Significance
Marek’s disease virus (MDV) serves as a versatile small-animal model for herpesvirus-induced oncogenesis. Infection of target cells in vitro was impossible due to the short-lived nature of B and T cells, and analysis of infected cells ex vivo was hampered by their low frequencies. To overcome these limitations, we established an in vitro system that allows infection of target cells with MDV using stimuli that prolong the survival of B and T cells. Our system recapitulates the situation in vivo, including transformation of T cells in vitro. In the future, our system will facilitate the analysis of viral and cellular factors that promote lytic replication, establishment of latency and transformation, processes ultimately resulting in deadly lymphomas in the natural host.
Keywords: Marek’s disease virus, B cells, T cells, lymphomagenesis, genomic integration
Abstract
Marek’s disease virus (MDV) is an alphaherpesvirus that causes deadly T-cell lymphomas in chickens and serves as a natural small animal model for virus-induced tumor formation. In vivo, MDV lytically replicates in B cells that transfer the virus to T cells in which the virus establishes latency. MDV also malignantly transforms CD4+ T cells with a Treg signature, ultimately resulting in deadly lymphomas. No in vitro infection system for primary target cells of MDV has been available due to the short-lived nature of these cells in culture. Recently, we characterized cytokines and monoclonal antibodies that promote survival of cultured chicken B and T cells. We used these survival stimuli to establish a culture system that allows efficient infection of B and T cells with MDV. We were able to productively infect with MDV B cells isolated from spleen, bursa or blood cultured in the presence of soluble CD40L. Virus was readily transferred from infected B to T cells stimulated with an anti-TCRαVβ1 antibody, thus recapitulating the in vivo situation in the culture dish. Infected T cells could then be maintained in culture for at least 90 d in the absence of TCR stimulation, which allowed the establishment of MDV-transformed lymphoblastoid cell lines (LCL). The immortalized cells had a signature comparable to MDV-transformed CD4+ α/β T cells present in tumors. In summary, we have developed a novel in vitro system that precisely reflects the life cycle of an oncogenic herpesivrus in vivo and will allow us to investigate the interaction between virus and target cells in an easily accessible system.
Marek’s disease virus (MDV) is a highly oncogenic alphaherpesvirus that infects chickens and causes paralysis, immunosuppression and visceral T-cell lymphomas (1, 2). The MDV-chicken system serves as a natural small-animal model for virus-induced tumor formation. Infection of susceptible animals with virulent MDV strains commonly results in tumor-induced mortality of 70–100% (1). Severity of disease is dependent on the virulence of the MDV strain and the genotype of the infected chicken (3). Over 40 y, modified-live virus vaccines have been successfully used to prevent disease and represent the first anticancer vaccines (4–7).
MDV infection is initiated by inhalation of infectious dust from contaminated environment. In the upper respiratory tract, infectious dust is taken up by phagocytic cells, including B cells (8, 9), which subsequently transport the virus to the primary lymphoid organs: the bursa of Fabricius, thymus and spleen. In these organs, MDV efficiently replicates in B cells that pass on the virus to T cells by direct cell-to-cell transfer (10). Infected T cells then deliver the virus to the feather follicle epithelium, where infectious virus is produced and shed into the environment (11). MDV primarily establishes latent infection in CD4+ T cells, which can become transformed and develop into deadly lymphomas (12). MDV-transformed cells exhibit a regulatory T-cell phenotype (Treg) based on their cytokine and cell surface marker profiles, including MHC class II, CD30 and CD25 (13–15). Factors that contribute to MDV-induced lymphomagenesis include the major MDV oncogene meq (MDV Eco Q-encoded protein), which encodes a basic leucine zipper (bZIP) transcription factor (16). The Meq protein is expressed in lytically infected cells and consistently in tumors and lymphoblastoid cell lines (LCL) derived from tumors, making it an optimal marker for the detection of both lytically and latently infected cells (16, 17).
Despite many advances in the understanding of MDV pathogenesis and the role of individual genes and gene products in lymphomagenesis, MDV research has been seriously hampered by the lack of an in vitro infection system for primary target cells in vivo. This was mainly due to the short-lived nature of B and T cells in culture. To overcome this limitation, Calnek and colleagues added fresh spleen lymphocytes every 2–3 d to infected chicken fibroblast and epithelial cells (18). They were able to maintain low levels of infection for more than 40 passages and initially showed that both B and T cells become infected (18, 19). Rarely, MDV-induced T-cell LCL were obtained, but were unstable in culture (20). None of the culture systems was further developed due to the limited availability of B- and T-cell growth and survival factors.
Since the initial publication of the chicken genome, significant progress has been made in avian immunology and cytokine research (21). Numerous cytokines and growth factors for B and T cells were identified. The avian homolog of B-cell activating factor of the tumor necrosis factor family (BAFF) was the first cytokine shown to prolong B-cell survival in vitro (22, 23). Although BAFF delayed apoptotic cell death of cultured B cells from bursa, blood, and spleen for 2–3 d, numbers of viable cells continued to decline. In contrast, a soluble form of chicken CD40 ligand (CD40L) strongly induced B-cell proliferation, allowing maintenance of the cultures for up to 2 wk (24). Several stimuli have been discovered that specifically induce proliferation and extend the life span of avian T cells in vitro. The most potent are αVβ1–T-cell receptor (TCR) cross-linking with the TCR-2 monoclonal antibody (25) as well as chicken IL-2 and IL-18 alone and in combination with TCR activation (26).
In this report, we harnessed, to our knowledge for the first time, these B- and T-cell stimuli to establish an in vitro infection system for the lymphotropic MDV. We could demonstrate that B cells can be efficiently infected in vitro. Virus was also transferred from infected B to T cells, in which MDV established latency. Furthermore, a subset of T cells infected in vitro underwent oncogenic transformation, resulting in the establishment of LCL harboring latent MDV. Our system recapitulates the infection model in vivo and will be useful to determine and test factors involved in efficient lytic replication, establishment of latency and transformation of T cells.
Results
Infection of Primary Chicken B Cells with MDV in Vitro.
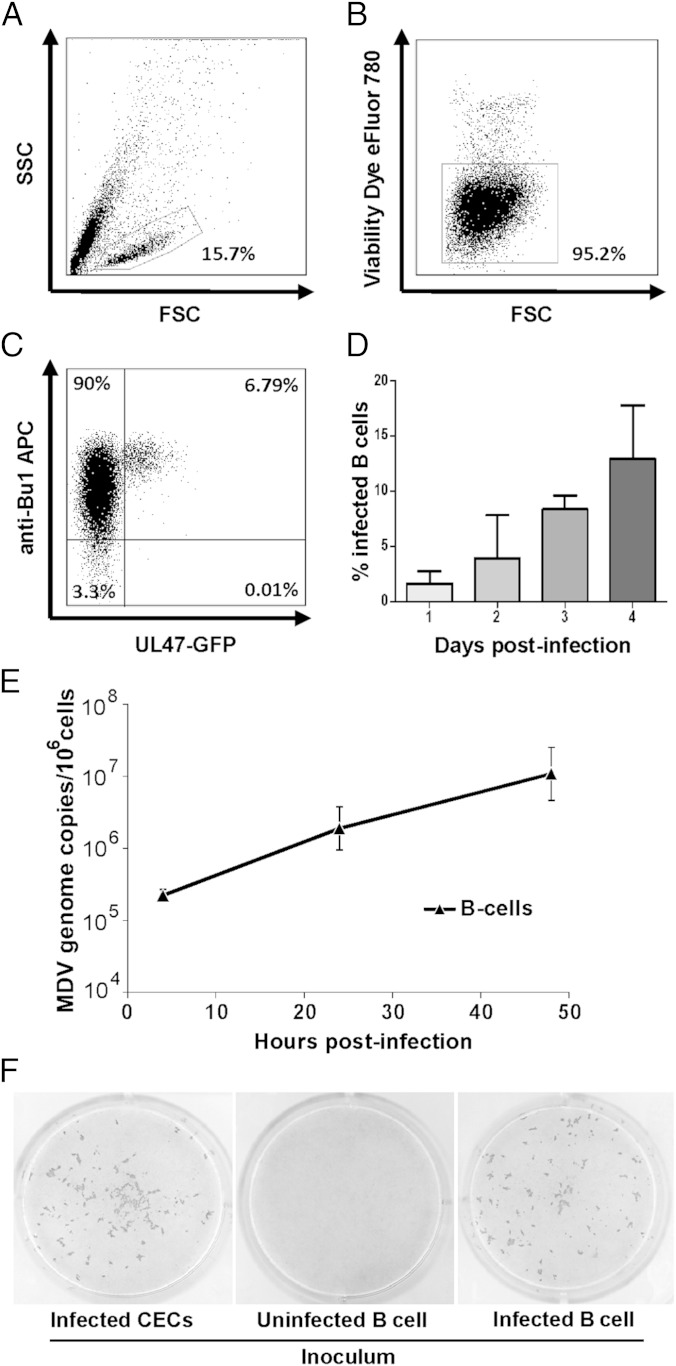
The main target cells of MDV lytic replication in vivo are B cells. Thorough analysis of infected B cells is virtually impossible due to the relatively small number of infected cells in vivo and the short-lived nature of the cells in vitro. We recently identified specific survival stimuli including chicken CD40L that allow maintenance of B cells in culture (25, 27). Primary chicken B cells from the bursa of Fabricius (Fig. 1), spleen or blood (Fig. S1) were cocultured in the presence of soluble CD40L with chicken embryo cells (CEC) that were infected by a recombinant MDV expressing green fluorescent protein (GFP) fused to the C terminus of the UL47 tegument protein (28). To analyze infection of B cells, we gated on the lymphocyte population (Fig. 1A), detected viable cells with eFluor780 dye (Fig. 1B), and stained B cells with the specific marker Bu1 to discriminate between infected B cells and CEC present in the inoculum (Fig. 1C). Within 24 h, fluorescent B cells expressing the UL47-GFP fusion protein could be detected (Fig. 1 C and D). Infection rates increased between day 1 and 4 from 2 to 13% (Fig. 1D). Similarly, B cells from spleen and peripheral blood could be efficiently infected with rates of up to 7% and 20%, respectively (Fig. S1 A–D). In contrast, B cells infected in the absence of CD40L showed rapid apoptotic cell death and no infected cells were observed as reported in earlier studies (19, 29).
Fig. 1.
MDV infection of primary B cells in vitro. Lymphocytes were isolated from the bursa of Fabricius, stimulated with soluble CD40L and cocultured with MDV-infected CEC (RB1B-UL47-GFP). Infected cells were analyzed by flow cytometry and successively gated. (A) The leukocyte population. (B) Viable cells according to eFluor780. (C) Infected B cells were detected using B-cell-specific anti-chBu1 and UL47-GFP fluorescence. (D) Infection kinetics of MDV in primary B cells. Percentages of infected B cells are shown as means of four independent experiments. (E) qPCR analysis of MDV genome copies in FACS-purified viable B cells (mean genome copies of three independent experiments). (F) Uninfected (Center) and infected B cells (Right) were sorted and seeded on CEC. MDV plaques were visualized by immunohistochemistry using anti-gB and anti-VP22 MAbs. Infected CEC were used as a positive control (Left).
Next, we addressed if the common vaccine strain CVI988/Rispens can also infect B cells in vitro using a recombinant CVI988 virus, which expresses GFP under the CMV IE promoter (CVI988-CMV-GFP). CVI988-CMV-GFP efficiently infected B cells and even higher infection rates (up to 50%) were observed compared with RB1B-UL47-GFP (14%; Fig. S2A). To confirm infection of B cells, we examined lymphocytes infected with CVI988-CMV-GFP and RB1B-UL47-GFP by fluorescence microscopy (Fig. S2B). Cells infected with either virus expressed the B-cell marker Bu1 (chB6), confirming that B cells are efficiently infected. Furthermore, we were also able to show that the infected cells undergo a full lytic cycle as evidenced by the expression of the late viral antigen glycoprotein B (gB) using a gB-specific monoclonal antibody (Fig. S3).
For subsequent studies, we isolated CD45+/Bu1+ B cells by FACS to exclude carryover of infected fibroblasts. To demonstrate that the cultured B cells are indeed productively infected, we determined MDV genome copy numbers by quantitative PCR (qPCR) and demonstrated that the MDV genome is efficiently replicated as genome copies increased almost 100-fold (Fig. 1E). To investigate whether the infected B cells produce infectious virus, GFP-positive B cells from infected cultures and GFP-negative B cells from uninfected cultures were sorted and plated on CEC monolayers. Infected (GFP-positive) B cells efficiently transmitted MDV to CEC resulting in plaque formation, whereas GFP-negative B cells failed to do so (Fig. 1F). Taken together, the established system allowed efficient lytic infection in vitro of primary chicken B cells from various organs as evidenced by lytic gene expression, robust genome replication and virus production, thereby mimicking the in vivo situation.
Infection of Primary Chicken T Cells with MDV in Vitro.
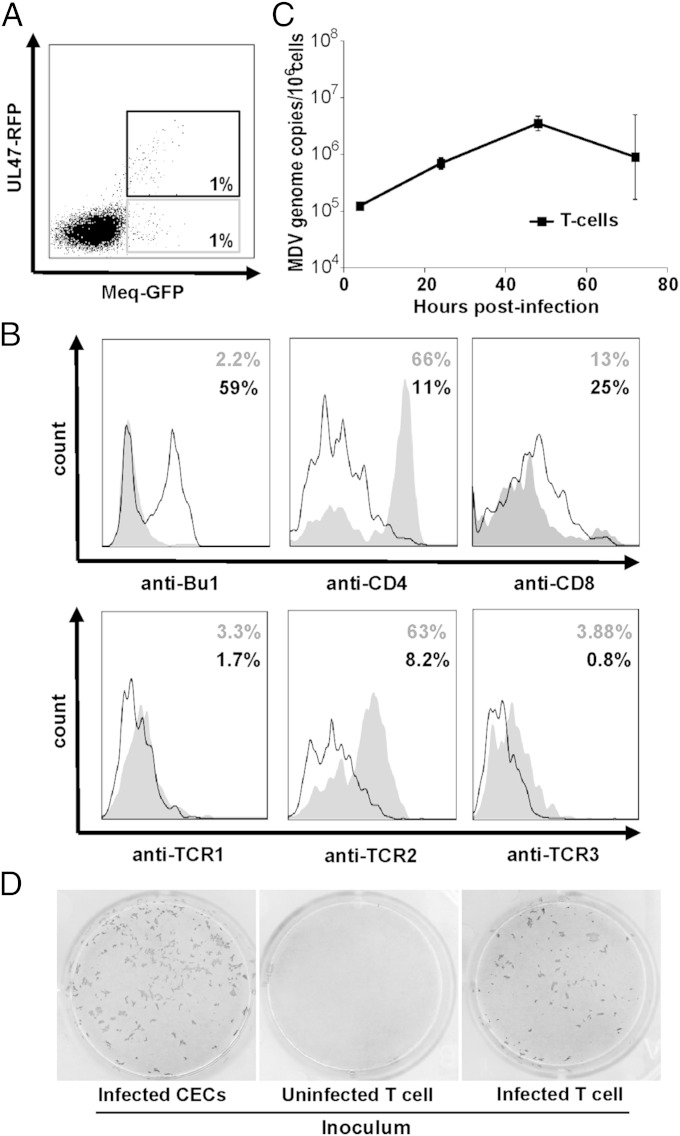
To determine whether primary chicken T cells can also be infected with MDV in vitro, we stimulated T cells by αVβ1-TCR cross-linking with the TCR-2 antibody and coseeded them with infected CEC. Because UL47-GFP is not expressed in latently infected cells, we generated a virus that expresses GFP fused to the C terminus of Meq, the major oncoprotein that is also expressed during latency. Red fluorescent protein (RFP) was fused to the C terminus of UL47, resulting in a virus that allows discrimination between lytically and latently infected cells based on the fluorescence emitted in infected cells. Upon infection of T cells, we gated on the Bu1 negative lymphocyte population and quantified the percentage of infected T cells by flow cytometry (Fig. 2A). Intriguingly, a subset of T cells only expressed Meq-GFP but not UL47-RFP, indicating that these cells may be latently infected (Fig. 2A). Most latently infected T cells were CD4+ CD8− and expressed the αVβ1-TCR, directly corresponding to the latently infected cells in infected animals (Fig. 2B). A larger proportion of T cells expressed both UL47-RFP and Meq-GFP (Fig. 2A), indicating that MDV replicated lytically in some T cells, which were predominantly CD4− and did not express TCR (41%). Rather, the majority of the lytically infected cells in the culture expressed the B-cell marker Bu1 (59%) indicating that the small population of thymic B cells was efficiently infected as well (Fig. 2B). For further analyses, we sorted the viable CD45+/Bu1− lymphocyte population from activated thymocyte cultures, thereby excluding CD45− fibroblasts and Bu1+ B cells from the preparation. qPCR analysis of these sorted viable and GFP-positive T cells suggested that MDV initially replicated productively in these cells until day 3, but that genome copy numbers stabilized afterward (Fig. 2C). To determine whether infectious virus was produced in lytically infected T cells (UL47-RFP and Meq-GFP double-positive cells), we sorted infected T cells (CD45+/Bu1-) and seeded them on CEC monolayers. Infected T cells could efficiently transmit the virus to CEC as evidenced by plaque formation (Fig. 2D).
Fig. 2.
MDV infection of primary T cells. (A) Lymphocytes were isolated from thymus, stimulated by TCR cross-linking and cocultured with CEC infected with RB1B-UL47-RFP_Meq-GFP. (B) Latently infected (UL47-RFP-negative, gray curve) and lytically infected cells (UL47-RFP-positive, black line) were analyzed for the expression of surface markers. (C) qPCR analysis of MDV genome copies in the FACS-sorted viable T-cell population. Data are shown as mean genome copies of three independent experiments. (D) Uninfected (Center) and infected T cells (Right) were sorted and seeded on CEC monolayers. MDV plaques were visualized as described in Fig. 1. Infected CECs were used as a positive control (Left).
To investigate whether MDV can also infect γδ T cells, we compared infection rates of αβ and γδ T cells upon TCR-1 and TCR-2 stimulation of thymocyte cultures (Fig. S4). In TCR-1–stimulated cell cultures, only very few infected γδ T cells were found, whereas a small population of infected αβ T cells (TCR-2+) were identified. Upon TCR-2 stimulation, higher infection rates of αβ T cells (4% were Meq-GFP positive) were obtained, whereas no γδ T cells were infected. Importantly, very few Meq-GFP positive cells were observed in unstimulated T-cell cultures, indicating that T-cell activation strongly enhances MDV infection. We concluded from the results that chicken T cells can be maintained in vitro and are susceptible to MDV infection. Moreover, we confirmed that T cells latently infected with MDV in vitro exhibit a signature indistinguishable from the targeted cells in vivo.
Transfer of MDV from B Cells to T Cells.
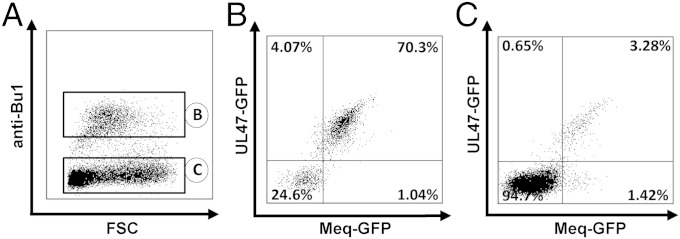
To determine whether B cells are capable of transferring virus directly to T cells in vitro in the established system, we infected B cells as described above and isolated infected B cells by FACS. Infected B cells were cocultured with primary thymus-derived T cells that had been TCR-2 stimulated (Fig. 3A). Infection rates of B cells (Fig. 3B) and non–B-cell thymocytes (predominantly T cells) (Fig. 3C) were monitored by flow cytometry, which revealed that virus was efficiently transferred from B to T cells; in the latter, infection rates of ∼5% were reached (Fig. 3C). The larger proportion of T cells was lytically infected as determined by coexpression of the lytic and latent markers (3.3%, Fig. 3C), whereas 1.4% of T cells expressed Meq-GFP but not UL47-RFP, indicating latent infection (Fig. 3C).
Fig. 3.
Transfer of MDV from B to T cells. (A) B cells were infected with RB1B-UL47-RFP_Meq-GFP for 24 h, infected B cells sorted by FACS and cocultured with TCR-2–stimulated thymic T cells for 2 d. Cultures were stained with anti-chBu1 to discriminate between B and non-B thymocytes (predominantly T cells). Analysis of B cells (B) and non-B cells (C) in the culture. Percentage of infected B cells is shown from one representative experiment.
Latent Infection and Transformation of T Cells in Culture.
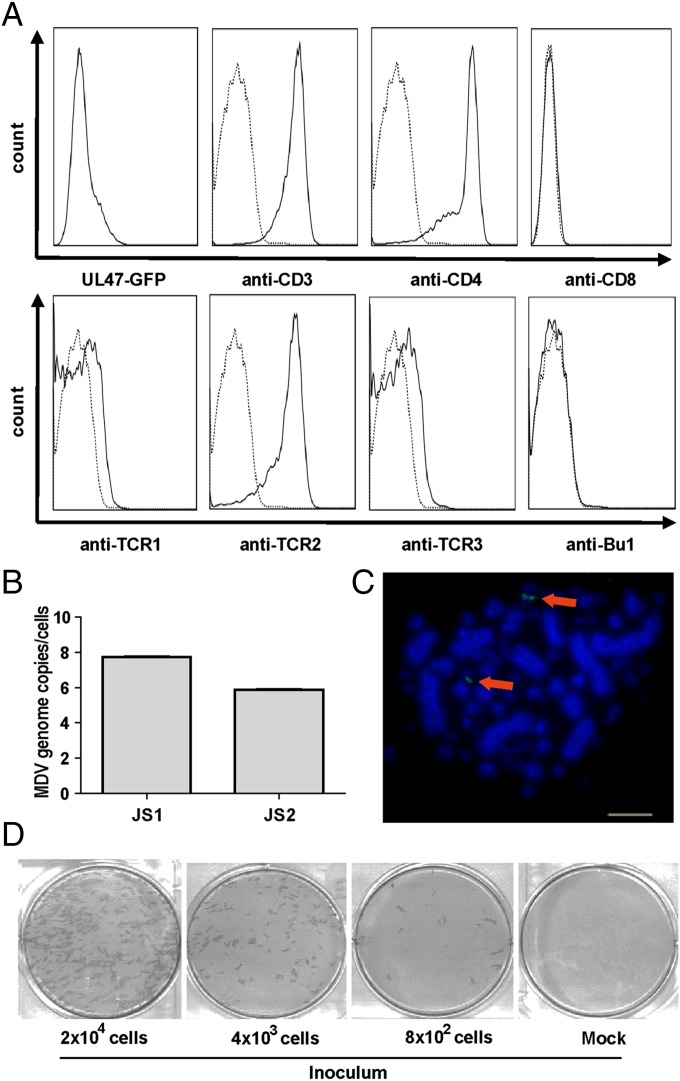
Next, we investigated whether infected T cells can be maintained in culture for extended periods of time. Infected T cells were cultured for 1 wk in the presence of the TCR-2 stimulus. Afterward, T cells were maintained in the absence of TCR-2 or any other growth factor supplementation. In several independent experiments, T-cell lines were established and maintained for almost half a year in culture. Cells were passaged every 3–5 d, resulting in 25–50 passages for each of the cell lines and stocks were frozen (Table 1). Frozen cell lines could also be thawed successfully and passaged for at least another 50 d. In contrast, control T cells rapidly died under comparable conditions. Analysis of the surface markers of the LCL established in vitro revealed that the pattern of their cell surface was very similar to MDV tumor cells taken directly ex vivo and expanded (CD3+, CD4+, CD28+, TCR2+), suggesting that the virus targets the same cells for transformation in vitro as it does in infected animals (Fig. 4A and Table 1). To determine if the established LCL harbored the virus genome, we measured MDV genome copy numbers in the cells. qPCR analyses revealed that each cell contains multiple MDV genome copies (Fig. 4B), as is commonly detected in MDV tumor cells.
Table 1.
Phenotypic characterization of four MDV transformed LCL
| Cell line | Days in culture* | CD4 | CD8 | TCR1 | TCR2 | TCR3 | MHCI | MHCII | Bu1 | CD28 | CD25 |
| JS1 | 167 | +++ | − | − | +++ | − | + | + | − | +++ | + |
| JS2 | 99 | +++ | − | − | +++ | − | +++ | + | − | ND | − |
| JS3 | 92 | − | + | − | +++ | ++ | +++ | + | − | ND | − |
| JS4 | 123 | +++ | − | − | +++ | − | +++ | − | − | +++ | + |
Cell lines were analyzed by flow cytometry for the indicated markers. Expression levels are shown: low (+), medium (++), high (+++), no expression (−), not done (ND).
Continuous days in culture.
Fig. 4.
Characterization of in vitro transformed T-cell lines. (A) T cells transformed with MDV in vitro were analyzed for the expression of cell surface markers by flow cytometry. (B) qPCR analysis of MDV genome copies in LCL shown as means of three independent experiments. (C) FISH of a representative T-cell line generated in vitro. Arrows indicate integration sites of the MDV genome (anti-DIG FITC, green) in host chromosomes stained with DAPI (blue). (Scale bar: 5 µm.) (D) Reactivation assay. The indicated number of cells from an LCL (JS1) was seeded on CEC and reactivated by serum starvation at RT. MDV plaques were visualized as described in Fig. 1.
A hallmark of MDV transformation is integration of the viral genome in transformed cells. We therefore analyzed the state of the MDV genome in the generated LCL by FISH. We found the MDV genome indeed integrated at the end of chromosomes of in vitro transformed T-cell lines (Fig. 4C). To determine whether MDV can be reactivated from the in vitro established LCL, we performed reactivation assays as described (Fig. 4D) (30). MDV was efficiently reactivated from these lines forming ∼4 plaques per 100 LCLs, indicating that the virus is able to readily mobilize its genome from the integrate state and reinitiate lytic replication. We concluded that MDV-infected T cells can be latently infected and survive in the absence of the TCR-2 stimulus. The T cells transformed in vitro have a phenotype identical to tumor cells taken ex vivo. In summary, the entire MDV replication cycle from lytic B-cell infection to T-cell transformation and reactivation from the integrated state could be replicated in vitro.
Discussion
Several herpesviruses including Epstein–Barr virus (EBV), Kaposi's sarcoma-associated herpesvirus (KSHV) and MDV cause tumors in animals and humans. However, only one natural small animal virus-host model for herpesvirus-induced tumor formation is available, which relies mostly on in vivo studies (1). In this model, infection of susceptible chickens with MDV results in a high tumor incidence of up to 100% (12). One drawback of the model has been that infection of B and T cells, the main targets for lytic replication and transformation, respectively, was not possible in vitro, due to the short-lived nature of lymphocytes in culture (18). To address this problem, we established an in vitro infection system that allowed us to efficiently infect B and T cells, facilitating lytic replication, latency and transformation of this oncogenic herpesvirus in vitro. Importantly, in vitro infection of lymphocytes was not only achieved with a highly pathogenic virus strain, but also with a nononcogenic vaccine virus. Prolonged survival of B and T cells was achieved by CD40L treatment and TCR–cross-linking, resulting in easily accessible and tunable culture system for infection with tumorigenic MDV.
During the establishment of the infection system, we tried various other avian specific survival stimuli, including BAFF, IL-10, and IL-21 for B cells and IL-2 and IL-18 for T cells. CD40L-mediated activation of B cells and T-cell receptor cross-linking proved to be most efficient in expanding cell numbers and prolonging survival. We reasoned that combinations of the stimuli and concerted (temporally staged) use could enhance survival of target cells and increase infection rates (26). On the other hand, multiple stimuli could possibly increase the likelihood that the phenotype of the cells was changing. Using CD40L and TCR cross-linking, the phenotype of infected B and T cells was virtually indistinguishable from cells infected in vivo, suggesting that the used stimuli did not reprogram target cells. In addition, the stimuli reflect the in vivo situation where virus transfers from B to T cells, most likely requiring direct cell-cell contact through MHC-TCR and CD40-CD40L–mediated interaction (31). With our infection system we could confirm the model of MDV infection in chickens proposed in the 1990s (2), which stipulated transfer of the virus from initially infected B cells to T cells, ultimately resulting in the establishment of latency in T cells followed by transformation in some cells. Unfortunately, in vivo studies do not allow infection of B and T cells in a synchronous manner, as cells in infected organs are at different stages of infection and transformation. In contrast, the established in vitro system permits the analysis of the kinetics of infection and the deliberate and coordinated use of target cells. Furthermore, analysis of the cells during the transformation process will be invaluable to examine in detail the sequence of events that lead to tumor formation. In combination with the genetic tools to manipulate the virus genome (32, 33), the in vitro infection system provides a unique tool to examine the interaction between MDV and its target cells.
In this study, we mainly used bursa and thymus as sources for B and T cells because the yield of target cells from these organs is very high. In addition, the bursa does not harbor relevant numbers of T cells (34), resulting in an almost pure B-cell population after isolation, making extensive purification steps obsolete. Similarly, in the thymus only few B cells (5%) are present. To remove the remaining B cells, magnetic sorting had been previously used. Importantly, these purified cultures could be equally infected with MDV.
It remains unclear whether a specific subpopulation of B cells becomes infected by MDV. Because we were able to infect lymphocytes from bursa, thymus, spleen and blood with similar efficiencies, the particular subpopulation would have to be present in all tissues at comparable levels. Unfortunately, the lack of cell surface markers has prevented further phenotypic characterization. In contrast, latently infected T cells were identified as αVβ1-TCR–expressing cells, which were CD4+ as described for latently infected cells in vivo. γδ T cells were poorly infectable, even upon strong stimulation and activation by TCR-1 crosslinking. In addition, activated T-helper cells could be transformed by MDV in vitro, giving rise to several cell lines. The established LCL could be continuously passaged for nearly half a year until stocks were frozen. Analysis of the surface markers on these LCLs revealed that their pattern was very similar to that of MDV tumor cell lines (CD3+, CD4+, CD28+, TCR2+). One T-cell line (JS3) was CD4− and CD8+, a phenotype that is also observed in vivo (31). One marker that was not detected on the in vitro transformed LCLs is CD30 (15). One explanation may be that many T cells are initially transformed in vivo, out of which only one or few give rise to the tumors (35). Hence, CD30 positivity may be a result of immune selection or possibly other selection mechanisms. We surmise that our in vitro transformed T cells likely correspond to the early transformed cells that have not undergone such selection. Two of cell the lines expressed the Treg marker CD25; however, as chickens do not encode Foxp3, a decisive marker for Tregs in mammals, it remains to be determined whether the generated T-cell lines indeed have a Treg phenotype.
Taken together, we present, to our knowledge for the first time, an in vitro infection model for MDV. We achieved prolonged survival of B and T cells, which allowed efficient infection of target cells with MDV. The established in vitro infection system could be used for a number of other lymphotropic (and partially oncogenic) pathogens including chicken anemia virus, infectious bursal disease virus and avian leukosis virus.
Materials and Methods
Preparation and Activation of Cells.
All animal work was approved by the appropriate government agency (Regierung von Oberbayern, Az.: 55.2-1-54-2532.6-12-09). CEC were generated and maintained as described (36). M11 (B2/B2) chickens were kindly provided by S. Weigend (Friedrich-Loeffler-Institut, Mariensee, Germany). Lymphocytes were obtained from bursa of Fabricius, thymus and spleen by dissociation of the organs and isolation of the cells by density gradient centrifugation as described (37). T cells were maintained in RPMI 1640 [supplemented with Glutamax, 10% (vol/vol) FBS, 1% penicillin/streptomycin], B cells in IMDM [with Glutamax, 8% (vol/vol) FBS, 2% (vol/vol) chicken serum, 50 mM β-mercaptoethanol, insulin-transferrin-sodium, selenite supplement and 1% penicillin/streptomycin] at 40 °C. B cells were activated using recombinant soluble chCD40L (24). Mouse CD8-chCD40L (m8c154) was expressed in HEK293 cells and purified as described (27). For T-cell activation, tissue culture plates were coated overnight at 4 °C with 1 µg/mL of TCR-1 or TCR-2 monoclonal antibody (MAb) (25, 26).
Generation of Recombinant Viruses and Lymphocyte Infection.
RB1B viruses with RFP and GFP fused to the C terminus of UL47 and Meq were engineered by two-step Red-mediated recombination (28, 32) using appropriate primers (Table S1). The viruses were termed RB1B-UL47-GFP and RB1B-UL47-RFP_Meq-GFP. A pCMV-GFP cassette was inserted into the pCVI988 (38) as described (32) resulting in CVI988-CMV-GFP. Viruses were reconstituted by transfection of BAC DNA and propagated in CEC (34).
B or T cells (1 × 106) were cocultured in 1 mL with 2.5 × 104 plaque-forming units (pfu) MDV in the presence of CD40L, TCR-1, or TCR-2 MAbs, respectively. For transfer of MDV from B to T cells, RFP-positive B cells were isolated by FACS sorting. Then, 4 × 105 infected B cells were cultured with TCR-2–stimulated thymic T cells for 24 h in 48-well plates.
Flow Cytometry and Cell Sorting.
Staining was performed as described (24). Monoclonal antibodies (MAb) specific for chicken CD45 (16-6) (39), Bu1 (AV20), CD25 (AV142), CD28 (2-4), CD40 (AV79), CD80 (IAH:F864:DC7) (Serotec), CD3 (CT3), CD4 (CT4), γδ-TCR (TCR-1), αVβ1-TCR (TCR-2) and αVβ2-TCR (TCR-3) (Southern Biotechnology) and for chicken CD8 (3-298) (40) were used. Isotype-matched MAbs were included as negative controls. Isotype-specific anti-mouse IgG1-APC, IgG2b-APC (Jackson ImmunoResearch), IgG2a Alexa-647 (Invitrogen), and IgG2b-RPE (Southern Biotechnology) antibodies were used for detection. Viability was determined using eFluor780 (Affymetrix eBioscience). After staining, cells were fixed (1% paraformaldehyde in PBS). For detection of gB, cells were stained with eFluor780 and anti-CD45-APC, incubated in fixation and permeabilization buffer (eBioscience), and stained with MAb K11 followed by anti-mouse IgG1-PE (Southern Biotechnology). Analyses were performed with a BD FACSCanto II (Becton Dickinson) using FACSDiva and FlowJo (Tree Star) software.
B and T cells were sorted using fluorescently labeled anti-Bu1 or anti-CD45 antibodies. Infected cells were detected by expression of UL47-RFP (or GFP) for lytic and Meq-GFP for latent infection. Dead cells were excluded by staining with PI or 7-aminoactinomycin D (each at 2.5 µg/mL). Sorting was performed on a FACS Aria III using the FACSDiva software (Becton Dickinson) and isolated cells were either directly used or stored at −80 °C until further analysis.
Quantification of MDV in Infected B and T Cells.
Infected B and T cells were isolated by FACS sorting and DNA was isolated using the E-Z96 96-well blood DNA isolation kit (Omega Biotek). MDV genome copies were determined by qPCR exactly as described (41–43).
Infectivity of B and T cells was analyzed by plaque assay. B or T cells (5 × 105) were seeded on CEC, fixed at 6 d postinfection, and stained with anti-VP22 (B12) (44) and/or anti-gB (K11) (45) MAbs. Plaques were visualized with goat anti-mouse–HRPO antibodies (Jackson ImmunoResearch) and NovaRed (Vector).
Indirect Immunofluorescence and Fluorescence in Situ Hybridization (FISH).
Infected B-cell cultures were harvested after 48 h postinfection. Viable cells were isolated by density gradient centrifugation and stained with the Bu1 antibody and anti-mouse IgG1 Alexa-568 (Invitrogen). Cell preparations were mounted using DAPI Vectashield (Vector Laboratories). For FISH, metaphase chromosomes were prepared from infected T cells. A DIG-labeled MDV whole genome probe and FITC-conjugated anti-DIG antibodies (Sigma-Aldrich) were used to visualize integration as described (30, 46). Fluorescent images were recorded using AxioImager M1 and AxioVision software (Carl Zeiss).
Supplementary Material
Acknowledgments
We thank Annemarie Engel, Ann Reum, Marina Kohn, and Beatrice Schärer for technical assistance as well as Jean-Francois Vautherot for providing antibodies. The study was supported by DFG Grant KA3492/3-1 (to B.B.K.), EU-EMIDA MADISPREAD (to B.K. and N.O.), and BMBF Grant FugatoPlus (to S.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1424420112/-/DCSupplemental.
References
- 1.Osterrieder N, Kamil JP, Schumacher D, Tischer BK, Trapp S. Marek’s disease virus: From miasma to model. Nat Rev Microbiol. 2006;4(4):283–294. doi: 10.1038/nrmicro1382. [DOI] [PubMed] [Google Scholar]
- 2.Calnek BW. Pathogenesis of Marek’s disease virus infection. Curr Top Microbiol Immunol. 2001;255:25–55. doi: 10.1007/978-3-642-56863-3_2. [DOI] [PubMed] [Google Scholar]
- 3.Witter RL, Calnek BW, Buscaglia C, Gimeno IM, Schat KA. Classification of Marek’s disease viruses according to pathotype: Philosophy and methodology. Avian Pathol. 2005;34(2):75–90. doi: 10.1080/03079450500059255. [DOI] [PubMed] [Google Scholar]
- 4.Churchill AE, Biggs PM. Herpes-type virus isolated in cell culture from tumors of chickens with Marek’s disease. II. Studies in vivo. J Natl Cancer Inst. 1968;41(4):951–956. [PubMed] [Google Scholar]
- 5.Churchill AE, Payne LN, Chubb RC. Immunization against Marek’s disease using a live attenuated virus. Nature. 1969;221(5182):744–747. doi: 10.1038/221744a0. [DOI] [PubMed] [Google Scholar]
- 6.Witter RL, Kreager KS. Serotype 1 viruses modified by backpassage or insertional mutagenesis: Approaching the threshold of vaccine efficacy in Marek’s disease. Avian Dis. 2004;48(4):768–782. doi: 10.1637/7203-050304R. [DOI] [PubMed] [Google Scholar]
- 7.Baigent SJ, Smith LP, Nair VK, Currie RJ. Vaccinal control of Marek’s disease: Current challenges, and future strategies to maximize protection. Vet Immunol Immunopathol. 2006;112(1-2):78–86. doi: 10.1016/j.vetimm.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Beasley JN, Patterson LT, McWade DH. Transmission of Marek's disease by poultry house dust and chicken dander. Avian Dis. 1970;14(1):45–53. [PubMed] [Google Scholar]
- 9.Baaten BJ, et al. Early replication in pulmonary B cells after infection with Marek’s disease herpesvirus by the respiratory route. Viral Immunol. 2009;22(6):431–444. doi: 10.1089/vim.2009.0047. [DOI] [PubMed] [Google Scholar]
- 10.Calnek BW. Marek’s disease—a model for herpesvirus oncology. Crit Rev Microbiol. 1986;12(4):293–320. doi: 10.3109/10408418509104432. [DOI] [PubMed] [Google Scholar]
- 11.Johnson EA, Burke CN, Fredrickson TN, DiCapua RA. Morphogenesis of Marek’s disease virus in feather follicle epithelium. J Natl Cancer Inst. 1975;55(1):89–99. doi: 10.1093/jnci/55.1.89. [DOI] [PubMed] [Google Scholar]
- 12.Jarosinski KW, Tischer BK, Trapp S, Osterrieder N. Marek’s disease virus: Lytic replication, oncogenesis and control. Expert Rev Vaccines. 2006;5(6):761–772. doi: 10.1586/14760584.5.6.761. [DOI] [PubMed] [Google Scholar]
- 13.Burgess SC, Davison TF. Identification of the neoplastically transformed cells in Marek’s disease herpesvirus-induced lymphomas: Recognition by the monoclonal antibody AV37. J Virol. 2002;76(14):7276–7292. doi: 10.1128/JVI.76.14.7276-7292.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shack LA, Buza JJ, Burgess SC. The neoplastically transformed (CD30hi) Marek’s disease lymphoma cell phenotype most closely resembles T-regulatory cells. Cancer Immunol Immunother. 2008;57(8):1253–1262. doi: 10.1007/s00262-008-0460-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burgess SC, et al. Marek’s disease is a natural model for lymphomas overexpressing Hodgkin’s disease antigen (CD30) Proc Natl Acad Sci USA. 2004;101(38):13879–13884. doi: 10.1073/pnas.0305789101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones D, Lee L, Liu JL, Kung HJ, Tillotson JK. Marek disease virus encodes a basic-leucine zipper gene resembling the fos/jun oncogenes that is highly expressed in lymphoblastoid tumors. Proc Natl Acad Sci USA. 1992;89(9):4042–4046. doi: 10.1073/pnas.89.9.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaufer BB, Trapp S, Jarosinski KW, Osterrieder N. Herpesvirus telomerase RNA(vTR)-dependent lymphoma formation does not require interaction of vTR with telomerase reverse transcriptase (TERT) PLoS Pathog. 2010;6(8):e1001073. doi: 10.1371/journal.ppat.1001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calnek BW, Schat KA, Shek WR, Chen CL. In vitro infection of lymphocytes with Marek’s disease virus. J Natl Cancer Inst. 1982;69(3):709–713. [PubMed] [Google Scholar]
- 19.Calnek BW, Schat KA, Ross LJ, Shek WR, Chen CL. Further characterization of Marek’s disease virus-infected lymphocytes. I. In vivo infection. Int J Cancer. 1984;33(3):389–398. doi: 10.1002/ijc.2910330318. [DOI] [PubMed] [Google Scholar]
- 20.Calnek BW, Schat KA. Proliferation of chicken lymphoblastoid cells after in vitro infection with Marek’s disease virus. Avian Dis. 1991;35(4):728–737. [PubMed] [Google Scholar]
- 21.Kaiser P, et al. A genomic analysis of chicken cytokines and chemokines. J Interferon Cytokine Res. 2005;25(8):467–484. doi: 10.1089/jir.2005.25.467. [DOI] [PubMed] [Google Scholar]
- 22.Schneider K, et al. Chicken BAFF—a highly conserved cytokine that mediates B cell survival. Int Immunol. 2004;16(1):139–148. doi: 10.1093/intimm/dxh015. [DOI] [PubMed] [Google Scholar]
- 23.Kothlow S, et al. Unique and conserved functions of B cell-activating factor of the TNF family (BAFF) in the chicken. Int Immunol. 2007;19(2):203–215. doi: 10.1093/intimm/dxl137. [DOI] [PubMed] [Google Scholar]
- 24.Kothlow S, Morgenroth I, Tregaskes CA, Kaspers B, Young JR. CD40 ligand supports the long-term maintenance and differentiation of chicken B cells in culture. Dev Comp Immunol. 2008;32(9):1015–1026. doi: 10.1016/j.dci.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Cihak J, et al. Characterization and functional properties of a novel monoclonal antibody which identifies a T cell receptor in chickens. Eur J Immunol. 1988;18(4):533–537. doi: 10.1002/eji.1830180407. [DOI] [PubMed] [Google Scholar]
- 26.Göbel TW, et al. IL-18 stimulates the proliferation and IFN-gamma release of CD4+ T cells in the chicken: Conservation of a Th1-like system in a nonmammalian species. J Immunol. 2003;171(4):1809–1815. doi: 10.4049/jimmunol.171.4.1809. [DOI] [PubMed] [Google Scholar]
- 27.Tregaskes CA, et al. Conservation of biological properties of the CD40 ligand, CD154 in a non-mammalian vertebrate. Dev Comp Immunol. 2005;29(4):361–374. doi: 10.1016/j.dci.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Jarosinski KW, Arndt S, Kaufer BB, Osterrieder N. Fluorescently tagged pUL47 of Marek’s disease virus reveals differential tissue expression of the tegument protein in vivo. J Virol. 2012;86(5):2428–2436. doi: 10.1128/JVI.06719-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobsen KA, Paramithiotis E, Ewert DL, Ratcliffe MJ. Apoptotic cell death in the chicken bursa of Fabricius. Adv Exp Med Biol. 1996;406:155–165. doi: 10.1007/978-1-4899-0274-0_17. [DOI] [PubMed] [Google Scholar]
- 30.Kaufer BB, Jarosinski KW, Osterrieder N. Herpesvirus telomeric repeats facilitate genomic integration into host telomeres and mobilization of viral DNA during reactivation. J Exp Med. 2011;208(3):605–615. doi: 10.1084/jem.20101402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schat KA, Chen CL, Calnek BW, Char D. Transformation of T-lymphocyte subsets by Marek’s disease herpesvirus. J Virol. 1991;65(3):1408–1413. doi: 10.1128/jvi.65.3.1408-1413.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tischer BK, von Einem J, Kaufer B, Osterrieder N. Two-step red-mediated recombination for versatile high-efficiency markerless DNA manipulation in Escherichia coli. Biotechniques. 2006;40(2):191–197. doi: 10.2144/000112096. [DOI] [PubMed] [Google Scholar]
- 33.Schumacher D, Tischer BK, Fuchs W, Osterrieder N. Reconstitution of Marek’s disease virus serotype 1 (MDV-1) from DNA cloned as a bacterial artificial chromosome and characterization of a glycoprotein B-negative MDV-1 mutant. J Virol. 2000;74(23):11088–11098. doi: 10.1128/jvi.74.23.11088-11098.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oláh I, Nagy N, Vervelde L. 2014. Structure of the Avian Lymphoid System. Avian Immunology, Chapter 2, ed Kaiser KASK (Academic Press, Boston), 2nd Ed, pp 11–44.
- 35.Mwangi WN, et al. Clonal structure of rapid-onset MDV-driven CD4+ lymphomas and responding CD8+ T cells. PLoS Pathog. 2011;7(5):e1001337. doi: 10.1371/journal.ppat.1001337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schat KA, Purchase HG. Cell-culture methods. In: Swayne DE, Glisson JR, Jackwood MW, Pearson JE, Reed WM, editors. A Laboratory Manual for the Isolation and Identification of Avian Pathogens. American Association of Avian Pathologists; Kennett Square, PA: 1998. pp. 223–234. [Google Scholar]
- 37.Martin A, Lillehoj HS, Kaspers B, Bacon LD. Antigen-specific T cell proliferation following coccidia infection. Poult Sci. 1993;72(11):2084–2094. doi: 10.3382/ps.0722084. [DOI] [PubMed] [Google Scholar]
- 38.Petherbridge L, et al. Replication-competent bacterial artificial chromosomes of Marek’s disease virus: Novel tools for generation of molecularly defined herpesvirus vaccines. J Virol. 2003;77(16):8712–8718. doi: 10.1128/JVI.77.16.8712-8718.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Viertlboeck BC, Göbel TW. Chicken thrombocytes express the CD51/CD61 integrin. Vet Immunol Immunopathol. 2007;119(1-2):137–141. doi: 10.1016/j.vetimm.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 40.Luhtala M, Lassila O, Toivanen P, Vainio O. A novel peripheral CD4+ CD8+ T cell population: Inheritance of CD8alpha expression on CD4+ T cells. Eur J Immunol. 1997;27(1):189–193. doi: 10.1002/eji.1830270128. [DOI] [PubMed] [Google Scholar]
- 41.Jarosinski KW, et al. Horizontal transmission of Marek’s disease virus requires US2, the UL13 protein kinase, and gC. J Virol. 2007;81(19):10575–10587. doi: 10.1128/JVI.01065-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jarosinski K, Kattenhorn L, Kaufer B, Ploegh H, Osterrieder N. A herpesvirus ubiquitin-specific protease is critical for efficient T cell lymphoma formation. Proc Natl Acad Sci USA. 2007;104(50):20025–20030. doi: 10.1073/pnas.0706295104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jarosinski KW, Osterrieder N, Nair VK, Schat KA. Attenuation of Marek’s disease virus by deletion of open reading frame RLORF4 but not RLORF5a. J Virol. 2005;79(18):11647–11659. doi: 10.1128/JVI.79.18.11647-11659.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dorange F, El Mehdaoui S, Pichon C, Coursaget P, Vautherot JF. Marek’s disease virus (MDV) homologues of herpes simplex virus type 1 UL49 (VP22) and UL48 (VP16) genes: High-level expression and characterization of MDV-1 VP22 and VP16. J Gen Virol. 2000;81(Pt 9):2219–2230. doi: 10.1099/0022-1317-81-9-2219. [DOI] [PubMed] [Google Scholar]
- 45.Blondeau C, et al. A full UL13 open reading frame in Marek’s disease virus (MDV) is dispensable for tumor formation and feather follicle tropism and cannot restore horizontal virus transmission of rRB-1B in vivo. Vet Res. 2007;38(3):419–433. doi: 10.1051/vetres:2007009. [DOI] [PubMed] [Google Scholar]
- 46.Kaufer BB. Detection of integrated herpesvirus genomes by fluorescence in situ hybridization (FISH) Methods Mol Biol. 2013;1064:141–152. doi: 10.1007/978-1-62703-601-6_10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.