in Ionized Areas of the Solar Nebula.
The origin of the refractory organic compounds found in chondrites is actively debated. Cold scenarios propose UV-induced photopolymerization of organic molecules in the icy mantles of dust grains, either in the ISM (23) or in the solar nebula (24). Low temperature conditions are consistent with models calling for ion–molecule reactions at the origin of the D and 15N enrichments of chondritic IOMs (37, 38). However, theoretical studies suggest that ion–molecule reactions are unable to produce the extreme 15N enrichments of several thousands of permils observed in some IOM hotspots, or under specific conditions only (38, 39). Other scenarios favor an origin of organics within the inner solar system via high-temperature condensation reactions, such as Fischer–Tropsch-like reactions (27, 28) or condensation reactions assisted by electric discharge, such as in the present experiment (40–42). More recently, a postaccretion scenario was proposed, via interstellar formaldehyde polymerization onto the parent body (25, 26). The similarity between the organic solids synthesized in the Nebulotron and chondritic IOM suggests that ionization is a sine qua non condition to obtain significant trapping and isotope fractionation of noble gases. However, Fischer–Tropsch-like reactions and formaldehyde polymerization do not induce ionization of the reactants, and therefore cannot fractionate noble gas or nitrogen isotopes significantly. Trapping of neutral noble gases into ices (e.g., akin to ISM dark clouds or midplane regions in the outer solar nebula) do not induce significant isotope fractionation (43).
However, our experiments do not reproduce the 15N enrichments observed in IOM, of ∼500–750‰ for bulk IOM [up to 1,100‰ for CR-type chondrites (31) and up to 5,800‰ in IOM hotspots in the Bells CM2-type chondrite (6)—all data relative to the PSN nitrogen isotopic composition (44)]. Indeed, we have shown in a previous study that nitrogen trapped in organics produced during plasma organosynthesis is moderately depleted in 15N by about 15–25‰ (29), consistent with kinetic isotope fractionation. Therefore, electron-driven reactions cannot account for solar system 15N enrichments. The only experiment done so far leading to strong 15N enrichments involves the illumination of N2+H2 gas mixtures by synchrotron-generated UV photons in the range 80–98 nm. Extreme 15N enrichments of ∼2,200‰ on average, up to 13,400‰ at 90 nm, were obtained in forming the photoproduct NH3 (45). Such enrichments were interpreted as the result of self-shielding and mutual shielding of N2 during photodissociation, associated with quantum perturbations that may explain the extreme 15N excursion at 90 nm (45, 46). None of these processes can take place in an electron-dominated environment because (i) ionization by electrons is volume-correlated, thus precluding self-shielding or mutual shielding, and (ii) contrary to photons, plasma electrons have a continuum spectrum of energy (30) that prohibits quantum effects. The noble gases are not affected by this limitation because their elemental and isotopic fractionations are not related to molecular dissociation as N2 is.
The 15N enrichments of solar system objects and reservoirs, together with noble gas fractionation, permit precise identification of the mode of ionization. Besides UV photon irradiation [requested to generate 15N-rich compounds (45)], other modes of ionization (e.g., electrons, radioactivity) could have contributed to noble gas fractionation, while not affecting nitrogen isotopes. Thus, variations of the N isotope composition in the solar system, together with near-constant noble gas fractionation, could be the result of heterogeneities in the respective modes of ionization. This possibility is consistent with D/H vs. 15N/14N variations (47). While some of the solar system objects and reservoirs present H and N isotope correlations that suggest a common ion–molecule reaction origin, in other cases including meteoritic organics, excesses of 15N and of D are uncorrelated, pointing to different isotope fractionation mechanisms. The 15N enrichments of solids in the inner solar system might have been very rapid, possibly 150 kya or less after the start of solar system condensation (48). The D enrichments could have then postdated the formation of organic precursors (5). Other processes could have resulted in the decoupling of D and 15N enrichments, such as H2 self-shielding at the very surface of the disk (49) or irradiation by electrons in the kiloelectronvolt energy range (50). These processes do not require low temperature, in agreement with our nebular scenario for the production of high-temperature noble gas-bearing organics.
Ionization in the Solar Nebula and Organosynthesis.
In protoplanetary disks, ionization sources are multiple, resulting in the common occurrence of plasma environments, i.e., partially ionized regions (51). Beyond 1 astronomical unit (AU), ionization is mainly controlled by irradiation sources, such as X-ray and UV photons from the central star and/or from the interstellar radiation field (52). In the very inner part of the disk, temperatures are high enough (>103 K) for thermal ionization to occur. However, it is unlikely that synthesized organics, if any, could survive there. Radioactive decay may also contribute, but to a lesser extent (51). Depending on disk parameters, the electron fraction, is expected to be in the range 10−12 (in dead zones) to 0.1 (in the most irradiated surface region close to the star), generating a stratified ionization structure of the disk (53). Hence, weakly to almost fully ionized plasmas constituted presumably the main fraction of the PSN. In these ionized regions, electrons generated by direct cosmic rays and by X-ray and UV photons are likely to be suprathermal. The exact energy spectrum of electrons is not known, but it has been suggested that the mean energy of the secondary electrons may be around 20 eV or higher (54, 55), well above the dissociation and ionization thresholds of H2, CO, N2, and noble gases. Therefore, these electrons may drive chemical reactions such as the ones occurring in laboratory plasmas and could be responsible for the synthesis of organic compounds.
While electron ionization is able to generate organosynthesis and noble gas isotopic fractionation, photon-driven reactions appear necessary to obtain large enrichments in 15N. Such modes of ionization are consistent with the so-called photon-dominated region (PDR) and the warm molecular layer (WML) in disk models. The PDR, at the very surface of the disk, is necessarily the place where N2 self-shielding occurs in the disk, because extreme UV photons may rapidly be absorbed by gas molecules and dust in the underlying layers. This region is almost fully ionized because of strong irradiation (53, 56) and is expected to be a place of synthesis by photochemistry, but also of destruction of organic molecules (57). As a consequence, the organic molecules that form in the PDR may have a short lifetime, but, in any case, the carrier of 15N enrichments must survive to a certain extent. The WML, although partly shielded from photon irradiation, is largely ionized too and is likely to be a place where organosynthesis and noble gas fractionation occur. Vertical transport, predicted to occur in a turbulent disk, may also help to conserve a part of the newly formed organics and the noble gas and nitrogen isotopic signatures by moving to deeper, colder, and more shielded parts of the disk (24). As a matter of fact, the PDR and the WML temperatures, within 50 AU of the central star, are expected to be >2,000 K and 100–1,000 K, respectively, and much lower beyond 50 AU (53, 56). Such “high” temperatures are generally not considered for organosynthesis in disk models, but our experiment demonstrates that temperatures up to 1,000 K promote, with satisfactory yields, the formation of compounds that present organic complexity that is required to account for IOM precursors. Simple organic molecules such as HCN, C2H2, CO2, H2O, and OH have been observed within 3 AU in the protoplanetary disk of AA Tauri (58), supporting also the hypothesis of an active organic chemistry in the hot and ionized inner part of the disk.
The densities invoked for protoplanetary disks in such active regions (∼108 cm−3) are orders of magnitude lower than those used in laboratory plasmas at ∼1 mbar (1015–1016 cm−3), likely resulting in much less efficient synthesis of organic compounds. Nonetheless, the time during which those processes operate would be much longer in the disk than in a laboratory, likely counterbalancing the lower gas density.
It has been observed that organic matter in chondrites, IDPs, and ultracarbonaceous micrometeorites is often intimately associated with crystalline and amorphous silicates (2, 59, 60). This suggests that gas–grain catalysis may have enhanced the synthesis of organic material in the solar nebula where inorganic dust grains were abundant, i.e., mainly in the inner part of the nebula. Such a process may be compatible with the organosynthesis route we suggest here. Interestingly, direct adsorption of organic material onto mineral surfaces as been advocated as an efficient way of producing more complex organic molecules of astrobiological interest, such as amino acids (61), and consequently may have strong implications on organic delivery to early Earth.
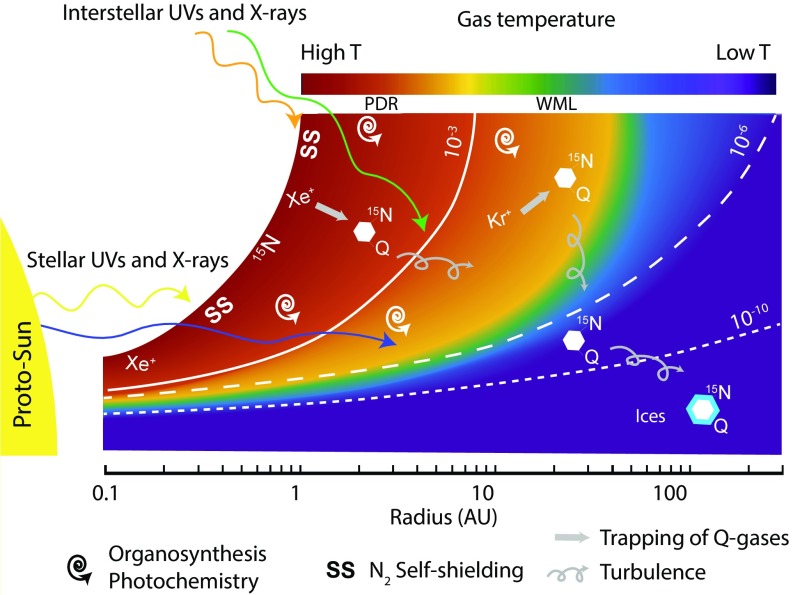
The present experimental study provides a challenging but nonetheless plausible scenario predicting that precursors of chondritic IOM, Q gases, and the 15N enrichments originated in a common environment from the interaction of photons and/or electrons with gas species, i.e., close to the surface of the disk (Fig. 5). Heterogeneities in the composition of refractory organics and radial variations of 15N/14N ratios among solar system objects and reservoirs with heliocentric distance could reflect heterogeneities in ionization processes. UV photon ionization and processing may have dominated in the outer solar system, whereas electron-dominated reactions may have been prevalent in hotter regions of the inner solar system.
Fig. 5.
Schematic diagram of the solar nebula (0.1–100 AU), irradiated by stellar and interstellar UVs and X-rays (height of the disk is scaled by the radius, i.e., Z/R). White lines represent electron fraction isolines. Both the electron fractions and the gas temperature scale are adapted from ref. 53. Synthesis of 15N- and noble gas-rich organics is possible in the most ionized areas of the disk, via photon and/or electron–gas interactions. The source of 15N enrichment is the UV photodissociation of N2 in the PDR region only. Dispersion of organics within the disk is possible thanks to turbulence and settlement. Organics may interact with ices in the cold and shielded middle part of the disk.
Supplementary Material
Acknowledgments
We warmly thank L. Zimmermann for help during noble gas analysis and G. Cernogora, E. Quirico, and F. R. Orthous-Daunay for helpful comments, discussions, and IR measurements. This study was funded by the European Research Council (FP/7 2007e2013, Grant Agreement 267255 to B.M.) and by the Programme National de Planétologie through grants to Y.M. and to L.T. This is Centre de Recherches Pétrographiques et Géochimiques CNRS Contribution 2368.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1502796112/-/DCSupplemental.
References
- 1.Pizzarello S, Cooper GF, Flynn G. The nature and distribution of the organic material in carbonaceous chondrites and interplanetary dust particles. In: Lauretta DS, McSween HY, editors. Meteorites and the Early Solar System II. Vol 1. Univ Arizon Press; Tucson, AZ: 2006. pp. 625–651. [Google Scholar]
- 2.Duprat J, et al. Extreme deuterium excesses in ultracarbonaceous micrometeorites from central Antarctic snow. Science. 2010;328(5979):742–745. doi: 10.1126/science.1184832. [DOI] [PubMed] [Google Scholar]
- 3.Cody GD, et al. Quantitative organic and light-element analysis of comet 81P/Wild 2 particles using C-, N-, and O-mu-XANES. Meteorit Planet Sci. 2008;43(1-2):353–365. [Google Scholar]
- 4.Derenne S, Robert F. Model of molecular structure of the insoluble organic matter isolated from Murchison meteorite. Meteorit Planet Sci. 2010;45(9):1461–1475. [Google Scholar]
- 5.Remusat L, Palhol F, Robert F, Derenne S, France-Lanord C. Enrichment of deuterium in insoluble organic matter from primitive meteorites: A solar system origin? Earth Planet Sci Lett. 2006;243(1-2):15–25. [Google Scholar]
- 6.Busemann H, et al. Interstellar chemistry recorded in organic matter from primitive meteorites. Science. 2006;312(5774):727–730. doi: 10.1126/science.1123878. [DOI] [PubMed] [Google Scholar]
- 7.Lewis RS, Srinivasan B, Anders E. Host phase of a strange xenon component in Allende. Science. 1975;190(4221):1251–1262. [Google Scholar]
- 8.Ott U, Mack R, Chang S. Noble-gas-rich separates from the Allende meteorite. Geochim Cosmochim Acta. 1981;45(10):1751–1788. [Google Scholar]
- 9.Marrocchi Y, Derenne S, Marty B, Robert F. Interlayer trapping of noble gases in insoluble organic matter of primitive meteorites. Earth Planet Sci Lett. 2005;236(3-4):569–578. [Google Scholar]
- 10.Huss GR, Lewis RS, Hemkin S. The “normal planetary” noble gas component in primitive chondrites: Compositions, carrier, and metamorphic history. Geochim Cosmochim Acta. 1996;60(17):3311–3340. [Google Scholar]
- 11.Busemann H, Baur H, Wieler R. Primordial noble gases in “phase Q” in carbonaceous and ordinary chondrites studied by closed system etching. Meteorit Planet Sci. 2000;35(5):949–973. [Google Scholar]
- 12.Spring NH, et al. Xenon in Antarctic micrometeorites (AMMs) and interplanetary dust particles (IDPs) Lunar Planet Sci Conf. 2014;45:2923. [Google Scholar]
- 13.Asplund M, Grevesse N, Sauval AJ, Scott P. 2009. The chemical composition of the Sun. Annu Rev Astron Astrophys, 47:481–522.
- 14.Heber VS, et al. 2012. Isotopic mass fractionation of solar wind: Evidence from fast and slow solar wind collected by the Genesis mission. Astrophys J 759(2): 121.
- 15.Pepin RO, Becker RH, Rider PE. Xenon and krytpon isotopes in extraterrestrial regolith soils and in the solar wind. Geochim Cosmochim Acta. 1995;59(23):4997–5022. [Google Scholar]
- 16.Meshik AP, Hohenberg CM, Pravdivtseva O, Burnett D. Heavy noble gases in solar wind delivered by Genesis mission. Geochim Cosmochim Acta. 2014;127:326–347. doi: 10.1016/j.gca.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frick U, Mack R, Chang S. Noble gas fractionation during synthesis of carbonaceous matter. Lunar Planet Sci Conf. 1979;10:1961–1972. [Google Scholar]
- 18.Dcizckniec M, Lumkin GR, Donohoe K, Chang S. Plasma synthesis of carbonaceous material with noble gas tracers. Lunar Planet Sci Conf. 1981;12:246–248. [Google Scholar]
- 19.Fukunaga K, Matsuda JI. Vapor-growth carbon and the origin of carbonaceous material in ureilites. Geochem J. 1997;31(5):263–273. [Google Scholar]
- 20.Marrocchi Y, Marty B, Reinhardt P, Robert F. Adsorption of xenon ions onto defects in organic surfaces: Implications for the origin and the nature of organics in primitive meteorites. Geochim Cosmochim Acta. 2011;75(20):6255–6266. [Google Scholar]
- 21.Hohenberg CM, Thonnard N, Meshik AP. Active capture and anomalous adsorption: New mechanisms for the incorporation of heavy noble gases. Meteorit Planet Sci. 2002;37(2):257–267. [Google Scholar]
- 22.Marrocchi Y, Marty B. Experimental determination of the xenon isotopic fractionation during adsorption. Geophys Res Lett. 2013;40(16):4165–4170. [Google Scholar]
- 23.Bernstein MP, Sandford SA, Allamandola LJ, Chang S, Scharberg MA. Organic compounds produced by photolysis of realistic interstellar and cometary ice analogs containing methanol. Astrophys J. 1995;454(1):327–344. [Google Scholar]
- 24.Ciesla FJ, Sandford SA. Organic synthesis via irradiation and warming of ice grains in the solar nebula. Science. 2012;336(6080):452–454. doi: 10.1126/science.1217291. [DOI] [PubMed] [Google Scholar]
- 25.Cody GD, et al. Establishing a molecular relationship between chondritic and cometary organic solids. Proc Natl Acad Sci USA. 2011;108(48):19171–19176. doi: 10.1073/pnas.1015913108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kebukawa Y, Kilcoyne ALD, Cody GD. Exploring the potential formation of organics solids in chondrites and comets through polymerization of interstellar formaldehyde. Astrophys J. 2013;771(1):19. [Google Scholar]
- 27.Nuth JA, Johnson NM, Manning S. A self-perpetuating catalyst for the production of complex organic molecules in protostellar nebulae. Astrophys J Lett. 2008;673(2):L225–L228. [Google Scholar]
- 28.Llorca J, Casanova I. Reaction between H2, CO, and H2S over Fe, Ni metal in the solar nebula: Experimental evidence for the formation of sulfur-bearing organic molecules and sulfides. Meteorit Planet Sci. 2000;35(4):841–848. [Google Scholar]
- 29.Kuga M, et al. Nitrogen isotopic fractionation during abiotic synthesis of organic solid particles. Earth Planet Sci Lett. 2014;393:2–13. [Google Scholar]
- 30.Fridman A. Plama Chemistry. Cambridge Univ Press; New York: 2008. [Google Scholar]
- 31.Alexander CMOD, Fogel M, Yabuta H, Cody GD. The origin and evolution of chondrites recorded in the elemental and isotopic compositions of their macromolecular organic matter. Geochim Cosmochim Acta. 2007;71(17):4380–4403. [Google Scholar]
- 32.Dobrica E, Engrand C, Quirico E, Montagnac G, Duprat J. Raman characterization of carbonaceous matter in CONCORDIA Antarctic micrometeorites. Meteorit Planet Sci. 2011;46(9):1363–1375. [Google Scholar]
- 33.Le Guillou C, et al. High resolution TEM of chondritic carbonaceous matter: Metamorphic evolution and heterogeneity. Meteorit Planet Sci. 2012;47(3):345–362. [Google Scholar]
- 34.Bonal L, Quirico E, Bourot-Denise M, Montagnac G. Determination of the petrologic type of CV3 chondrites by Raman spectroscopy of included organic matter. Geochim Cosmochim Acta. 2006;70(7):1849–1863. [Google Scholar]
- 35.Busemann H, Alexander CMOD, Nittler LR. Characterization of insoluble organic matter in primitive meteorites by microRaman spectroscopy. Meteorit Planet Sci. 2007;42(7-8):1387–1416. [Google Scholar]
- 36.Ott U. Planetary and pre-solar noble gases in meteorites. Chem Erde. 2014;74(4):519–544. [Google Scholar]
- 37.Yang J, Epstein S. Interstellar organic-matter in meteorites. Geochim Cosmochim Acta. 1983;47(12):2199–2216. [Google Scholar]
- 38.Terzieva R, Herbst E. The possibility of nitrogen isotopic fractionation in interstellar clouds. Mon Not R Astron Soc. 2000;317(3):563–568. [Google Scholar]
- 39.Rodgers SD, Charnley SB. Nitrogen superfractionation in dense cloud cores. Mon Not R Astron Soc. 2008;385(1):L48–L52. [Google Scholar]
- 40.Sagan C, Khare BN. Tholins: Organic chemistry of interstellar grains and gas. Nature. 1979;227(5692):102–107. [Google Scholar]
- 41.Morgan WA, Jr, Feigelson ED, Wang H, Frenklach M. A new mechanism for the formation of meteoritic kerogen-like material. Science. 1991;252(5002):109–112. doi: 10.1126/science.252.5002.109. [DOI] [PubMed] [Google Scholar]
- 42.Saito M, Kimura Y. Origin of organic globules in meteorites: Laboratory simulations using aromatic hydrocarbons. Astrophys J Lett. 2009;703(2):L147–L151. [Google Scholar]
- 43.Notesco G, Bar-Nun A, Owen T. Gas trapping in water ice at very low deposition rates and implications for comets. Icarus. 2003;162(1):183–189. [Google Scholar]
- 44.Marty B, Chaussidon M, Wiens RC, Jurewicz AJG, Burnett DS. A 15N-poor isotopic composition for the solar system as shown by Genesis solar wind samples. Science. 2011;332(6037):1533–1536. doi: 10.1126/science.1204656. [DOI] [PubMed] [Google Scholar]
- 45.Chakraborty S, et al. Massive isotopic effect in vacuum UV photodissociation of N2 and implications for meteorite data. Proc Natl Acad Sci USA. 2014;111(41):14704–14709. doi: 10.1073/pnas.1410440111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clayton RN. Solar System: Self-shielding in the solar nebula. Nature. 2002;415(6874):860–861. [Google Scholar]
- 47.Aleon J. Multiple origins of nitrogen isotopic anomalies in meteorites and comets. Astrophys J. 2010;722(2):1342–1351. [Google Scholar]
- 48.Füri E, Chaussidon M, Marty B. Evidence for an early nitrogen isotopic evolution in the solar nebula from volatile analyses of a CAI from the CV3 chondrite NWA 8616. Geochim Cosmochim Acta. 2015;153:183–201. [Google Scholar]
- 49.Cleeves LI, et al. The ancient heritage of water ice in the solar system. Science. 2014;345(6204):1590–1593. doi: 10.1126/science.1258055. [DOI] [PubMed] [Google Scholar]
- 50.Laurent B, et al. Isotopic and structural signature of experimentally irradiated organic matter. Geochim Cosmochim Acta. 2014;142:522–534. [Google Scholar]
- 51.Fromang S, Terquem C, Balbus SA. The ionization fraction in alpha models of protoplanetary discs. Mon Not R Astron Soc. 2002;329(1):18–28. [Google Scholar]
- 52.Glassgold AE, Najita J, Igea J. X-ray ionization of protoplanetary disks. Astrophys J. 1997;480(1):344–350. [Google Scholar]
- 53.Walsh C, Nomura H, Millar TJ, Aikawa Y. Chemical processes in protoplanetary disks. II. On the importance of photochemistry and X-ray ionization. Astrophys J. 2012;747(2):114. [Google Scholar]
- 54.Gredel R, Lepp S, Dalgarno A, Herbst E. Cosmic-ray induced photodissociation and photoionization rates of interstellar molecules. Astrophys J. 1989;347(1):289–293. [Google Scholar]
- 55.Inutsuka S, Sano T. Self-sustained ionization and vanishing dead zones in protoplanetary disks. Astrophys J. 2005;628(2):L155–L158. [Google Scholar]
- 56.Walsh C, Millar TJ, Nomura H. Chemical processes in protoplanetary disks. Astrophys J. 2010;722(2):1607–1623. [Google Scholar]
- 57.Henning T, Semenov D. Chemistry in protoplanetary disks. Chem Rev. 2013;113(12):9016–9042. doi: 10.1021/cr400128p. [DOI] [PubMed] [Google Scholar]
- 58.Carr JS, Najita JR. Organic molecules and water in the planet formation region of young circumstellar disks. Science. 2008;319(5869):1504–1506. doi: 10.1126/science.1153807. [DOI] [PubMed] [Google Scholar]
- 59.Garvie L, Baumgardner G, Buseck PR. Scanning electron microscopical and cross sectional analysis of extraterrestrial carbonaceous nanoglobules. Meteorit Planet Sci. 2008;43(5):899–903. [Google Scholar]
- 60.Zega TJ, et al. Mineral associations and character of isotopically anomalous organic material in the Tagish Lake carbonaceous chondrite. Geochim Cosmochim Acta. 2010;74(20):5966–5983. [Google Scholar]
- 61.Asaduzzaman AM, et al. A computational investigation of adsorption of organics on mineral surfaces: Implications for organics delivery in the early solar system. Earth Planet Sci Lett. 2014;408:355–361. [Google Scholar]
- 62.Orthous-Daunay FR, et al. Mid-infrared study of the molecular structure variability of insoluble organic matter from primitive chondrites. Icarus. 2013;223(1):534–543. [Google Scholar]
- 63.Sciamma-O'Brien E, Carrasco N, Szopa C, Buch A, Cernogora G. Titan's atmosphere: An optimal gas mixture for aerosol production? Icarus. 2010;209(2):704–714. [Google Scholar]
- 64.Dartois E, et al. UltraCarbonaceous Antarctic micrometeorites, probing the Solar System beyond the nitrogen snow-line. Icarus. 2013;224(1):243–252. [Google Scholar]
- 65.Quirico E, et al. Origin of insoluble organic matter in type 1 and 2 chondrites: New clues, new questions. Geochim Cosmochim Acta. 2014;136:80–99. [Google Scholar]
- 66.Quirico E, Borg J, Raynal PI, Montagnac G, d'Hendecourt L. A micro-Raman survey of 10 IDPs and 6 carbonaceous chondrites. Planet Space Sci. 2005;53(14-15):1443–1448. [Google Scholar]
- 67.Rotundi A, et al. Combined micro-Raman, micro-infrared, and field emission scanning electron microscope analyses of comet 81P/Wild 2 particles collected by Stardust. Meteorit Planet Sci. 2008;43(1-2):367–397. [Google Scholar]
- 68.Sandford SA, et al. Organics captured from comet 81P/Wild 2 by the Stardust spacecraft. Science. 2006;314(5806):1720–1724. doi: 10.1126/science.1135841. [DOI] [PubMed] [Google Scholar]
- 69.Bonal L, Bourot-Denise M, Quirico E, Montagnac G, Lewin E. Organic matter and metamorphic history of CO chondrites. Geochim Cosmochim Acta. 2007;71(6):1605–1623. [Google Scholar]
- 70.Brunetto R, et al. Comparison of the Raman spectra of ion irradiated soot and collected extraterrestrial carbon. Icarus. 2009;200(1):323–337. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.