Significance
Cholesterol-derived steroid hormones are critical mediators in development, reproduction, behavior, and stress response. Steroid biosynthesis occurs in hormone-dependent steroidogenic cells, which are defined by their ability to cleave cholesterol to pregnenolone in mitochondria, the adrenal cortex, and gonads. The rate-limiting step in steroid biosynthesis is the transport of cholesterol from intracellular stores into mitochondria. Translocator protein (TSPO) is a high-affinity drug- and cholesterol-binding protein important for cholesterol import into mitochondria. Two distinct genetically engineered Tspo mouse mutants were generated to induce gonadal and steroidogenic cell-specific Tspo deletion. Our studies show the importance of TSPO for murine development as well as for hormone-induced steroidogenesis, suggesting a key role for TSPO in the development and function of the mammalian acute stress response.
Keywords: translocator protein, anti-Mullerian hormone receptor type II, nuclear receptor subfamily 5 group A member 1, knockout mice, steroidogenesis
Abstract
Translocator protein (TSPO) is a key member of the mitochondrial cholesterol transport complex in steroidogenic tissues. To assess the function of TSPO, we generated two lines of Cre-mediated Tspo conditional knockout (cKO) mice. First, gonadal somatic cell-targeting Amhr2-Cre mice were crossed with Tspo-floxed mice to obtain F1 Tspo Amhr2 cKO mice (Tspofl/fl;Amhr2-Cre/+). The unexpected Mendelian ratio of 4.4% cKO mice was confirmed by genotyping of 12.5-day-postcoitum (dpc) embryos. As Amhr2-Cre is expressed in gonads at 12.5 dpc, these findings suggest preimplantation selection of embryos. Analysis of expression databases revealed elevated levels of Amhr2 in two- and eight-cell zygotes, suggesting ectopic Tspo silencing before the morula stage and demonstrating elevated embryonic lethality and involvement of TSPO in embryonic development. To circumvent this issue, steroidogenic cell-targeting Nr5a1-Cre mice were crossed with Tspo-floxed mice. The resulting Tspofl/fl;Nr5a1-Cre/+ mice were born at a normal Mendelian ratio. Nr5a1-driven Tspo cKO mice exhibited highly reduced Tspo levels in adrenal cortex and gonads. Treatment of mice with human chorionic gonadotropin (hCG) resulted in increased circulating testosterone levels despite extensive lipid droplet depletion. In contrast, Nr5a1-driven Tspo cKO mice lost their ability to form corticosterone in response to adrenocorticotropic hormone (ACTH). Important for ACTH-dependent steroidogenesis, Mc2r, Stard1, and Cypa11a1 levels were unaffected, whereas Scarb1 levels were increased and accumulation of lipid droplets was observed, indicative of a blockade of cholesterol utilization for steroidogenesis. TSPO expression in the adrenal medulla and increased epinephrine production were also observed. In conclusion, TSPO was found necessary for preimplantation embryo development and ACTH-stimulated steroid biosynthesis.
The 18-kDa translocator protein (TSPO) is an outer mitochondrial membrane (OMM) protein, originally named the peripheral benzodiazepine receptor (PBR) due to its discovery as a high-affinity binding site for the benzodiazepine diazepam outside of the central nervous system (1). Pharmacological, biochemical, and structural research has revealed TSPO’s ability to bind numerous classes of drugs (1–4). Additional investigations also demonstrated that TSPO is a high-affinity cholesterol-binding protein. Cholesterol binding is localized to the C-terminal portion of its fifth transmembrane helix at a conserved cholesterol recognition/association amino acid consensus motif (1, 5). This finding was further supported by recent structural data (6). A search for additional physiological ligands of TSPO revealed that both the tetrapyrole protoporphyrin IX, a heme precursor, and the polypeptide endozepine, a GABAA modulator, bind TSPO, suggesting a role for TSPO in numerous physiological processes (1, 2, 5). Interestingly, a catalytic role of TSPO in protoporphyrin IX degradation was recently proposed (4).
The association of TSPO ligand effects with TSPO physiological functions has ascribed a protean nature to the protein, including roles in cancer, apoptosis, and neuroinflammation (1, 2). One such process supported by multiple lines of corroborating evidence is steroidogenesis (1, 5). During steroid synthesis in vertebrates, the substrate cholesterol moves from intracellular stores to the OMM and translocates from the OMM to the inner mitochondrial membrane (IMM). Cholesterol is metabolized at the IMM to pregnenolone by the cytochrome P450 enzyme cytochrome P450 family 11 subfamily A polypeptide 1 (CYP11A1) (7). TSPO is abundantly expressed in steroidogenic cells of the adrenal cortex and gonads, and its OMM localization, as well as its cholesterol-binding ability, position this protein favorably for a role in intramitochondrial cholesterol transport (5). Indeed, TSPO ligands have been shown to stimulate or inhibit steroidogenesis and mitochondrial cholesterol distribution in model cell systems as well as in animals (5). Moreover, numerous proteins that directly bind and functionally associate with TSPO have been shown to regulate steroidogenesis. These proteins include the above-mentioned endozepine; acyl-CoA–binding domain protein 3; the cAMP-dependent protein kinase A; the voltage-dependent anion channel; the ATPase family, AAA domain-containing 3A; 14–3-3 adaptor proteins; and the steroidogenic acute regulatory protein (STAR) (8). These findings led to biochemical characterization of a hormone-regulated macromolecular protein complex machinery that assists in the intramitochondrial translocation of cholesterol to CYP11A1 (9). Pharmacological manipulation of TSPO expression with gingkolides further supports the requirement of TSPO in steroidogenesis, although effects were more pronounced in the adrenal cortex than in the gonad (10).
Although the pharmacology of TSPO is broadly accepted, the genetics of TSPO is more controversial. TSPO appears to be an ancient protein highly conserved in all domains of life (11), suggesting an essential role. Moreover, although no natural deleterious mutations in TSPO have been reported, an identified protein-coding polymorphism alters the drug-binding properties of TSPO and pregnenolone biosynthesis (6, 12, 13). Homologous recombination-based deletion of TSPO in a steroidogenic tumor cell line ablated steroidogenesis (14), and oligonucleotide antisense-based knockdown in a different steroidogenic cell model severely reduced hormone-stimulated steroid synthesis (15). Moreover, attempts to generate a TSPO-null mouse by removing the entire 11-kb gene resulted in embryonic lethality (16). Other reports, first with testis-specific deletion of TSPO (17) and subsequently in whole-body TSPO deletion (18, 19), generated by floxing Tspo exons 2 and 3, directly contradicted previous observations by reporting a lack of effects of TSPO genetic deletion on viability or steroidogenesis, suggesting that TSPO is either not essential for development and steroidogenesis or that functional robustness compensates for genetic TSPO loss.
Here, we present data from genetically engineered TSPO mouse mutants, generated using Cre-Lox combinatorial genetics to drive conditional anti-Mullerian hormone type-II receptor (Amrh2) and nuclear receptor subfamily 5 group A member 1 (Nr5a1; encoding steroidogenic factor 1) expression-dependent deletion of TSPO. Our studies of the F1 generation of mutant mice highlight the importance of this enigmatic protein for murine development as well as for hormone-driven steroidogenesis, extending our knowledge of steroidogenesis and suggesting a key role for TSPO in the development and function of the mammalian acute stress response.
Results
Unexpected Mendelian Ratio in Amhr2-Cre–Driven Tspo Conditional Knockout Mice.
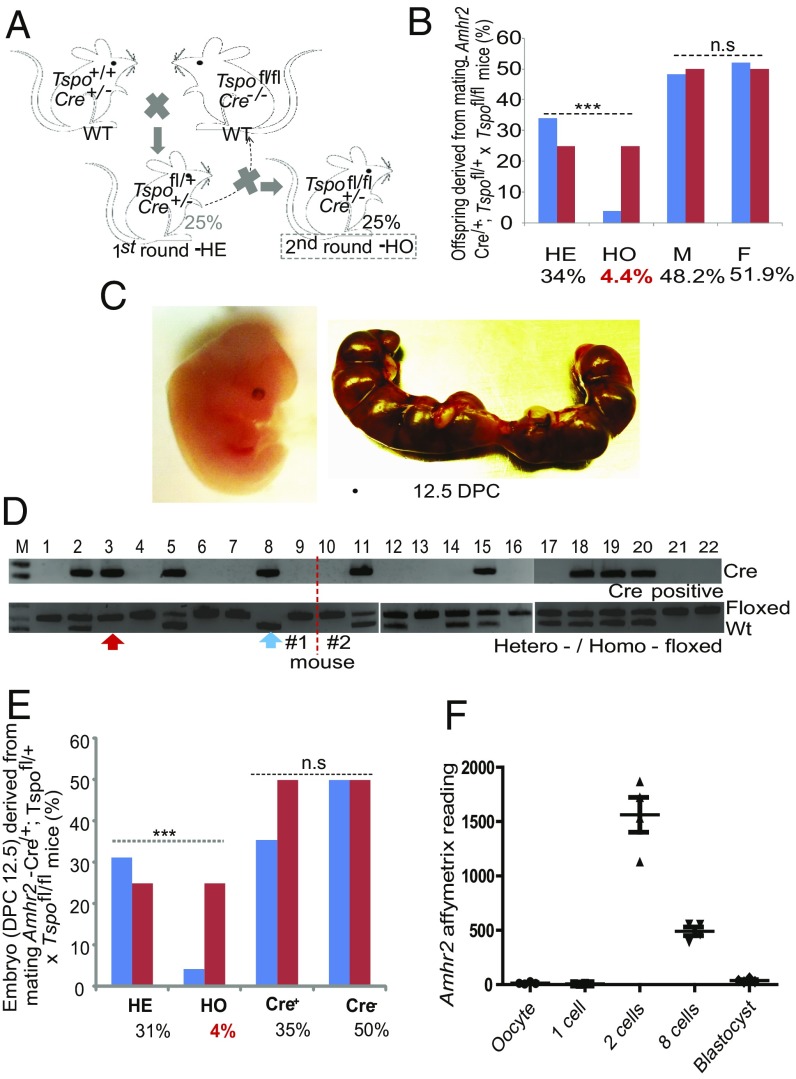
Genetically engineered mice carrying loxP sites flanking exons 2 and 3 of the Tspo locus were used in the current studies (20). The integrity of these sites was routinely confirmed in our breeding colony by PCR and sequencing (SI Appendix, Fig. S1). To examine the effect of Tspo deletion in the steroidogenic cells of the gonad, we bred homofloxed Tspo mice (Tspofl/fl) with mice carrying a cre recombinase gene at one allele of the Amhr2 gene [Amhr2cre/+ (21)] for conditional deletion of testis Leydig and Sertoli cell genes. To obtain F1 homozygous tissue-targeted Tspo knockout mice with maximal retention of the original WT Tspo allele, we performed two rounds of breeding. In the first, we bred Tspofl/fl,Amhr2+/+ mice with Tspo+/+,Amhr2cre/+ mice to generate heterozygous mice (HE; Tspofl/+,Amhr2cre/+). In the second round, the HE (Tspofl/+,Amhr2cre/+) mice were backcrossed to Tspofl/fl,Amhr2+/+ mice to generate an F1 generation in which 25% of the offspring were theoretically expected to be HE (Tspofl/+,Amhr2cre/+) mice and 25% to be the homozygous mice (HO; Tspofl/fl,Amhr2cre/+) in genotypes (Fig. 1A and SI Appendix, Fig. S2). Although no differences in the ratio of males and females were observed, only 4.4% of the animals exhibited an HO genotype, significantly lower than the expected 25% (Fig. 1B). These results suggest an embryonic lethal phenotype for the HO mice.
Fig. 1.
Breeding scheme and abnormal Mendelian ratio observed in Tspo-deficient mice (Cre+/−; Tspofl/fl). (A) Breeding scheme: WT, Cre-positive, and Cre-negative Tspo homofloxed mice were generated and mated to generate Tspo tissue-specific HE mice, expected to represent 25% of the offspring. The second round of breeding that crossed HE mice with WT Tspo homofloxed mice was expected to produce 25% Tspo tissue-specific HO mice. (B) Mendelian ratio of Amhr2-Cre × Tspo homofloxed mice from 180 mice. The observed values (blue bars) are compared with the theoretical values (red bars) for male and female. χ2-Test; ***P < 0.001; n.s., not significant; n = 180. (C) Morphology of an embryo at 12.5 dpc and one uterus extirpated on 12.5 dpc. (D) Genotyping of 22 representative embryos from two uteri. (Top) PCR showing which embryos are Cre-positive. (Bottom) PCR showing WT, HE, or HO Tspo floxed genotypes. Rare cases of Cre-positive Tspo homofloxed embryo (red arrow) and Cre-positive Tspo WT embryo (blue arrow) are indicated. (E) Bar graph of the Mendelian and sex ratios of 48 embryos showing the expected ratios (red bars) and experimental results (blue bars). χ2-Test; ***P < 0.001; n = 48. (F) Expression profile of the Amhr2 gene during preimplantation mouse embryo development. The scatter plot of the Affymetrix reading of Amhr2 gene using probe 1457021_×_at is shown against each preimplantation developmental stage: oocyte, 1 cell, 2 cells, 8 cells, and blastocyst. Affymetrix data were retrieved from GSE1749 with the platform Affymetrix mouse expression 430B array. n = 4.
To validate this unexpectedly lower Mendelian ratio, we examined embryos at 12.5 days postcoitum (dpc), an age at which Amhr2-Cre is expressed in a tissue-specific manner (21). Examination of 48 embryos revealed that all morphologies appeared normal with no apparent uterine resorption sites (Fig. 1C). Of these 48 embryos, the HO genotype, determined as the copresence of Cre and homofloxed Tspo (Fig. 1D), accounted for only 4% of embryos, in agreement with the abnormal Mendelian ratios of the adult animals (Fig. 1E). In addition, analysis of embryos with Amhr2cre/+, Tspofl/+ or Amhr2cre/+, Tspo−/+ genotypes confirmed the early stage of Amrh2-Cre–mediated Tspo deletion (Fig. 1D). Further analysis of the surviving 4% of HO mice indicated that TSPO expression was not significantly reduced in testes (SI Appendix, Fig. S3A), suggesting incomplete Cre activity. Furthermore, TSPO expression in the ovary was reduced 2.6-fold (SI Appendix, Fig. S3B), suggesting that sex-dependent factors were influencing recombinase activity.
To gain insight into the mechanism of this early embryonic phenotype, we analyzed several transcriptomic datasets generated during murine preimplantation embryogenesis. We found that the Amhr2 gene (probe 1457021_×_at) is briefly expressed during the two- and eight-cell embryonic stages (22), suggesting that Amhr2-Cre is capable of ectopically excising a floxed gene during very early development before the targeted ages of gonadal-specific expression of the Amhr2 gene (Fig. 1F).
Nr5a1-Driven Disruption of Tspo Reduces TSPO Levels in Gonads and Adrenal but Not in Brain.
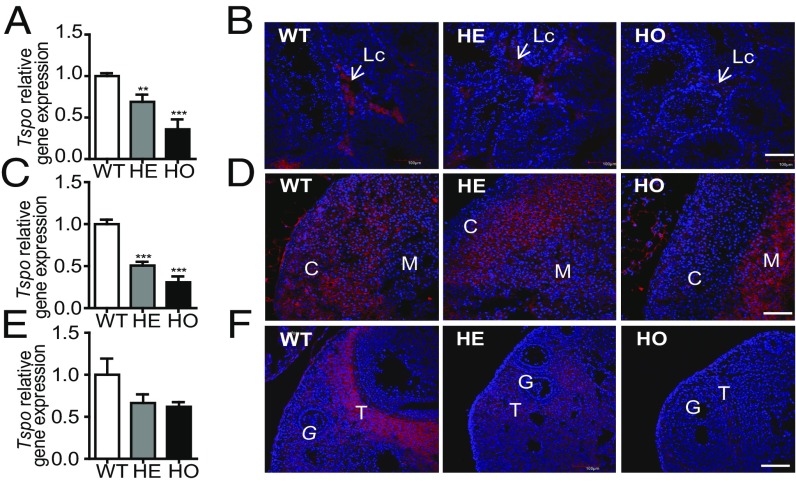
Considering the high embryonic lethality observed with Amhr2-Cre mice, we sought to generate tissue-specific Tspo gene knockout mice using Nr5a1-Cre used for conditional deletion of genes in steroidogenic cells where the expression of steroidogenic enzymes is driven by the transcription factor Nr5a1 (23). Nr5a1-Cre mice were bred with Tspo-homofloxed mice using the breeding scheme described above. The 185 mice genotyped conformed to the expected Mendelian ratio (SI Appendix, Fig. S4). To examine the effectiveness of the Cre-loxP system, we amplified the targeted Tspo area and found that the 196-bp amplification product was present in HE and HO genotypes, suggesting that the system works in a cell-specific manner (SI Appendix, Fig. S4). Quantitative RT-PCR analysis indicated that testis Tspo levels were decreased in HE and HO animals by 1.45- and 2.79-fold (P < 0.01 and P < 0.001; Fig. 2A), respectively. Tspo levels in the male adrenal glands were significantly decreased in both the HE and HO animals by 1.97- and 3.25-fold (P < 0.001), respectively (Fig. 2C). Similar results were observed in the female adrenal (SI Appendix, Fig. S5A). Although a reduction of Tspo levels in the ovary was observed, the difference was not statistically significant (Fig. 2E). Immunofluorescence staining of testes showed reduced expression of TSPO in the HO mouse interstitial compartment where Leydig cells reside (Fig. 2B). Immunofluorescence staining of the adrenal gland showed that TSPO expression was dramatically reduced in both the zona glomerulosa and zona fasciculata and was completely depleted in the zona reticularis in HO mice. TSPO immunoreactivity was curiously increased in the medulla area, which is typically devoid of TSPO in WT mice (Fig. 2D). Despite the lack of significant reduction of Tspo transcript levels in the ovary, TSPO protein expression was strikingly reduced in ovaries of the HO mice, particularly in the theca cells, which exhibit high TSPO expression in WT mice (Fig. 2F). Notably, HE mice presented an intermediary phenotype in all three tissues. No obvious differences in brain TSPO expression were observed among WT, HE, and HO mice (SI Appendix, Fig. S6).
Fig. 2.
Conditional reduction of Tspo mRNA levels and TSPO protein expression in WT, HE, and HO mice. (A and B) Real-time PCR of Tspo mRNA expression and confocal immunofluorescence images of TSPO protein in mouse testis (A and B), male adrenal gland (C and D), and ovary (E and F). Arrows point to Leydig cell (Lc). C, cortex; M, medulla. T, theca cells; G, granulosa cells. The β-actin (Actb) gene was used for normalization of real-time PCR data. In the confocal microscopy images, red indicates anti-TSPO immunoreactivity, and blue indicates DAPI staining. [Scale bar: 100 μm (A, B, E, and F); 50 μm (C and D).] Student’s t test; **P < 0.01; ***P < 0.001.
Conditional Targeted Disruption of Tspo Gene in Nr5a1-Cre–Driven Conditional Knockout Mice Blocks Adrenocorticotropic Hormone-Induced Steroidogenesis.
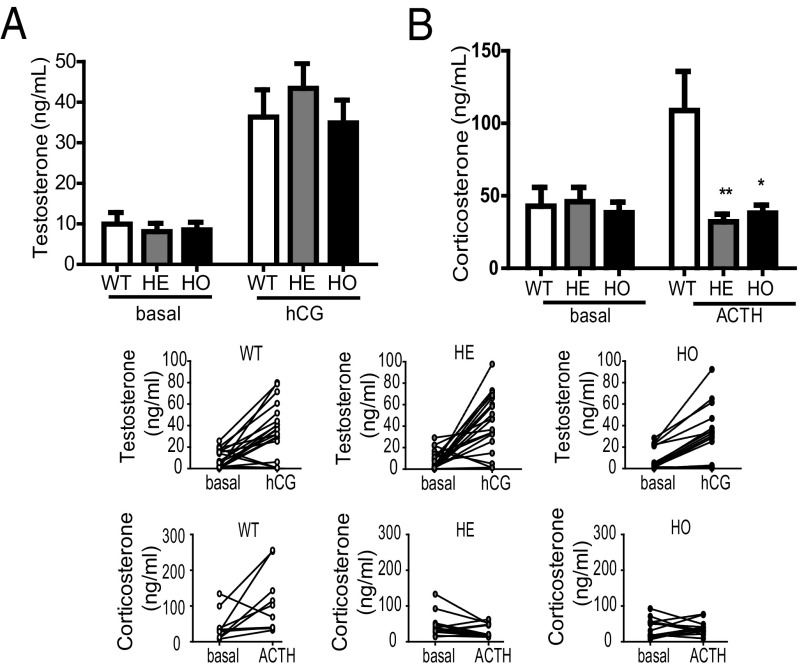
The reduced expression of TSPO in steroidogenic cells of the gonads and adrenals in Nr5a1-Cre conditional knockout (cKO) mice allowed us to examine the role of TSPO in steroidogenesis. Basal levels of circulating testosterone, progesterone, and corticosterone were measured to assess the baseline steroidogenic capacity of the gonads and adrenals. No significant differences were observed between circulating testosterone or progesterone levels in WT, HE, and HO mice (Fig. 3A and SI Appendix, Fig. S7A). Moreover, no significant differences between the circulating corticosterone levels in male WT, HE, and HO mice were observed (Fig. 3B), although a reduction was observed in female HE and HO adrenals compared with WT (SI Appendix, Fig. S8). To examine the effect of TSPO knockdown on hormone-stimulated steroidogenesis, we treated the same mice with human chorionic gonadotropin (hCG) to stimulate gonadal steroidogenesis in males and females or adrenocorticotropic hormone (ACTH) to stimulate adrenal steroidogenesis. Plasma was collected after 1 h. No significant differences were observed in circulating gonadal steroids between the groups (Fig. 3B and Fig. S7A). Although the hCG response of the testes was lost in some mice, corticosterone production in response to ACTH was lost in all male and female HE and HO mice (Fig. 3B and SI Appendix, Fig. S8). Interestingly, the adrenal medulla, which is devoid of TSPO in WT mice, exhibited increased TSPO expression (Fig. 2D) coupled with increased epinephrine levels in ACTH-treated HO animals compared with that in WT mice (SI Appendix, Fig. S9).
Fig. 3.
Circulating testosterone and corticosterone levels in WT, HE, and HO Tspo cKO mice treated with and without hCG or ACTH. (A) Plasma testosterone levels from basal and hCG-treated WT, HE, and HO mice. No significant differences compared with the control were found. Mann–Whitney U test, P > 0.05; n = 14–20 animals per group. (B) Plasma corticosterone levels from basal and ACTH-treated WT, HE, and HO mice. Mann–Whitney U test; *P < 0.05, **P < 0.01; n = 10–13 animals per group. Lower plots show basal and ACTH-induced corticosterone levels in individual WT, HE, and HO mice.
Gene Expression and Lipid Changes in Nr5a1-Cre–Driven Tspo cKO Mice.
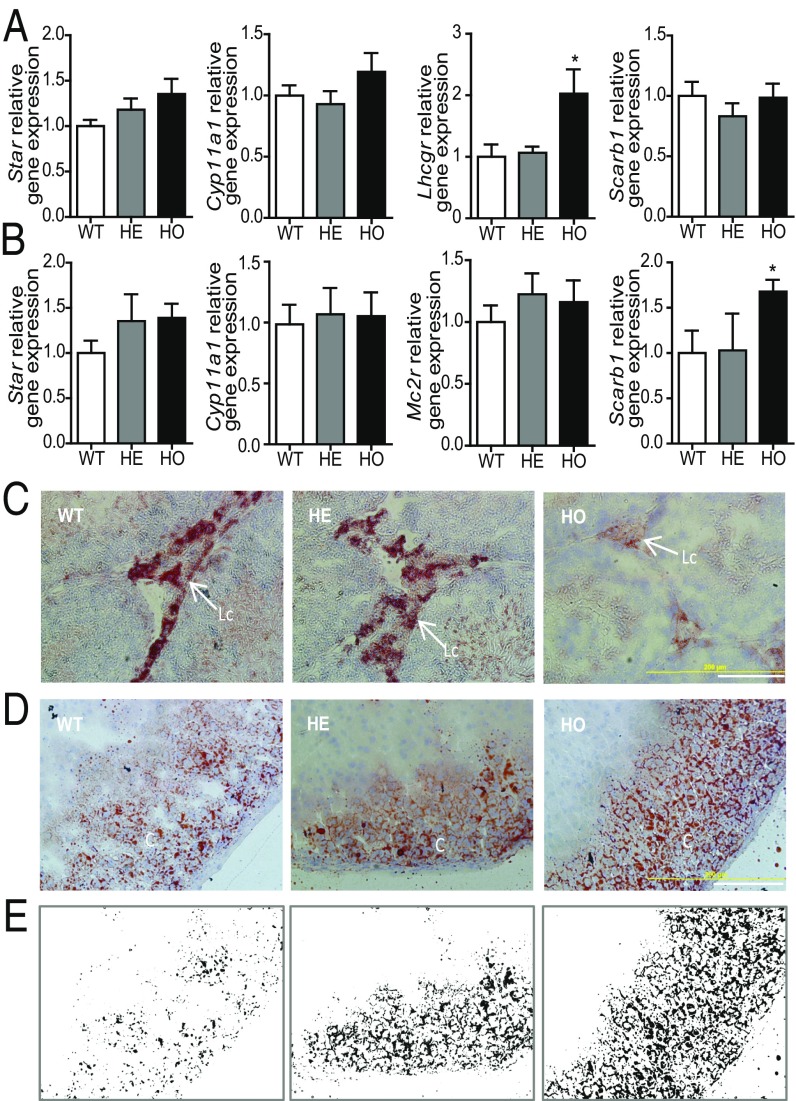
We examined the expression levels of genes important for steroidogenesis. No significant differences were observed in levels of the Stard1, encoding STAR, and Cyp11a1 genes in the gonadal and adrenal tissues (Fig. 4 A and B and SI Appendix, Fig. S5D). The same was true for Vdac1 and Atad3a, members of the mitochondrial transduceosome protein complex (SI Appendix, Fig. S10). The LH receptor (Lhcgr) gene was significantly up-regulated in HO mice testes (2.03-fold, P < 0.05; Fig. 4A) but not in the ovaries (SI Appendix, Fig. S7B). The ACTH receptor (Mcr2r) gene levels were not affected in HO mice adrenals (Fig. 4B). The levels of the scavenger receptor class B member 1 (Scarb1) gene, which facilitates the uptake of cholesteryl esters from high-density lipoproteins (24), were not affected in the gonads but were increased in the adrenals by 1.68-fold (P < 0.05; Fig. 4 A and B). This latter observation suggested the presence of a compensatory mechanism in adrenals to increase the availability of cholesterol for steroidogenesis. To determine whether the observed altered steroidogenic capacity by the adrenals and testes of HO mice had an effect on lipid accumulation, we stained tissues for neutral lipids. Oil Red O staining of both male and female adrenals revealed increased accumulation of lipid droplets (Fig. 4 D and E and SI Appendix, Fig. S5 B and C) in HO mice, suggesting decreased lipid metabolism in the adrenals. By contrast, Oil Red O staining in HO mice showed depletion of the lipid storage in testes (Fig. 4C) and ovaries (SI Appendix, Fig. S7C), suggesting an accelerated use of lipid stores with implications for steroidogenic substrate exhaustion.
Fig. 4.
Effect of Tspo deficiency on mRNA levels of steroidogenic genes and esterified cholesterol. (A and B) Real-time PCR of mRNA expression of Star, Cyp11a1, Mc2r, and Scarb1 in testes (A) and adrenal glands (B). The β-actin mRNA (Actb) was used for normalization. Student’s t test; *P < 0.01. (C) Oil Red O staining of testes from WT, HE, and HO mice. HO mice exhibited a notable decrease in Oil Red O staining of neutral lipids. Lc, Leydig cells. (Scale bar: 200 μm.) (D and E) Oil Red O staining of adrenal glands in WT, HE, and HO mice. A notable increase in accumulation of neutral lipid staining is observed as highlighted in the masked image, created using Image-Pro Plus software on the images shown in D. C, cortex. (Scale bar: 200 μm.)
Discussion
The ability of various chemically distinct TSPO ligands to stimulate steroidogenesis in model and physiological systems as well as the identification of a number of TSPO-associated proteins able to regulate steroidogenesis led to a model of steroidogenesis in which TSPO is part of an OMM protein complex that facilitates intramitochondrial cholesterol transport (9). Our current work extends our understanding of TSPO in an in vivo physiological setting. First, our attempts to generate an Amrh2-driven Tspo deletion unexpectedly resulted in a skewed Mendelian ratio with a lack of embryonic development of Tspo-null mice. This bias in Tspo genotypes was traced to early embryonic development, consistent with an essential role for TSPO in mouse development. Further analysis of available microarray datasets suggested that Amrh2 expression, despite its key role in gonadal development, spikes early in embryogenesis in preimplantation zygotes, thus providing a mechanism for early Tspo deletion and embryonic development. These findings suggest incomplete Amrh2 activity or another compensatory mechanism in 4% of the surviving fetuses. Second, Nr5a1-mediated deletion of Tspo in gonadal and adrenal steroidogenic tissues, but not in brain, reduced TSPO expression in a gene dosage-dependent manner. Surprisingly, this deletion had little effect on gonadal steroidogenesis. In contrast, although basal adrenal steroidogenesis was unaffected in the male and reduced in the female by TSPO deletion, ACTH stimulation of corticosterone production in Tspo-depleted adrenals was severely compromised in both sexes, supporting an important role for TSPO in adrenal hormone-mediated steroidogenesis. The lack of TSPO deletion by Nr5a1-Cre in brain suggests that Nr5a1 is insufficient to drive adequate Cre expression in this tissue or transcriptional mechanisms exist regulating steroidogenesis distinct from those used in the periphery.
To date, three mouse models of Tspo genetic mutation have been published. The first used a mutational strategy similar to ours. Specifically, this strategy used the Amhr2-cre mouse line in conjunction with Tspo mice with LoxP sites flanking exons 2 and 3 to generate a gonad-specific deletion of Tspo (17). In contrast to the embryonic lethal phenotype observed for our Tspo homozygote deletions, these mice were viable. Tspo was deleted in the somatic cells of the testis, and no other phenotypic changes were observed. Such divergent phenotypes have often been ascribed to background genetic differences between strains of mice (25). For example, deletion of Vdac1 is lethal on the C56Bl6 strain background but viable with a minor metabolic phenotype on other backgrounds (26). In addition to the possibility that strain differences may play a role in these phenotypic differences, ectopic activity of Amhr2-cre deletion has been documented, further supporting our observations. Researchers investigating the phenotypes of Amhr2-cre–driven deletion of the phosphatase and tensin homolog (Pten) and the orphan nuclear receptor Nr2f2, both of which had been implicated in embryonic lethality (27, 28), observed significant embryonic lethality in their Amhr2Cre/+Ptenfl/fl and Amhr2cre/+Nr2f2fl/fl mice (29, 30). Petit et al. (30) even reported Mendelian ratios of 7% for Nr2f2−/− mice following deletion via Amhr2cre/+, which is quantitatively similar to the results presented here. In conjunction with the data indicating that Amhr2 expression spikes briefly during early embryonic development, these observations strongly support a role for TSPO in early vertebrate development and underscore the importance of fully reporting breeding strategies as well as Mendelian ratios in gene knockout studies. Similar comparisons of the viability of our Amhr2-cre/+,Tspofl/fl mice and that of the previously reported whole-body Tspo deletion mutants (18, 19) can be made, although differing methodologies make direct comparisons a challenge. Interestingly, adaptation to genetic manipulation may underlie some of the observed phenotypes, as evidenced by the gene expression changes observed in our Nr5a1-Cre/+, Tspofl/fl mice. Indeed, although the Tspo−/− mice were viable and physiologically functional, Banati et al. reported a severe bioenergetic deficit in glial cells isolated from these Tspo−/− animals (19). Although adaptive responses in genetically engineered organisms have been reported (31, 32), systematic exploration of this question has only just begun to be explored in genetically characterized organisms such as yeast, indicating that genetic adaptation to targeted gene deletion is highly reproducible and widespread (33). Understanding the commonalities and differences between the Tspo-deletion mouse models may provide insight into such processes in higher metazoan genetic models such as mice.
Because the discovery that high-flux steroid synthesis by the adrenal glands and gonads is limited by the availability of cholesterol to the CYP11A1 enzyme, research into this process has been framed in terms of intramitochondrial cholesterol transport from OMM to IMM. This research focus has been aided greatly by the discovery of mutations in the STAR protein in humans, resulting in compromise of high-flux adrenal steroidogenesis (7) and contributing to congenital lipoid adrenal hyperplasia (34). The molecular mechanisms underlying mitochondrial cholesterol transport, however, have remained elusive. The pharmacologically well-characterized TSPO is an attractive participant in this process due to its high expression in steroidogenic cells, localization in OMM, cholesterol-binding properties, and the observation that drug ligands were able to stimulate cholesterol movement and steroidogenesis both in vitro and in vivo (5). Recent work has suggested that TSPO acts as a member of an OMM protein complex that facilitates intramitochondrial cholesterol movement (9). The work presented here, along with that of others, provides important insight into this model of steroidogenesis. To our surprise, TSPO appears nonessential for low-flux basal steroidogenesis in the gonads and adrenals and for hCG-induced steroid production in the gonad, as no significant differences between the WT, HE, and HO mice were observed. Taken together, these data suggest that the testis, unlike the adrenal, was able to maintain testosterone production via an adjustment to the lack of TSPO through compensatory mechanisms, such as increased expression of LHCGR. Single mutation studies such as this, however, do not preclude a lack of involvement of a gene in a process, as has been clearly demonstrated by a century of research into epistatic genetic relationships (35). Indeed, the Nr5a1-driven Tspo cKO HO mice surprisingly exhibited depleted stores of cholesterol (lipid droplets), suggesting that Tspo may affect lipid homeostasis in Leydig cells and Tspo-null cells may be at risk to steroidogenic exhaustion.
The observation that Tspo null mutations in the Nr5a1-driven Tspo cKO HE and HO mice contributed to the inability of the adrenal to respond to ACTH suggests that mitochondrial cholesterol transport is a complex phenotype that is characterized by incomplete penetrance by null mutations in a tissue-specific manner. Such a phenotype has also been reported for the Star-null mouse, which exhibits a severe adrenal phenotype but a relatively minor gonadal phenotype (36, 37), even in the presence of exogenous glucocorticoids, which are known steroidogenic inhibitors (38). Furthermore, human patients with STAR mutations have been reported to exhibit divergent testicular and adrenal phenotypes (39). Moreover, biochemical evidence linking TSPO to multimeric OMM protein complexes provides a physical mechanism for the robustness of TSPO loss, as distribution of robustness within the macromolecular complexes may compensate for the loss of function of individual components, although no changes in Vdac1 and Atad3a mRNA levels were observed in Nr5a1-driven Tspo cKO HO mice. Interestingly, we previously showed that ginkgolides reduced Tspo levels in the adrenals but not in testes and reduced the ACTH-induced corticosteroid production in rats, providing a mechanism to explain the reported antistress effects of these compounds (10).
Impairment of mitochondrial cholesterol transport and steroidogenesis underlies congenital adrenal lipoid hyperplasia, which is linked to mutations in the STAR gene (34). The adrenal glands of these patients display an elevated intracellular accumulation of cholesterol and lipid deposits, contributing to loss of steroid synthesis. Consistent with an impaired steroidogenic phenotype in the adrenal glands that lack TSPO, the adrenal fasciculata layer of HO animals displayed elevated levels of neutral lipids and an increased number of lipid droplets compared with their WT littermates. These mice also exhibited up-regulation of Scarb1, which is involved in the main pathway of cholesterol uptake from the periphery (24), indicating an adaptive response to the lack of substrate availability for steroidogenesis. Surprisingly, similar analysis of the gonads, which did not exhibit significant changes in their steroidogenic profile, showed a significant reduction in the neutral lipid content of HO mice in comparison with WT mice. Thus, although TSPO deletion in the gonad results in a minor steroidogenic phenotype, this loss exerts a significant effect on lipid homeostasis. Collectively, these findings support an important role for TSPO in cellular cholesterol and/or lipid homeostasis in steroidogenic tissues.
Although we have not yet performed behavioral studies, the Nr5a1-driven Tspo cKO HO mice appear to act normally. The lack of response to ACTH is indicative of a lack of stress response in these animals. It is interesting to note that the adrenal medulla, which is normally devoid of TSPO, had robust TSPO expression in HO mice, which was coupled with increased epinephrine levels. As epinephrine is another mediator of the stress response (40), we propose that this increase in epinephrine may serve as an adaptive mechanism developed to function in the presence of Tspo deletion.
In conclusion, genetic models serve as powerful tools for probing the structure of biological systems, especially when used in conjunction with biochemical and pharmacological methodologies. Caution must be exerted when using such models, however, as the complex interactions of the thousands of genes in a multicellular organism may obscure the functions of any individual gene (25). The tissue-specific Tspo conditional knockout mouse models that we describe here expand our knowledge of the function of this protein and suggest a critical role for TSPO in preimplantation embryogenesis as well as in hormone-mediated adrenal steroid production. Moreover, the viability of the Nr5a1-driven Tspo cKO mice coupled with their inability to mount a glucocorticoid response in response to ACTH offers a fascinating model of stress response and allostatic load.
Materials and Methods
Animals were handled according to protocols approved by the McGill University Animal Care and Use Committee. Tspo-homofloxed mice (Tspofl/fl) were maintained via crossing of two Tspofl/+ mice (a gift from Michael Forte, Vollum Institute, Oregon Health and Science University, Portland, OR), and every 10 generations, the Tspofl/+ females were crossed back with male wild-type mice (Tspo+/+; C57BL/6J) (Fig. 1A). The two lines of Cre mouse colonies—Amhr2-Cre/+ or Amhr2Cre/+ mice (B6;129S7-Amhr2tm3(cre)Bhr/Mmnc) from the Mutant Mouse Regional Resource Center at University of North Carolina at Chapel Hill and Nr5a1-Cre/+ mice [FVB-Tg(Nr5a1-Cre)2Lowl/J] from the Jackson Laboratory—were maintained via crossing Cre-positive male mice with wild-type females (Tspo+/+; C57BL/6J) (30, 35). Tspo cKO mice (Tspofl/fl; Amhr2-Cre/+; HO) were generated by first-round mating of Tspofl/fl mice to Amhr2-Cre/+ mice, resulting in HE mice with genotype Tspofl/+; Amhr2-Cre/+, and then second-round breeding was performed via crossing the HE with Tspofl/fl mice (Fig. 1B and SI Appendix, Fig. S1).
The same breeding scheme was used for Nr5a1-driven Tspo cKO mice. Tail biopsies were collected for genotyping using standard PCR protocols with the oligonucleotides listed in SI Appendix, Table S1. For embryonic analyses, uteri were removed from pregnant dams at 12.5 dpc, and whole embryos were collected for genotyping and cryotissue sectioning. For adult analyses, F1 mice from two rounds of breeding between the corresponding Tspo-homofloxed mice and Cre-positive mice, and then Tspo-heterofloxed mice with Cre-positive (HE; Fig. 1B), were used for phenotype analysis and steroid measurement.
Hormone treatment, blood plasma sample collection, measurement of circulating steroids and epinephrine, immunofluorescence and confocal microscopy, Oil Red O staining, quantitative real-time PCR, microarray analysis data mining, and statistical analysis of the data were performed using established methods and are described in detail in SI Appendix.
Supplementary Material
Acknowledgments
We thank Dr. M. Forte (Oregon Health Science University) for providing us with the Tspo floxed mice; Dr. A. F. Parlow (National Hormone and Peptide Program) for the gift of hCG; Ms. C. Therrien for tissue cryosectioning; and and Ms. N. Laughren and Mr. C. Essagian for help with handling the laboratory animals. This work was supported by a grant from the Canadian Institutes of Health Research (MOP125983) and a Canada Research Chair in Biochemical Pharmacology. The Research Institute of McGill University Health Centre was supported by a center grant from Les Fonds de la Recherche du Québec Santé.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1502670112/-/DCSupplemental.
References
- 1.Papadopoulos V, et al. Translocator protein (18kDa): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27(8):402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Rupprecht R, et al. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov. 2010;9(12):971–988. doi: 10.1038/nrd3295. [DOI] [PubMed] [Google Scholar]
- 3.Jaremko L, Jaremko M, Giller K, Becker S, Zweckstetter M. Structure of the mitochondrial translocator protein in complex with a diagnostic ligand. Science. 2014;343(6177):1363–1366. doi: 10.1126/science.1248725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo Y, et al. Protein structure. Structure and activity of tryptophan-rich TSPO proteins. Science. 2015;347(6221):551–555. doi: 10.1126/science.aaa1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lacapère JJ, Papadopoulos V. Peripheral-type benzodiazepine receptor: Structure and function of a cholesterol-binding protein in steroid and bile acid biosynthesis. Steroids. 2003;68(7-8):569–585. doi: 10.1016/s0039-128x(03)00101-6. [DOI] [PubMed] [Google Scholar]
- 6.Li F, Liu J, Zheng Y, Garavito RM, Ferguson-Miller S. Protein structure. Crystal structures of translocator protein (TSPO) and mutant mimic of a human polymorphism. Science. 2015;347(6221):555–558. doi: 10.1126/science.1260590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jefcoate C. High-flux mitochondrial cholesterol trafficking, a specialized function of the adrenal cortex. J Clin Invest. 2002;110(7):881–890. doi: 10.1172/JCI16771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Issop L, Rone MB, Papadopoulos V. Organelle plasticity and interactions in cholesterol transport and steroid biosynthesis. Mol Cell Endocrinol. 2013;371(1-2):34–46. doi: 10.1016/j.mce.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Rone MB, et al. Identification of a dynamic mitochondrial protein complex driving cholesterol import, trafficking, and metabolism to steroid hormones. Mol Endocrinol. 2012;26(11):1868–1882. doi: 10.1210/me.2012-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amri H, Ogwuegbu SO, Boujrad N, Drieu K, Papadopoulos V. In vivo regulation of peripheral-type benzodiazepine receptor and glucocorticoid synthesis by Ginkgo biloba extract EGb 761 and isolated ginkgolides. Endocrinology. 1996;137(12):5707–5718. doi: 10.1210/endo.137.12.8940403. [DOI] [PubMed] [Google Scholar]
- 11.Fan J, Lindemann P, Feuilloley MGJ, Papadopoulos V. Structural and functional evolution of the translocator protein (18 kDa) Curr Mol Med. 2012;12(4):369–386. doi: 10.2174/1566524011207040369. [DOI] [PubMed] [Google Scholar]
- 12.Costa B, et al. The spontaneous Ala147Thr amino acid substitution within the translocator protein influences pregnenolone production in lymphomonocytes of healthy individuals. Endocrinology. 2009;150(12):5438–5445. doi: 10.1210/en.2009-0752. [DOI] [PubMed] [Google Scholar]
- 13.Owen DR, et al. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab. 2012;32(1):1–5. doi: 10.1038/jcbfm.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papadopoulos V, et al. Targeted disruption of the peripheral-type benzodiazepine receptor gene inhibits steroidogenesis in the R2C Leydig tumor cell line. J Biol Chem. 1997;272(51):32129–32135. doi: 10.1074/jbc.272.51.32129. [DOI] [PubMed] [Google Scholar]
- 15.Hauet T, et al. Peripheral-type benzodiazepine receptor-mediated action of steroidogenic acute regulatory protein on cholesterol entry into leydig cell mitochondria. Mol Endocrinol. 2005;19(2):540–554. doi: 10.1210/me.2004-0307. [DOI] [PubMed] [Google Scholar]
- 16.Papadopoulos V, et al. Peripheral benzodiazepine receptor in cholesterol transport and steroidogenesis. Steroids. 1997;62(1):21–28. doi: 10.1016/s0039-128x(96)00154-7. [DOI] [PubMed] [Google Scholar]
- 17.Morohaku K, et al. Translocator protein/peripheral benzodiazepine receptor is not required for steroid hormone biosynthesis. Endocrinology. 2014;155(1):89–97. doi: 10.1210/en.2013-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tu LN, et al. Peripheral benzodiazepine receptor/translocator protein global knock-out mice are viable with no effects on steroid hormone biosynthesis. J Biol Chem. 2014;289(40):27444–27454. doi: 10.1074/jbc.M114.578286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banati RB, et al. Positron emission tomography and functional characterization of a complete PBR/TSPO knockout. Nat Commun. 2014;5:5452. doi: 10.1038/ncomms6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Šileikytė J, et al. Regulation of the mitochondrial permeability transition pore by the outer membrane does not involve the peripheral benzodiazepine receptor (Translocator Protein of 18 kDa (TSPO)) J Biol Chem. 2014;289(20):13769–13781. doi: 10.1074/jbc.M114.549634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Genetic studies of the AMH/MIS signaling pathway for Müllerian duct regression. Mol Cell Endocrinol. 2003;211(1-2):15–19. doi: 10.1016/j.mce.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Zeng F, Baldwin DA, Schultz RM. Transcript profiling during preimplantation mouse development. Dev Biol. 2004;272(2):483–496. doi: 10.1016/j.ydbio.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 23.Bingham NC, Verma-Kurvari S, Parada LF, Parker KL. Development of a steroidogenic factor 1/Cre transgenic mouse line. Genesis. 2006;44(9):419–424. doi: 10.1002/dvg.20231. [DOI] [PubMed] [Google Scholar]
- 24.Shen WJ, Hu J, Hu Z, Kraemer FB, Azhar S. Scavenger receptor class B type I (SR-BI): A versatile receptor with multiple functions and actions. Metabolism. 2014;63(7):875–886. doi: 10.1016/j.metabol.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbaric I, Miller G, Dear TN. Appearances can be deceiving: Phenotypes of knockout mice. Brief Funct Genomics Proteomics. 2007;6(2):91–103. doi: 10.1093/bfgp/elm008. [DOI] [PubMed] [Google Scholar]
- 26.Raghavan A, Sheiko T, Graham BH, Craigen WJ. Voltage-dependant anion channels: Novel insights into isoform function through genetic models. Biochim Biophys Acta. 2012;6(1818):1477–1485. doi: 10.1016/j.bbamem.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19(4):348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- 28.Pereira FA, Qiu Y, Zhou G, Tsai MJ, Tsai SY. The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev. 1999;13(8):1037–1049. doi: 10.1101/gad.13.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laguë MN, et al. Synergistic effects of Pten loss and WNT/CTNNB1 signaling pathway activation in ovarian granulosa cell tumor development and progression. Carcinogenesis. 2008;29(11):2062–2072. doi: 10.1093/carcin/bgn186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petit FG, et al. Deletion of the orphan nuclear receptor COUP-TFII in uterus leads to placental deficiency. Proc Natl Acad Sci USA. 2007;104(15):6293–6298. doi: 10.1073/pnas.0702039104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torres EM, et al. Identification of aneuploidy-tolerating mutations. Cell. 2010;143(1):71–83. doi: 10.1016/j.cell.2010.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng TS, et al. Deficiency in caspase-9 or caspase-3 induces compensatory caspase activation. Nat Med. 2000;6(11):1241–1247. doi: 10.1038/81343. [DOI] [PubMed] [Google Scholar]
- 33.Teng X, et al. Genome-wide consequences of deleting any single gene. Mol Cell. 2013;52(4):485–494. doi: 10.1016/j.molcel.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bose HS, Sugawara T, Strauss JF, III, Miller WL. International Congenital Lipoid Adrenal Hyperplasia Consortium The pathophysiology and genetics of congenital lipoid adrenal hyperplasia. N Engl J Med. 1996;335(25):1870–1878. doi: 10.1056/NEJM199612193352503. [DOI] [PubMed] [Google Scholar]
- 35.Phillips PC. Epistasis: The essential role of gene interactions in the structure and evolution of genetic systems. Nat Rev Genet. 2008;9(11):855–867. doi: 10.1038/nrg2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caron KM, et al. Targeted disruption of the mouse gene encoding steroidogenic acute regulatory protein provides insights into congenital lipoid adrenal hyperplasia. Proc Natl Acad Sci USA. 1997;94(21):11540–11545. doi: 10.1073/pnas.94.21.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hasegawa T, et al. Developmental roles of the steroidogenic acute regulatory protein (StAR) as revealed by StAR knockout mice. Mol Endocrinol. 2000;14(9):1462–1471. doi: 10.1210/mend.14.9.0515. [DOI] [PubMed] [Google Scholar]
- 38.Hardy MP, et al. Stress hormone and male reproductive function. Cell Tissue Res. 2005;322(1):147–153. doi: 10.1007/s00441-005-0006-2. [DOI] [PubMed] [Google Scholar]
- 39.Flück CE, et al. Characterization of novel StAR (steroidogenic acute regulatory protein) mutations causing non-classic lipoid adrenal hyperplasia. PLoS ONE. 2011;6(5):e20178. doi: 10.1371/journal.pone.0020178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kvetnansky R, Lu X, Ziegler MG. Stress-triggered changes in peripheral catecholaminergic systems. Adv Pharmacol. 2013;68:359–397. doi: 10.1016/B978-0-12-411512-5.00017-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.