Significance
Dendritic cells are critical in regulating immune responses. DEC205 (CD205) is an endocytotic receptor on dendritic cells with antigen presentation function and has been widely used in immune therapies. Here, we report that DEC205 is an immune receptor that recognizes apoptotic and necrotic cells specifically through a pH-dependent mechanism. The ectodomain of DEC205 forms a double-ringed conformation at acidic pH and becomes extended at basic pH. DEC205 only recognizes apoptotic and necrotic cells at acidic conditions with its N-terminal small ring and has no binding activities to healthy cells at either acidic or basic conditions, thus representing a novel pathway for immune clearance of dead cells and a potential mechanism for tumor scavenging.
Keywords: DEC205, pH dependence, apoptosis, cryoEM, mannose receptor family
Abstract
Dendritic cells play important roles in regulating innate and adaptive immune responses. DEC205 (CD205) is one of the major endocytotic receptors on dendritic cells and has been widely used for vaccine generation against viruses and tumors. However, little is known about its structure and functional mechanism. Here we determine the structure of the human DEC205 ectodomain by cryoelectron microscopy. The structure shows that the 12 extracellular domains form a compact double ring-shaped conformation at acidic pH and become extended at basic pH. Biochemical data indicate that the pH-dependent conformational change of DEC205 is correlated with ligand binding and release. DEC205 only binds to apoptotic and necrotic cells at acidic pH, whereas live cells cannot be recognized by DEC205 at either acidic or basic conditions. These results suggest that DEC205 is an immune receptor that recognizes apoptotic and necrotic cells specifically through a pH-dependent mechanism.
In living organisms such as humans, billions of cells are turned over through apoptosis or killed by pathological infections or inflammation every day. Therefore, clearance of dead cells is critical for maintaining tissue homeostasis and preventing autoimmunity and inflammation (1–3). Dead cells are usually removed by the host immune system through phagocytosis by phagocytes. Antigen-presenting cells (APCs) such as dendritic cells and macrophages are professional phagocytes that can engulf target cells or fragments by recognizing specific ligands through their cell surface receptors (4), and after antigen uptake, processing, and presentation, they can lead to either immune activation or tolerance (5–8).
DEC205 (CD205 or Ly75, MW 205 kDa) is an endocytotic receptor with wide tissue distribution and is highly expressed on dendritic cells and thymic epithelial cells (8, 9). It has been shown that DEC205 is involved in antigen uptake and can induce either tolerance or immunity in the absence or presence of inflammatory stimulus (10). It has also been suggested that DEC205 may bind apoptotic and necrotic cells (11) and oligonucleotides (12); however, neither the structure nor the functional mechanism of DEC205 has been identified.
In contrast, the role of DEC205 in generating protective immunity has been studied extensively. It is probably the most widely used receptor target in dendritic cell-based immune therapies. The high efficiency of antigen delivery and presentation makes DEC205 an ideal vehicle for vaccine generation against various antigens such as tumors and viruses (13, 14), mainly through DEC205-specific antibodies fused with a fragment or intact protein of the target antigen (15–17). This strategy has been shown to be successful in generate protective immune responses and reveals good potentials in clinical applications (18).
DEC205 belongs to the mannose receptor family (19). To date, five proteins have been classified as the mannose receptor family members, including the mannose receptor itself (20), DEC205 (9), Endo180 (21), PLA2R (22), and FcRY (23). These receptors share similar structural features, but their physiological functions are made diverse by recognizing different ligands. The ectodomain of mannose receptor family members begins with a cysteine-rich domain (CysR), followed by a fibronectin type II domain (FNII) and eight (10 for DEC205) C-type lectin-like domains (CTLDs) (Fig. 1A). Probably because of the potential internal flexibility, no high-resolution structures have been determined for this family. Currently known structural information of this family comes from electron microscopy. A negatively stained image reconstruction shows that the mannose receptor has a compact conformation (24). The cryoelectron microscopy (cryoEM) reconstruction of FcRY indicates that its ectodomain adopts a double-ringed conformation at acidic pH (25). However, because of limited resolution, the detailed domain arrangements of these molecules, especially the interactions among these domains, are unclear.
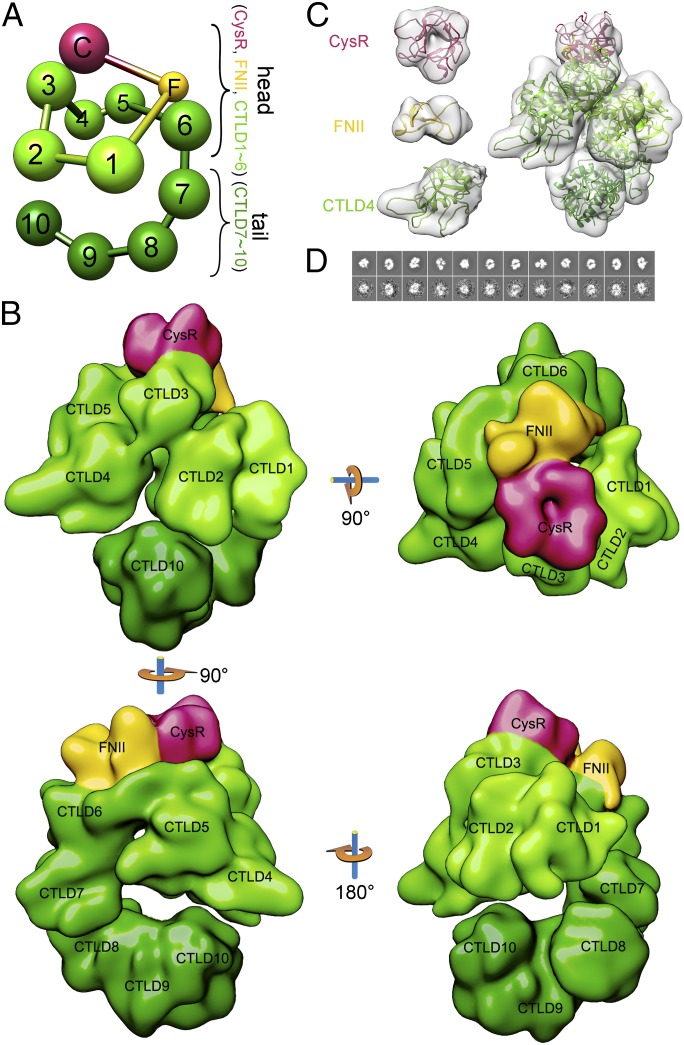
Fig. 1.
The pH-dependent conformational change of DEC205. (A) A schematic representation of DEC205 domain arrangement. (B) SEC profiles of DEC205 at pH 6 and pH 8. (C) Dynamic light scattering analysis of DEC205 at pH 6 and pH 8. (D) A negatively stained EM micrograph (Top) and the representative reference-free 2D classes (Bottom) of DEC205 at basic pH. (E) A negatively stained EM micrograph (Top) and the representative reference-free 2D classes (Bottom) of DEC205 at acidic pH. (F) A cryoEM micrograph (Top) and the representative reference-free 2D classes (Bottom) of DEC205 at acidic pH. (Scale bars, 50 nm for micrographs and 10 nm for the reference-free 2D classes.)
Here we determined the 3D structure of human DEC205 ectodomain by cryoEM single-particle reconstruction, identified its pH-dependent conformational change, and also investigated the mechanism of the conformational change and its correlation with ligand binding and release. These results indicate that DEC205 is an immune receptor that recognizes apoptotic and necrotic cells specifically through a pH-dependent mechanism.
Results
pH-Dependent Conformational Change of DEC205.
The human DEC205 ectodomain (residues 1–1,668) was expressed in HEK293 cells. The purified protein was analyzed by size-exclusion chromatography (SEC) at both acidic (pH 6) and basic (pH 8) conditions. The DEC205 ectodomain eluted earlier at pH 8 than at pH 6 (Fig. 1B), suggesting a more extended conformation at pH 8. Consistent with this interpretation, dynamic light scattering showed an increase in the hydrodynamic radius at basic pH compared with acidic pH (Fig. 1C).
To visualize the conformational change of DEC205 directly, the DEC205 ectodomain was negatively stained at both acidic (pH 6.0) and basic (pH 8.0) conditions and was imaged by electron microscopy. The images obtained at acidic pH showed that DEC205 had a homogeneous conformation with a globular shape (Fig. 1E), whereas the sample stained at basic pH showed mostly linear and extended particles with heterogeneous conformations (Fig. 1D). These results confirmed that DEC205 underwent a conformational change between acidic and basic conditions.
CryoEM Reconstruction of DEC205.
To investigate the 3D structure of DEC205 at higher resolution, we collected images from the unstained frozen hydrated samples of human DEC205 ectodomain by cryoEM at pH 6 (Fig. 1F) and pH 8. 3D reconstruction of DEC205 at pH 8 was not successful, probably because of the flexibility and heterogeneity of the open conformation at basic conditions, as observed in the negatively stained images (Fig. 1D). The compact conformation at pH 6 was reconstructed and refined to 14.6 Å resolution, the highest resolution achieved for a mannose receptor family member to date (Fig. 2). The overall structure of DEC205 can be roughly divided into a head and a tail region. The head adopts a double-ringed conformation. The small ring contains CysR, FNII, and CTLD1∼CTLD3, with CysR interacting with CTLD3. The larger ring is formed by FNII and CTLD1∼CTLD6, with FNII interacting with CTLD6 (Fig. 2 and Fig. 3A). The tail of DEC205 includes domains from CTLD7 to CTLD10. A similar compact double ring-shaped conformation at acidic pH has been found for FcRY (25), suggesting the conformation might be conserved within the mannose receptor family.
Fig. 2.
3D reconstruction of DEC205 by cryoEM. (A) A ball-and-stick model of DEC205 domain arrangement from cryoEM. (B) Views of the cryoEM reconstruction of DEC205. (C) CryoEM densities (gray) of the individual domains (CysR, FNII, and CTLD4) (Left) and DEC205 (Right) fitted with the corresponding homology models. (D) Back projections of DEC205 reconstruction (Top) and the corresponding reference-based 2D classes (Bottom).
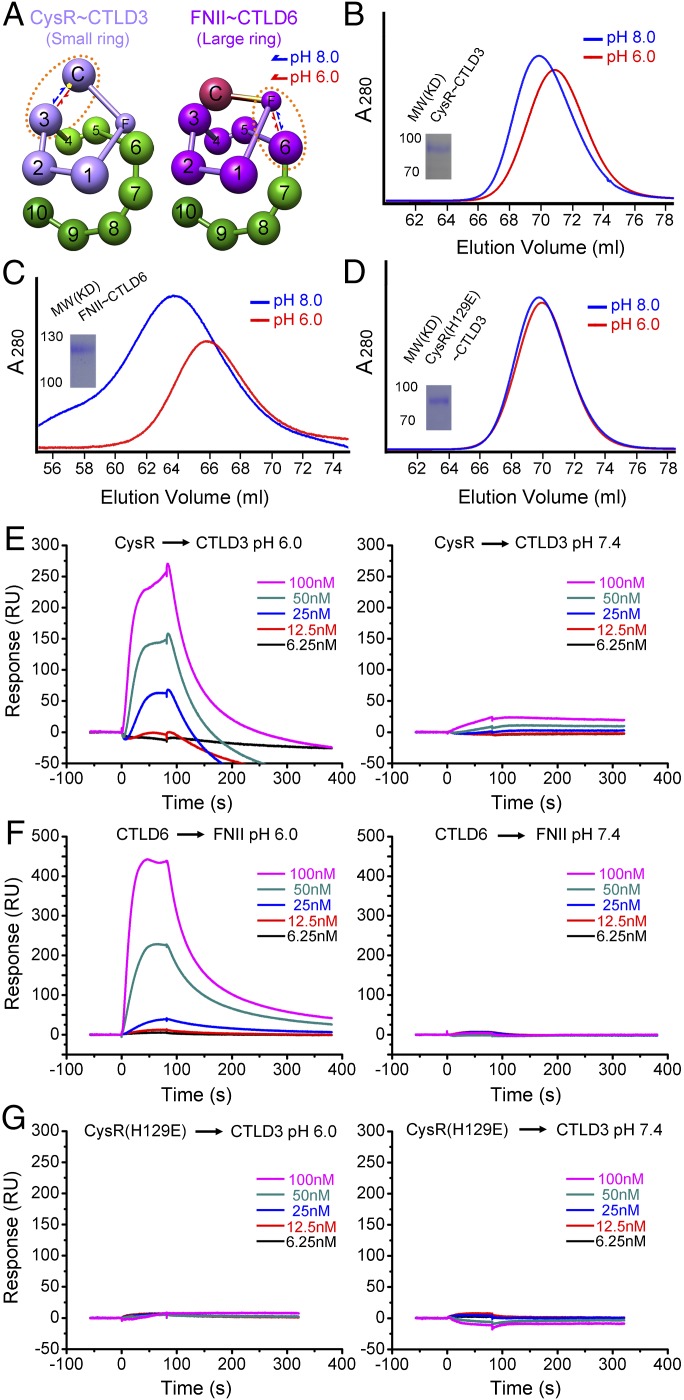
Fig. 3.
The mechanism of DEC205 conformational change. (A) The conformational change of the small ring (CysR∼CTLD3, light purple, Left) and the large ring (FNII∼CTLD6, dark purple, Right) of DEC205 between acidic and basic pH. The approximate position of H129 is shown as a yellow dot. The domains involved in the intramolecular interactions are circulated by the brown dotted lines. (B) SEC profiles of the small ring at pH 6 and pH 8. (C) SEC profiles of the large ring at pH 6 and pH 8. (D) SEC profiles of the small ring H129E mutant at pH 6 and pH 8. (E) Sensorgrams for the interactions of CysR with CTLD3 at acidic (Left) and basic (Right) conditions. (F) Sensorgrams for the interactions of CTLD6 with FNII at acidic (Left) and basic (Right) conditions. (G) Sensorgrams for the interactions of CysR(H129E) with CTLD3 at acidic (Left) and basic (Right) conditions.
The cryoEM reconstruction also allows the individual domain of DEC205 to be recognized (Fig. 2B). For example, the pseudo threefold structure of CysR (26) and the adjacent saddle-like shape consistent with the structure of FNII domain (27) are visualized in the reconstruction (Fig. 2 B and C). Crystal structures of CTLDs suggest these domains include a large loop region of about 20 residues (28), which may introduce internal flexibility and reduce the resolution of reconstruction. However, the boundary of each CTLD is recognizable in the reconstruction and can be fit with CTLD models (Fig. 2C). Notably, the adjacent CTLDs of DEC205 can form groups, such as CTLD1∼CTLD2, CTLD4∼CTLD5, and CTLD8∼CTLD10. The CTLDs within the same group associate with each other tightly, consistent with the results from protease digestions of the mannose receptor (29). In contrast, CTLD3, CTLD6, and CTLD7 are loosely associated with neighboring domains (Figs. 1A and 2B). The tight or loose association among neighboring CTLDs may contribute to the formation of the overall conformation and the transition between the open and the closed states.
Mutagenesis Studies of the DEC205 Conformational Change.
To investigate the pH-dependent conformational change of DEC205, a series of mutants was constructed to probe the interactions among the extracellular domains. A truncation mutant containing the domains that form the small ring, including CysR, FNII, and CTLD1∼CTLD3, was constructed and expressed. The purified protein of this mutant exhibited a pH-dependent elution shift by SEC at pH 6 and pH 8 (Fig. 3 A and B), suggesting the small ring is involved in the pH-dependent conformational change. Another mutant comprising the domains forming the large ring, including FNII and CTLD1∼CTLD6, was also expressed and purified, and a similar pH-dependent elution shift was observed (Fig. 3 A and C). These results suggest that both rings are open at basic pH, driving DEC205 to adopt a linear and extended structure as observed by SEC and negatively stained EM.
The pH-dependent properties of proteins are commonly associated with histidine residues that undergo charge transitions between acidic and basic environments. We therefore investigated the potential locations of histidine residues in the ectodomain of DEC205 based on the cryoEM reconstruction. For forming the small ring, CysR and CTLD3 need to interact with each other, and histidine 129 of CysR locates at the potential interface between CysR and CTLD3 in the fitting model (Fig. 3A). Indeed, the SEC elution profiles at pH 6 and pH 8 of the purified H129E (Fig. 3D) and H129A mutants were almost identical, suggesting the pH-dependent conformational change was abolished for these mutants. These results also validate the cryoEM model. Identification of the histidines that regulate the conformational change of the large ring was not successful because of the low expression level of the large ring mutants.
To further validate the intramolecular interactions of DEC205 that are critical for forming the double ring-shaped conformation at acidic pH, four single domains, including CysR, CTLD3, FNII, and CTLD6, were expressed individually fused to an IgG Fc region, and the interactions of CysR with CTLD3 and FNII with CTLD6 were monitored by surface plasmon resonance (Fig. 3 E and F). The results showed that both interactions, CysR with CLTD3 and FNII with CTLD6, were pH-dependent, thus validating the structural model from cryoEM. We also tested the interaction between the CysR (H129E) and CTLD3, and no detectable interaction was found at either acidic or basic pH (Fig. 3G), confirming the importance of this residue in the DEC205 conformational change.
pH-Dependent Recognition of Apoptotic and Necrotic Cells by DEC205.
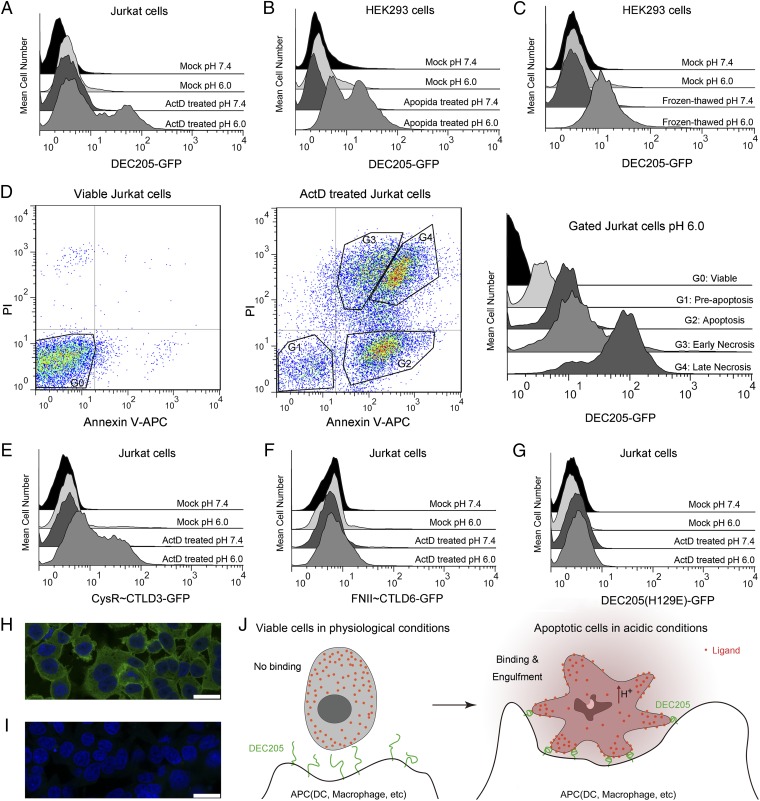
We further explored functional correlation of the DEC205 conformational change by a series of cell assays. Binding of the GFP-tagged DEC205 to Jurkat and HEK293 cells were assayed at different pH conditions by flow cytometry and confocal microscopy. The flow cytometry data showed that DEC205 had no binding to healthy Jurkat cells at either acidic or basic pH (Fig. 4A). However, when these cells were treated with actinomycin D (ActD) to induce apoptosis and necrosis, DEC205 exhibited binding activities under acidic, but not basic, conditions (Fig. 4A). Similar binding characteristics were also observed for DEC205-GFP interactions with HEK293 cells (Fig. 4B), suggesting common ligands were expressed on both cell types. In addition, DEC205-GFP also bound frozen-thawed HEK293 cells at acidic pH (Fig. 4C), indicating that the ligand of DEC205 was likely to be a naturally expressed cellular component before apoptosis. To further investigate binding differences between apoptotic and necrotic cells, both viable and ActD-treated Jurkat cells were stained by DEC205-GFP at acidic pH (Fig. 4D). DEC205 showed only weak binding to the preapoptotic Jurkat cells, but strong binding to the apoptotic and the early necrotic cells, and the strongest binding to the late necrotic cells. These results suggested that the cellular ligands of DEC205 were gradually exposed as the apoptotic process continued until the stage of necrosis.
Fig. 4.
The pH-dependent recognition of apoptotic and necrotic cells by DEC205. (A) DEC205-GFP binds to the ActD-treated Jurkat cells at acidic pH. (B) DEC205-GFP binds to the Apopida-treated HEK293 cells at acidic pH. (C) DEC205-GFP binds to the frozen-thawed HEK293 cells at acidic pH. (D) Triple staining of the viable Jurkat cells (Left) and the ActD-treated Jurkat cells (Middle) by Annexin V-APC, PI, and DEC205-GFP at acidic pH. The binding of DEC205 to the gated subsets of the viable and the ActD-treated Jurkat cells are shown on the right. (E) CysR∼CTLD3-GFP (the small ring) binds to the ActD-treated Jurkat cells at acidic conditions. (F) FNII∼CTLD6-GFP (the large ring) does not bind to the ActD-treated Jurkat cells at either acidic or basic conditions. (G) DEC205 (H129E)-GFP does not bind to the ActD-treated Jurkat cells at either acidic or basic conditions. (H) The fixed and permeabilized HEK293 cells stained by DEC205-GFP and DAPI at pH 6.0 by confocal microscopy. (I) The fixed and permeabilized HEK293 cells stained by DEC205-GFP and DAPI at pH 7.4 by confocal microscopy. (Scale bars, 25 μm.) (J) A cartoon representation of the pH-dependent recognition of apoptotic cells by DEC205.
The truncation mutants of DEC205, including the small ring fragment (CysR to CTLD3) and the large ring fragment (FNII to CTLD6) (Fig. 3), were also used for cell staining. The results showed that the small ring bound only to the apoptotic and necrotic cells at acidic pH and had no binding to healthy cells (Fig. 4E), similar to the results obtained from the intact DEC205 ectodomain (Fig. 4A). In contrast, the large ring fragment showed no binding to both ActD-treated and untreated cells at either acidic or basic pH (Fig. 4F). Furthermore, the H129E mutant of DEC205 was also tested for staining and showed no binding activity to both treated and untreated cells at either acidic or basic conditions (Fig. 4G). These data together demonstrated that the small ring of DEC205 was required and sufficient for recognizing apoptotic and necrotic cells, and the binding and release of ligand were correlated with the pH-dependent conformational change. To visualize cell staining by DEC205, fixed and permeabilized HEK293 cells were stained with DEC205-GFP and imaged by confocal microscopy at both pH 6 and pH 8. The images showed that DEC205 stained most of the cellular regions except nucleus at pH 6 (Fig. 4H), but at pH 8, DEC205-GFP showed only background level of cell staining (Fig. 4I), suggesting DEC205 only recognized cellular components at acidic conditions.
Discussion
Similar to other mannose receptor family members, DEC205 has a relatively large ectodomain that contains 12 domains; however, instead of having a flexible conformation, the cryoEM structure of DEC205 shows a rather compact double ring-shaped conformation at acidic pH. Similar conformation has been found for FcRY (25), another member of mannose receptor family, and it may also be true for the mannose receptor, according to the negatively stained EM results (24). It is noteworthy that this unique conformational feature has not been found outside this family, suggesting a common ancestor shared by the family members during evolution.
The high-resolution structural determination of the mannose receptor family members is not successful up to date, which could be because of the internal flexibility of the molecule; for example, the CTLD domains usually have large flexible loops, which would reduce the reconstruction resolution. Nevertheless, the 14.6-Å-resolution structure of DEC205 obtained by cryoEM is able to differentiate the individual domains of DEC205 clearly (Fig. 2), thus locating the interacting interfaces among these domains. The head of DEC205 contains two ring-shaped structures. The small ring is formed by CysR, FNII, and CTLD1∼CTLD3, whereas the large ring is formed by FNII and CTLD1∼CTLD6. The tail starts from CTLD7 to CTLD10. Consistent with the protease digestion results of the mannose receptor (29), the EM reconstruction shows that there are three tightly associated groups of CTLDs in DEC205, including CTLD1∼CTLD2, CTLD4∼CTLD5, and CTLD8∼CTLD10, which may act as a scaffold for maintaining the double-ringed conformation (Fig. 1A). The domains that are loosely associated with neighboring domains such as CTLD3 and CTLD6 may have more flexibility in locating their binding partners. Indeed, the small ring is formed by the interaction between CTLD3 and CysR, and the large ring is formed by CTLD6 interacting with FNII. Similar domain arrangements and intramolecular interactions have been found in the EM structures of other mannose receptor family members (24, 25).
The pH-dependent conformational change has been observed for FcRY (23) and mannose receptor (24), suggesting pH dependence might be a conserved feature for the family. However, the mechanism and the residues involved in the conformational changes have not been identified in both cases. The mutagenesis studies of DEC205 show that both the small ring and the large ring undergo conformational changes as pH changes, resulting in a linear conformation at basic or physiological pH (∼7.4). The cell binding results indicate that only the small ring is involved in ligand binding at its closed state; therefore, the opening and closing of the large ring may act as a facilitating factor for the conformational change of the small ring. It is not surprising that a histidine (His129) is identified as an important residue for the conformational change of the small ring and ligand binding. However, whether histidine residues are directly involved in ligand recognition remains unclear.
It seems unexpected that DEC205 is activated for apoptotic cell recognition at acidic pH, rather than at physiological pH. In fact, the intracellular acidification has been found to be common for apoptotic cells and occurs as an early event of apoptosis (30–32). As apoptosis proceeds, the extracellular environment around the apoptotic cells may also be acidified; for example, through hydrogen exchangers (33) or when cell membrane starts leaking. The acidification can then induce the conformational change of DEC205 and make it ready for ligand binding (Fig. 4J). The requirements for both acidification and ligand exposure increase the selectivity of DEC205 for target recognition. Unfortunately, the natural ligand or ligands of DEC205 still remain unknown. Nevertheless, the finding of the pH-dependent binding characteristic of DEC205 would help ligand identification in the future.
The extracellular acidification is usually associated with inflammation and tumorigenesis and treated as a “danger signal” by immune system (34, 35). For dendritic cells, extracellular acidification can affect their maturation and differentiation, especially antigen uptake and presentation (36). It has been shown that the antigen uptake of dendritic cells at pH 6.5 is almost 10-fold higher than at pH 7.3 (37), suggesting more receptors are activated at acidic pH, which is well consistent with the finding of DEC205.
To date, a number of receptors of phagocytes such as CD14 (38), CD36 (39), and integrin (40) are identified to be involved in removing apoptotic cells. The phagocyte receptors bind to dead cells through a variety of cell surface markers (3). For example, PtdSerR recognizes phosphatidylserine on the membrane of apoptotic cells (41). LRP of engulfing cells recognizes calreticulin on apoptotic cell surface for clearance (42). Clec9A recognizes actin filaments of damaged cells (43–45). However, none of these receptors has been shown to have pH-dependent activities. Therefore, the pH-dependent recognition of apoptotic and necrotic cells by DEC205 represents a novel mechanism for the immune system to regulate cell clearance and immune responses. Considering the high expression level of DEC205 on dendritic cells, DEC205 might be involved in routine screening over cells, detecting apoptosis at early stages by sensing pH changes and ligand exposure and triggering phagocytosis. Alternatively, DEC205 may also be able to access the internalized dead cells or fragments at phagosome and be activated under the acidic environment for antigen binding and presentation.
Unlike normal cells, tumor cells have relatively higher intracellular pH and lower extracellular pH, which could facilitate their proliferation, apoptosis evasion, and extracellular matrix remodeling for invasion (46). Therefore, the pH-dependent activities of DEC205 may give it advantages to target tumors specifically for immune recognition and clearance. Because DEC205 has been used for tumor vaccine generation (17, 18), a combination of the pH-dependent feature of DEC205 with its high antigen presentation efficiency may provide more potential to the immune therapies against tumors.
Materials and Methods
HEK293F cells were cultured with HyClone SFM4 HEK293 medium (HyClone Laboratories, Inc.) supplemented with penicillin and streptomycin. Five hours before transfection, the cells were collected by centrifugation and cultured in suspension with FreeStyle 293 expression medium (Gibco, Inc.) at 1 × 106 cells/mL for transfection.
Further experimental details can be found in SI Materials and Methods.
SI Materials and Methods
Protein Expression and Purification.
Constructs encoding the ectodomain of DEC205 (including the native signal sequence and residues 1–1,668 of the mature protein), CysR∼CTLD3 (the small ring, including the native signal sequence and residues 1–630 of the mature protein), and FNII∼CTLD6 (the large ring, including the interleukin 3 signal sequence and residuals 155–1,094 of the mature protein) with a C-terminal six-His tag were subcloned into the pTT5 expression vectors. The single domains of DEC205 (including CysR, FNII, CTLD3, and CTLD6) were constructed with a C-terminal Fc-tag and a six-His tag into the pTT5 vectors. DEC205-GFP, CysR∼CTLD3-GFP, FNII∼CTLD6-GFP, and DEC205(H129E)-GFP were also constructed with a C-terminal six-His tag into the pTT5 vectors. The supernatants of the transfected HEK293F cells were buffer-exchanged with 50 mM Tris, 150 mM NaCl at pH 8.0 by dialysis, then applied to Ni-NTA chromatography (Ni-NTA Superflow, Qiagen). The imidazole elutes were further purified by gel filtration chromatography with a Superdex 200 column. All DEC205 samples were prepared following similar procedures.
Dynamic Light Scattering.
Dynamic light scattering was monitored with a DynaPro NanoStar (Wyatt Technology, Inc.). The hydrodynamic radii of DEC205 were measured at pH 6.0 and pH 8.0 with specimen concentrations of ∼0.5 mg/mL Data were collected and plotted by DYNAMICS7.1.
SEC.
SEC was performed on a Superdex 200 16/600 column (GE Healthcare) with a flow rate of 1.0 mL/min. The column was eluted with 50 mM Bis⋅Tris, 150 mM NaCl at pH 6.0, or 50 mM Tris, 150 mM NaCl at pH 8.0. DEC205 and the mutants were injected at concentrations of ∼0.5 mg/mL.
Negative Stain and 2D Class Average.
Ten microliters of purified DEC205 ectodomain in acidic buffer (150 mM NaCl, 50 mM Bis⋅Tris pH 6.0) and basic buffer (150 mM NaCl, 50 mM Tris pH 8.0) were applied to the glow-discharged EM carbon grids and stained with 0.75% (wt/vol) uranyl formate and 2% (wt/vol) phosphotungstic acid (pH 8.0), respectively. Negatively stained EM grids were imaged on a Tecnai T12 microscope (FEI) operated at 120 kV. Images were recorded at a nominal magnification of 67,000×, using a 4k × 4k Eagle CCD camera, corresponding to a pixel size of 1.74 Å per pixel on the specimen. e2boxer.py program in EMAN2 suite was used to pick 26,589 particles in acidic conditions and 11,829 particles in basic conditions. e2refine2d.py of EMAN2 was used to generate 2D classifications (47).
CryoEM Data Collection and Reconstruction.
A total of ∼2 μL purified DEC205 ectodomain (∼3.0 mg/mL, pH 6.0) was loaded onto glow-discharged Quantifoil Holey Carbon grids and vitrified in liquid ethane using an FEI vitrobot with a 6-s blotting time at 100% humidity. Frozen grids were transferred to a JEOL microscope for imaging on a 4k × 4k Gatan US4000 camera with a nominal magnification of 80,000× corresponding to a pixel size of 1.36 Å per pixel on the specimen at a dose of ∼20e−/ Å2. Imaging was performed at 200 kV at defocus values of 2∼5 μm. In total, 15,723 particles were picked by boxer in EMAN suite (48). Contrast transfer functions were determined using ctfit of EMAN. 2D classifications were calculated by refine2d.py. The initial model of DEC205 was generated using the program startAny of EMAN, and the refine program was used for the initial refinement, and then e2refine_easy.py of EMNA2 (47) was used for the further refinement. The final resolution was estimated based on the gold standard criterion.
Homology Modeling and Structural Fitting.
Homology models of the each domain of DEC205 were created using the program MODELLER9.12 (49). Crystal structure of the cysteine-rich domain of the mannose receptor (PDB 1DQO) was used as a template for the CysR domain of DEC205. An NMR structure of fibronectin (PDB 2FN2) was used as a template for FNII domain of DEC205, and the crystal structure of the CTLD of tenascin (PDB code 1TDQ) was used as a template for the CTLDs of DEC205. Fifty models were generated by MODELER for each domain, and the best model was selected by the multivariate model assessment scores. The models were fitted manually into the cryoEM density, using Chimera (50).
Surface Plasmon Resonance.
Analyses of interactions between the domains of DEC205 were performed on a BIAcore T100 surface plasmon resonance instrument (GE Healthcare) at 25 °C. FNII-Fc and CysR-Fc were covalently immobilized to a flow cell on a CM5 biosensor chip (GE Healthcare), using standard primary amine coupling chemistry (BIACORE manual) at a concentration of 100 ng/mL. The control cell was mock coupled using the Fc tag. The test proteins were injected in PBS (pH 6.0) or PBS (pH 7.4) with a series of concentrations. After dissociation, the bound analytes were removed by a 120-s wash with PBS (pH 7.4). The resulting data after subtracting the control values were analyzed using the BIAcore T100 evaluation software.
Apoptosis and Necrosis Assay.
Jurkat cells were cultured in 1640 medium (Gibco, Inc.) supplemented with 10% (vol/vol) FCS (HyClone Laboratories, Inc). To induce apoptosis and necrosis, Jurkat cells were incubated in tissue culture flasks for 12 h with 1 μg/mL ActD until use. For inducing apoptosis and necrosis of HEK293F cells, the cells were cultured in FreeStyle 293 medium including apoptosis inducer A (Apopida) (1:1,000, Beyotime) for 16 h. For freezing-thawing of HEK293F cells, the cells were incubated in a dry ice bath for 10 min and thawed immediately in a 37 °C water bath for 10 min.
Cell Staining by Flow Cytometry.
Apoptosis and necrosis was measured using Annexin V Apoptosis Detection Kit APC (eBioscience, Inc.). Briefly, cells were washed in PBS and binding buffer (10 mM Hepes at pH 7.4, 140 mM NaCl, 2.5 mM CaCl2), and then resuspended in binding buffer at 1–5 × 106 cells/mL with 5 μL Annexin V-APC and incubated for 20 min at 4 °C. Then the cells were washed in binding buffer and resuspended in 400 μL binding buffer including 5 μL propidium iodide staining solution and analyzed by flow cytometry. For GFP staining, the cells were washed with PBS (pH 7.4) first and then washed with either PBS at pH 7.4 or PBS at pH 6.0 for different assays. The cells were incubated with the GFP-tagged DCE205 fragments in PBS (pH 7.4 or 6.0) for 20 min at room temperature and then washed by PBS (pH 7.4 or pH 6) again, and analyzed by flow cytometry. For the triple staining assays, the cells were washed once in PBS (pH 7.4) and then in PBS (pH 6.0), resuspended in PBS (pH 6.0, 2.5 mM CaCl2) including the GFP-tagged protein and 5 μL Annexin V-APC solution, and incubated at 4 °C for 30 min. Then the cells were washed in PBS (pH 6.0, 2.5mM CaCl2) and resuspended in 400 μL PBS (pH 6.0, 2.5 mM CaCl2), including 5 μL propidium iodide staining solution, and analyzed by flow cytometry. Data were acquired using a Becton Dickenson FACSCaliber flow cytometer and CELLQuest software. Data analysis was performed using FlowJo software (Tree Star, Inc.).
Confocal Microscopy.
HEK293 cells grown on a coverslip were fixed by 4% paraformaldehyde, permeabilized with 0.25% Triton X-100 in PBS (pH 7.4), washed twice in PBS at pH 6.0 or PBS at pH 7.4, and stained with DEC205-GFP for 2 h at pH 6.0 or pH 7.4. Then the cells were washed twice in PBS at pH 6.0 or PBS at pH 7.4, incubated with 5 μM DAPI for 30 min, and then washed for confocal microscopy. The confocal images were taken on a Leica SP8 microscope.
Acknowledgments
We thank the National Centre for Protein Science Shanghai (Electron Microscopy, Integrated Laser Microscopy and Protein Expression and Purification systems) for their instrumental support and technical assistance, Meng Wu (Core Facility of Molecular Biology at Shanghai Institute of Biochemistry and Cell Biology) for the help with surface plasmon resonance experiments, Wah Chiu and Steven Ludtke for the help of EM reconstruction, and the National Center for Macromolecular Imaging at Baylor College of Medicine for CryoEM data collections. This work is supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB08020102), the National Natural Science Foundation of China (31270772), the “One Hundred Talents” Program of the Chinese Academy of Sciences (2012OHTP03), and the Shanghai Pujiang Program (13PJ1409700) (to Y.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The cryoEM map has been deposited in the PDB EM Data Bank with entry EMD-6333.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1505924112/-/DCSupplemental.
References
- 1.Poon IK, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol. 2014;14(3):166–180. doi: 10.1038/nri3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lauber K, Blumenthal SG, Waibel M, Wesselborg S. Clearance of apoptotic cells: getting rid of the corpses. Mol Cell. 2004;14(3):277–287. doi: 10.1016/s1097-2765(04)00237-0. [DOI] [PubMed] [Google Scholar]
- 3.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2(12):965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 4.Ravichandran KS. Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. J Exp Med. 2010;207(9):1807–1817. doi: 10.1084/jem.20101157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392(6671):86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 6.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 7.van Vliet SJ, García-Vallejo JJ, van Kooyk Y. Dendritic cells and C-type lectin receptors: coupling innate to adaptive immune responses. Immunol Cell Biol. 2008;86(7):580–587. doi: 10.1038/icb.2008.55. [DOI] [PubMed] [Google Scholar]
- 8.Geijtenbeek TB, van Vliet SJ, Engering A, ’t Hart BA, van Kooyk Y. Self- and nonself-recognition by C-type lectins on dendritic cells. Annu Rev Immunol. 2004;22:33–54. doi: 10.1146/annurev.immunol.22.012703.104558. [DOI] [PubMed] [Google Scholar]
- 9.Jiang W, et al. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature. 1995;375(6527):151–155. doi: 10.1038/375151a0. [DOI] [PubMed] [Google Scholar]
- 10.Hawiger D, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194(6):769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shrimpton RE, et al. CD205 (DEC-205): a recognition receptor for apoptotic and necrotic self. Mol Immunol. 2009;46(6):1229–1239. doi: 10.1016/j.molimm.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lahoud MH, et al. DEC-205 is a cell surface receptor for CpG oligonucleotides. Proc Natl Acad Sci USA. 2012;109(40):16270–16275. doi: 10.1073/pnas.1208796109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinman RM. The control of immunity and tolerance by dendritic cell. Pathol Biol (Paris) 2003;51(2):59–60. doi: 10.1016/s0369-8114(03)00096-8. [DOI] [PubMed] [Google Scholar]
- 14.Bonifaz LC, et al. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199(6):815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbuto S, et al. Induction of innate and adaptive immunity by delivery of poly dA:dT to dendritic cells. Nat Chem Biol. 2013;9(4):250–256. doi: 10.1038/nchembio.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheong C, et al. Improved cellular and humoral immune responses in vivo following targeting of HIV Gag to dendritic cells within human anti-human DEC205 monoclonal antibody. Blood. 2010;116(19):3828–3838. doi: 10.1182/blood-2010-06-288068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhodapkar MV, et al. 2014. Induction of antigen-specific immunity with a vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205. Sci Transl Med 6(232):232ra51.
- 18.Sehgal K, Dhodapkar KM, Dhodapkar MV. Targeting human dendritic cells in situ to improve vaccines. Immunol Lett. 2014;162(1 Pt A):59–67. doi: 10.1016/j.imlet.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.East L, Isacke CM. The mannose receptor family. Biochim Biophys Acta. 2002;1572(2-3):364–386. doi: 10.1016/s0304-4165(02)00319-7. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Pomares L. The mannose receptor. J Leukoc Biol. 2012;92(6):1177–1186. doi: 10.1189/jlb.0512231. [DOI] [PubMed] [Google Scholar]
- 21.Engelholm LH, et al. The collagen receptor uPARAP/Endo180. Front Biosci (Landmark Ed) 2009;14:2103–2114. doi: 10.2741/3365. [DOI] [PubMed] [Google Scholar]
- 22.Bernard D, Vindrieux D. PLA2R1: expression and function in cancer. Biochim Biophys Acta. 2014;1846(1):40–44. doi: 10.1016/j.bbcan.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 23.West AP, Jr, Herr AB, Bjorkman PJ. The chicken yolk sac IgY receptor, a functional equivalent of the mammalian MHC-related Fc receptor, is a phospholipase A2 receptor homolog. Immunity. 2004;20(5):601–610. doi: 10.1016/s1074-7613(04)00113-x. [DOI] [PubMed] [Google Scholar]
- 24.Boskovic J, et al. Structural model for the mannose receptor family uncovered by electron microscopy of Endo180 and the mannose receptor. J Biol Chem. 2006;281(13):8780–8787. doi: 10.1074/jbc.M513277200. [DOI] [PubMed] [Google Scholar]
- 25.He Y, Bjorkman PJ. Structure of FcRY, an avian immunoglobulin receptor related to mammalian mannose receptors, and its complex with IgY. Proc Natl Acad Sci USA. 2011;108(30):12431–12436. doi: 10.1073/pnas.1106925108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, et al. Crystal structure of the cysteine-rich domain of mannose receptor complexed with a sulfated carbohydrate ligand. J Exp Med. 2000;191(7):1105–1116. doi: 10.1084/jem.191.7.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sticht H, Pickford AR, Potts JR, Campbell ID. Solution structure of the glycosylated second type 2 module of fibronectin. J Mol Biol. 1998;276(1):177–187. doi: 10.1006/jmbi.1997.1528. [DOI] [PubMed] [Google Scholar]
- 28.Lundell A, et al. Structural basis for interactions between tenascins and lectican C-type lectin domains: evidence for a crosslinking role for tenascins. Structure. 2004;12(8):1495–1506. doi: 10.1016/j.str.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 29.Napper CE, Dyson MH, Taylor ME. An extended conformation of the macrophage mannose receptor. J Biol Chem. 2001;276(18):14759–14766. doi: 10.1074/jbc.M100425200. [DOI] [PubMed] [Google Scholar]
- 30.Matsuyama S, Llopis J, Deveraux QL, Tsien RY, Reed JC. Changes in intramitochondrial and cytosolic pH: early events that modulate caspase activation during apoptosis. Nat Cell Biol. 2000;2(6):318–325. doi: 10.1038/35014006. [DOI] [PubMed] [Google Scholar]
- 31.Gottlieb RA, Nordberg J, Skowronski E, Babior BM. Apoptosis induced in Jurkat cells by several agents is preceded by intracellular acidification. Proc Natl Acad Sci USA. 1996;93(2):654–658. doi: 10.1073/pnas.93.2.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lagadic-Gossmann D, Huc L, Lecureur V. Alterations of intracellular pH homeostasis in apoptosis: origins and roles. Cell Death Differ. 2004;11(9):953–961. doi: 10.1038/sj.cdd.4401466. [DOI] [PubMed] [Google Scholar]
- 33.De Vito P. The sodium/hydrogen exchanger: a possible mediator of immunity. Cell Immunol. 2006;240(2):69–85. doi: 10.1016/j.cellimm.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Rajamäki K, et al. Extracellular acidosis is a novel danger signal alerting innate immunity via the NLRP3 inflammasome. J Biol Chem. 2013;288(19):13410–13419. doi: 10.1074/jbc.M112.426254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lardner A. The effects of extracellular pH on immune function. J Leukoc Biol. 2001;69(4):522–530. [PubMed] [Google Scholar]
- 36.Vermeulen ME, et al. The impact of extracellular acidosis on dendritic cell function. Crit Rev Immunol. 2004;24(5):363–384. doi: 10.1615/critrevimmunol.v24.i5.40. [DOI] [PubMed] [Google Scholar]
- 37.Vermeulen M, et al. Acidosis improves uptake of antigens and MHC class I-restricted presentation by dendritic cells. J Immunol. 2004;172(5):3196–3204. doi: 10.4049/jimmunol.172.5.3196. [DOI] [PubMed] [Google Scholar]
- 38.Devitt A, et al. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature. 1998;392(6675):505–509. doi: 10.1038/33169. [DOI] [PubMed] [Google Scholar]
- 39.Urban BC, Willcox N, Roberts DJ. A role for CD36 in the regulation of dendritic cell function. Proc Natl Acad Sci USA. 2001;98(15):8750–8755. doi: 10.1073/pnas.151028698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Albert ML, Kim JI, Birge RB. alphavbeta5 integrin recruits the CrkII-Dock180-rac1 complex for phagocytosis of apoptotic cells. Nat Cell Biol. 2000;2(12):899–905. doi: 10.1038/35046549. [DOI] [PubMed] [Google Scholar]
- 41.Fadok VA, et al. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405(6782):85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 42.Gardai SJ, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123(2):321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 43.Zhang JG, et al. The dendritic cell receptor Clec9A binds damaged cells via exposed actin filaments. Immunity. 2012;36(4):646–657. doi: 10.1016/j.immuni.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 44.Sancho D, et al. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458(7240):899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahrens S, et al. F-actin is an evolutionarily conserved damage-associated molecular pattern recognized by DNGR-1, a receptor for dead cells. Immunity. 2012;36(4):635–645. doi: 10.1016/j.immuni.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer. 2011;11(9):671–677. doi: 10.1038/nrc3110. [DOI] [PubMed] [Google Scholar]
- 47.Tang G, et al. EMAN2: an extensible image processing suite for electron microscopy. J Struct Biol. 2007;157(1):38–46. doi: 10.1016/j.jsb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 48.Ludtke SJ, Baldwin PR, Chiu W. EMAN: semiautomated software for high-resolution single-particle reconstructions. J Struct Biol. 1999;128(1):82–97. doi: 10.1006/jsbi.1999.4174. [DOI] [PubMed] [Google Scholar]
- 49.Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234(3):779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 50.Goddard TD, Huang CC, Ferrin TE. Visualizing density maps with UCSF Chimera. J Struct Biol. 2007;157(1):281–287. doi: 10.1016/j.jsb.2006.06.010. [DOI] [PubMed] [Google Scholar]