Significance
Netrin-1 has been considered a pro-oncogene due to its oncogenic activity. However, the mechanism by which netrin-1 modulates cancer progression is not well understood. Here, we show that netrin-1 promotes cancer cell proliferation and migration through up-regulating Yes-associated protein (YAP), a downstream target of the Hippo signaling pathway. Further studies suggest that, via uncoordinated-5 homolog B (UNC5B)/deleted in colorectal cancer receptors, netrin-1 enhances YAP stability via promoting phosphatase 1A-induced YAP dephosphorylation, leading to high levels of YAP activity. These results provide a mechanism of netrin-1 oncogenic activity and identify a cross-talk between the Hippo pathway and netrin signaling.
Keywords: netrin-1, YAP, cancer progression
Abstract
Yes-associated protein (YAP), a transcription coactivator, is the major downstream effector of the Hippo pathway, which plays a critical role in organ size control and cancer development. However, how YAP is regulated by extracellular stimuli in tumorigenesis remains incompletely understood. Netrin-1, a laminin-related secreted protein, displays proto-oncogenic activity in cancers. Nonetheless, the downstream signaling mediating its oncogenic effects is not well defined. Here we show that netrin-1 via its transmembrane receptors, deleted in colorectal cancer and uncoordinated-5 homolog, up-regulates YAP expression, escalating YAP levels in the nucleus and promoting cancer cell proliferation and migration. Inactivating netrin-1, deleted in colorectal cancer, or uncoordinated-5 homolog B (UNC5B) decreases YAP protein levels, abrogating cancer cell progression by netrin-1, whereas knockdown of mammalian STE20-like protein kinase 1/2 (MST1/2) or large tumor suppressor kinase 1/2 (Lats1/2), two sets of upstream core kinases of the Hippo pathway, has no effect in blocking netrin-1–induced up-regulation of YAP. Netrin-1 stimulates phosphatase 1A to dephosphorylate YAP, which leads to decreased ubiquitination and degradation, enhancing YAP accumulation and signaling. Hence, our findings support that netrin-1 exerts oncogenic activity through YAP signaling, providing a mechanism coupling extracellular signals to the nuclear YAP oncogene.
The Hippo signaling pathway, first discovered in Drosophila, is a conserved regulator of organ size. A core kinase cascade leading from the tumor suppressor Hippo [mammalian STE20-like protein kinase 1/2 (MST1/2)] to the oncoprotein Yki [Yes-associated protein (YAP) and PDZ-binding motif (TAZ) in mammals], a transcriptional coactivator of target genes involved in cell proliferation and survival, is central to this pathway (1). YAP and TAZ function predominantly through binding to nuclear TEAD transcription factors to initiate expression of genes that promote proliferation and migration (2). YAP is not only a key regulator of organ size/homeostasis but also a well-characterized human oncogene. It is highly expressed via chromosome amplification (3). Transgenic overexpression of YAP in mice liver results in enlarged liver and liver carcinomas (4). During cancer progression, YAP protein levels and its nuclear residency, the key indicator of YAP activity, are also elevated (5). Notably, the active mutation in YAP induces oncogenic cell transformation (6), supporting the oncogenic functions of YAP protein.
Netrin-1, a secreted laminin-related protein, is a bifunctional molecule (7). It acts as a bifunctional regulator on cell survival through binding to or not binding to its receptors [mainly to deleted in colorectal cancer (DCC) and the UNC5 family], which are named dependence receptors (8). In the absence of netrin-1, both DCC and UNC5 trigger apoptosis. However, accumulating evidence supports that binding of netrins and their receptors is engaged in tumorigenesis. This loss of apoptosis as a selective advantage for tumor development could, in the context of dependence receptors, be conferred by at least two events: either by losing receptor expression or by gaining netrin-1 expression. As a matter of fact, DCC and UNC5 receptors are down-regulated in numerous human cancers (9). On the other hand, netrin-1 functions as an oncogene and is up-regulated in various kinds of cancers (10). In netrin-1 transgenic mice, netrin-1 expression in the gastrointestinal tract is associated with tumor initiation and progression (11). Moreover, netrin-1 induces proinvasive tumor-promoting signals in colon cancer cells (12). Clearly, netrins and their dependence receptors are important regulators of tumorigenesis and cancer progression. It should be noticed that upon netrin-1 binding, multiple pathways are activated, including integrins/FAK, PI3K/Akt, ERK1/2, and small GTPase RhoA/Rac-1/Cdc-42 (13).
Although numerous upstream components of the Hippo pathway have been identified, the extracellular ligands and cell surface receptors mediating Hippo pathways remain incompletely understood. Accumulating evidence provides the rationale linking netrin and YAP. For instance, (i) both are highly expressed in cancers, (ii) both mediate cancer cell invasion and migration, (iii) both are related to the key oncogenic kinase Akt, and (iv) both are linked to Rho GTPases (14). In the current report, we show that netrin-1 up-regulates YAP signaling, although DCC and uncoordinated-5 homolog B (UNC5B), mediating netrin-1’s oncogenic activities, are important for understanding the signaling transduction induced by netrin-1.
Results
YAP is Required for Netrin-1 to Promote Cell Proliferation and Migration.
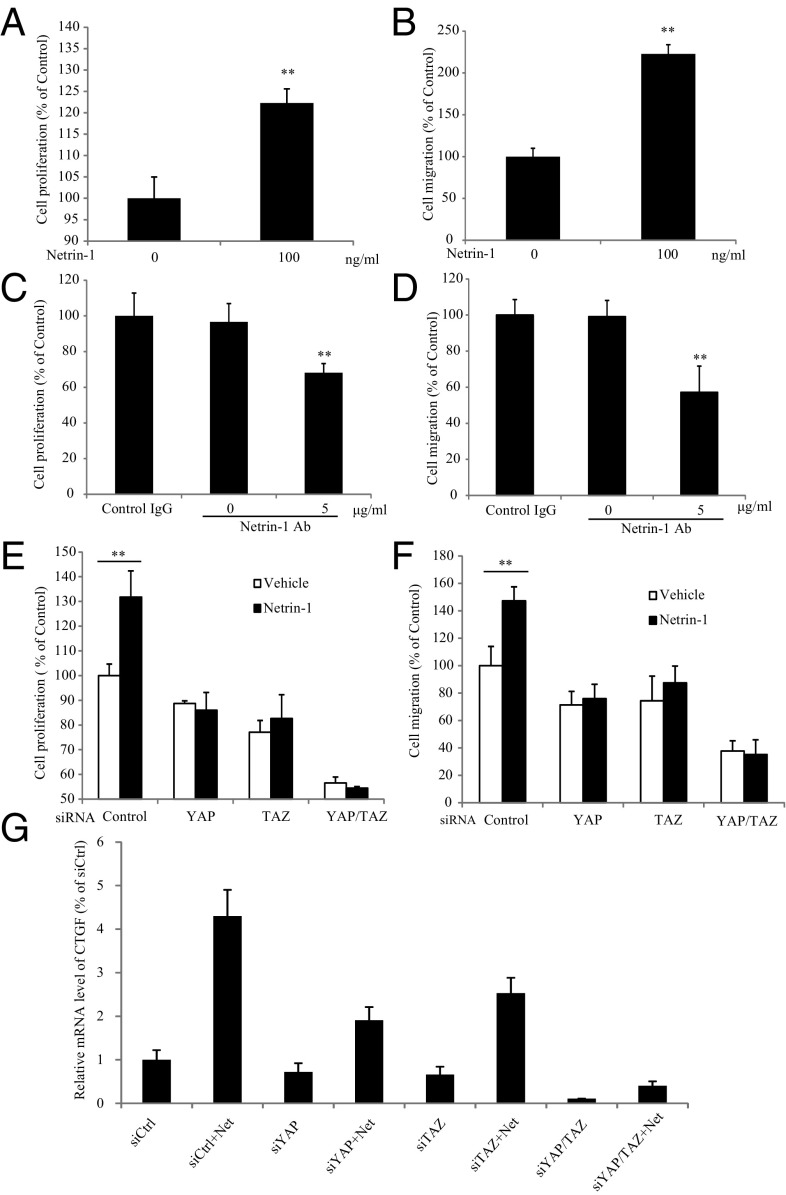
To explore whether netrin-1 mediates cancer cell progression, we monitored netrin-1 and its canonical receptor expression patterns in various cell lines by RT-PCR. UNC5s and DCC were differentially expressed in different cell lines, whereas netrin-1 was expressed in all of the tested cell lines (Fig. S1A). Netrin-1 (100 ng/mL) treatment increased Huh7 hepatocyte carcinoma cell proliferation and migration (Fig. 1 A and B). Neutralization of the secreted netrin-1 in the medium via its specific antibody repressed cell proliferation and migration (Fig. 1 C and D). Netrin-1 also dose-dependently elevated HepG2 hepatocyte carcinoma cell and LN229 glioblastoma cell proliferation and migration (Fig. S1 B and C). The effects of netrin-1 on cell proliferation were also confirmed by BrdU incorporation assay (Fig. S1D). Inhibition of netrin-1 with its specific antibody decreased cell proliferation/migration in LN229, HepG2, and HEK293 cells as well (Fig. S1 E and F). To explore whether the oncogenic proteins YAP and TAZ play any role in netrin-mediated cancer cell progression, we depleted YAP or TAZ or both with their specific siRNAs in Huh7 cells. Knocking down these proteins decreased cell proliferation and migration and abolished the stimulatory effect by netrin-1 compared with vehicle-treated groups (Fig. 1 E and F). The regulatory effect of netrin-1 on YAP/TAZ activity was also confirmed by examining CTGF (connective tissue growth factor), a readout gene whose transcription is initiated by YAP/TAZ (15). As shown in Fig. 1G, netrin-1 elicited a fourfold increase of CTGF expression at the mRNA level, whereas knocking down either YAP or TAZ or both evidently reduced this effect. The activity was strongly eradicated when both YAP and TAZ were depleted. We made the same observation in LN229 cells (Fig. S1 G–J).
Fig. 1.
YAP/TAZ is required for netrin-1 to promote cell proliferation and migration. (A and B) Netrin-1 treatment enhances cell proliferation and migration in Huh7 cells. Following treatment with netrin-1 (100 ng/mL) for 48 h, cell proliferation was examined by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay (A) and transwell migration assay (B), respectively. Data are presented as mean ± SEM (**P < 0.01, two-tailed Student’s t test; n = 3). (C and D) Netrin is required for cell proliferation and migration. Huh7 cells were treated with the antibody of netrin-1 (5 μg/mL) for 48 h. Cell proliferation and migration were examined by MTT assay (C) and transwell cell migration assay (D). Data are presented as mean ± SEM (**P < 0.01, one-way ANOVA; n = 3). (E and F) YAP/TAZ are required for netrin-1 to stimulate cell proliferation and migration. YAP/TAZ were knocked down by their specific siRNAs in Huh7 cells, followed by treatment with or without 100 ng/mL netrin-1 for 48 h. Netrin-1 was replenished every day. Cell proliferation and migration were determined by MTT assay (E) and transwell cell migration assay (F), respectively. Data are presented as mean ± SEM (*P < 0.05, **P < 0.01, two-tailed Student’s t test; n = 3). (G) YAP/TAZ are required for netrin-1 to induce their responsive CTGF gene expression. Following transfection with the indicated siRNA, Huh7 cells were treated by netrin-1 (100 ng/mL) for 2 h. The mRNA levels of the CTGF were measured by RT-PCR as described in Methods. Data are presented as mean ± SEM.
Netrin-1 Up-Regulates YAP Signaling and Induces Its Accumulation in the Nucleus.
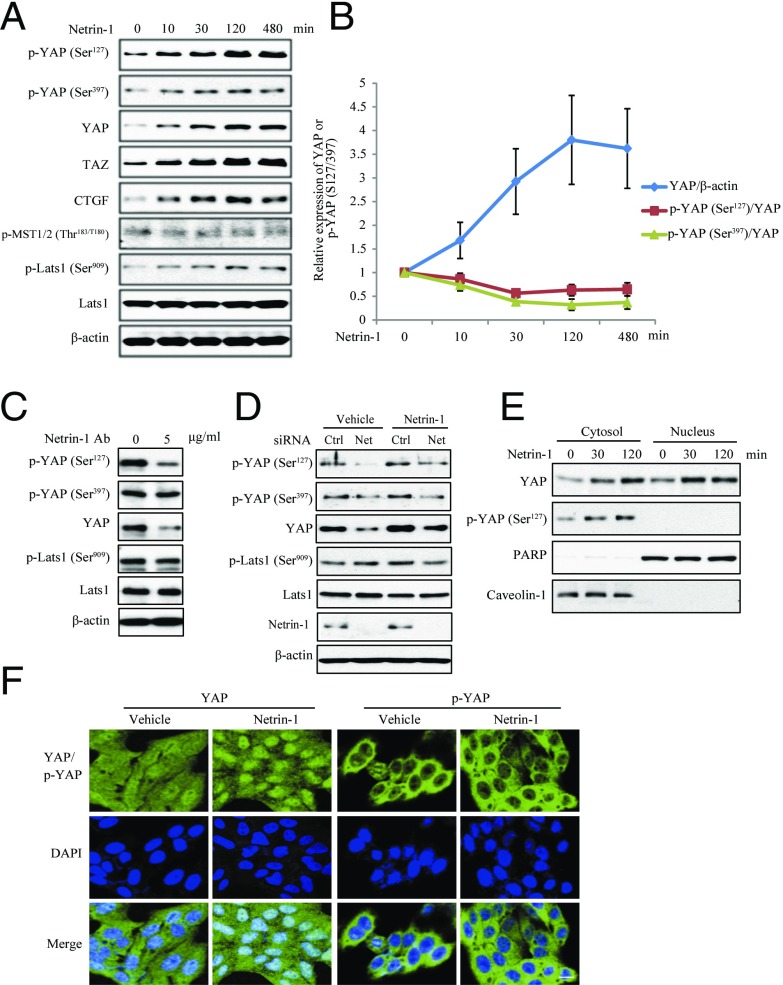
Netrin-1 treatment elicited prominent phospho-YAP S127 signals. By contrast, phospho-YAP S397 was slightly changed (Fig. 2A, Top and Second panels). Remarkably, total levels of YAP and TAZ were robustly elevated in response to netrin treatment, as was the downstream target CTGF (Fig. 2A, Third to Fifth panels). Quantitative analysis indicated that upon netrin-1 treatment, total YAP protein levels were potently escalated, whereas the ratios of phospho-YAP/total YAP were decreased and the reduction of phospho-YAP S397 was much more than that of phospho-YAP S127 (Fig. 2B). To validate indeed that netrin-1 regulates YAP signaling, we antagonized netrin-1 with its specific antibody, which incurred an evident reduction in total YAP and a modest decrease in phospho-YAP S127 (Fig. 2C). To further test the role of netrin-1 in regulating YAP signaling, we knocked down endogenous netrin-1 with its specific siRNA. Depletion of netrin-1 led to suppression of YAP levels and adding the exogenous netrin-1 in the medium partially rescued YAP expressions (Fig. 2D). Subcellular fractionation assay demonstrated that phosphorylated YAP exclusively resided in the cytoplasmic fraction and its signals were increased upon netrin-1 treatment, and YAP levels were augmented in both cytoplasmic and nuclear fractions (Fig. 2E). Immunofluorescent staining also confirmed the accumulation of YAP in the nucleus induced by netrin-1 (Fig. 2F). These similar effects exerted by netrin-1 were observed in HepG2 and LN229 cells (Fig. S2). Thus, netrin-1 increases total YAP levels and up-regulates its nuclear accumulation.
Fig. 2.
Netrin-1 up-regulates YAP expression and accumulation in the nucleus. (A) Netrin-1 induces YAP expression and activity in Huh7 cells. Cells were treated with netrin-1 (100 ng/mL) for the indicated times. Cell lysates were subjected to Western blotting with the indicated antibodies. (B) Quantification of the effect of netrin-1 on YAP expression and YAP (S127/S397) phosphorylation. Data are presented as mean ± SEM from three experiments. (C) Netrin-1 antibody decreases YAP expression in Huh7 cells. The netrin-1 antibody (5 μg/mL) was added into cell culture medium for 48 h. Cell lysates were subjected to Western blotting with the indicated antibodies. (D) Knocking down netrin-1 reduces YAP phosphorylation and its expression. Huh7 cells were transfected with control siRNA or netrin-1 siRNA, followed by treatment with vehicle or netrin-1 (100 ng/mL) for 2 h. Cell lysates were subjected to Western blotting with the indicated antibodies. (E and F) Netrin-1 induced YAP accumulation in the nucleus. Cells were treated with netrin-1 for 30 or 120 min and subjected to subcellular fractionation. Equal amounts of proteins from the nuclear and cytosolic fractions were subjected to immunoblotting with antibodies of PARP and Caveolin-1 (E). Netrin-1–treated cells (120 min) were stained with anti–phospho-YAP (S127) or anti-YAP antibodies, respectively, followed by analysis with immunofluorescence assay under a confocal microscope (F). (Scale bar, 25 μm.)
Netrin-1 Regulates YAP Expression Through UNC5B/DCC.
To explore whether the canonical netrin receptors play any role in up-regulating YAP signaling, we depleted UNC5B in Huh7 cells, as UNC5B is the only detected netrin receptor expressed. Knocking down UNC5B reduced the levels of phospho-YAP S127, phospho-YAP S397, and total YAP regardless of netrin-1 treatment (Fig. 3A). Noticeably, netrin-1 oncogenic activities were diminished when UNC5B was depleted (Fig. 3B and Fig. S3A). Next, we extended the study into LN229 cells that express both DCC and UNC5C. Strikingly, knocking down DCC completely abolished netrin-1–induced YAP up-regulation. Moreover, phospho-large tumor suppressor kinase 1 (Lats1) S909 and phospho-Lats1 S1079 signals were prominently abrogated regardless of netrin-1 treatment (Fig. 3C). In addition, depletion of UNC5C inhibited netrin-1’s activity on phospho-Lats1 S909/phospho-Lats1 S1079 and subsequent phospho-YAP S397, whereas phospho-YAP S127 and total YAP levels were barely affected (Fig. 3C), fitting with the observation that depletion of DCC but not UNC5C abolished netrin-1’s stimulatory activity in cell proliferation and migration (Fig. 3D and Fig. S3 B–D). Remarkably, overexpression of netrin receptors alone enhanced the levels of YAP/TAZ, and netrin-1 treatment further elevated the up-regulation (Fig. 3E). Similar results were observed in HepG2 cells (Fig. S4). To further explore whether the UNC5B receptor is necessary for netrin-1 to exert its stimulatory effects on YAP signaling, we knocked out the UNC5B receptor in the liver of UNC5Bflox/flox mice by infecting the mice with the Cre recombinase lentivirus and i.v. injecting the mice with netrin-1. Knockout of UNC5B in the liver evidently suppressed netrin-1’s effect on phospho-YAP S127 and total YAP and TAZ levels. By contrast, phospho-YAP S397 was only slightly affected regardless of whether UNC5B was knocked out or not (Fig. 3F). Together, these findings support that netrin-1 up-regulates YAP signaling via its canonical receptors.
Fig. 3.
Netrin-1 regulates YAP through netrin receptor UNC5B/DCC. (A) The UNC5B receptor is required for netrin-1–induced YAP up-regulation. Huh7 cells were transfected with control siRNA or UNC5B siRNA and subsequently treated with netrin-1 for 2 h, and cell lysates were subjected to immunoprecipitation/Western blotting with the indicated antibodies. (B) Knockdown of UNC5B abolishes netrin-1–induced cell proliferation in Huh7 cells. Cell proliferation was determined by MTT assay as described in Fig. 1. Data are presented as mean ± SEM (**P < 0.01, two-tailed Student’s t test; n = 3). (C) LN229 cells were transfected with control siRNA or UNC5C/DCC siRNA, followed by treatment with netrin-1 for 2 h, and cell lysates were subjected to immunoprecipitation/Western blotting with the indicated antibodies. (D) Knockdown of DCC abolished netrin-1–induced cell proliferation in LN229 cells. Cell proliferation was determined by MTT assay as described above. Data are presented as mean ± SEM (**P < 0.01, two-tailed Student’s t test; n = 3). (E) Overexpression of the netrin receptor induces YAP phosphorylation and expression. Huh7 cells were transfected with the indicated netrin receptors, followed by treatment with vehicle or netrin-1 for 2 h, and cell lysates were subjected to Western blotting with the indicated antibodies. (F) UNC5B is required for the up-regulation of YAP by netrin-1 in vivo. UNC5Bflox/flox mice were injected with the Cre virus and 8 d later were treated with either vehicle or netrin-1 (1 μg per mouse). Livers were harvested 2 h after treatment and analyzed by Western blotting with the indicated antibody.
Mst1/Mst2 Is Not Required for Netrin-1 to Mediate YAP Signaling.
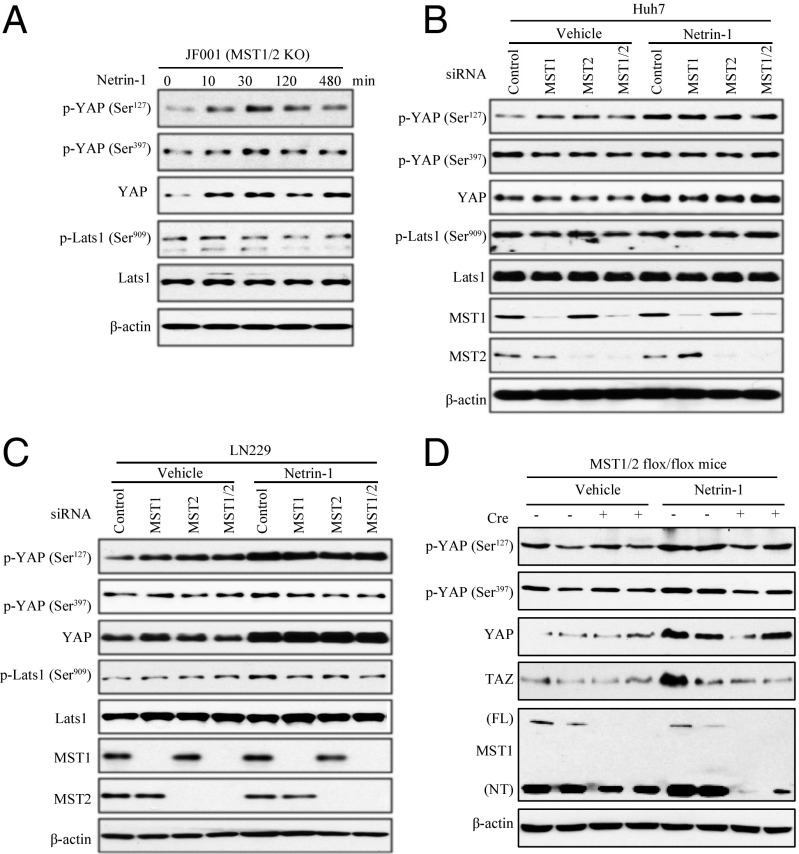
To test whether Mst1/Mst2 plays any role in mediating netrin-1’s effect in YAP up-regulation, we used Mst1/Mst2-null JF001 cells. Netrin-1 treatment elicited a marked up-regulation of YAP protein levels, associated with robust phospho-YAP S127 activation. However, phospho-Lats S909 and phospho-YAP S397, downstream signaling of Mst1/2, were scarcely activated (Fig. 4A), suggesting that Mst1/Mst2 might not be required for netrin-1 to enhance YAP expression. To further explore the role of Mst1/Mst2 in netrin-1’s stimulatory effect on YAP, we depleted Mst1 or Mst2 or both using specific siRNAs. Noticeably, knockdown of either Mst1 or Mst2 or both slightly down-regulated phospho-Lats S909 and phospho-YAP S397 in Huh7 cells (Fig. 4B). Netrin-1 treatment prominently activated phospho-YAP S127 without agitating phospho-Lats1 or phospho-YAP S397. Accordingly, YAP protein levels were strongly increased regardless of whether Mst1 or Mst2 or both were depleted (Fig. 4B). We made the same observation in LN229 (Fig. 4C). Furthermore, we examined the role of MST1/2 in netrin-1–induced YAP up-regulation in vivo with MST1/2flox/flox mice. We knocked out MST1/2 in the liver by infecting the mice with the Cre recombinase lentivirus, and then we treated the mice with netrin-1 intravenously. As shown in Fig. 4D, netrin-1 up-regulated YAP expressions in the livers regardless of MST1/2 status. Thus, these data indicate that Mst1 and Mst2 are not implicated in netrin-1–induced up-regulation of YAP signaling.
Fig. 4.
Regulation of YAP by netrin-1 is independent of Mst1/2. (A) JF001 hepatocyte carcinoma cells (deficiency of MST1/2) were treated with netrin-1 (100 ng/mL) for the indicated time points, and cell lysates were subjected to Western blotting with the indicated antibodies. (B and C) Knocking down Mst1/2 does not affect the up-regulation of YAP by netrin-1. Huh7 (B) or LN229 (C) cells were transfected with control siRNA or MST1 or MST2-specific siRNAs, respectively, followed by treatment with netrin-1 (100 ng/mL) for 2 h, and cell lysates were subjected to Western blotting with the indicated antibodies. (D) Netrin-1–up-regulated YAP expression is independent of MST1/2 in vivo. MST1/2flox/flox mice were injected with the Cre virus and 8 d later were treated with either vehicle or netrin-1 (1 μg per mouse). Livers were harvested 2 h after treatment and analyzed by Western blotting with the indicated antibody.
Netrin-1 Up-Regulates YAP Expression Through Enhancing Its Stability.
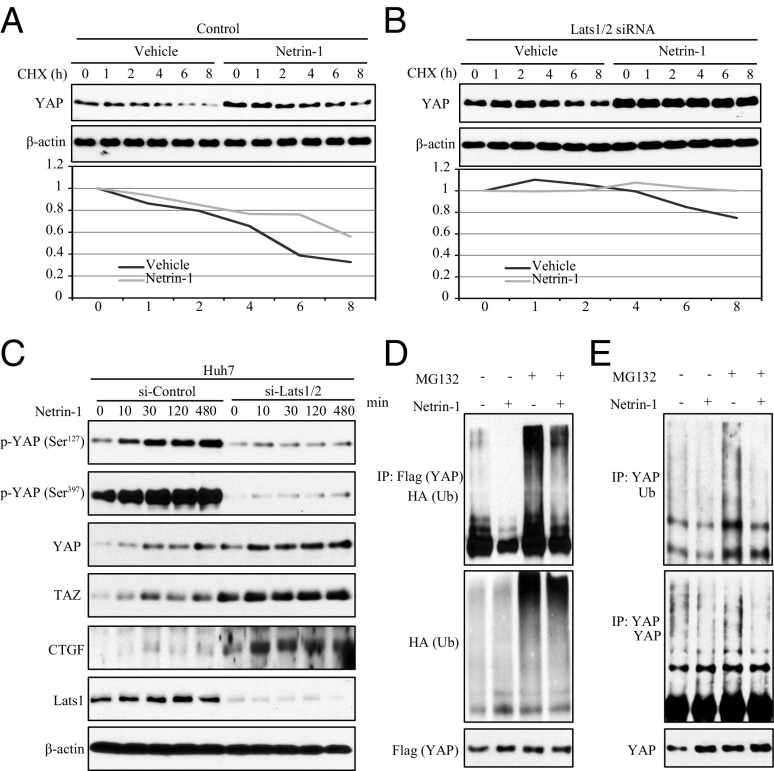
Netrin-1 treatment up-regulated total YAP, whereas the ratios of phospho-YAP S127 and phospho-YAP S397 against total YAP were decreased. Moreover, the decrease of phospho-YAP S397 was much more than that of phospho-YAP S127 (Fig. 2B). It is reported that phosphorylation of YAP Ser127 and YAP Ser397 contributes to YAP degradation and that YAP Ser397 phosphorylation by Lats primes its subsequent phosphorylation by CK1 δ/ε in a phosphordegron, leading to YAP polyubiquitination and degradation (6). Therefore, a plausible assumption is that netrin-1 represses YAP ubiquitination and enhances its stability, leading to YAP accumulation. Quantitative RT-PCR analysis demonstrated that netrin-1 did not regulate either YAP or TAZ mRNA levels in Huh7 or LN229 cells (Fig. S5). Cycloheximide (CHX, a protein synthesis inhibitor) chase assay was then used to study the effect of netrin-1 on YAP stability. Clearly, netrin-1 treatment elevated endogenous YAP protein levels in comparison to the vehicle control (Fig. 5A). As expected, depletion of Lats increased YAP stability. In addition, netrin-1 treatment further escalated the accumulation of YAP (Fig. 5B). Remarkably, netrin-1 up-regulated YAP expression in both control and Last1/2 siRNA-treated groups (Fig. 5C), indicating that Last1/2 is not required for the activity of netrin-1 in stabilizing YAP. Because YAP degradation occurs through the ubiquitin–proteasome proteolytic pathway, we further investigated the regulatory effect of netrin-1 on YAP ubiquitination. As shown in Fig. 5D, netrin-1 treatment decreased YAP ubiquitination in cells independent of MG132. Furthermore, netrin-1 suppressed endogenous YAP ubiquitination, and the inhibition was much more evident in cells treated with MG132 (Fig. 5E). Collectively, these data demonstrate that netrin-1 up-regulates YAP expression but inhibits its ubiquitination and enhances its stability.
Fig. 5.
Netrin-1 suppresses YAP ubiquitination and enhances its stability. (A) Netrin-1 increases the stability of YAP in Huh7 cells. Cells were treated with CHX (50 μg/mL) for the indicated times in the presence or absence of netrin-1 (100 ng/mL). Cell lysates were collected and the expression of YAP was determined by Western blotting. (B) Depletion of Lats1/2 elongates YAP stability by netrin-1. Cells were pretreated with siRNAs of Lats1/2 and then treated with CHX (50 μg/mL) for the indicated times in the presence or absence of netrin-1 (100 ng/mL). Cell lysates were collected, and the expression of YAP was determined by Western blotting. (C) YAP up-regulation by netrin-1 is independent of Lats1/2. Following knockdown of Last1/2 by using siRNA, Huh7 cells were treated with netrin-1 for the indicated times. Cell lysates were collected and subjected to Western blotting with the indicated antibodies. (D) Netrin-1 inhibits exogenous YAP ubiquitination in HEK293 cells. Flag-YAP– and HA-Ub–transfected cells were treated with netrin-1 (100 ng/mL) in the presence and absence of MG132 (25 μM) for 5 h. Flag-YAP–associated proteins were collected using immunoprecipitation with anti-Flag antibody and examined by immunoblotting with the indicated antibodies. (E) Netrin-1 suppresses endogenous YAP ubiquitination in Huh7 cells. Cells were treated as described above, and YAP-associated proteins were collected using immunoprecipitation with anti-YAP antibody and examined by immunoblotting with the indicated antibodies.
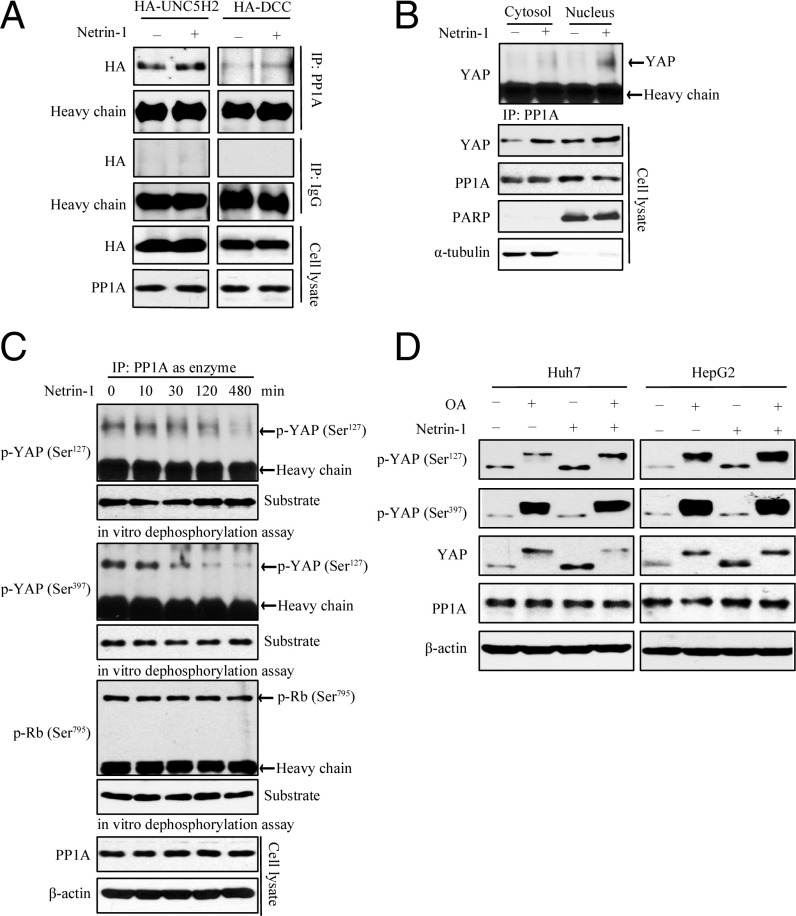
Netrin-1 Stimulates PP1A-Induced YAP Dephosphorylation.
Because phosphorylation of YAP Ser127 and YAP Ser397 plays an important role in YAP nuclear translocation and stability, we further examined the effect of netrin-1 on protein phosphatase 1A (PP1A), which acts as the phosphatase dephosphorylating YAP/TAZ at S127/S89, leading to their oncogenic activity (16). We observed that PP1A interacted with UNC5B and DCC in cells and netrin-1 enhanced the binding of PP1A to UNC5B/DCC receptors (Fig. 6A). The binding of YAP and PP1A has been demonstrated, and we further observed that netrin-1 treatment induced the binding of YAP and PP1A in both the cytoplasm and the nucleus (Fig. 6B). The in vitro phosphatase assay also showed that PP1A activity was induced by netrin-1, leading to dephosphorylation of both phospho-YAP S127 and phospho-YAP S397 (Fig. 6C), correlating with temporal patterns of YAP nuclear translocation and protein up-regulation. However, phospho-Rb Ser795, another substrate of PP1A (17), did not respond to netrin-1 (Fig. 6C), indicating that netrin-1–induced PP1A specifically dephosphorylates YAP but not any other substrates. Remarkably, dephosphorylation of phospho-YAP S397 was more prominent than that of phospho-YAP S127, indicating that netrin-1–induced PP1A might preferentially dephosphorylate phospho-YAP S397, which is the main phosphorylation event responsible for YAP ubiquitination and degradation (6). To further explore the role of PP1A regulated by netrin-1 in YAP up-regulation, we treated cells with netrin-1 in the presence or absence of okadaic acid (OA, a PP1A phosphatase inhibitor). We found that OA blocked the effect of netrin-1 on the up-regulation of YAP and YAP Ser397 was highly phosphorylated compared with YAP Ser127 in cells treated with OA (Fig. 6D), indicating that netrin-1–provoked PP1A activity mediates YAP expression and phospho-YAP Ser397 is more sensitive to the phosphatase activity. Collectively, these data support that netrin-1 enhances PP1A activity and induces YAP dephosphorylation, leading to YAP stabilization and accumulation.
Fig. 6.
Netrin-1 induces YAP dephosphorylation through PP1A. (A) PP1A is associated with UNC5H2/B and DCC in cells. HEK293 cells were transfected with HA-UNC5H2 or HA-DCC. PP1A-associated proteins were pulled down through immunoprecipitation with anti-PP1A antibody and examined with the indicated antibodies. (B) Netrin-1 stimulates the interaction between YAP and PP1A both in the cytoplasm and in the nucleus. Huh7 cells were incubated in the presence or absence of netrin-1 (100 ng/mL), and the cytosolic and the nuclear lysates were immuoprecipitated with the anti-PP1A antibody. The binding of YAP was analyzed by Western blotting. Equal amounts of proteins from the nuclear and cytosolic fractions were determined by antibodies of PARP and α-tubulin, respectively. (C) Netrin-1 enhances the dephosphorylation of YAP via PP1A. Using the antibody against p-YAP Ser127/Ser397-immunoprecipitated samples from Huh7 cells as a substrate, regulation of the activity of PP1A by netrin-1 was determined by in vitro dephosphorylation assay, as described in Methods. (D) OA blocks netrin-1–induced YAP up-regulation, and phosphorylation of YAP Ser397 is highly induced by OA. Huh7 cells were incubated with vehicle or netrin-1 (100 ng/mL) for 120 min in the presence or absence of OA (200 nM). Cell lysates were collected and analyzed by immunoblotting with the indicated antibodies.
Discussion
In the present study, we provide several lines of evidence demonstrating that netrin-1 via its transmembrane canonical receptors UNC5B and DCC enhances YAP activity. Depletion of either YAP or TAZ or both cripples netrin-1’s oncogenic activities on both cell proliferation and migration (Fig. 1). This finding provides an innovative molecular mechanism explaining how netin-1 exerts its oncogenic activity and how YAP signaling is regulated by extracellular signals.
Here we show that levels of phospho-YAP S127 and phopho-YAP S397 were decreased upon netrin-1 treatment when normalized to total YAP (Fig. 2B). However, Lats is also weakly agitated by netrin-1 (Fig. 2 and Fig. S2). Currently, it remains unknown how netrin-1 provokes Lats activation. It has been reported before that netrin-1 regulates PP2A phosphatase activity. The association of the UNC5B/DAP kinase complex with the PP2A complex is negatively regulated by netrin-1 (18). Because PP1A dephosphorylates YAP/TAZ at S127/S89 and induces YAP/TAZ stability and nuclear translocation, leading to their oncogenic activity (19), we then investigated the effect of netrin-1 on PP1A. Interestingly, we observed that UNC5B/DCC associated with PP1A (Fig. 6A). Although the escalation of binding between UNC5B/DCC and PP1A by netrin-1 was not dramatic, netrin-1 significantly induced the binding between YAP and PP1A (Fig. 6B) and triggered PP1A phosphatase activity to selectively dephosphorylate YAP Ser127 and YAP Ser397 (Fig. 6C). Hence, our data support the notion that there may be a complex among UNC5B/DCC, PP1A, and YAP that is involved in the regulation of YAP activity by netrin-1. Upon netrin-1 treatment, PP1A activity was escalated, leading to YAP S127/S397 dephosphorylation and YAP nuclear translocation and stabilization.
Netrin-1 plays a crucial role in neuronal navigation during nervous system development mainly through its interaction with its receptors DCC and UNC5B. Upon netrin-1 binding, DCC receptors mediate chemo-attraction, whereas the UNC5 receptor elicits chemo-repulsive activity (20). However, accumulating evidence demonstrates that both of them belong to a family of dependence receptors that transmit either pro- or antiapoptosis signals depending on the availability of the ligand. In the absence of netrin-1, both DCC and UNC5B act as tumor suppressors through the cleavages of their intracellular portion by caspase activity, and subsequent cellular apoptosis is induced. By contrast, caspase-dependent cellular apoptosis is inhibited upon netrin-1 binding (21). Therefore, it is rational that elimination of netrin-1 suppresses cancer cell proliferation and migration and interferes in YAP signaling (Fig. 2C and Fig. S1 E and F).
In the presence of netrin-1, the multimerized receptors recruit different adapter proteins and activate PI3K/Akt, ERK1/2, and small GTPase RhoA/Rac-1/Cdc-42 to affect cell survival, motility, invasion, and morphogenesis through cytoskeletal rearrangements (22–24). It is reported that PP1A acts downstream of RhoA in LPA-induced YAP dephosphoryaltion (25) and PI3K and ERK1/2 signaling is involved in the regulation of PP1A activity in cancer cells (26, 27). Conceivably, netrin-1 elevates YAP stability through regulating PP1A activity induced by the signaling transduced from the binding of netrin and its receptors. Except for the UNC5 family and DCC, dozens of netrin receptors, such as neogenin, integrin, adenosine A2b, and DSCAM, have been reported (13). Presumably, signaling transmitted from these receptors may exert a different regulatory effect on YAP signaling in a pattern that looks similar to the up-regulation and down-regulation of YAP activity through activating different GPCRs. Indeed, as shown in Fig. 3, UNC5B and UNC5C function differentially in mediating netrin-1’s signaling cascade to YAP. Imaginably, the cancer cells can secrete the intracellular netrin-1 and activate the receptors on the plasma membrane via autocrine, leading to YAP signaling regulation. It is also reported that YAP activity in modulation of proliferation and senescence is regulated by Ras association domain family 1A (RASSF1A) (28) and RASSF4 (29), respectively. The RASSF family of proteins consists of 10 members, most of which are considered as tumor suppressor proteins that undergo loss of expression through promoter methylation in various types of cancers. RASSFs can associate via the SARAH domain with downstream kinases such MST1/MST2 to promote apoptosis (30). Although our data indicate that MST1 and MST2, key members of Hippo pathways, are not required for netrin-1–induced YAP activity (Fig. 4), the regulation of the upstream signaling of MST1/2 by netrin-1 is not excluded. Moreover, RASSF5 plays an important role in mediating apoptosis in response to death receptor ligands (31). Thus, netrin-1, as the ligand of dependence receptors, through up-regulation of YAP activity, may exert the antagonistic action against RASSF.
In summary, we provide compelling evidence demonstrating that netrin-1 provokes YAP activation through its canonical receptors. Moreover, netrin-1 also elevates PP1 phosphatase activity and dephosphorylates YAP, leading to its accumulation in the nucleus and to its enhanced stability. Our findings not only provide insight into the mechanism regulating the complicated YAP signaling but also delineate the downstream signaling for the even more complicated dependence receptors. Certainly, YAP/TAZ oncoproteins, combined with netrin-1 and its dependence receptors, are promising targets for cancer therapy (13).
Methods
Cell Lines and Animal Treatments.
Human liver cancer cells Huh7 and HepG2, human glioblastoma LN229 cells, and human embryonic kidney 293 cells were maintained in DMEM with 10% (vol/vol) FBS and 1× pen/strep/glutamine at 37 °C in a 5% (vol/vol) CO2 atmosphere in a humidified incubator. JF001 cells are typically grown in type I collagen-coated plates, in DMEM:F12 medium supplemented with 5% (vol/vol) FBS, 2.5% (vol/vol) 1× pen/strep, 1 mM sodium pyruvate, 10 mM nicotinamide, insulin (10 μg/mL), IGFII (60 μg/mL), and hEGF (50 ng/mL). The UNC5Bflox/flox (6 wk old; a gift from Dean Y. Li, University of Utah, Salt Lake City, UT) or MST1/2flox/flox mice (The Jackson Laboratory, stock no. 017635) received 200 μL of the phosphate-buffered Cre recombinant lentivirus (1 × 108 pfu per mouse) by tail vein injection. The protocol was reviewed and approved by the Emory Institutional Animal Care and Use Committee. After 8 d, the mice were treated with either vehicle or netrin-1 (1 μg per mouse) via tail vein injection. Livers were harvested 2 h after treatment and analyzed by Western blotting.
Statistics Analysis.
Data are presented as mean ± SEM from three independent experiments. Statistical evaluation was carried out by Student’s t test or one-way ANOVA. Data were considered statistically significant at *P < 0.05 and **P < 0.01. All statistical analyses were performed by Prism (GraphPad Software).
Supplementary Material
Acknowledgments
We thank Dr. Nabeel Bardeesy (Massachusetts General Hospital Cancer Center, Center for Regenerative Medicine, Harvard Medical School) for the JF001 cells. This project was funded in whole or in part with federal funds from National Cancer Institute, National Institutes of Health Grants R01 CA127119 and CA186918 (to K.Y.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1505917112/-/DCSupplemental.
References
- 1.Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19(4):491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao B, Lei QY, Guan KL. The Hippo-YAP pathway: New connections between regulation of organ size and cancer. Curr Opin Cell Biol. 2008;20(6):638–646. doi: 10.1016/j.ceb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Overholtzer M, et al. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci USA. 2006;103(33):12405–12410. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camargo FD, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17(23):2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 5.Steinhardt AA, et al. Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008;39(11):1582–1589. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP) Genes Dev. 2010;24(1):72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serafini T, et al. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78(3):409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- 8.Mehlen P, et al. The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature. 1998;395(6704):801–804. doi: 10.1038/27441. [DOI] [PubMed] [Google Scholar]
- 9.Bernet A, et al. Inactivation of the UNC5C Netrin-1 receptor is associated with tumor progression in colorectal malignancies. Gastroenterology. 2007;133(6):1840–1848. doi: 10.1053/j.gastro.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumartin L, et al. Netrin-1 mediates early events in pancreatic adenocarcinoma progression, acting on tumor and endothelial cells. Gastroenterology. 2010;138(4):1595–1606, 1606.e1–8. doi: 10.1053/j.gastro.2009.12.061. [DOI] [PubMed] [Google Scholar]
- 11.Mazelin L, et al. Netrin-1 controls colorectal tumorigenesis by regulating apoptosis. Nature. 2004;431(7004):80–84. doi: 10.1038/nature02788. [DOI] [PubMed] [Google Scholar]
- 12.Rodrigues S, De Wever O, Bruyneel E, Rooney RJ, Gespach C. Opposing roles of netrin-1 and the dependence receptor DCC in cancer cell invasion, tumor growth and metastasis. Oncogene. 2007;26(38):5615–5625. doi: 10.1038/sj.onc.1210347. [DOI] [PubMed] [Google Scholar]
- 13.Mehlen P, Guenebeaud C. Netrin-1 and its dependence receptors as original targets for cancer therapy. Curr Opin Oncol. 2010;22(1):46–54. doi: 10.1097/CCO.0b013e328333dcd1. [DOI] [PubMed] [Google Scholar]
- 14.Feng X, et al. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer Cell. 2014;25(6):831–845. doi: 10.1016/j.ccr.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan SW, et al. Hippo pathway-independent restriction of TAZ and YAP by angiomotin. J Biol Chem. 2011;286(9):7018–7026. doi: 10.1074/jbc.C110.212621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Royer C, et al. ASPP2 links the apical lateral polarity complex to the regulation of YAP activity in epithelial cells. PLoS ONE. 2014;9(10):e111384. doi: 10.1371/journal.pone.0111384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolupaeva V, Janssens V. PP1 and PP2A phosphatases—Cooperating partners in modulating retinoblastoma protein activation. FEBS J. 2013;280(2):627–643. doi: 10.1111/j.1742-4658.2012.08511.x. [DOI] [PubMed] [Google Scholar]
- 18.Guenebeaud C, et al. The dependence receptor UNC5H2/B triggers apoptosis via PP2A-mediated dephosphorylation of DAP kinase. Mol Cell. 2010;40(6):863–876. doi: 10.1016/j.molcel.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 19.Wang P, et al. PP1A-mediated dephosphorylation positively regulates YAP2 activity. PLoS ONE. 2011;6(9):e24288. doi: 10.1371/journal.pone.0024288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai Wing Sun K, Correia JP, Kennedy TE. Netrins: Versatile extracellular cues with diverse functions. Development. 2011;138(11):2153–2169. doi: 10.1242/dev.044529. [DOI] [PubMed] [Google Scholar]
- 21.Llambi F, Causeret F, Bloch-Gallego E, Mehlen P. Netrin-1 acts as a survival factor via its receptors UNC5H and DCC. EMBO J. 2001;20(11):2715–2722. doi: 10.1093/emboj/20.11.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang X, et al. Netrin-1 mediates neuronal survival through PIKE-L interaction with the dependence receptor UNC5B. Nat Cell Biol. 2008;10(6):698–706. doi: 10.1038/ncb1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He K, Jang SW, Joshi J, Yoo MH, Ye K. Akt-phosphorylated PIKE-A inhibits UNC5B-induced apoptosis in cancer cell lines in a p53-dependent manner. Mol Biol Cell. 2011;22(11):1943–1954. doi: 10.1091/mbc.E10-11-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forcet C, et al. Netrin-1-mediated axon outgrowth requires deleted in colorectal cancer-dependent MAPK activation. Nature. 2002;417(6887):443–447. doi: 10.1038/nature748. [DOI] [PubMed] [Google Scholar]
- 25.Cai H, Xu Y. The role of LPA and YAP signaling in long-term migration of human ovarian cancer cells. Cell Commun Signal. 2013;11(1):31. doi: 10.1186/1478-811X-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geetha T, Langlais P, Caruso M, Yi Z. Protein phosphatase 1 regulatory subunit 12A and catalytic subunit δ, new members in the phosphatidylinositide 3 kinase insulin-signaling pathway. J Endocrinol. 2012;214(3):437–443. doi: 10.1530/JOE-12-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monick MM, et al. Active ERK contributes to protein translation by preventing JNK-dependent inhibition of protein phosphatase 1. J Immunol. 2006;177(3):1636–1645. doi: 10.4049/jimmunol.177.3.1636. [DOI] [PubMed] [Google Scholar]
- 28.Gordon M, et al. The tumor suppressor gene, RASSF1A, is essential for protection against inflammation -induced injury. PLoS ONE. 2013;8(10):e75483. doi: 10.1371/journal.pone.0075483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crose LE, et al. Alveolar rhabdomyosarcoma-associated PAX3-FOXO1 promotes tumorigenesis via Hippo pathway suppression. J Clin Invest. 2014;124(1):285–296. doi: 10.1172/JCI67087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Avruch J, et al. Rassf family of tumor suppressor polypeptides. J Biol Chem. 2009;284(17):11001–11005. doi: 10.1074/jbc.R800073200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park J, et al. Tumor suppressor ras association domain family 5 (RASSF5/NORE1) mediates death receptor ligand-induced apoptosis. J Biol Chem. 2010;285(45):35029–35038. doi: 10.1074/jbc.M110.165506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.