Significance
Global change and regime shifts of ecosystems are a major concern today. It has been suggested that the end-Cretaceous impact of an asteroid not only caused mass extinction but also involved the transgression of a global tipping point. We demonstrate that the ecological structure of pre- and postextinction assemblages of marine bivalves and gastropods differ far more than expected from usual background fluctuations. In-depth ecological analyses suggest site-specific rather than globally uniform shifts to different dynamic regimes. At more inclusive ecological levels, parallel increases emerge in mobility levels, in species burrowing into the sediment, and in deposit feeders and carnivores.
Keywords: end-Cretaceous mass extinction, regime shift, functional groups, mollusks, ecospace
Abstract
Contemporary biodiversity loss and population declines threaten to push the biosphere toward a tipping point with irreversible effects on ecosystem composition and function. As a potential example of a global-scale regime shift in the geological past, we assessed ecological changes across the end-Cretaceous mass extinction based on molluscan assemblages at four well-studied sites. By contrasting preextinction and postextinction rank abundance and numerical abundance in 19 molluscan modes of life—each defined as a unique combination of mobility level, feeding mode, and position relative to the substrate—we find distinct shifts in ecospace utilization, which significantly exceed predictions from null models. The magnitude of change in functional traits relative to normal temporal fluctuations at far-flung sites indicates that molluscan assemblages shifted to differently structured systems and faunal response was global. The strengths of temporal ecological shifts, however, are mostly within the range of preextinction site-to-site variability, demonstrating that local ecological turnover was similar to geographic variation over a broad latitudinal range. In conjunction with varied site-specific temporal patterns of individual modes of life, these spatial and temporal heterogeneities argue against a concerted phase shift of molluscan assemblages from one well-defined regime to another. At a broader ecological level, by contrast, congruent tendencies emerge and suggest deterministic processes. These patterns comprise the well-known increase of deposit-feeding mollusks in postextinction assemblages and increases in predators and predator-resistant modes of life, i.e., those characterized by elevated mobility and infaunal life habits.
Recognizing nonlinear responses and tipping points in complex biological systems has raised concerns over the effects of global change, the extinction of species, and future global-scale shifts in the state of ecosystems (1, 2). Severe perturbations of the earth system, coupled with significant losses of biodiversity, have occurred a few times in earth history. These episodes of mass extinction provide the opportunity to study the ecological and evolutionary dynamics of the earth system when exposed to critical stress. Mass extinctions do not only devastate biodiversity—they also fundamentally restructure the variety of functions performed by the biota (3, 4). The analysis of ancient mass extinctions allows establishing the circumstances and the degree of system-level change in the geological past and thus could be informative of future changes in ecosystems due to anthropogenically driven biodiversity loss.
The extinction event at the Cretaceous–Paleogene boundary (KPB, 66 million years before present) was the most recent mass extinction with an estimated 70% species loss (5). Similar to other mass extinctions it was associated with a profound disruption of the global carbon cycle (6). The ultimate trigger was probably the impact of an asteroid at Chicxulub in present-day Mexico (7), whereas Deccan Trap volcanism may have been an additional stressor (8). The most likely proximate killing mechanism was a crisis in primary productivity and global collapse of food webs owing to the suppression of photosynthesis (9–11). Other factors with devastating effects for marine ecosystems may have been metal poisoning (12), the acidification of oceanic surface waters (13), and short-lived global cooling (14).
Major biotic changes associated with regime shifts can involve diversity loss, changes in biomass and trophic interactions, and the establishment of novel species assemblages (1). The contrast among different states in ecosystems is usually caused by a shift in dominance among organisms with different modes of life (15). Here we quantify the ecological change across the KPB in shallow marine benthic soft-bottom assemblages. These fossil assemblages are dominated by bivalve and gastropod mollusks, which not only have an excellent fossil record but also represent many different modes of life (MOLs), yielding unique insights into the ecological dynamics of the extinction and subsequent recovery. We compare molluscan ecospace occupation—as defined by the mobility, feeding mechanisms, and living positions of species (16)—in latest Cretaceous (Late Maastrichtian) preextinction times with that in earliest Paleogene (Danian) postextinction times.
Previous studies showed an Early Danian increase in infaunal deposit feeders and mobility levels in some environments at some sites (17–21), whereas at other sites, either infaunal or epifaunal suspension feeders dominated and mobility levels and benthic tiering structure displayed no trends (21–25). Increased predation pressure after the KPB has been inferred from the radiation of predatory carnivores, in particular neogastopods (3, 18, 21, 26–28), elevated gastropod drilling frequencies (29, 30), and a trend toward deeper burrowing in bivalves (31), but predatory interactions did not increase universally (21, 25). One study demonstrated that Cretaceous–Paleogene spatial variation in functional group composition exceeded any changes through time (32), but otherwise this topic is largely unexplored. Some workers have questioned the presence of general ecological patterns (25), whereas others argued that ecological effects were habitat specific, with significant restructuring occurring in offshore assemblages and siliciclastic environments but not in shallow subtidal habitats and oligotrophic carbonate settings (20, 21).
We studied mollusk-dominated siliciclastic shelf ecosystems at four well-studied sites before and after the KPB (SI Text S1). At each site, sedimentological evidence suggests that external environmental conditions were similar before and after the KPB (SI Text S1 and SI Text S2). The successions at three sites (Brazos River, Bajada del Jagüel, and Seymour Island) formed in a middle-to-outer shelf environment, whereas the fourth site (San Ramón) represents a tide-dominated delta. We estimated the ecological importance of each MOL in pre- and postextinction assemblages by its respective proportion based on counts of individuals. For this purpose, all Late Maastrichtian samples at a site were combined into a preextinction assemblage and all Danian samples into a postextinction assemblage. Specimen-level data were not available for the Maastrichtian of Seymour Island, and here the KPB comparison resides on the number of occurrences, i.e., counts of the presence of species of a particular MOL. In addition to comparing aggregate pre- and postextinction assemblages we used permutation tests and ordination techniques to compare the between-sample variation in ecological structure before and after the extinction event. Specifically, we tested whether these ecosystems experienced large, temporally abrupt, and persistent changes in ecological structure across the KPB. First, we examined whether postextinction assemblages constitute a fundamentally different assembly of functional groups. Differences to preextinction assemblages can be expected from previous work, but it is not clear whether they were beyond those of ordinary background fluctuations. Second, we explored whether any ecological disparity of postextinction assemblages reflects the ecospace occupation displayed by those taxa of preextinction assemblages that survived the extinction event. This scenario would suggest extinctions as the primary cause of ecological shifts. Third, we analyzed how consistently ecological patterns changed across sites. Congruence would suggest that the ecological systems responded deterministically to environmental change. Finally, we contrasted faunal shifts in time with the site-to-site variability to evaluate the spatiotemporal dimensions of ecological change.
To test the significance of our results, we generated random species assemblages from the individual samples at each site for which we then calculated their ecological dissimilarity. By repeating this procedure many times we obtained a null distribution of ecological dissimilarity for “pre-” and “postextinction” assemblages (Materials and Methods). Comparison of the observed pattern with the prediction of the permuted null model allowed us to evaluate the significance of ecological change.
Results
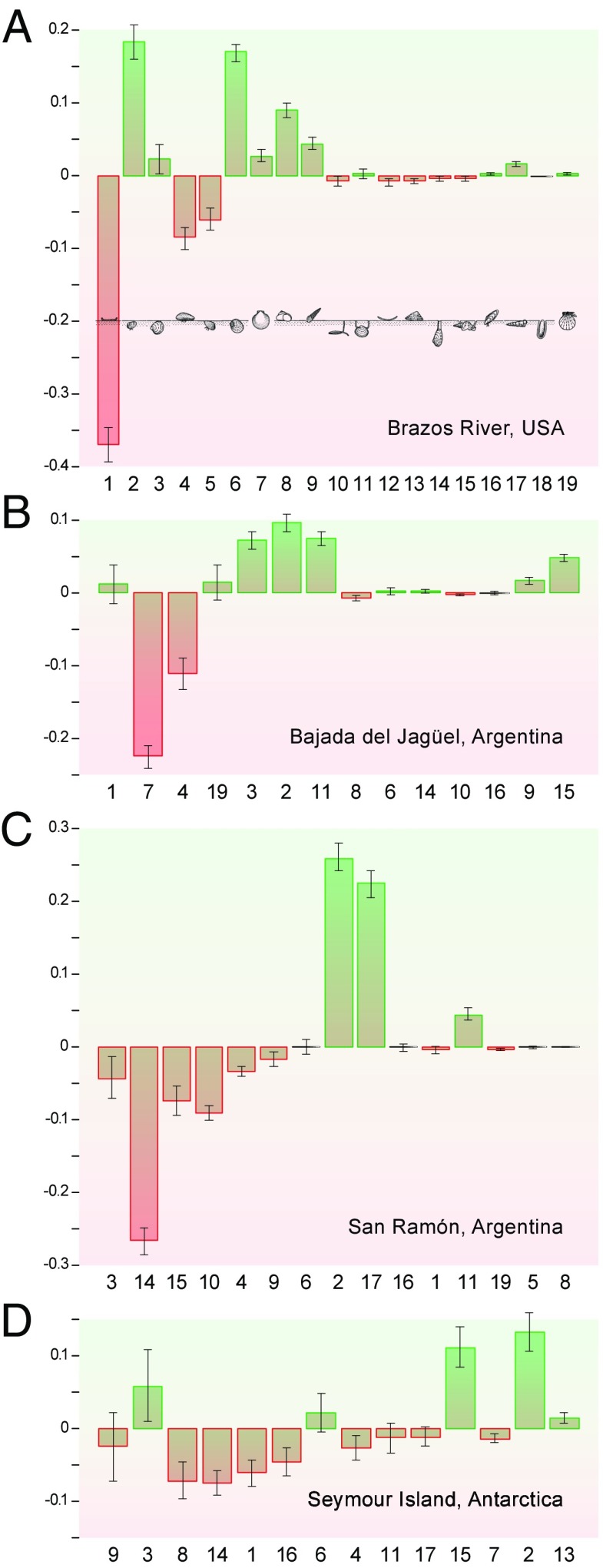
Of 18 unique MOLs in the preextinction assemblages, none was lost across the boundary when all four sites are viewed jointly (Fig. 1), and only one MOL (MOL18) is restricted to the postextinction assemblage. At this level of functional diversity the complexity of realized ecospace remained virtually unchanged.
Fig. 1.
Changes in the proportional abundance of modes of life (MOLs 1–19) represented in marine molluscan assemblages across the Cretaceous–Paleogene boundary. The four analyzed sites (A–D) are arranged from north (A) to south (D) and also reflect increasing distances from the Chicxulub impact site. Modes of life are rank ordered according to their preextinction abundance, decreasing from Left to Right. Negative values indicate proportionally declining modes of life and positive values, expanding modes of life across the KPB. Error bars represent the summed SEs of pre- and postextinction proportions. 1, epifaunal, stationary, cemented, suspension feeders; 2, shallow infaunal, motile, deposit feeders; 3, shallow infaunal, facultatively motile, unattached, suspension feeders; 4, epifaunal, stationary, byssate, suspension feeders; 5, shallow infaunal, facultatively motile, byssate, suspension feeders; 6, shallow infaunal, motile, carnivores; 7, epifaunal, facultatively motile, unattached, suspension feeders; 8, epifaunal, motile, herbivores; 9, epifaunal, motile, carnivores; 10, deep infaunal, facultatively motile, surface deposit feeders; 11, deep infaunal, facultatively motile, chemosymbiosis; 12, epifaunal, stationary, unattached, suspension feeders; 13, epifaunal, facultatively motile, herbivores; 14, deep infaunal, facultatively motile, suspension feeders; 15, shallow infaunal, motile, surface deposit feeders; 16, semiinfaunal, stationary, byssate, suspension feeders; 17, shallow infaunal, motile, suspension feeders; 18, infaunal, stationary, boring, suspension feeders; and 19, epifaunal, facultatively motile, byssate, suspension feeders.
The individual sites experienced complete losses from the Maastrichtian to the Danian of at least one and up to three MOLs (Table S1). These MOLs were mostly rare in the preextinction assemblages and their disappearance could be stochastic noise (Fig. S1). A notable exception occurred at Bajada del Jagüel, where the second most abundant MOL disappeared at the boundary. This exception is attributable to the extinction of a single, albeit abundant, species of pectinoid bivalves, indicating a lack of redundancy within this MOL at that site. Pectinoids, however, did return to Argentinian offshore environments after they recovered from a global evolutionary bottleneck at the KPB (33). The one or two MOLs that are restricted to the Danian at individual sites (Table S1) only occupy subordinate ranks of the postextinction assemblage, again suggesting stochasticity rather than ecologic signal (Fig. S1).
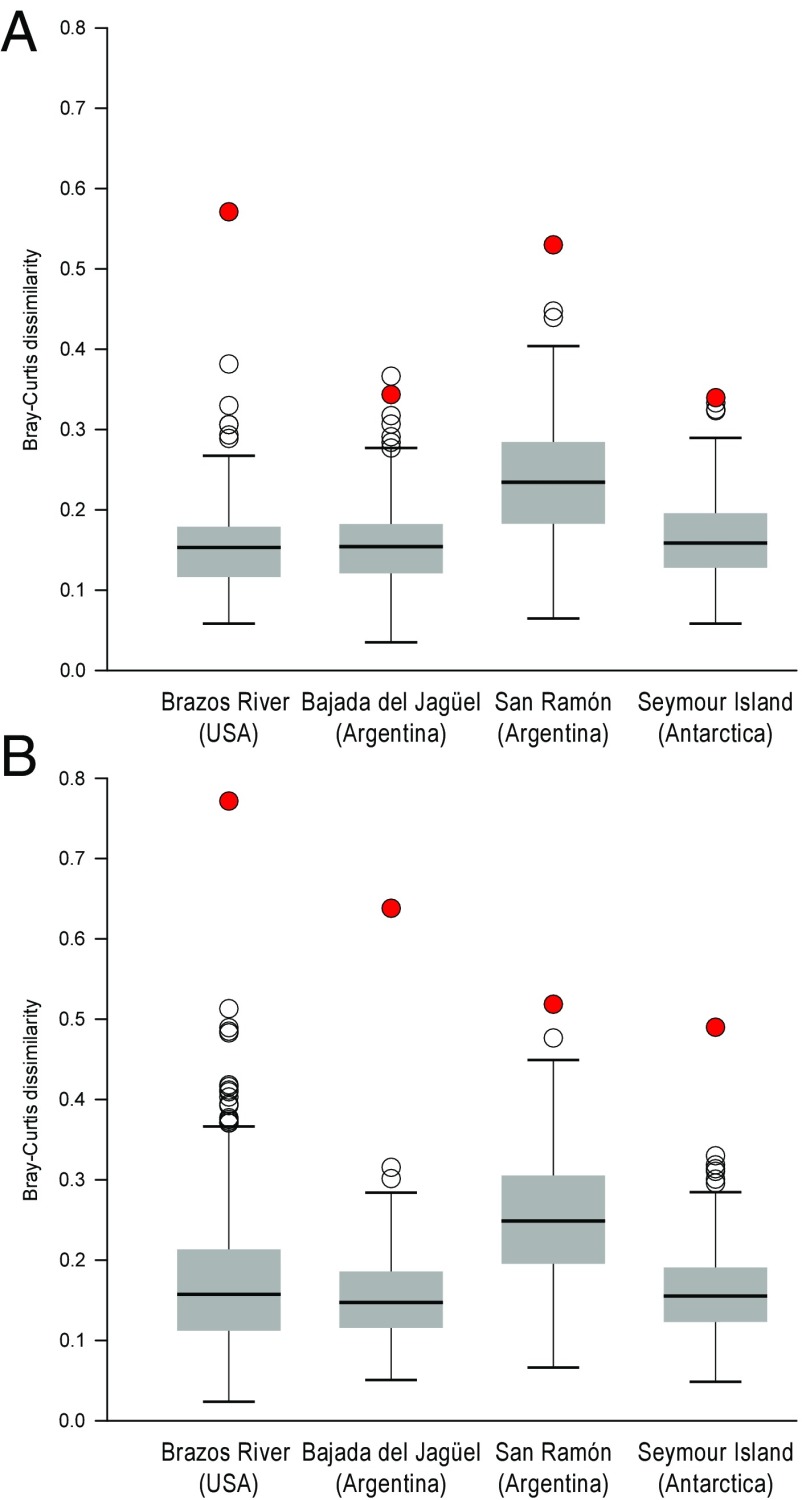
Despite this general stability in the presence of MOLs, distinct shifts in abundance within MOLs are evident at all sites. These shifts are commonly expressed as decreases within previously top-ranked groups and increases in lower-ranked ones (Fig. 1). Comparison with the null distribution indicates that the ecological dissimilarities between pre- and postextinction assemblages are significantly greater than the expectation of a random sampling of the available sampling pool (Fig. 2A). Additional analyses that control for potential heterogeneities between Maastrichtian and Danian environments confirm the nature and the significance of ecological changes (SI Text S2 and Fig. S2). Ordinations show that samples from pre- and postextinction times form discrete, nonoverlapping groups (Fig. S3), indicating that extensive and persistent ecological reorganizations across the KPG are evident at the community scale as well. We thus reject a scenario in which the ecospace vacated through extinctions or through declines in species abundances was simply refilled during recovery. Likewise, postextinction assemblages exhibit pronounced changes in rank positions and proportional abundances compared with preextinction assemblages from which species that disappeared in the latest Maastrichtian have been removed before analysis (Fig. S4). These differences are also significant as indicated by the corresponding permutation tests (Fig. 2B). We therefore also reject the hypothesis that the postextinction assemblages reflect the ecological residuals of preextinction assemblages after accounting for the extinction-induced losses within MOLs.
Fig. 2.
Ecological dissimilarity between preextinction and postextinction molluscan assemblages compared with patterns based on randomized data. (A) Comparison based on full datasets. (B) Comparison after removing species that disappeared in the latest Maastrichtian. Filled circles indicate the observed Bray-Curtis dissimilarity. The box plots show the distribution of dissimilarity values in 500 permutation trials. The solid black lines inside the boxes represent the medians, the Top and Bottom edges of the boxes correspond to the first and third quartiles, and whiskers represent 1.5 times the interquartile range. Points outside the whiskers are outliers (open circles).
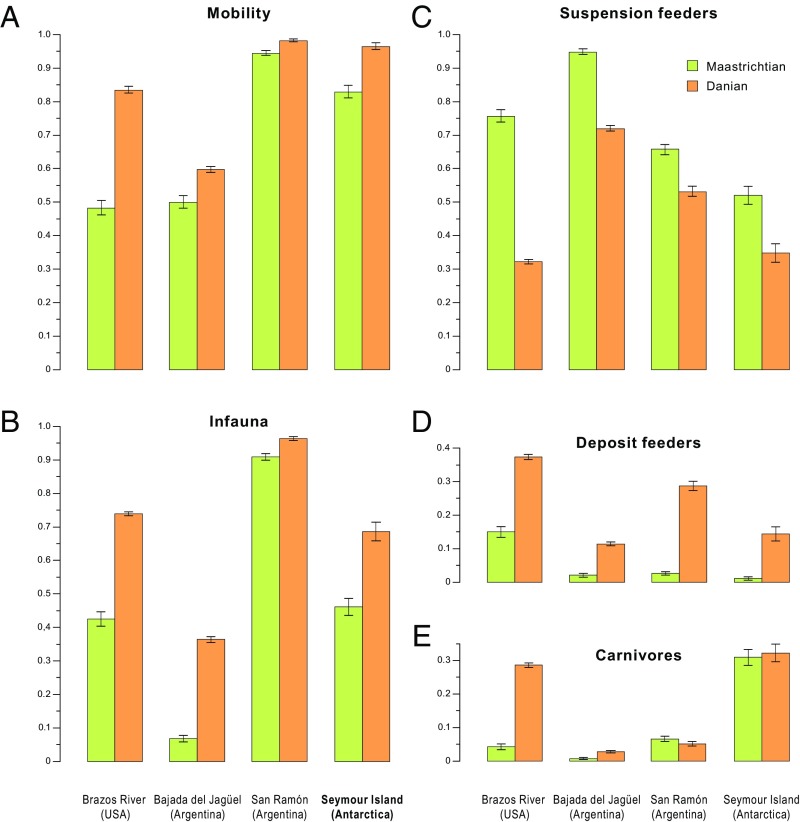
Ecological structure varies among sites and neither the preextinction nor the postextinction faunas represent a single well-defined state (Figs. 1 and 3 and Fig. S1). Only MOL2 (shallow infaunal, motile, deposit feeders) increased and MOL4 (epifaunal, stationary, byssate, suspension feeders) decreased consistently across the KPG. Congruent trends across sites, however, emerge when the three main ecological factors—mobility, feeding, and tiering—are analyzed separately (Fig. 3). Elevated proportions of motile and infaunal mollusks in postextinction assemblages remain significant even if we remove shallow infaunal, motile deposit feeders from our analysis, i.e., the MOL with the most prominent abundance increase. The use of trophic resources changed in unison, and the major feeding categories became more evenly distributed (Fig. 3). Along with increased carnivory, these patterns suggest more complex postextinction food web structures. Spatial and temporal heterogeneities at the detailed autecological level aside, benthic marine mollusks responded in a similar manner to disturbances associated with the KPB mass extinction event when change is quantified within broader ecological categories.
Fig. 3.
Proportional abundance changes in the ecological composition of molluscan assemblages across the Cretaceous–Paleogene boundary at the four studied sites. Consistent shifts across all sites are evident as increase in mobility levels (A); increase in the degree of infaunality (B); decrease in suspension feeding (C); and increase in deposit feeding (D). Carnivores (E) increased at two sites and remained stable at two other sites. Error bars represent one SE in each direction.
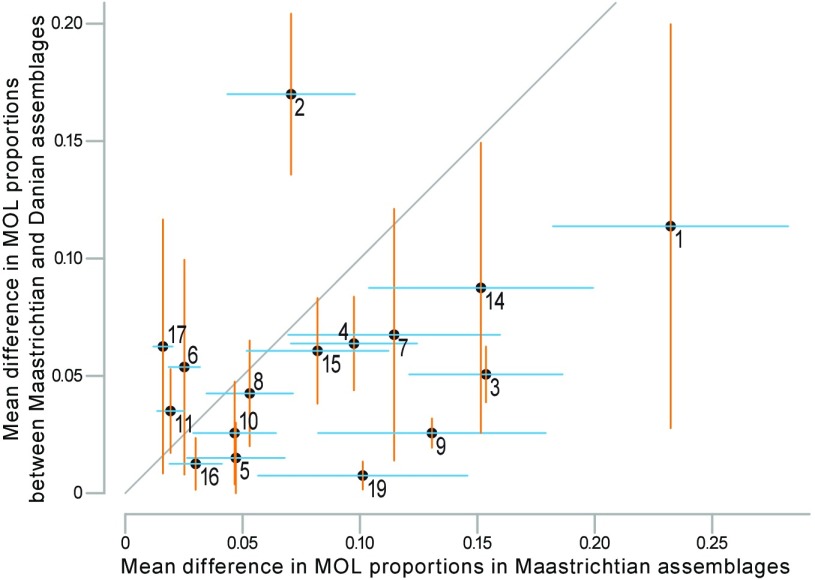
Contrasting the strength of ecological shifts in time with the site-to-site variability in ecological composition demonstrates that for most MOLs the average change in time was less profound than the change in space. Only MOL2 showed significantly more pronounced temporal change than is evident in the geographic variation of Maastrichtian assemblages (Fig. 4). Along with MOL14, it also exhibits a more significant temporal shift compared with the geographic variation of Danian assemblages (Fig. S5). In summary, changes are characterized by significant local ecological turnover compared with background fluctuations, but do not involve unusually intense modifications relative to ecological variation between sites, although it should be kept in mind that our sites span a large geographic range from subtropical to polar latitudes.
Fig. 4.
Comparison of ecological change in time with ecological variability in space. The mean difference in proportional abundance of modes of life (MOLs 1–19, see key in Fig. 1) between pre- and postextinction assemblages at each site is plotted against the mean difference across the four studied sites in Maastrichtian assemblages. The diagonal line indicates equality of mean temporal change and mean spatial variation. The horizontal and vertical lines associated with each MOL represent one SE in each direction.
Discussion
Despite the substantial loss of taxonomic diversity during the end-Cretaceous mass extinction, we find that the primary modes of molluscan life were maintained. Such a stability of functional diversity in benthic shallow marine ecosystems was also observed on the global scale for the end-Permian (34) and the end-Triassic mass extinctions (35). Although these three mass extinctions are considered to be the ecologically most severe events of the Phanerozoic (36), the persistence of the principal benthic marine life habits seems to be a general feature across mass extinctions. Furthermore, the Paleogene postextinction molluscan assemblages lack evidence for the acquisition of any novel key adaptation that would allow adopting a new lifestyle. Similarly, just one novel MOL emerged in the aftermath of the end-Permian event: erect, facultatively motile, attached suspension feeders appeared with the evolution of motile crinoids in the Early Triassic (34). Hence, mass extinctions do not seem to promote the production of new lifestyles.
Nonetheless, we demonstrate significant changes in the quantitative representation of molluscan lifestyles in the aftermath of the KPB. The observed changes cannot merely be explained by the extinction-driven vacation of ecospace. Furthermore, evidence is lacking for major shifts in the external context of the examined ecosystems that may have amplified these differences. Although not all environmental components can be retrieved from facies analysis, sedimentological data indicate that environmental conditions were similar before and after the KPB (SI Text S1 and SI Text S2). Various proxy data suggest that Late Maastrichtian and Danian climatic variations in the studied regions were similar (37–39). Temperature oscillations were possibly related to pulses of Deccan volcanism (40) but had only minor effects compared with those of the bolide impact (14, 41, 42), and at the planetary scale the earth's climate–ocean system remained in a greenhouse state.
Previous work had found interregional and environmental variation in the biogeographic and evolutionary dynamics during the recovery of mollusks from the end-Cretaceous extinction (26, 43). Similarly, our study demonstrates site-dependent ecological variation across the KPG for most MOLs, but simultaneously reveals high consistency in the direction of change when measured at broader ecological levels. It appears that consistent changes in mobility, trophic structure, and benthic tiering can be generated by multiple combinations of lower-level ecological properties that define MOLs, which individually might even exhibit opposite trends among sites. The abundance dynamics of the species of any MOL depend in part on that of interacting species from other MOLs in the same assemblage, thus giving rise to a complex dynamic at the MOL level but evidently relative conformity at higher hierarchical levels. Because these emergent ecological patterns are similar in shallow and deeper shelf environments and at various latitudes and distances from the Chicxulub impact site, our findings support the view of a global shift to different ecological dynamics after the extinction event.
Such shifts are reminiscent of regime shifts in modern, anthropogenically disturbed ecosystems. A catastrophic disruption may lead to large, persistent reconfigurations of ecosystem structure outside the range of fluctuations in the previous regime, and a return to previous external conditions is not necessarily matched by a return of the system to its previous state (44). Regime shifts in modern ecosystems usually involve change in the dominant internal feedbacks of a system. Apart from difficulties in detecting and understanding regime shifts even in present-day systems (44), the nonuniform spatial and temporal patterns of individual MOLs at the KPB argue against a concerted phase shift of molluscan assemblages from one well-defined, internally modulated system to another. Rather it seems that the global effects of the KPB bolide impact caused changes in varied local systems, and the forcing was sufficiently strong to override local factors that controlled the preextinction states.
A critical question is whether the ecological differences between pre- and postextinction assemblages reflect deterministic processes in community assembly or just stochasticity (45, 46). The observation that ecological shifts are significantly greater than expected from background variations suggests that they were dominated by ecological selection rather than random variations in the abundances of species and MOLs, i.e., ecological drift. In combination with the uniform direction of higher-level ecological shifts in mobility, life habit, and feeding mode across sites (Fig. 3) this is evidence that benthic marine ecosystems responded deterministically to some degree.
Two large-scale ecological shifts may reveal underlying processes: changes in feeding strategies and in energy budgets. The well-known increase of deposit feeders at the expense of suspension feeders is consistent with a KPB collapse of primary productivity as a major stress factor because suspension feeders—relying directly on an adequate supply of photosynthetically produced phytoplankton—should be more strongly affected by phytoplankton collapse during an impact winter than organisms in more buffered, detritus-based food webs (e.g., refs. 20, 47). It is striking that the crisis in primary algal production in neritic ecosystems probably lasted only a brief period—less than a century after the impact event (11, 48, 49)—whereas the studied postextinction assemblages represent much longer time spans, covering several hundred thousand to a few million years. This temporal mismatch suggests that if a short-lived productivity crash was the main driver of the shift in the trophic composition of molluscan assemblages, it had a long-term effect far beyond the time at which the ecosystems’ productivity had recovered.
The proportional increase in energy-intensive MOLs such as motile and predatory mollusks might be viewed as opposing the previous trend. The energetic needs of an assemblage, however, also depend on the mean body size and the absolute, not just relative, local abundance. In principle, an increase in the number of individuals with energy-intensive MOLs could have been overcompensated by reductions in mean body size or in population densities (50, 51) such that overall benthic energy budgets were actually lower than before the extinction event. Although a definitive quantitative energetic comparison is not feasible with the available data, postextinction assemblages exhibit clear signatures of an intensified top-down control on ecological structure. More numerous and intense interspecific interactions are apparent from an increased proportion of predatory gastropods at two of the studied sites (Fig. 3E) and elsewhere (21, 28, 29) and a global diversity increase of marine metazoan predators in the wake of the mass extinction (3). Antipredatory behavior is also evident as an escape strategy in motile organisms and as an avoidance strategy in those that burrowed into the sediment, whereas morphological adaptations showed little effects at the KPB (52).
Carnivores are relatively abundant and diverse in other, shallow subtidal Maastrichtian assemblages (21), and their Danian rise in mid-to-outer shelf assemblages might have involved the spread of a community type already established in shallower water (as discussed in ref. 53). However, other ecological attributes of Maastrichtian shallow-water assemblages—specifically mobility levels and the percentage of deposit feeders—were not elevated relative to those of coeval offshore assemblages (21), and their postextinction rise requires additional reasons. Onshore–offshore evolutionary expansion of innovation across the shelf (53) may have been an important factor in the observed postextinction restructuring but cannot fully explain it.
Even though we argue that deterministic ecological processes are involved in the ecological Cretaceous–Paleogene reorganizations—triggered by the short-term environmental forcing of a bolide impact—it would not have been possible to predict its exact nature and magnitude. Whereas the general increase in deposit feeding conforms to the impact winter scenario, the escalatory increases apparently do not. For the studied marine molluscan assemblages across the KPB, we conclude that major environmental perturbation deterministically caused irreversible long-term system change, but that important features of the new dynamic regimes, in which species interacted substantially different from before, are not yet fully explained. A combination of empirical, modeling, and theoretical approaches is required to better understand how evolution and ecology will interact and what form of ecoevolutionary dynamics will result.
Materials and Methods
The four analyzed sections across the KPB are located on a north–south trending transect ranging from paleolatitude 32°N in the Gulf of Mexico to paleolatitude 65°S in the Southern Ocean. Environmental and faunal data have been retrieved from the literature [Brazos River, Texas (17–19) and Seymour Island, Antarctica (54, 55)] and our own collections from Patagonia (Argentina) at Bajada del Jagüel in Neuquén (20, 56) and at San Ramón in Chubut (38). We selected these localities because they yield quantitative bed-by-bed sampling data of well-preserved molluscan faunas with both aragonitic and calcitic shells present; they represent similar depositional environments, i.e., soft, siliciclastic substrates of the marine shelf; and their chronostratigraphy, including the position of the KPB, is well constrained (SI Text S1 and Dataset S1). The amount of time spanned by faunal samples varies among sites and reaches up to about 2 million years (My) in the Maastrichtian and about 4 My in the Danian (SI Text S1). Differences in pre- and postextinction time spans did not affect the basic results (SI Text S1 and SI Text S2).
We assigned each taxon (species or genus) to a unique MOL within a slightly modified version of the ecospace model of Bambach et al. (16). MOL was inferred from analogy with living relatives, functional morphology, and previous publications (Dataset S1). Apart from counts of individuals as a measure of the ecological importance of each MOL, we also used counts of the presence of species of a particular MOL in individual samples (occurrences) and the total number of species within each MOL. These three metrics were mostly significantly correlated with each other (Table S2).
We designed a permutation test to decide whether the observed ecological shifts in the postextinction assemblages are outside the range of usual fluctuations among assemblages during Maastrichtian and Danian background times. Observed ecological changes of the real data were here compared with changes in a permuted dataset in which observed species occurrences were randomly assigned to pre- or postextinction levels. The total sample size of each level was determined by the sample sizes of the original dataset. Changes were measured by Bray-Curtis dissimilarity of pre- and postextinction faunas using 500 permutation trials. Using box plot statistics, we tested whether the observed Bray-Curtis dissimilarity is a significant outlier of the randomized distribution. This approach is similar to the permutational multivariate analysis as implemented in the anosim function in R’s “vegan” package (57) that we also applied (see legend for Fig. S3). Here individual samples are grouped by horizon and differences in group means are tested for significance. To test whether postextinction assemblages are a functionally depleted relic of preextinction assemblages we repeated all tests with preextinction assemblages from which species that disappeared in the latest Maastrichtian have been removed. To further characterize the between-sample variation in ecological structure before and after the KPG, we performed nonmetric multidimensional scaling on the Bray-Curtis distances among samples based on the abundances of MOLs. Finally, we contrasted the magnitude of Maastrichtian to Danian changes of MOLs with their spatial variability. For each MOL, we calculated the mean proportional difference between the four Maastrichtian assemblages (and the four Danian assemblages, respectively) and compared this value with the mean proportional Maastrichtian–Danian difference at the four sites. All analyses were performed in the R programming environment (www.r-project.org).
Supplementary Material
Acknowledgments
We thank Dave Lazarus for discussions and the editor and two journal reviewers for their constructive suggestions; Francisco Medina, Roberto Scasso, Henning Scholz, and Sven Weidemeyer for assistance in the field; and Elke Siebert for support in figure preparation. This study was funded by the Deutsche Forschungsgemeinschaft (AB 109/8-1, Ki 806/1-1).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 7111.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1422248112/-/DCSupplemental.
References
- 1.Barnosky AD, et al. Approaching a state shift in Earth’s biosphere. Nature. 2012;486(7401):52–58. doi: 10.1038/nature11018. [DOI] [PubMed] [Google Scholar]
- 2.Scheffer M, et al. Anticipating critical transitions. Science. 2012;338(6105):344–348. doi: 10.1126/science.1225244. [DOI] [PubMed] [Google Scholar]
- 3.Bambach RK, Knoll AH, Sepkoski JJ., Jr Anatomical and ecological constraints on Phanerozoic animal diversity in the marine realm. Proc Natl Acad Sci USA. 2002;99(10):6854–6859. doi: 10.1073/pnas.092150999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jablonski D. Mass extinctions and macroevolution. Paleobiology. 2005;31(Suppl 2):192–210. [Google Scholar]
- 5.Jablonski D. Extinctions in the fossil record. Philos Trans R Soc Lond B Biol Sci. 1994;344:11–16. [Google Scholar]
- 6.D’Hondt S, Donaghay P, Zachos JC, Luttenberg D, Lindinger M. Organic carbon fluxes and ecological recovery from the Cretaceous-Tertiary mass extinction. Science. 1998;282(5387):276–279. doi: 10.1126/science.282.5387.276. [DOI] [PubMed] [Google Scholar]
- 7.Schulte P, et al. The Chicxulub asteroid impact and mass extinction at the Cretaceous-Paleogene boundary. Science. 2010;327(5970):1214–1218. doi: 10.1126/science.1177265. [DOI] [PubMed] [Google Scholar]
- 8.Renne PR, et al. Time scales of critical events around the Cretaceous-Paleogene boundary. Science. 2013;339(6120):684–687. doi: 10.1126/science.1230492. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez LW, Alvarez W, Asaro F, Michel HV. Extraterrestrial cause for the Cretaceous-Tertiary extinction. Science. 1980;208(4448):1095–1108. doi: 10.1126/science.208.4448.1095. [DOI] [PubMed] [Google Scholar]
- 10.Arthur MA, Zachos JC, Jones DS. Primary productivity and the Cretaceous/Tertiary boundary event in the oceans. Cretac Res. 1987;8:43–54. [Google Scholar]
- 11.Pope KO, Baines KH, Ocampo AC, Ivanov BA. Energy, volatile production, and climatic effects of the Chicxulub Cretaceous/Tertiary impact. J Geophys Res. 1997;102(E9):21645–21664. doi: 10.1029/97je01743. [DOI] [PubMed] [Google Scholar]
- 12.Jiang S, Bralower TJ, Patzkowsky ME, Kump LR, Schueth JD. Geographic controls on nannoplankton extinction across the Cretaceous/Palaeogene boundary. Nat Geosci. 2010;3:280–285. [Google Scholar]
- 13.Alegret L, Thomas E, Lohmann KC. End-Cretaceous marine mass extinction not caused by productivity collapse. Proc Natl Acad Sci USA. 2012;109(3):728–732. doi: 10.1073/pnas.1110601109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vellekoop J, et al. Rapid short-term cooling following the Chicxulub impact at the Cretaceous–Paleogene boundary. Proc Natl Acad Sci USA. 2014;111(21):7537–7541. doi: 10.1073/pnas.1319253111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheffer M, Carpenter S, Foley JA, Folke C, Walker B. Catastrophic shifts in ecosystems. Nature. 2001;413(6856):591–596. doi: 10.1038/35098000. [DOI] [PubMed] [Google Scholar]
- 16.Bambach RK, Bush AM, Erwin DH. Autecology and the filling of ecospace: Key metazoan radiations. Palaeontology. 2007;50:1–22. [Google Scholar]
- 17.Hansen T, Farrand RB, Montgomery HA, Billman HG, Blechschmidt G. Sedimentology and extinction patterns across the Cretaceous-Tertiary boundary interval in east Texas. Cretac Res. 1987;8:229–252. [Google Scholar]
- 18.Hansen TA, Farrell BR, Upshaw B. The first 2 million years after the Cretaceous-Tertiary boundary in east Texas: Rate and paleoecology of the molluscan recovery. Paleobiology. 1993;19:251–265. [Google Scholar]
- 19.Hansen TA, Upshaw B, Kauffman EG, Gose W. Patterns of molluscan extinction and recovery across the Cretaceous–Tertiary boundary in east Texas; reports on new outcrops. Cretac Res. 1993;14:685–706. [Google Scholar]
- 20.Aberhan M, Weidemeyer S, Kiessling W, Scasso RA, Medina FA. Faunal evidence for reduced productivity and uncoordinated recovery in Southern Hemisphere Cretaceous-Paleogene boundary sections. Geology. 2007;35:227–230. [Google Scholar]
- 21.Sessa JA, Bralower TJ, Patzkowsky ME, Handley JC, Ivany LC. Environmental and biological controls on the diversity and ecology of Late Cretaceous through early Paleogene marine ecosystems in the U.S. Gulf Coastal Plain. Paleobiology. 2012;38:218–239. [Google Scholar]
- 22.Bryan JR, Jones DS. Fabric of the Cretaceous–Tertiary marine macrofaunal transition at Braggs, Alabama. Palaeogeogr Palaeoclimatol Palaeoecol. 1989;69:279–301. [Google Scholar]
- 23.Gallagher WB. Selective extinction and survival across the Cretaceous-Tertiary boundary in the northern Atlantic Coastal Plain. Geology. 1991;19:967–970. [Google Scholar]
- 24.Gallagher WB. Faunal changes across the Cretaceous-Tertiary (K-T) boundary in the Atlantic coastal plain of New Jersey: Restructuring the marine community after the K-T mass-extinction event. Spec Pap Geol Soc Am. 2002;356:291–301. [Google Scholar]
- 25.Heinberg C. Lower Danian bivalves, Stevns Klint, Denmark: Continuity across the K/T boundary. Palaeogeogr Palaeoclimatol Palaeoecol. 1999;154:87–106. [Google Scholar]
- 26.Jablonski D. Geographic variation in the molluscan recovery from the end-Cretaceous extinction. Science. 1998;279(5355):1327–1330. doi: 10.1126/science.279.5355.1327. [DOI] [PubMed] [Google Scholar]
- 27.Stilwell JD. Patterns of biodiversity and faunal rebound following the K–T boundary extinction event in Austral Palaeocene molluscan faunas. Palaeogeogr Palaeoclimatol Palaeoecol. 2003;195:319–356. [Google Scholar]
- 28.Crame JA, et al. The early origin of the Antarctic marine fauna and its evolutionary implications. PLoS ONE. 2014;9(12):e114743. doi: 10.1371/journal.pone.0114743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelley PH, Hansen TA. Recovery of the naticid gastropod predator-prey system from the Cretaceous-Tertiary and the Eocene-Oligocene extinction. Geol Soc Lond Spec Publ. 1996;102:373–386. [Google Scholar]
- 30.Kelley PH, Hansen TA. Comparisons of class- and lower taxon-level patterns in naticid gastropod predation, Cretaceous to Pleistocene of the U.S. Coastal Plain. Palaeogeogr Palaeoclimatol Palaeoecol. 2006;236:302–320. [Google Scholar]
- 31.Lockwood R. The K/T event and infaunality: Morphological and ecological patterns of extinction and recovery in veneroid bivalves. Paleobiology. 2004;30:507–521. [Google Scholar]
- 32.Kosnik MA. Changes in Late Cretaceous-early Tertiary benthic marine assemblages: Analyses from the North American coastal plain shallow shelf. Paleobiology. 2005;31:459–479. [Google Scholar]
- 33.Waller TR. Evolutionary relationships among commercial scallops (Mollusca: Bivalvia: Pectinidae) Dev Aquacult Fish Sci. 1991;21:1–73. [Google Scholar]
- 34.Foster WJ, Twitchett RJ. Functional diversity of marine ecosystems after the Late Permian mass extinction event. Nat Geosci. 2014;7:233–238. [Google Scholar]
- 35.Kiessling W, Aberhan M, Brenneis B, Wagner PJ. Extinction trajectories of benthic organisms across the Triassic–Jurassic boundary. Palaeogeogr Palaeoclimatol Palaeoecol. 2007;244:201–222. [Google Scholar]
- 36.McGhee GR, Clapham ME, Sheehan PM, Bottjer DJ, Droser ML. A new ecological-severity ranking of major Phanerozoic biodiversity crises. Palaeogeogr Palaeoclimatol Palaeoecol. 2013;370:260–270. [Google Scholar]
- 37.Schulte P, Speijer R, Mai H, Kontny A. The Cretaceous–Paleogene (K–P) boundary at Brazos, Texas: Sequence stratigraphy, depositional events and the Chicxulub impact. Sediment Geol. 2006;184:77–109. [Google Scholar]
- 38.Scasso RA, et al. Integrated bio- and lithofacies analysis of coarse-grained, tide-dominated deltaic environments across the Cretaceous/Paleogene boundary in Patagonia, Argentina. Cretac Res. 2012;36:37–57. [Google Scholar]
- 39.Kemp DB, et al. A cool temperate climate on the Antarctic Peninsula through the latest Cretaceous to early Paleogene. Geology. 2014;42:583–586. [Google Scholar]
- 40.Tobin TS, et al. Extinction patterns, δ18 O trends, and magnetostratigraphy from a southern high-latitude Cretaceous–Paleogene section: Links with Deccan volcanism. Palaeogeogr Palaeoclimatol Palaeoecol. 2012;350–352:180–188. [Google Scholar]
- 41.Wilf P, Johnson KR, Huber BT. Correlated terrestrial and marine evidence for global climate changes before mass extinction at the Cretaceous-Paleogene boundary. Proc Natl Acad Sci USA. 2003;100(2):599–604. doi: 10.1073/pnas.0234701100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Witts JD, et al. Evolution and extinction of Maastrichtian (Late Cretaceous) cephalopods from the López de Bertodano Formation, Seymour Island, Antarctica. Palaeogeogr Palaeoclimatol Palaeoecol. 2015;418:193–212. [Google Scholar]
- 43.Aberhan M, Kiessling W. Rebuilding biodiversity of Patagonian marine molluscs after the end-Cretaceous mass extinction. PLoS ONE. 2014;9(7):e102629. doi: 10.1371/journal.pone.0102629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biggs R, et al. Regime shifts. In: Hastings A, Gross L, editors. Encyclopedia of Theoretical Ecology. University of California Press; Berkeley: 2012. pp. 609–617. [Google Scholar]
- 45.Jackson JBC, Erwin DH. What can we learn about ecology and evolution from the fossil record? Trends Ecol Evol. 2006;21(6):322–328. doi: 10.1016/j.tree.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 46.Fisher CK, Mehta P. The transition between the niche and neutral regimes in ecology. Proc Natl Acad Sci USA. 2014;111(36):13111–13116. doi: 10.1073/pnas.1405637111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sheehan PM, Hansen TA. Detritus feeding as a buffer to extinction at the end of the Cretaceous. Geology. 1986;14:868–870. [Google Scholar]
- 48.Sepúlveda J, Wendler JE, Summons RE, Hinrichs KU. Rapid resurgence of marine productivity after the Cretaceous-Paleogene mass extinction. Science. 2009;326(5949):129–132. doi: 10.1126/science.1176233. [DOI] [PubMed] [Google Scholar]
- 49.Robertson DS, Lewis WM, Sheehan PM, Toon OB. K-Pg extinction patterns in marine and freshwater environments: The impact winter model. J Geophys Res Biogeosci. 2013;118:1006–1014. [Google Scholar]
- 50.Twitchett RJ. Incompleteness of the Permian–Triassic fossil record: A consequence of productivity decline? Geol J. 2001;36:341–353. [Google Scholar]
- 51.Finnegan S, McClain CR, Kosnik MA, Payne JL. Escargots through time: An energetic comparison of marine gastropod assemblages before and after the Mesozoic Marine Revolution. Paleobiology. 2011;37:252–269. [Google Scholar]
- 52.Reinhold ME, Kelley PH. The influence of antipredatory morphology on survivorship of the Owl Creek Formation molluscan fauna through the end-Cretaceous extinction. Palaeogeogr Palaeoclimatol Palaeoecol. 2005;217:143–153. [Google Scholar]
- 53.Jablonski D, Sepkoski JJ, Jr, Bottjer DJ, Sheehan PM. Onshore-offshore patterns in the evolution of Phanerozoic shelf communities. Science. 1983;222(4628):1123–1125. doi: 10.1126/science.222.4628.1123. [DOI] [PubMed] [Google Scholar]
- 54.Zinsmeister WJ, Macellari CE. Bivalvia (Mollusca) from Seymour Island, Antarctic Peninsula. Geol Soc Am. 1988;169:253–284. [Google Scholar]
- 55.Stilwell JD, Zinsmeister WJ, Oleinik AE. Early Paleocene mollusks of Antarctica: Systematics, paleoecology and paleobiogeographic significance. Bull Am Paleontol. 2004;367:1–89. [Google Scholar]
- 56.Scasso RA, et al. A tsunami deposit at the Cretaceous-Paleogene boundary in the Neuquén Basin of Argentina. Cretac Res. 2005;26:283–297. [Google Scholar]
- 57.Oksanen J, et al. 2015. Vegan: Community Ecology Package, R Package version 2.0.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.