Significance
In proliferative diabetic retinopathy (PDR), the most vision-threatening sequela of diabetic eye disease, retinal ischemia leads to increased expression of angiogenic factors that promote neovascularization. Although therapies targeting the potent angiogenic mediator vascular endothelial growth factor have been remarkably successful for the treatment of diabetic macular edema, this approach has not proven sufficient to prevent the development of retinal neovascularization, implicating additional angiogenic factor(s) in PDR pathogenesis. We demonstrate here that angiopoietin-like 4 is a potent angiogenic mediator with markedly increased expression in the eyes of PDR patients. Our studies identify a novel therapeutic target for the treatment of ocular neovascular disease and may have broad implications for the treatment of other diseases dependent on pathologic angiogenesis.
Keywords: diabetes, neovascularization, hypoxia inducible factor-1, angiopoietin-like 4, vascular endothelial growth factor
Abstract
Diabetic eye disease is the most common cause of severe vision loss in the working-age population in the developed world, and proliferative diabetic retinopathy (PDR) is its most vision-threatening sequela. In PDR, retinal ischemia leads to the up-regulation of angiogenic factors that promote neovascularization. Therapies targeting vascular endothelial growth factor (VEGF) delay the development of neovascularization in some, but not all, diabetic patients, implicating additional factor(s) in PDR pathogenesis. Here we demonstrate that the angiogenic potential of aqueous fluid from PDR patients is independent of VEGF concentration, providing an opportunity to evaluate the contribution of other angiogenic factor(s) to PDR development. We identify angiopoietin-like 4 (ANGPTL4) as a potent angiogenic factor whose expression is up-regulated in hypoxic retinal Müller cells in vitro and the ischemic retina in vivo. Expression of ANGPTL4 was increased in the aqueous and vitreous of PDR patients, independent of VEGF levels, correlated with the presence of diabetic eye disease, and localized to areas of retinal neovascularization. Inhibition of ANGPTL4 expression reduced the angiogenic potential of hypoxic Müller cells; this effect was additive with inhibition of VEGF expression. An ANGPTL4 neutralizing antibody inhibited the angiogenic effect of aqueous fluid from PDR patients, including samples from patients with low VEGF levels or receiving anti-VEGF therapy. Collectively, our results suggest that targeting both ANGPTL4 and VEGF may be necessary for effective treatment or prevention of PDR and provide the foundation for studies evaluating aqueous ANGPTL4 as a biomarker to help guide individualized therapy for diabetic eye disease.
Diabetic eye disease is the most common microvascular complication in the diabetic population and remains the leading cause of blindness among working-age adults in the developed world (1). Diabetic retinopathy (DR) is classified as either nonproliferative (NPDR) or proliferative (PDR). Although sustained hyperglycemia is the major stimulus for the development of NPDR (2), retinal ischemia is the prerequisite for PDR and results in the up-regulation of angiogenic factors that promote retinal neovascularization (3). If left untreated, neovascularization can lead to vitreous hemorrhage, retinal detachment, or glaucoma and result in profound and often irreversible loss of vision. For the last 4 decades, the standard of care for PDR has been panretinal photocoagulation (PRP), a process in which the peripheral ischemic retina is killed (burned) with a laser to protect the patient’s central vision (4). Although effective in reducing the risk of central vision loss, PRP results in decreased peripheral and night vision in treated patients. Moreover, PDR can progress in patients despite appropriate treatment. This emphasizes the importance of understanding the mechanism(s) promoting the development of retinal neovascularization to identify targeted therapeutic approaches for the prevention or treatment of PDR.
In this regard, hypoxia-inducible factors (HIFs) activate the transcription of multiple genes encoding angiogenic cytokines that promote retinal neovascularization in PDR (5). Among the angiogenic genes regulated by HIFs in PDR, considerable attention has focused on the contribution of vascular endothelial growth factor (VEGF). Several multicenter randomized controlled clinical trials have demonstrated the benefit of monthly injections with biologic molecules directed against VEGF to treat diabetic macular edema (DME) (6). These studies have demonstrated a significant reduction in the progression to PDR in some—but not all—patients receiving anti-VEGF therapy (7, 8), suggesting that other mediator(s) may participate in the development of PDR in many diabetic patients. Over the last 2 decades, several angiogenic cytokines, growth factors, and inflammatory mediators have been implicated in the pathogenesis of PDR (9). Despite these efforts, PRP treatment for PDR remains unchanged. In an effort to identify an alternative therapeutic approach for the treatment of retinal neovascularization, we set out to identify an ischemia-driven mediator that directly contributes to the angiogenic phenotype in patients with PDR.
Results
Angiogenic Potential of Aqueous Fluid from PDR Patients Is Elevated but Independent of VEGF Levels.
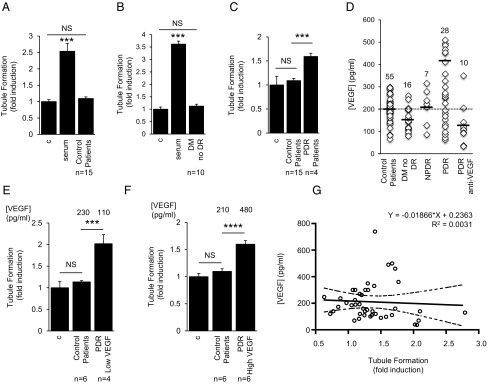
The levels of angiogenic growth factors in the aqueous fluid of diabetic patients correlate with the severity of DR (10). To study the angiogenic potential of aqueous fluid from diabetic patients with and without PDR, we assessed the ability of aqueous fluid to stimulate endothelial cell tubule formation. Aqueous fluid from control patients (Fig. 1A, Table 1, and SI Appendix, Fig. S1A) or diabetic patients without DR (Fig. 1B and SI Appendix, Fig. S1B) did not have a significant effect on tubule formation. In contrast, aqueous fluid from diabetic patients with PDR stimulated tubule formation (Fig. 1C and SI Appendix, Fig. S1C). This effect was correlated with an approximately twofold increase in the concentration of VEGF in aqueous fluid from PDR patients compared with control patients or diabetic patients without DR (SI Appendix, Fig. S2 and Table S1). These results are consistent with the hypothesis that the increased induction of tubule formation by aqueous fluid from PDR patients was due to elevated levels of VEGF. However, VEGF levels in the aqueous fluid from PDR patients demonstrated considerable variability (Fig. 1D and SI Appendix, Fig. S3A); 10/28 (36%) of patients with PDR had VEGF levels in the aqueous fluid that were less than the mean value measured for control (nondiabetic) patients. These VEGF levels were similar to those detected in aqueous fluid from PDR patients who had received anti-VEGF therapy within 2 wk of sample collection (Fig. 1D). Nonetheless, stimulation of tubule formation by these low-VEGF PDR aqueous fluid samples (Fig. 1E and SI Appendix, Fig. S3B) was similar to that by high-VEGF PDR aqueous fluid samples (Fig. 1F and SI Appendix, Fig. S3C). Indeed, there was no correlation between the VEGF concentration and stimulation of tubule formation by aqueous fluid samples (Fig. 1G and SI Appendix, Fig. S3D).
Fig. 1.
Angiogenic potential of aqueous fluid from PDR patients is elevated but independent of VEGF levels. (A and B) Aqueous fluid from nondiabetic patients (control patients; A) and diabetic patients without DR (DM no DR; B) does not stimulate tubule formation. (C) Aqueous fluid from diabetic patients with PDR (PDR Patients) stimulates tubule formation. Media without serum (control) or with 10% (vol/vol) FBS (serum) serve as controls. (D) Levels of VEGF in the aqueous fluid from diabetic and nondiabetic patients. Aqueous fluid levels from 10/28 PDR patients measure below the average levels observed for nondiabetic patients (dashed line). Note: PDR samples with [VEGF] > 600 pg/mL are not displayed to adequately demonstrate the variability within the control samples. (E and F) Aqueous fluid from PDR patients with VEGF levels below the average level for control (nondiabetic) patients (PDR low VEGF; E) stimulate tubule formation similar to aqueous fluid from PDR patients with VEGF levels higher than the average level for control patients (PDR high VEGF; F). (G) Aqueous fluid stimulation of tubule formation does not correlate with the concentration of VEGF. One-way ANOVA. Student’s t test and Pearson correlation.
Table 1.
Patient samples for tubule formation assays
| Patient | Age, y | Sex | Phakic status* | Prior vitrectomy | DM type | DM duration, y | Prior PRP | Anti-VEGF within 2–6 wk | Anti-VEGF within 2 wk |
| Control | |||||||||
| 1 | 70 | F | P | No | – | – | – | – | – |
| 2 | 62 | F | P | No | – | – | – | – | – |
| 3 | 83 | M | P | No | – | – | – | – | – |
| 4 | 70 | F | P | No | – | – | – | – | – |
| 5 | 75 | M | P | No | – | – | – | – | – |
| 6 | 71 | F | P | No | – | – | – | – | – |
| 7 | 64 | F | P | No | – | – | – | – | – |
| 8 | 49 | F | P | No | – | – | – | – | – |
| 9 | 50 | M | P | No | – | – | – | – | – |
| 10 | 55 | F | P | No | – | – | – | – | – |
| 11 | 65 | F | P | No | – | – | – | – | – |
| 12 | 73 | F | P | No | – | – | – | – | – |
| 13 | 55 | F | P | No | – | – | – | – | – |
| 14 | 62 | F | P | No | – | – | – | – | – |
| 15 | 59 | M | P | No | – | – | – | – | – |
| Diabetic (no DR) | |||||||||
| 1 | 83 | F | P | No | II | 9 | – | – | – |
| 2 | 70 | M | P | No | I | 27 | – | – | – |
| 3 | 68 | F | P | No | II | 12 | – | – | – |
| 4 | 76 | F | PP | No | II | 5 | – | – | – |
| 5 | 65 | M | P | No | II | 41 | – | – | – |
| 6 | 73 | F | P | No | II | 4 | – | – | – |
| 7 | 91 | M | P | No | II | 30 | – | – | – |
| 8 | 63 | F | P | No | I | 36 | – | – | – |
| 9 | 68 | F | P | No | II | 11 | – | – | – |
| 10 | 63 | F | P | No | II | 9 | – | – | – |
| PDR | |||||||||
| 1 | 31 | F | P | No | II | 19 | Yes | No | No |
| 2 | 57 | F | P | No | II | 25 | No | No | No |
| 3 | 46 | M | P | No | II | 20 | Yes | No | No |
| 4 | 58 | M | P | No | II | 20 | No | No | No |
| High-VEGF PDR | |||||||||
| 1 | 31 | M | P | No | I | 25 | No | No | No |
| 2 | 37 | F | P | Yes | I | 27 | Yes | No | No |
| 3 | 58 | M | P | No | II | 20 | No | No | No |
| 4 | 57 | F | P | No | II | 25 | No | No | No |
| 5 | 50 | M | P | No | II | 4 | No | No | No |
| 6 | 55 | M | P | No | II | 2 | Yes | No | No |
| Low-VEGF PDR | |||||||||
| 1 | 55 | M | P | Yes | II | 2 | Yes | No | No |
| 2 | 58 | M | P | Yes | II | 29 | Yes | No | No |
| 3 | 52 | F | PP | No | I | 26 | Yes | No | No |
| 4 | 43 | M | P | No | I | Unknown | Yes | No | No |
| Anti-VEGF PDR | |||||||||
| 1 | 50 | M | P | No | II | 4 | No | No | Yes |
| 2 | 35 | M | P | No | I | Unknown | Yes | No | Yes |
| 3 | 33 | F | P | No | I | 23 | No | No | Yes |
| 4 | 33 | F | P | No | I | 23 | Yes | No | Yes |
| 5 | 42 | M | P | No | II | 12 | Yes | No | Yes |
| 6 | 68 | M | P | No | II | 15 | No | No | Yes |
| 7 | 58 | F | P | No | II | Unknown | Yes | No | Yes |
| 8 | 57 | M | P | No | II | Unknown | Yes | No | Yes |
| 9 | 45 | M | P | No | II | Unknown | Yes | No | Yes |
At time of sample collection. DM, diabetes mellitus; DR, diabetic retinopathy; F, female; M, male; P, phakic; PDR, proliferative diabetic retinopathy; PP, pseudophakic; PRP, panretinal photocoagulation; VEGF, vascular endothelial growth factor.
Angiogenic Potential of Aqueous Fluid from PDR Patients Is Not Affected by Anti-VEGF Therapy.
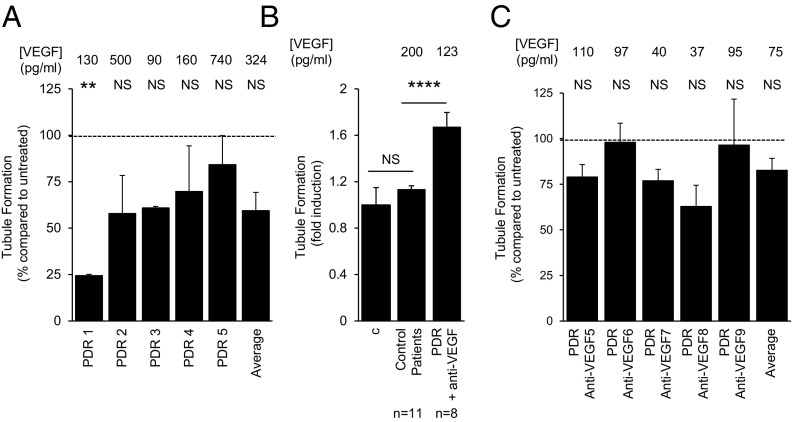
Despite the variability in VEGF concentrations in PDR aqueous fluid samples, the presence of VEGF in these samples could still be responsible for the induction of tubule formation. To assess whether the angiogenic potential of aqueous fluid from PDR patients was influenced by anti-VEGF therapy, we tested the ability of aqueous fluid from PDR patients (with high, average, or low levels of VEGF) to stimulate tubule formation in the presence of bevacizumab at a 10-fold higher concentration than the dose which effectively neutralizes the highest levels of VEGF detected in the aqueous fluid of PDR patients (SI Appendix, Fig. S4 and Table 1). Treatment with bevacizumab did not significantly affect tubule formation stimulation by aqueous fluid from PDR patients (Fig. 2A and SI Appendix, Fig. S5A). Moreover, aqueous fluid from patients who received anti-VEGF therapy by intravitreal injection within 2 wk of sample collection had VEGF levels lower than those measured in aqueous fluid from control patients and diabetics without DR (SI Appendix, Fig. S2), yet these samples still stimulated tubule formation (Fig. 2B and SI Appendix, Fig. S5B) and were also unaffected by treatment with additional bevacizumab (Fig. 2C and SI Appendix, Fig. S5C).
Fig. 2.
Angiogenic potential of aqueous fluid from PDR patients is unaffected by anti-VEGF therapy. (A) Addition of the VEGF neutralizing monoclonal antibody, bevacizumab, to aqueous fluid in the tubule formation assay does not significantly affect the ability of aqueous fluid from PDR patients to stimulate tubule formation, regardless of [VEGF]. Data presented as percent induction of tubule formation compared with tubule formation induced by aqueous fluid in the absence of bevacizumab. (B) Aqueous fluid from PDR patients who received an intravitreal injection with anti-VEGF therapy within 2 wk of sample collection (PDR + anti-VEGF) is still able to stimulate tubule formation. (C) Addition of bevacizumab to aqueous fluid in the tubule formation assay does not significantly affect the ability of aqueous fluid from PDR anti-VEGF patients to stimulate tubule formation. Data presented as percent induction of tubule formation compared with tubule formation induced by aqueous fluid from PDR anti-VEGF patients in the absence of bevacizumab. Wilcoxon test.
HIF Promotes the Secretion of VEGF and Other Angiogenic Factors in Hypoxic Retinal Cells.
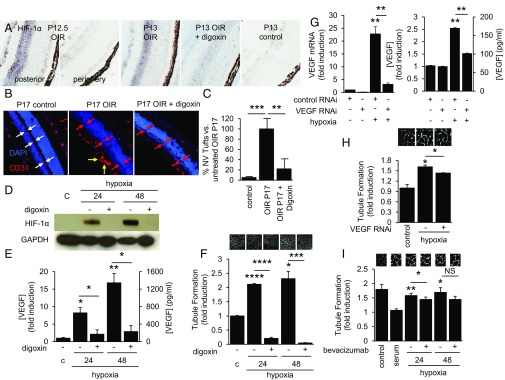
These results suggested that VEGF is not the only angiogenic mediator responsible for stimulation of tubule formation by aqueous fluid from PDR patients, providing a unique opportunity to examine the contribution of other angiogenic mediator(s) to the development of PDR. To interrogate the role of novel angiogenic factors in mediating retinal neovascularization in PDR, we used the oxygen-induced retinopathy (OIR) model of ischemic retinal disease (11). During the ischemic stage of the OIR model, ischemia in the posterior retina promotes stabilization of HIF-1α (Fig. 3A), which results in the promotion of retinal neovascularization (Fig. 3B). Inhibition of HIF-1α accumulation by treatment with digoxin (Fig. 3A) prevents the development of retinal neovascularization in the OIR model (Fig. 3 B and C) (12). We have previously reported that hypoxic retinal Müller cells in the ischemic inner retina play a critical role in the expression of VEGF and the promotion of vascular permeability and angiogenesis by stabilizing HIF-1α (13, 14). Stabilization of HIF-1α in hypoxic Müller cells resulted in the up-regulation of VEGF expression (Fig. 3 D and E). In turn, conditioned media from these cells were potently angiogenic, a property that was completely blocked following pretreatment of the Müller cells with digoxin (300 nM) to inhibit HIF-1α accumulation (Fig. 3F), demonstrating the importance of this transcription factor in the regulation of angiogenic genes in retinal Müller cells. These results suggested that HIF-1α was necessary to promote the angiogenic potential of retinal Müller cells. By contrast, RNA interference (RNAi) targeting VEGF (Fig. 3 G and H) or blocking antibody to VEGF (Fig. 3I) only partially inhibited the angiogenic potential of conditioned media from retinal Müller cells.
Fig. 3.
HIF promotes the secretion of VEGF and other angiogenic factors in hypoxic retinal cells. (A) Immunohistochemical analysis demonstrates accumulation of HIF-1α in the retina 12 h following return of postnatal day (P)12 OIR pups from hyperoxic [75% (vol/vol) O2] to normoxic [20% (vol/vol) O2] conditions. Daily i.p. injection of the HIF-inhibitor digoxin inhibits HIF-1α protein accumulation in OIR mice. (B and C) Immunofluorescent analysis demonstrates the presence (white double arrows) or absence (red double arrows) of inner retinal vessels in the P17 control vs. OIR mice, respectively (B). Preretinal (pathological) neovascularization (yellow arrows) is observed in P17 OIR mice but not in digoxin-treated mice, despite the absence of inner retinal vessels (red double arrows). Inhibition of HIF-1α accumulation with daily i.p. injections of digoxin prevents the development of retinal neovascularization (C). (D and E) Exposure of human retinal Müller cells to 1% O2 (hypoxia) promotes HIF-1α protein accumulation (D) and VEGF secretion (E), which are both blocked by treatment with digoxin. (F) Stimulation of tubule formation by conditioned media from retinal Müller cells exposed to hypoxia is blocked with digoxin. (G and H) RNAi targeting VEGF inhibits mRNA expression and protein secretion of VEGF (G) but only partially inhibits the stimulation of tubule formation by conditioned media from Müller cells exposed to hypoxia (H). Similar results were obtained using a VEGF neutralizing antibody (bevacizumab; I). One-way ANOVA.
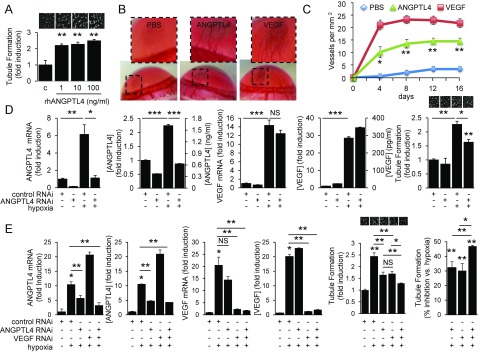
HIF-Dependent ANGPTL4 Expression Is Induced by Hypoxia in Vitro and Retinal Ischemia in Vivo.
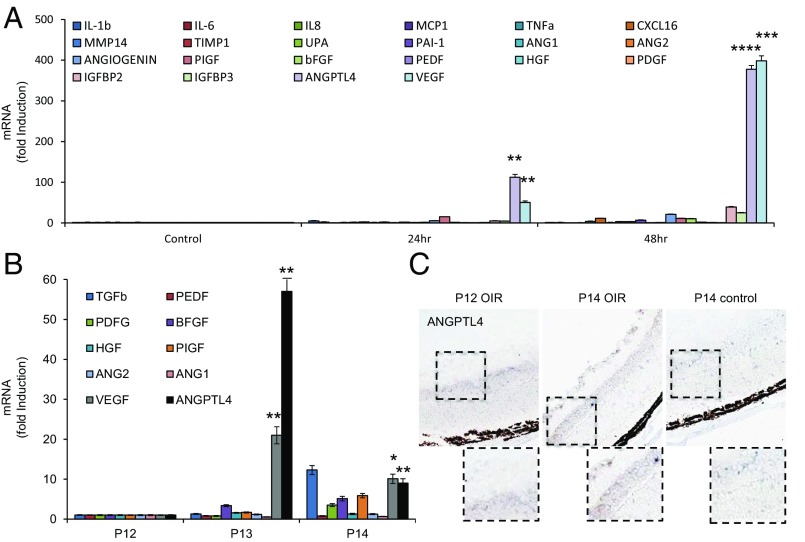
We set out to identify another candidate angiogenic mediator that may play a role in the promotion of angiogenesis in the aqueous fluid of PDR patients with low VEGF levels. To this end, we exposed retinal Müller cells to hypoxia and examined the expression of mRNAs encoding 22 inflammatory cytokines, proteases, and angiogenic cytokines previously reported to be regulated (directly or indirectly) by HIF-1 and proposed to promote angiogenesis. IL-1β, angiogenin, PIGF, IGFBP2, and IGFBP3 mRNA levels were increased within 24 h of exposure to hypoxia, whereas TNFα, PAI-1, and bFGF were induced after 48 h of exposure to hypoxia (Fig. 4A and SI Appendix, Fig. S6). However, the fold induction of these mRNAs was modest compared with VEGF mRNA. Only the induction of angiopoietin-like 4 (ANGPTL4) mRNA was comparable to that of VEGF mRNA (Fig. 4A). HIF-dependent expression of ANGPTL4 has been shown to mediate the ability of conditioned media from Kaposi’s sarcoma and hypoxic breast cancer cells to impair EC–EC interaction (15, 16). We have recently reported that ANGPTL4 may play an important role in vascular permeability in ischemic retinal disease, including DME (13). ANGPTL4 has also been implicated in the up-regulation of retinal neovascularization by peroxisome proliferator-activated receptor (PPAR-β/δ) (17). However, a role for ANGPTL4 as an angiogenic factor in patients with PDR has not been explored. We first investigated whether ANGPTL4 mRNA expression was also increased in vivo in the OIR model and found that the fold induction of ANGPTL4 mRNA was similar or greater than that observed for VEGF mRNA (Fig. 4B). By contrast, mRNA levels of several other known HIF-regulated angiogenic factors were only modestly increased. ANGPTL4 protein expression in the OIR model was confirmed by immunohistochemistry (Fig. 4C).
Fig. 4.
Hypoxia potently promotes up-regulation of ANGPTL4 mRNA and protein expression at levels similar to VEGF. (A and B) The mRNA expression of 22 known inflammatory cytokines, proteases, and angiogenic cytokines regulated (directly or indirectly) by HIF-1α and previously reported to play a role in angiogenesis in hypoxic human retinal Müller cells (A) and in the OIR model (B). Fold induction of ANGPTL4 mRNA expression is comparable to the fold induction for VEGF mRNA. All mRNA levels were normalized to β-actin (for cell culture) or cyclophilin A (for OIR) mRNA and reported as fold induction compared with cells exposed to 20% (vol/vol) O2 (control). (C) Representative images from immunohistochemical analysis of ANGPTL4 expression in the ischemic inner retina at P12 and P14 in the OIR model compared with P14 control mice. Student’s t test.
ANGPTL4 Is Necessary and Sufficient to Promote Angiogenesis.
We next investigated whether ANGPTL4 contributes to the angiogenic phenotype in PDR. Recombinant human (rh)ANGPTL4 promoted EC survival and migration (SI Appendix, Fig. S7 A and B) but did not affect EC proliferation (SI Appendix, Fig. S7C). Increasing doses of rhANGPTL4 potently stimulated tubule formation in vitro (Fig. 5A) and corneal neovascularization in vivo (Fig. 5 B and C). To directly assess whether ANGPTL4 contributed to the angiogenic potential of hypoxic retinal Müller cells, we transfected Müller cells with RNAi targeting ANGPTL4 and observed a marked reduction in ANGPTL4—but not VEGF—mRNA and protein expression (Fig. 5D). Conditioned media from these cells had reduced angiogenic potential compared with controls (Fig. 5D). Inhibition of either ANGPTL4 or VEGF expression alone resulted in an ∼30% reduction in the stimulation of tubule formation by hypoxic retinal Müller cells (Fig. 5E). However, inhibiting both ANGPTL4 and VEGF with RNAi resulted in an almost 50% reduction in the stimulation of tubule formation by hypoxic retinal Müller cells (Fig. 5E).
Fig. 5.
ANGPTL4 is up-regulated by hypoxia and HIF in vitro and retinal ischemia in vivo and is necessary and sufficient to promote angiogenesis. (A) rhANGPTL4 potently stimulates tubule formation in vitro in a dose-dependent manner. (B and C) Representative photographs 1 wk following bead implantation (B) and quantitation of vessels per mm2 (C) demonstrating that ANGPTL4 potently stimulates corneal neovascularization in vivo, similar to VEGF. (D) RNAi targeting ANGPTL4 causes a marked reduction in ANGPTL4—but not VEGF—mRNA and protein expression in human retinal Müller cells. This, in turn, results in reduced angiogenic potential of aqueous fluid from Müller cells pretreated with RNAi targeting ANGPTL4 compared with scrambled controls. (E) Inhibition of ANGPTL4 or VEGF mRNA and protein expression results in an ∼30% reduction in the stimulation of tubule formation by hypoxic retinal Müller cells. However, combined inhibition of both ANGPTL4 and VEGF mRNA and protein expression using RNAi in hypoxic retinal Müller cells results in an almost 50% reduction in the stimulation of tubule formation. One-way ANOVA.
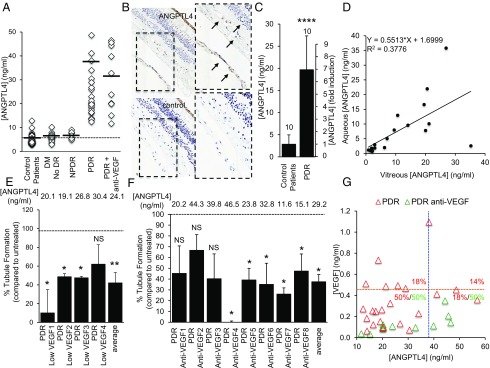
ANGPTL4 Is an Angiogenic Factor Expressed in the Eyes of Diabetic Patients with PDR.
We hypothesized that ANGPTL4 may be an angiogenic factor present in low-VEGF aqueous fluid from PDR patients. To test this hypothesis, we measured levels of ANGPTL4 in aqueous fluid from diabetic patients and observed that ANGPTL4 levels were increased in the aqueous fluid of PDR patients compared with the aqueous fluid of control patients, diabetic patients without DR, and diabetic patients with NPDR (Fig. 6A and SI Appendix, Table S2). Levels of ANGPTL4 were increased even in the aqueous fluid of patients who had been treated with anti-VEGF therapy within 2 wk of sample collection and in which VEGF levels were markedly reduced (SI Appendix, Fig. S8), suggesting that ANGPTL4 expression was independent of VEGF expression. Immunohistochemical analysis of eyes from 5/5 PDR patients demonstrated ANGPTL4 expression in areas of preretinal neovascularization (Fig. 6B). We also observed elevated ANGPTL4 levels in the vitreous of PDR patients compared with control patients (Fig. 6C and SI Appendix, Table S3). Interestingly, we observed a close correlation between the aqueous and vitreous levels of ANGPTL4 in PDR patients (Fig. 6D).
Fig. 6.
ANGPTL4 is an angiogenic factor expressed in the eyes of diabetic patients with PDR. (A) Levels of ANGPTL4 in the aqueous fluid from diabetic (with and without diabetic retinopathy) and nondiabetic patients. (B) Immunohistochemical analysis of eyes from patients with known PDR demonstrates expression of ANGPTL4 in areas of preretinal neovascularization; similar results were observed in 5/5 PDR eyes. (C) ANGPTL4 protein levels are elevated in the vitreous of PDR patients compared with control patients. (D) Control and PDR patient samples demonstrate a correlation between levels of ANGPTL4 in aqueous fluid and levels of ANGPTL4 in vitreous (P = 0.007). (E and F) ANGPTL4 blocking antibody reduces the ability of low-VEGF PDR aqueous fluid (E) or PDR anti-VEGF aqueous fluid (F) to stimulate tubule formation. (G) Two-dimensional scatter plot demonstrating the [VEGF] and [ANGPTL4] in the aqueous fluid from PDR patients. The percentage of PDR (red) or PDR anti-VEGF (green) eyes within each quadrant defined by the average aqueous fluid [VEGF] (orange dashed line) and [ANGPTL4] (blue dashed line) levels is shown. Note: PDR samples with [ANGPTL4] > 60 ng/mL and/or with [VEGF] > 1.2 ng/mL are not depicted to adequately demonstrate the variability within the samples. One-way ANOVA. Wilcoxon test and Pearson correlation.
To investigate the potential of inhibiting ANGPTL4 as a therapeutic approach for the treatment of PDR, we identified a monoclonal antibody to ANGPTL4 that could effectively block the stimulation of tubule formation by rhANGPTL4 but not by rhVEGF (SI Appendix, Fig. S9 A and B). Using this ANGPTL4 neutralizing antibody, we were able to inhibit the angiogenic potential of conditioned media from hypoxic retinal Müller cells (SI Appendix, Fig. S9C), similar to the VEGF neutralizing antibody, bevacizumab (see Fig. 3I). To assess whether blocking ANGPTL4 could be an effective approach for PDR patients who do not respond adequately to anti-VEGF therapy, we next analyzed the effect of ANGPTL4 neutralizing antibody on the ability of low-VEGF aqueous fluid from PDR patients to stimulate tubule formation and observed a potent inhibition of angiogenic potential (Fig. 6E and SI Appendix, Fig. S10A). The limited volume of aqueous fluid that could be safely removed from PDR patients did not allow us to assess the effect of using neutralizing antibodies to VEGF and ANGPTL4 together, compared with either antibody alone. To overcome this obstacle, we analyzed whether ANGPTL4 neutralizing antibody could prevent tubule formation by aqueous fluid from PDR patients who received recent anti-VEGF therapy within 2 wk of sample collection. These aqueous fluid samples had markedly reduced detectable VEGF levels, and their ability to stimulate tubule formation was not affected by pretreatment with VEGF blocking antibody (see Fig. 2 B and C). ANGPTL4 neutralizing antibody reduced the angiogenic potential of aqueous fluid from PDR patients, despite treatment with anti-VEGF therapy within 2 wk of sample collection (Fig. 6F and SI Appendix, Fig. S10B).
Unlike VEGF, there was minimal overlap between the ANGPTL4 levels in the aqueous fluid from diabetic patients with and without PDR and with diabetic patients with NPDR (Fig. 6A and SI Appendix, Fig. S11), suggesting that aqueous fluid levels of ANGPTL4 may further help define the subpopulation of patients with diabetic eye disease. Indeed, examination of the aqueous fluid levels of ANGPTL4 compared with VEGF resulted in a clear separation between control patients, diabetics without DR, and diabetics with NPDR or PDR (SI Appendix, Fig. S12 A and B). Aqueous fluid from PDR patients could be further stratified into four groups based on the relative levels of VEGF and ANGPTL4 (Fig. 6G and SI Appendix, Table S4). Moreover, although VEGF levels were markedly lower in the aqueous fluid from patients who underwent treatment with anti-VEGF therapy within 2 wk of sample collection, the aqueous fluid levels of ANGPTL4 remained elevated in these patients (Fig. 6G).
Discussion
In a recent clinical trial, it was demonstrated that although over one-third of patients with severe NPDR progress to PDR within 2 y, this number was reduced by up to two-thirds in patients who receive monthly anti-VEGF therapy (8). However, for many of the patients who respond to anti-VEGF therapy, the response may only be temporary; the number of diabetic patients with NPDR who continue to progress to PDR increases over time (18), and this is likely to continue despite treatment with anti-VEGF therapy. This suggests that additional factors participate in the development of PDR and highlights major gaps in our understanding of the underlying biological processes that promote the persistence (and growth) of retinal neovascularization despite anti-VEGF treatment.
Equally troublesome are growing concerns regarding the consequences of chronic VEGF inhibition. VEGF produced by the retinal pigment epithelium is essential to maintaining the health of the underlying choriocapillaris, the vascular bed that supplies the metabolically active retinal photoreceptors (19–22). It is postulated that VEGF may also play a more direct role as a neurotrophic factor for the neurosensory retina (23). Chronic inhibition of VEGF may impair its normal physiologic roles in the eye (24, 25). Indeed, it has recently been reported that anti-VEGF therapy is associated with the development of retinal atrophy in some patients with macular degeneration (26). Recent data raise the additional concern that anti-VEGF therapy may contribute to the development of glaucoma in vulnerable patients (27). Collectively, these observations emphasize the importance of ongoing efforts to identify other angiogenic factors that promote the development of diabetic eye disease and that may therefore serve as effective—and perhaps safer—targets for patients with DR.
In this regard, studies using animal models have demonstrated that expression of a constitutively active HIF-1α mutant was sufficient to promote retinal neovascularization in vivo (28), whereas expression of VEGF alone was not sufficient to mediate this effect (29–31). These results implicate additional HIF-regulated angiogenic factor(s) in the promotion of retinal neovascularization in PDR patients. Of interest, erythropoietin (EPO)—the first identified HIF-regulated gene—has been implicated in the development of PDR (32, 33). However, PDR patients often have end-stage renal disease and require parenteral injection of rhEPO on a regular basis. Additionally, in the eye, EPO has been found to reduce neuronal, glial, and vascular damage or dysfunction and to inhibit the breakdown of the blood–retinal barrier in a diabetic rodent model (34–36). EPO appears to play a complex and multifaceted role in diabetic patients, as well as in diabetic eye disease, and modulation of EPO in this population should be approached with caution.
The results of our study implicate an alternative angiogenic factor, ANGPTL4, in the promotion of retinal neovascularization in PDR patients. ANGPTL4 has been implicated in the up-regulation of retinal neovascularization by PPAR-β/δ (17). We show here that HIF-1 also plays an important role in regulating expression of ANGPTL4 in ischemic retinal disease. Expression of ANGPTL4 was markedly increased in the aqueous and vitreous of PDR patients, and ANGPTL4 expression was localized to retinal neovascularization in PDR eyes. Inhibition of ANGPTL4 reduced the angiogenic potential of hypoxic retinal cells; this effect was additive, with simultaneous inhibition of VEGF. A neutralizing antibody for ANGPTL4 inhibited the angiogenic potential of aqueous fluid from PDR patients, including aqueous fluid from PDR patients who had received anti-VEGF therapy within 2 wk of sample collection. Although we cannot rule out a contribution from other angiogenic factors in the promotion of retinal neovascularization in patients with PDR, our results suggest that targeting both ANGPTL4 and VEGF may be necessary for effective treatment or prevention of PDR. An essential next step will be the development of specific and effective anti-ANGPTL4 therapies. This will require the identification of the relevant endothelial cell receptor through which ANGPTL4 mediates its pathological effects in the retina. Of note, as ANGPTL4 has also been implicated in the promotion of vascular permeability (and macular edema) in diabetic patients (13), therapies targeting ANGPTL4 may also be an effective approach for the treatment of patients with DME.
These studies may have more broad implications. The anticipated increase in the worldwide diabetic population emphasizes the need for novel approaches to identify which diabetic patients would benefit from access to higher levels of ophthalmic care (37–39). Access to eye care specialists remains limited in parts of the developing world where the diabetic population is expected to double over the next 2 decades (38). In this regard, we demonstrate here that aqueous fluid levels of ANGPTL4 correlate very well with the levels of ANGPTL4 in the vitreous. Acquiring aqueous fluid from patients is relatively straightforward and poses limited risks on patients, and although there is considerable overlap of VEGF levels in aqueous fluid of control patients, diabetic patients without DR, and diabetics with NPDR or PDR, ANGPTL4 levels were strongly predictive of the presence or absence of PDR. This suggests that ANGPTL4 levels in the aqueous fluid could serve as a diagnostic biomarker to help predict the level of diabetic eye disease in vulnerable populations.
Interestingly, aqueous fluid from PDR patients fell into four categories: modestly high levels of both VEGF and ANGPTL4 (50%), very high VEGF levels but only modestly high ANGPTL4 levels (18%), very high ANGPTL4 levels but only modestly high VEGF levels (18%), and very high VEGF and ANGPTL4 levels (14%). We speculate that the aqueous fluid levels of ANGPTL4 (and VEGF) could help define subpopulations of PDR patients who may be more (or less) susceptible to anti-VEGF therapy. Indeed, the aqueous fluid levels of ANGPTL4 remain elevated despite treatment with anti-VEGF therapy, further suggesting that aqueous fluid levels of ANGPTL4 could be used as a biomarker in patients receiving anti-VEGF therapy to help guide individualized therapy. This is particularly important given the rising cost of anti-VEGF therapies, which now account for 1/6 of the total Medicare Part B drug budget (40). This includes the cost of treating patients who do not respond adequately to this therapeutic approach. Ongoing work is currently focused on determining whether ANGPTL4 levels in aqueous fluid could help predict which patients are more (or less) likely to respond to anti-VEGF therapy.
As HIF-1 is a critical mediator of ocular neovascular disease, ANGPTL4 may play a key role in other vision-threatening diseases in which hypoxia (e.g., retinal ischemia) is a driving force, such as in retinal vein occlusion, sickle cell retinopathy, and retinopathy of prematurity. Our results suggest that ANGPTL4 may contribute to retinal neovascularization in these patients. Ultimately, our observations provide the foundation for studies to assess inhibition of ANGPTL4—alone or in combination with inhibition of VEGF—as a therapeutic approach for the treatment of PDR and other ocular neovascular diseases as well as for studies investigating the use of aqueous fluid ANGPTL4 as a diagnostic and/or therapeutic biomarker for these diseases.
Materials and Methods
Constructs and Reagents.
Recombinant human ANGPTL4, VEGF, and ANGPTL4 (DuoSet) and VEGF (Quantikine) ELISA kits were purchased from R&D Systems. Predesigned control (scrambled), ANGPTL4, and VEGF siRNA were obtained from Santa Cruz. Lipofectamine RNAiMAX transfection reagent and Opti-MEM medium were obtained from Life Technologies. Hypoxia chambers were used to expose MIO-M1 cells (1% oxygen) and primary murine Müller cells [3% (vol/vol) oxygen; exposure of primary murine Müller cells to lower oxygen concentrations resulted in cell death]. Digoxin and desferrioxamine (DFO) were obtained from Sigma. Dimethyloxalylglycine (DMOG) was obtained from Cayman Pharmaceuticals.
Cell Culture.
MIO-M1 cells were a generous gift from Astrid Limb (University College London Institute of Ophthalmology) and cultured with DMEM (Invitrogen) containing 1 g/L glucose with 10% (vol/vol) FBS (Invitrogen) and 1% penicillin/streptomycin (Cellgro). Immortalized human dermal microvascular endothelial cells (HMEC1) were obtained from the CDC and cultured with DMEM containing 4.5 g/L glucose with 10% (vol/vol) FBS and 1% penicillin/streptomycin. Before treatments, the growth media were replaced with serum starvation media containing 1% FBS.
siRNA Transfection.
Cells were seeded at 60–80% confluence at transfection. Lipofectamine RNAiMAX reagent was diluted in Opti-MEM medium. Thirty picomoles of siRNA from stock of 10 μM was diluted in Opti-MEM medium. Diluted siRNA was added to diluted Lipofectamine RNAiMAX reagent (1:1 ratio) and incubated for 5 min at room temperature. siRNA–lipid complex was added to cells and incubated at 37 °C for 24 h. The media were then washed out, and cells were ready for experiments.
Mice.
Timed pregnant C57BL/6 mice (E14) (Charles River Laboratories) were treated in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and the guidelines of the Johns Hopkins University Animal Care and Use Committee. OIR experiments were performed as previously described (11). Briefly, 7-d-old (P7) C57BL/6 mice and their mothers were exposed to 75% (vol/vol) oxygen for 5 d. The mice were returned to room air at P12. The mice were killed and the eyes were collected at P12 (2 h after return to normoxia), P13, P14, and/or P17. A subset of mice was given daily i.p. injection of vehicle or 2 mg/kg digoxin.
Western Blot.
Cells in culture dishes were washed with PBS and lysed using RIPA buffer (Sigma) with 10% (vol/vol) protease inhibitor mixture (Sigma). Cell lysates were then solubilized in LDS-sample buffer (Life Technologies) and incubated for 5 min at 95 °C. Lysates were subjected to 4–15% (wt/vol) gradient SDS/PAGE (Invitrogen). After blocking the membrane with 5% (wt/vol) milk (Bio-Rad), the membrane was then incubated with mouse anti-HIF-1α (BD, 610959) or rabbit anti-HIF-1α (Abcam, 2185) or with mouse anti-GAPDH monoclonal antibody (Fitzgerald) overnight at 4 °C. After washing, the membrane was incubated with HRP-conjugated anti-mouse or anti-rabbit IgG (Cell Signaling) for 1 h and then visualized with ECL Super Signal West Femto (Thermo). Western blot scans are representative of at least three independent experiments.
Quantitative RT-PCR.
mRNA was isolated from cultured cells or isolated retinas with RNeasy Mini Kit (Qiagen), and cDNA was prepared with MuLV Reverse Transcriptase (Applied Biosystems). Quantitative real-time PCR was performed with Power SYBR Green PCR Master Mix (Applied Biosystems) and MyiQ Real-Time PCR Detection System (Bio-Rad). Cyclophilin A and β-actin were used for normalization of mouse tissue and cell lines or for human cell lines, respectively. Primers are listed in SI Appendix, Tables S5 and S6.
Immunohistochemistry and Immunofluorescence.
Immunohistochemical detection of HIF-1α (Abcam, 2185) and ANGPTL4 (Abcam, 115798) was performed in paraffin-embedded human tissue and cryopreserved mouse tissue sections using ABC system (Dako) as previously described (13). CD31 antibody was obtained from BD (550274). Images were captured using a confocal microscope LSM 710 META (Carl Zeiss).
Tubule Formation Assay.
Tubule formation assay was performed using growth factor-reduced Matrigel (BD Biosciences; 356231). Sixty to eighty microliters of Matrigel was added into a prechilled 96-well plate and placed in a 37 °C CO2 incubator for 30 min. HMECs were then counted and plated at 2 × 104 cells per well on the Matrigel in a 96-well plate. Eighteen hours later, images were captured and analyzed using ImageJ software. Tubule formation assay with patient samples was performed with an addition of 5 μL/well of aqueous fluid to the cell suspension before adding into the Matrigel-coated wells. VEGF neutralization was performed using 100 μg/mL of bevacizumab (Johns Hopkins University Pharmacy). ANGPTL4 neutralization was performed using 10 μg/mL of an ANGPTL4 monoclonal antibody (Enzo, 804–732-C100).
Patient Samples.
Institutional Review Board approval from the Johns Hopkins University School of Medicine was obtained for all patient samples used in this study. An a priori power analysis using a Cohen’s d effect size of 0.8 based on results from in vivo studies and preliminary results, power of 0.8, and ⍺ of 0.05 yielded an estimated sample size of 26 control and PDR patients. Aqueous and vitreous samples were collected from consenting patients at the Wilmer Eye Institute undergoing cataract and/or vitrectomy surgery. Vitreous samples were immediately centrifuged at 16,000 × g for 5 min at 4 °C, and supernatant was removed. Aqueous and vitreous samples were then aliquoted and stored at −80 °C before analysis.
ELISA.
Aqueous diluted 1:10 and undiluted vitreous were analyzed for ANGPTL4 and VEGF with ELISAs performed according to the manufacturer’s protocols (R&D Systems).
Statistical Analysis.
Results from cell culture and animal models are shown as mean ± SEM from at least three independent experiments. Results from clinical samples are shown as mean ± SD. Statistical differences between groups were determined by Student’s t test, Wilcoxon signed-rank test, and one-way ANOVA when indicated. Correlation was tested using Pearson’s method. Statistical analysis was performed using Microsoft Office and Prism 6.0 software (GraphPad). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; NS, nonsignificant.
Supplementary Material
Acknowledgments
This work was supported by the National Eye Institute Grant K08-EY021189 (to A.S.), the Microscopy and Imaging Core Module and the Animal Core Module of the Wilmer Core Grant EY001765, and an Unrestricted Grant from Research to Prevent Blindness (to A.S.). A.S. gratefully acknowledges the support he receives from the William and Ella Owens Medical Research Foundation, as a Career Development Award recipient from the Research to Prevent Blindness Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1423765112/-/DCSupplemental.
References
- 1.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 2.Stitt AW. AGEs and diabetic retinopathy. Invest Ophthalmol Vis Sci. 2010;51(10):4867–4874. doi: 10.1167/iovs.10-5881. [DOI] [PubMed] [Google Scholar]
- 3.Durham JT, Herman IM. Microvascular modifications in diabetic retinopathy. Curr Diab Rep. 2011;11(4):253–264. doi: 10.1007/s11892-011-0204-0. [DOI] [PubMed] [Google Scholar]
- 4.Bressler NM, Beck RW, Ferris FL., 3rd Panretinal photocoagulation for proliferative diabetic retinopathy. N Engl J Med. 2011;365(16):1520–1526. doi: 10.1056/NEJMct0908432. [DOI] [PubMed] [Google Scholar]
- 5.Kurihara T, Westenskow PD, Friedlander M. Hypoxia-inducible factor (HIF)/vascular endothelial growth factor (VEGF) signaling in the retina. Adv Exp Med Biol. 2014;801:275–281. doi: 10.1007/978-1-4614-3209-8_35. [DOI] [PubMed] [Google Scholar]
- 6.Krispel C, Rodrigues M, Xin X, Sodhi A. Ranibizumab in diabetic macular edema. World J Diabetes. 2013;4(6):310–318. doi: 10.4239/wjd.v4.i6.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bressler SB, et al. Exploratory analysis of the effect of intravitreal ranibizumab or triamcinolone on worsening of diabetic retinopathy in a randomized clinical trial. JAMA Ophthalmol. 2013;131(8):1033–1040. doi: 10.1001/jamaophthalmol.2013.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ip MS, Domalpally A, Hopkins JJ, Wong P, Ehrlich JS. Long-term effects of ranibizumab on diabetic retinopathy severity and progression. Arch Ophthalmol. 2012;130(9):1145–1152. doi: 10.1001/archophthalmol.2012.1043. [DOI] [PubMed] [Google Scholar]
- 9.Wang S, Park JK, Duh EJ. Novel targets against retinal angiogenesis in diabetic retinopathy. Curr Diab Rep. 2012;12(4):355–363. doi: 10.1007/s11892-012-0289-0. [DOI] [PubMed] [Google Scholar]
- 10.Funatsu H, et al. Aqueous humor levels of cytokines are related to vitreous levels and progression of diabetic retinopathy in diabetic patients. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2005;243(1):3–8. doi: 10.1007/s00417-004-0950-7. [DOI] [PubMed] [Google Scholar]
- 11.Smith LE, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35(1):101–111. [PubMed] [Google Scholar]
- 12.Yoshida T, et al. Digoxin inhibits retinal ischemia-induced HIF-1alpha expression and ocular neovascularization. FASEB J. 2010;24(6):1759–1767. doi: 10.1096/fj.09-145664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xin X, et al. Hypoxic retinal Muller cells promote vascular permeability by HIF-1-dependent up-regulation of angiopoietin-like 4. Proc Natl Acad Sci USA. 2013;110(36):E3425–E3434. doi: 10.1073/pnas.1217091110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodrigues M, et al. VEGF secreted by hypoxic Müller cells induces MMP-2 expression and activity in endothelial cells to promote retinal neovascularization in proliferative diabetic retinopathy. Diabetes. 2013;62(11):3863–3873. doi: 10.2337/db13-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, et al. HIF-1-dependent expression of angiopoietin-like 4 and L1CAM mediates vascular metastasis of hypoxic breast cancer cells to the lungs. Oncogene. 2012;31(14):1757–1770. doi: 10.1038/onc.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Ma T, et al. Viral G protein-coupled receptor up-regulates Angiopoietin-like 4 promoting angiogenesis and vascular permeability in Kaposi’s sarcoma. Proc Natl Acad Sci USA. 2010;107(32):14363–14368. doi: 10.1073/pnas.1001065107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capozzi ME, McCollum GW, Savage SR, Penn JS. Peroxisome proliferator-activated receptor-β/δ regulates angiogenic cell behaviors and oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2013;54(6):4197–4207. doi: 10.1167/iovs.13-11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fong DS, et al. Retinopathy in diabetes. Diabetes Care. 2004;27(Suppl 1):S84–S87. doi: 10.2337/diacare.27.2007.s84. [DOI] [PubMed] [Google Scholar]
- 19.Blaauwgeers HG, et al. Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris. Evidence for a trophic paracrine relation. Am J Pathol. 1999;155(2):421–428. doi: 10.1016/S0002-9440(10)65138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marneros AG, et al. Vascular endothelial growth factor expression in the retinal pigment epithelium is essential for choriocapillaris development and visual function. Am J Pathol. 2005;167(5):1451–1459. doi: 10.1016/S0002-9440(10)61231-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saint-Geniez M, Maldonado AE, D’Amore PA. VEGF expression and receptor activation in the choroid during development and in the adult. Invest Ophthalmol Vis Sci. 2006;47(7):3135–3142. doi: 10.1167/iovs.05-1229. [DOI] [PubMed] [Google Scholar]
- 22.Saint-Geniez M, Kurihara T, Sekiyama E, Maldonado AE, D’Amore PA. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc Natl Acad Sci USA. 2009;106(44):18751–18756. doi: 10.1073/pnas.0905010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Bao S, Hambly BD, Gillies MC. Vascular endothelial growth factor-A: A multifunctional molecular player in diabetic retinopathy. Int J Biochem Cell Biol. 2009;41(12):2368–2371. doi: 10.1016/j.biocel.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 24.McLeod DS, et al. Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50(10):4982–4991. doi: 10.1167/iovs.09-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurihara T, Westenskow PD, Bravo S, Aguilar E, Friedlander M. Targeted deletion of Vegfa in adult mice induces vision loss. J Clin Invest. 2012;122(11):4213–4217. doi: 10.1172/JCI65157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grunwald JE, et al. Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121(1):150–161. doi: 10.1016/j.ophtha.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bakri SJ, et al. Intraocular pressure in eyes receiving monthly ranibizumab in 2 pivotal age-related macular degeneration clinical trials. Ophthalmology. 2014;121(5):1102–1108. doi: 10.1016/j.ophtha.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 28.Kelly BD, et al. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. 2003;93(11):1074–1081. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- 29.Tolentino MJ, et al. Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology. 1996;103(11):1820–1828. doi: 10.1016/s0161-6420(96)30420-x. [DOI] [PubMed] [Google Scholar]
- 30.Ozaki H, et al. Intravitreal sustained release of VEGF causes retinal neovascularization in rabbits and breakdown of the blood-retinal barrier in rabbits and primates. Exp Eye Res. 1997;64(4):505–517. doi: 10.1006/exer.1996.0239. [DOI] [PubMed] [Google Scholar]
- 31.Ohno-Matsui K, et al. Inducible expression of vascular endothelial growth factor in adult mice causes severe proliferative retinopathy and retinal detachment. Am J Pathol. 2002;160(2):711–719. doi: 10.1016/S0002-9440(10)64891-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watanabe D, et al. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N Engl J Med. 2005;353(8):782–792. doi: 10.1056/NEJMoa041773. [DOI] [PubMed] [Google Scholar]
- 33.Katsura Y, et al. Erythropoietin is highly elevated in vitreous fluid of patients with proliferative diabetic retinopathy. Diabetes Care. 2005;28(9):2252–2254. doi: 10.2337/diacare.28.9.2252. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, et al. Intravitreal injection of erythropoietin protects both retinal vascular and neuronal cells in early diabetes. Invest Ophthalmol Vis Sci. 2008;49(2):732–742. doi: 10.1167/iovs.07-0721. [DOI] [PubMed] [Google Scholar]
- 35.Wang Q, et al. Long-term treatment with suberythropoietic Epo is vaso- and neuroprotective in experimental diabetic retinopathy. Cell Physiol Biochem. 2011;27(6):769–782. doi: 10.1159/000330085. [DOI] [PubMed] [Google Scholar]
- 36.McVicar CM, et al. Intervention with an erythropoietin-derived peptide protects against neuroglial and vascular degeneration during diabetic retinopathy. Diabetes. 2011;60(11):2995–3005. doi: 10.2337/db11-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burgess PI, Msukwa G, Beare NA. Diabetic retinopathy in sub-Saharan Africa: Meeting the challenges of an emerging epidemic. BMC Med. 2013;11:157. doi: 10.1186/1741-7015-11-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng Y, He M, Congdon N. The worldwide epidemic of diabetic retinopathy. Indian J Ophthalmol. 2012;60(5):428–431. doi: 10.4103/0301-4738.100542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sivaprasad S, Gupta B, Crosby-Nwaobi R, Evans J. Prevalence of diabetic retinopathy in various ethnic groups: A worldwide perspective. Surv Ophthalmol. 2012;57(4):347–370. doi: 10.1016/j.survophthal.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Hutton D, Newman-Casey PA, Tavag M, Zacks D, Stein J. Switching to less expensive blindness drug could save medicare part B $18 billion over a ten-year period. Health Aff (Millwood) 2014;33(6):931–939. doi: 10.1377/hlthaff.2013.0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.