Significance
Declining mobility levels following the Pleistocene had profound effects on human demography, social organization, and health, but the exact timing and pace of this critical change are unknown. Here we examine direct evidence for changing mobility levels from limb bone structural characteristics in a large sample of European skeletons spanning the past 30,000 y. Our results show that mobility first declined during the Neolithic, at the onset of food production, but that the decline was gradual, continuing for several thousand years as agriculture intensified. No change in relative limb strength occurred during the past 2,000 y. Thus, the more gracile modern human skeleton is a result of increased sedentism tied to food production, not subsequent mechanization and industrialization.
Keywords: mobility, Europe, Neolithic, bone strength
Abstract
Increased sedentism during the Holocene has been proposed as a major cause of decreased skeletal robusticity (bone strength relative to body size) in modern humans. When and why declining mobility occurred has profound implications for reconstructing past population history and health, but it has proven difficult to characterize archaeologically. In this study we evaluate temporal trends in relative strength of the upper and lower limb bones in a sample of 1,842 individuals from across Europe extending from the Upper Paleolithic [11,000–33,000 calibrated years (Cal y) B.P.] through the 20th century. A large decline in anteroposterior bending strength of the femur and tibia occurs beginning in the Neolithic (∼4,000–7,000 Cal y B.P.) and continues through the Iron/Roman period (∼2,000 Cal y B.P.), with no subsequent directional change. Declines in mediolateral bending strength of the lower limb bones and strength of the humerus are much smaller and less consistent. Together these results strongly implicate declining mobility as the specific behavioral factor underlying these changes. Mobility levels first declined at the onset of food production, but the transition to a more sedentary lifestyle was gradual, extending through later agricultural intensification. This finding only partially supports models that tie increased sedentism to a relatively abrupt Neolithic Demographic Transition in Europe. The lack of subsequent change in relative bone strength indicates that increasing mechanization and urbanization had only relatively small effects on skeletal robusticity, suggesting that moderate changes in activity level are not sufficient stimuli for bone deposition or resorption.
Declining mobility levels since the Terminal Pleistocene contributed to fundamental changes in demography, health and disease, and social organization among many human populations (1–6). The Neolithic Demographic Transition, characterized by increased fertility, population size, and density, may be partially attributable to decreased energy expenditure associated with greater sedentism (2, 7). Paradoxically, in many cases increased sedentism also may have led to declines in overall health and increased morbidity within populations by facilitating transmission of infectious diseases and through other negative consequences of more dense settlements (3, 4, 6). Reductions in mechanical loading of the skeleton associated with a more sedentary lifestyle may have contributed to the etiology of modern skeletal disorders such as osteoporosis (8–10). Declines in mobility also had significant effects on sociopolitical organization, including sexual division of labor, social hierarchy, and territoriality (1, 11, 12). However, despite its broad evolutionary significance, the timing and patterning of declining mobility during the Holocene and its relationship to changing subsistence economies has proven difficult to characterize from material archaeological remains (1, 5, 7, 13, 14), leaving many unanswered questions. For example, were declines in mobility relatively abrupt at the onset of food production in the Early Neolithic, as suggested by some demographic studies (15), or did they begin earlier, during the Mesolithic (5, 16)? Did mobility continue to decrease after the Neolithic in response to intensification of agriculture and other factors? Is there evidence for continuing declines in mobility to the present time, with recent industrialization and mechanization?
An alternative approach to addressing such issues is to assess directly the evidence preserved in human skeletal remains (17). Although skeletal morphology is determined by a complex interplay between various genetic and environmental factors (18, 19), there is abundant evidence that mechanical loading during life has a strong influence on skeletal structure (20–23). Earlier studies identified declines in skeletal robusticity (strength relative to body size) in Homo throughout the Pleistocene (24, 25), but recent analyses suggest that the major decrease in robusticity occurred later, at the end of the Pleistocene, between early anatomically modern H. sapiens and Holocene populations (26, 27). This suggestion in turn strongly implicates increased sedentism as a major driver in producing the more gracile modern human skeleton (10, 25, 26) and focuses attention on the Holocene as the critical period during which this transformation took place. However, the timing and pace of this change relative to major subsistence and lifestyle transitions cannot be determined from these studies, given their sparse sampling of terminal Pleistocene and Holocene populations.
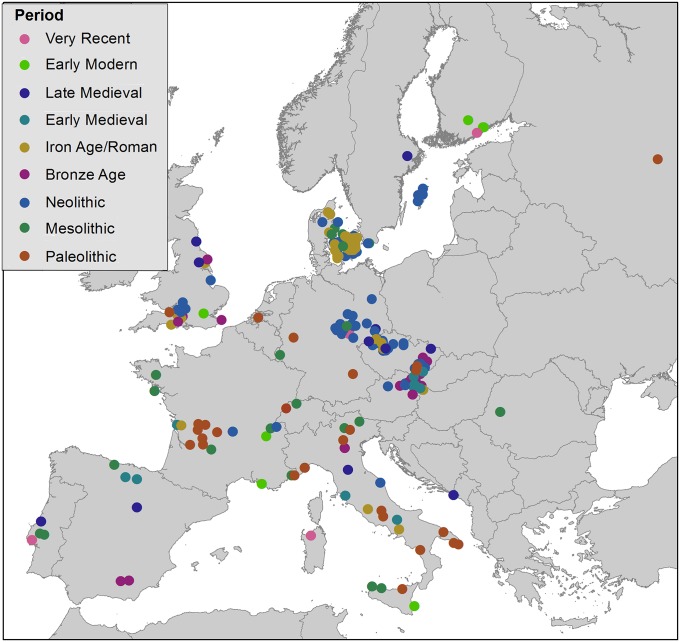
Here we use a sample of 1,842 individuals distributed across Europe to investigate changes in skeletal robusticity and mobility from the Upper Paleolithic [11,000–33,000 calibrated years (Cal y) B.P.] through the 20th century (Fig. 1, Table 1, and Dataset S1). Europe is an appropriate region to carry out such an analysis because of the abundance of well-provenienced skeletal remains and rich archaeological context (28) over the time range of interest. The structural characteristics evaluated here are anteroposterior (A–P) and mediolateral (M–L) section moduli of the midshaft regions of long bones, which are measures of A–P and M–L bending strength (29). It is known that long bone diaphyseal cortices react to imposed mechanical loadings throughout life, changing their cross-sectional geometry to adapt to altered strain magnitudes and distributions, which in turn are dependent on behavioral use of the limbs (20–22, 30, 31). For example, vigorous exercise in humans greatly increases bending strains in the tibia (32, 33) and is associated with preferential strengthening in the direction of movement, i.e., running and jumping lead to increases in A–P/M–L strength (30, 34, 35). Increased A–P/M–L bending strength of the lower limb bones also characterizes more terrestrially mobile populations or subpopulations (36–38). Following this rationale, several previous studies have examined temporal trends in lower limb bone cross-sectional shape, as a proxy for mobility, within Late Pleistocene or Holocene archaeological samples, generally demonstrating declines in A–P/M–L (or maximum/minimum) rigidity or strength (39–42). However, all these studies were limited to particular regions (42), temporal ranges [e.g., Pleistocene through early Holocene (39) or post-Pleistocene (42)], and/or very limited population sampling within the Holocene (40, 41). In addition, none directly compared temporal changes in upper and lower limb bone strength. In this study we examine temporal changes in relative strength of the femur, tibia, and right humerus. Inclusion of the upper limb provides an important control over possible general systemic (e.g., dietary, general activity level) effects on skeletal structure.
Fig. 1.
Location of study sites, by temporal period (see Table 1). For individual listing of sites, see Dataset S1.
Table 1.
Study sample sizes by temporal period
| Period | Date range, Cal y | Males, n | Females, n |
| Very recent | ≥1900 AD | 96 | 58 |
| Early Modern | 1500–1850 AD | 87 | 56 |
| Late Medieval | 1000–1450 AD | 211 | 185 |
| Early Medieval | 600–950 AD | 159 | 122 |
| Iron/Roman | 2,250–1,650 B.P. | 147 | 137 |
| Bronze | 4,350–2,950 B.P. | 120 | 93 |
| Neolithic | 7,300–4,000 B.P. | 170 | 111 |
| Mesolithic | 10,500–5900 B.P. | 39 | 16 |
| Upper Paleolithic | 33,000–11,000 B.P. | 23 | 12 |
| Total | 1,052 | 790 |
We use this large study sample to test the following hypotheses: (i) Changes in skeletal robusticity throughout the Holocene were more marked in the lower limb than in the upper limb, because of the specific effects of mobility on mechanical loading of the lower limb. (ii) Skeletal evidence for declining mobility in the Holocene begins before the Neolithic, in conjunction with subsistence changes associated with the Mesolithic Broad Spectrum Revolution. (iii) The major decline in mobility in the Holocene occurred during the Neolithic, at the initiation of food production. (iv) Declines in mobility during the European Holocene were gradual, extending from early food production in the Neolithic through intensification of food production in the Bronze and Iron Ages. (v) Mobility levels continued to decline through the later Holocene with increasing mechanization and industrialization in Europe.
Results
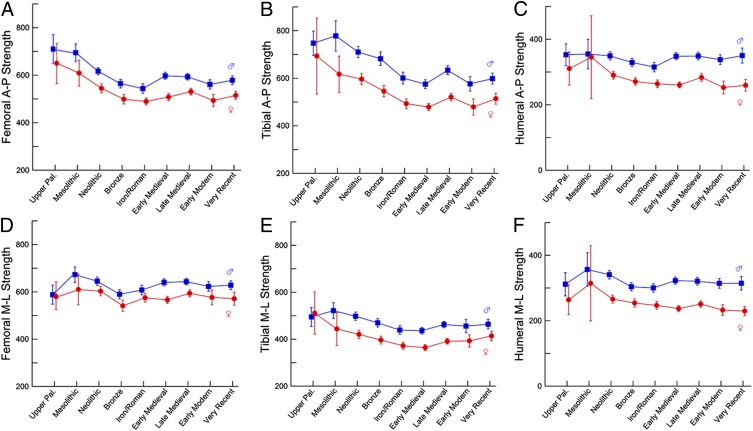
Differences in relative bone strengths between temporal periods are shown graphically in Fig. 2 (also see Table S1). The femur and tibia both show consistent declines in relative A–P bending strength of 20–30% between the Upper Paleolithic and Iron/Roman periods, in both sexes (Fig. 2 A and B). Relative M–L strength of the femur shows no consistent change over the same temporal span, with a 2% decline in females and a 3% increase in males (Fig. 2D). The decline in relative tibial M–L strength through the first five temporal periods is modest in males (12%) and greater in females (27%) (Fig. 2E). There is no consistent change in relative A–P or M–L strength of either bone from the Iron/Roman period through very recent samples (≤10% for all properties). Temporal changes in relative humeral strength are modest in both sexes, with a 10–15% decline in humeral A–P strength between the Upper Paleolithic and Iron/Roman periods (Fig. 2C) and smaller (<10%) changes in M–L strength over this time span (Fig. 2F) and in the strength in either plane after the Iron/Roman period.
Fig. 2.
Temporal trends in bending strength relative to body size [mm3/(kg·mm)·104]. (A) Femoral A–P strength. (B) Tibial A–P strength. (C) Humeral A–P strength. (D) Femoral M–L strength. (E) Tibial M–L strength. (F) Humeral M–L strength. Data are shown as mean ± 95% confidence interval (CI). Males: blue trace; females: red trace. Summary statistics by period are given in Table S1.
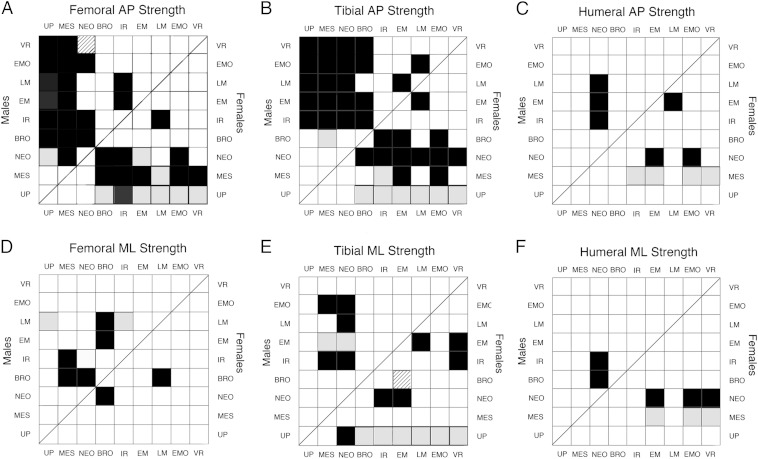
These observations are supported by pairwise comparisons between temporal periods (Fig. 3). The most consistently significant differences, in both sexes, are in femoral and tibial relative A–P strength between the three earliest periods (Upper Paleolithic, Mesolithic, and Neolithic) and the five latest periods (Bronze, Iron/Roman, Early and Late Medieval, early modern, and very recent) (Fig. 3 A and B). Small sample sizes and greater within-period variance in the female Upper Paleolithic sample (Fig. 2 and Table S1) led to nonsignificant Games–Howell test results for most comparisons involving this group. However, if the Upper Paleolithic and Mesolithic groups are combined to increase sample sizes, virtually all Upper Paleolithic/Mesolithic tibial and femoral A–P strength comparisons with later periods are significant using Games–Howell tests in both males and females (Fig. S1). Intertemporal period differences in femoral and tibial M–L strength are less consistent (Fig. 3 D and E and Fig. S1). Humeral relative strengths show only a few, sparsely distributed significant differences between temporal periods (Fig. 3 C and F and Fig. S1). Differences between the Upper Paleolithic and Mesolithic periods are nonsignificant for any property.
Fig. 3.
Results of pairwise comparisons between temporal periods. (A) Femoral A–P strength. (B) Tibial A–P strength. (C) Humeral A–P strength. (D) Femoral M–L strength. (E) Tibial M–L strength. (F) Humeral M–L strength. In all panels, males: Upper Left; females: Lower Right. Black filled squares: significant (P < 0.05) Tukey and Games–Howell; Gray filled squares: significant Tukey only; striped filled squares: significant Games–Howell only. BRO, bronze; EM, Early Medieval; IR, Iron/Roman; LM, Late Medieval; MES, Mesolithic; NEO, Neolithic; UP, Upper Paleolithic; VR, very recent.
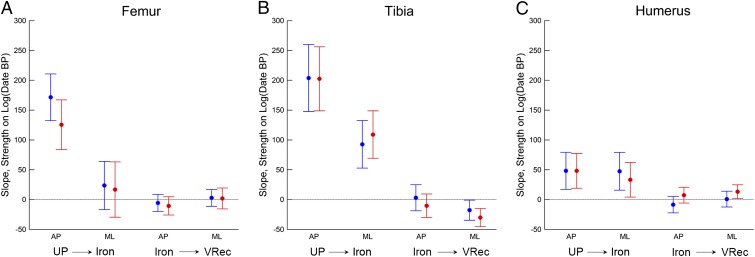
Regressions of bone structural properties on log(date) demonstrate the same general temporal trends (Fig. 4 and Table S2). There are large, highly significant (P < 0.0001) declines in relative A–P strength of the femur and tibia between the Upper Paleolithic and Iron/Roman periods, with no significant decline thereafter (Fig. 4 A and B). Relative M–L strength of the femur shows no significant decline either before or after the Iron/Roman period (Fig. 4A). Relative M–L strength of the tibia shows a significant decline between the Upper Paleolithic and Iron/Roman periods, but the magnitude of this decline is about half that of the decline in A–P strength (Fig. 4B). Relative A–P and M–L strengths of the humerus show significant but much smaller declines between the Upper Paleolithic and Iron/Roman periods and very little change thereafter (Fig. 4C).
Fig. 4.
Regression slopes ± 95% CI for femoral, tibial, and humeral relative strengths against log10(Cal y B.P.) for two temporal ranges: Upper Paleolithic through Iron Age, and Iron Age through very recent (see Table 1). (A) Femur. (B) Tibia. (C) Humerus. Males: blue; females: red. Because time is represented in reverse (years before present), positive slopes indicate negative chronological trends. See Table S2 for associated statistics. UP, Upper Paleolithic; VRec, very recent.
Discussion
Our results support hypotheses i and iv but not ii, iii, or v. We demonstrate a large decline in lower limb bone A–P strength relative to body size beginning in the Neolithic (∼4,000–7,000 Cal y B.P.) and continuing through the Iron/Roman period (∼2,000 Cal y B.P.). Dietary or other systemic influences are unlikely to explain this trend. First, such systemic factors should affect the entire appendicular skeleton, i.e., the upper limbs as well as the lower limbs. However, temporal changes in the humerus over this time range were much smaller and less consistent. It also is difficult to envision how general systemic factors would preferentially affect bone deposition in specific anatomical planes, as we found here for the femur and tibia. Changes in bone shape of this kind strongly imply localized modeling/remodeling resulting from specific mechanical stimuli (20). Despite some partially contrary evidence in cursorial animals (43), which may be under special constraints (20), a number of both experimental and observational studies of humans support the association between preferential strengthening of the lower limb bones in the A–P plane and greater mobility (30, 34–38). Thus, our results imply a major reduction in mobility in both sexes beginning in the Neolithic.
In addition to behavioral differences, another factor that potentially could influence these results is systematic temporal variation in body shape. Linear body proportions, which are known to vary among both Late Pleistocene and Holocene humans (44), do not appear to affect the cross-sectional shape of the lower limb bone significantly (45). However, relative body breadth (pelvic breadth/stature) does affect cross-sectional diaphyseal shape, particularly of the femur (45–47), by altering mechanical loadings in the M–L relative to A–P planes (48). There is significant variation in pelvic breadth/stature among our temporal samples (males, F = 3.07, P = 0.002; females, F = 2.53, P = 0.01), although temporal period means, within sex, vary by less than 5%, and only a very few (5 of 72) pairwise comparisons between periods are significant. Controlling for pelvic breadth/stature has very little effect on regression slopes of relative strengths on log(date) (Fig. S2 and Table S3). Thus, variation in body shape does not explain temporal changes in lower limb bone structure in our sample.
Changes in the European gene pool over the past 30,000 y (49, 50) also might account, at least in part, for the observed variation in limb bone structure. However, unlike craniometric variables (51), limb bone dimensions do not appear to track population history in Europe (52). Recent work also indicates that the general genetic pool from which all Holocene Europeans were derived is ancient, extending back before the earliest specimens in our sample (53) and implying overall continuity despite periodic migrational events.
Thus, the most parsimonious explanation for the temporal patterns observed in our sample is that they reflect a systematic decline in mobility across Europe following the Mesolithic. We found little evidence for any change in relative A–P bending strength of the femur and tibia before the Neolithic, thus arguing against significant reductions in mobility during the Mesolithic, at least in Europe (16). Rather, the first skeletal evidence for increased sedentism appears to be tied to the initiation of food production. However, the reduction in mobility beginning in the Neolithic was gradual, continuing for several thousand years through the Bronze and Iron/Roman period. This result supports archaeological evidence for mixed subsistence economies including significant residential as well as logistical mobility throughout the Neolithic in Europe (54, 55) as well as gradual increases in settlement sizes and other evidence for increased sedentism throughout the Bronze and Iron/Roman periods, as agriculture intensified (56, 57). The introduction of the horse and wheeled vehicles to Europe in the later Neolithic (12, 58–60) likely also reduced lower limb loading in subsequent temporal periods.
This gradual change in mobility does not match the predictions of a relatively abrupt Neolithic Demographic Transition in Europe (15) and thus only partially supports the proposed impact of increased sedentism on this transition (2). The largest previous study of European Holocene samples (n = 248) also documented gradual declines in A–P/M–L bending rigidity of the femur and tibia from the Neolithic through Iron Age or Early Medieval samples in Central Europe (42). No comparisons with earlier (Upper Paleolithic or Mesolithic) or later (post-Early Medieval) samples were carried out in that study, and section properties were estimated only from external bone contours and thus did not account for possible variation occurring on the endosteal surface (61). (In fact, there are significant temporal changes in endosteal relative to periosteal area in our sample; P < 0.0001, ANOVA, all three sections.)
The lack of change found here in relative A–P bending strength of the femur between the Upper Paleolithic and Mesolithic is partially at variance with some previous studies that have documented an increase in circularity of the femoral midshaft in the European Mesolithic (39–41). However, it can be seen from Fig. 2 A and D that such increases in circularity are as much or more a product of increases in relative M–L strength than of decreases in relative A–P strength. The approach adopted here is more effective in partitioning out specific changes in the mechanical variable of most interest from a behavioral standpoint, i.e., relative A–P bending strength. For the same reason, although measures of average bending or torsional strength (i.e., the polar section modulus, Zp) can be used to assess overall limb mechanical loading, they also are less specific with regard to loadings associated with differences in mobility per se.
“Mobility” itself encompasses a number of concepts (1, 5), and it is likely that a combination of distance traveled, speed of travel, and terrain all have effects on relative A–P bending of the lower limb bones. In fact, we have found that terrain itself has an independent effect on femoral and tibial cross-sectional shape in our sample (with increasing A–P bending strength in more mountainous regions), both before and after the Neolithic (62). Such factors likely contributed to local variation in mechanical loading of the lower limb but do not obscure overall temporal patterns across Europe.
The lack of any continuing decrease in relative lower limb bone strength since the Iron/Roman period suggests that the less robust skeleton of modern as compared with Pleistocene humans (25) is a product primarily of the transition from foraging to farming, i.e., increased sedentism, rather than the result of other subsequent cultural/technological innovations such as increasing mechanization. This finding is consistent with recently reported results based on limb bone trabecular structure (10, 26), in which significant differences in bone density were found between Holocene foragers and farmers but not between farmers and a modern industrial sample. Our results for a much more comprehensive Holocene sample indicate that moderate variation in activity level, i.e., between the Iron/Roman period and 20th century, does not affect relative bone strength significantly. This finding has implications for understanding the historical prevalence and etiology of osteoporosis in relation to reduced physical activity among modern populations (8, 9, 63). Studies of age-related changes in trabecular density or cortical bone relative thickness in Iron/Roman through early industrial European samples have produced mixed results, with some reporting less decline than in modern populations (64–66) and others finding no difference or even more negative trends in the earlier samples (67–69). The results of the present study suggest that only very vigorous exercise is a sufficient stimulus for increasing bone strength, as a possible protective mechanism for age-related bone loss (21, 22).
Our findings are strictly relevant to European populations and may not apply to all populations undergoing similar economic and behavioral transitions. However, application of the direct biomechanical approach used in this study has the potential to elucidate patterns of changing mobility in other regions of the world (e.g., ref. 5) that can be inferred only indirectly from archaeological evidence.
Materials and Methods
Skeletal material was obtained from archaeological and historical samples throughout Europe. The locations of the study sample sites, by temporal period, are shown in Fig. 1. Individual sites, locations, and sample sizes are given in Dataset S1. Temporal periods were defined using traditional cultural criteria (28, 70), with chronological overlap between some of the earlier periods (Table 1) because of variation in cultural transition times in different parts of Europe. Geographic representation within each temporal period varies, but every period includes samples from several widely distributed geographic regions. The earliest paleontological or archaeological sites are small, with one or a few individual burials. Later samples are generally larger and derive from cemeteries or, in some very recent samples, autopsy material. Only adults, defined on the basis of epiphyseal fusion of the major long bones, were included. In nonautopsy material, sex was determined using standard osteological features of the pelvis and skull (71). Individuals showing evidence of pathological conditions that could affect long bone diaphyseal structure were eliminated.
A femur, tibia, and right humerus from each individual were included when available. All specimens were positioned relative to standardized anatomical axes (72), and cross-sectional geometric properties determined at 50% of bone length (length'; ref. 72) in the femur and tibia and 35% of bone length from the distal end in the humerus. Not all three diaphyseal sections were preserved in all individuals: A femoral section was preserved in 95%, a tibial section in 85%, and a right humeral section in 69%. Cross-sectional diaphyseal images were obtained using CT or external molding combined with biplanar radiography, and structural properties were determined using custom-designed software (73, 74).
To control for the effects of body size, body mass was estimated from femoral head breadth or reconstructed stature and pelvic breadth (75). Priority was given to the latter technique, which was possible in 58% of the total sample (40–73% within temporal periods). Stature was reconstructed using the anatomical method (76) when possible (39% of total sample; 24–52% within temporal periods) or from long bone lengths using previously derived formulae for Holocene Europeans (75), including consideration of geographic differences in proportions (44, 75). Section moduli were divided by the product of body mass × bone length to derive relative bone strengths (77).
Temporal trends in relative bone strength were assessed both by comparisons between periods and linear regressions of relative strength on log10(Cal y B.P.). Logarithmic transformation of dates was carried out for the latter analyses because of the greatly skewed temporal distribution of samples (Table 1). Given the evidence for different temporal trends before and after the Iron/Roman period (Fig. 2), regression analyses were carried out for temporal periods up through this period and from this period onwards. Both Tukey multiple comparison tests and Games–Howell post hoc tests were used for pairwise comparisons between individual periods. Games–Howell tests do not assume equal variances within temporal groups and so are particularly relevant for some of the smaller earlier samples.
Supplementary Material
Acknowledgments
We thank Erik Trinkaus and Steven Churchill for making available previously collected Upper Paleolithic and Mesolithic data; Trang Diem Vu, Sarah Reedy, Quan Tran, Andrew Merriweather, Danielle Tompkins, Anna-Kaisa Salmi, Tiina Väre, Heli Maijanen, Sirpa Ninimaki, Kati Salo, Jaroslav Roman, and Petra Spevackova for assistance in collecting and/or processing of data; and Andrew Chamberlain, Rob Kruszynski, Jay Stock, Mercedes Okumura, Jane Ellis-Schön, Jacqueline McKinley, Lisa Webb, Jillian Greenaway, Alison Brookes, Jo Buckberry, Chris Knusel, Horst Bruchhaus, Ronny Bindl, Hugo Cardoso, Sylvia Jiménez-Brobeil, Maria Dolores Garralda, Michèle Morgan, Clive Bonsall, Adina Boroneant, Alexandru Vulpe, Monica Zavattaro, Elsa Pacciani, Fulvia Lo Schiavo, Maria Giovanna Belcastro, Alessandro Riga, Nico Radi, Giorgio Manzi, Maryanne Tafuri, Pascal Murail, Patrice Courtaud, Dominique Castex, Fredérik Léterlé, Emilie Thomas, Aurore Schmitt, Aurore Lambert, Sandy Parmentier, Alessandro Canci, Gino Fornaciari, Davide Caramella, Jan Storå, Anna Kjellström, Petra Molnar, Niels Lynnerup, Pia Bennike, Leena Drenzel, Torbjörn Ahlström, Per Karsten, Bernd Gerlach, Lars Larsson, Petr Veleminsky, Maria Teschler-Nicola, and Anna Pankowska for providing access to skeletal collections and facilitating data acquisition. This project was supported by National Science Foundation Grants BCS-0642297 and BCS-0642710, Grant Agency of the Czech Republic Grant 206/09/0589, and the Academy of Finland and Finnish Cultural Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1502932112/-/DCSupplemental.
References
- 1.Kelly RL. Mobility/sedentism – Concepts, archaeological measures, and effects. Annu Rev Anthropol. 1992;21:43–66. [Google Scholar]
- 2.Bocquet-Appel J-P. Explaining the Neolithic demographic transition. In: Bocquet-Appel J-P, Bar-Yosef O, editors. The Neolithic Demographic Transition and its Consequences. Springer; New York: 2008. pp. 35–55. [Google Scholar]
- 3.Cohen MN. Health and the Rise of Civilization. Yale Univ Press; New Haven, CT: 1989. [Google Scholar]
- 4. Cohen MN, Crane-Kramer GMM, eds (2007) Ancient Health: Skeletal Indicators of Agricultural and Economic Intensification (Univ Press of Florida, Gainesville, FL)
- 5.Bar -Yosef O, Belfer-Cohen A. The origins of sedentism and farming communities in the Levant. J World Prehist. 1989;3(4):447–498. [Google Scholar]
- 6.Larsen CS. The agricultural revolution as environmental catastrophe: Implications for health and lifestyle in the Holocene. Quat Int. 2006;150:12–20. [Google Scholar]
- 7.Cohen MN. The Food Crisis in Prehistory. Yale Univ Press; New Haven, CT: 1977. [Google Scholar]
- 8.Mays SA. Osteoporosis in earlier human populations. J Clin Densitom. 1999;2(1):71–78. doi: 10.1385/jcd:2:1:71. [DOI] [PubMed] [Google Scholar]
- 9.Nelson DA, Sauer NJ, Agarwal SC. Evolutionary aspects of bone health. Clin Rev Bone Min Metabol. 2002;1(3-4):169–179. [Google Scholar]
- 10.Ryan TM, Shaw CN. Gracility of the modern Homo sapiens skeleton is the result of decreased biomechanical loading. Proc Natl Acad Sci USA. 2015;112(2):372–377. doi: 10.1073/pnas.1418646112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marean CW. The origins and significance of coastal resource use in Africa and Western Eurasia. J Hum Evol. 2014;77:17–40. doi: 10.1016/j.jhevol.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 12.Milisauskas S, Kruk J. 2002. The Middle Neolithic. European Prehistory: A Survey, ed Milisauskas S (Kluwer Academic/Plenum, New York), pp 193–246.
- 13.Bocquet-Appel J-P, Bar-Yosef O. Prehistoric demography in a time of globalization. In: Bocquet-Appel J-P, Bar-Yosef O, editors. The Neolithic Demographic Transition and its Consequences. Springer; New York: 2008. pp. 1–10. [Google Scholar]
- 14.Bellwood P. First Farmers: The Origin of Agricultural Societies. Blackwell; Oxford: 2005. [Google Scholar]
- 15.Bocquet-Appel JP. Paleoanthropological traces of a neolithic demographic transition. Curr Anthropol. 2002;43:637–650. [Google Scholar]
- 16.Jochim M. The Mesolithic. In: Milisauskas S, editor. European Prehistory: A Survey. Kluwer Academic/Plenum; New York: 2002. pp. 115–141. [Google Scholar]
- 17.Larsen CS. Bioarchaeology: Interpreting Behavior from the Human Skeleton. 2nd Ed Cambridge Univ Press; Cambridge, UK: 2015. [Google Scholar]
- 18.Pearson OM, Lieberman DE. The aging of Wolff’s “law”: Ontogeny and responses to mechanical loading in cortical bone. Am J Phys Anthropol. 2004;47(Suppl 39):63–99. doi: 10.1002/ajpa.20155. [DOI] [PubMed] [Google Scholar]
- 19.Wallace IJ, Tommasini SM, Judex S, Garland T, Jr, Demes B. Genetic variations and physical activity as determinants of limb bone morphology: An experimental approach using a mouse model. Am J Phys Anthropol. 2012;148(1):24–35. doi: 10.1002/ajpa.22028. [DOI] [PubMed] [Google Scholar]
- 20.Ruff C, Holt B, Trinkaus E. Who’s afraid of the big bad Wolff?: “Wolff’s law” and bone functional adaptation. Am J Phys Anthropol. 2006;129(4):484–498. doi: 10.1002/ajpa.20371. [DOI] [PubMed] [Google Scholar]
- 21.Warden SJ, et al. Physical activity when young provides lifelong benefits to cortical bone size and strength in men. Proc Natl Acad Sci USA. 2014;111(14):5337–5342. doi: 10.1073/pnas.1321605111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erlandson MC, et al. Former premenarcheal gymnasts exhibit site-specific skeletal benefits in adulthood after long-term retirement. J Bone Miner Res. 2012;27(11):2298–2305. doi: 10.1002/jbmr.1689. [DOI] [PubMed] [Google Scholar]
- 23.Trinkaus E, Churchill SE, Ruff CB. Postcranial robusticity in Homo. II: Humeral bilateral asymmetry and bone plasticity. Am J Phys Anthropol. 1994;93(1):1–34. doi: 10.1002/ajpa.1330930102. [DOI] [PubMed] [Google Scholar]
- 24.Ruff CB. Mechanical determinants of bone form: Insights from skeletal remains. J Musculoskelet Neuronal Interact. 2005;5(3):202–212. [PubMed] [Google Scholar]
- 25.Ruff CB, Trinkaus E, Walker A, Larsen CS. Postcranial robusticity in Homo. I: Temporal trends and mechanical interpretation. Am J Phys Anthropol. 1993;91(1):21–53. doi: 10.1002/ajpa.1330910103. [DOI] [PubMed] [Google Scholar]
- 26.Chirchir H, et al. Recent origin of low trabecular bone density in modern humans. Proc Natl Acad Sci USA. 2015;112(2):366–371. doi: 10.1073/pnas.1411696112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trinkaus E, Ruff CB. Femoral and tibial diaphyseal cross-sectional geometry in Pleistocene Homo. PaleoAnthropol. 2012;2012:13–62. [Google Scholar]
- 28. Milisauskas S, ed (2002) European Prehistory: A Survey (Kluwer Academic/Plenum, New York)
- 29.Ruff CB. Biomechanical analyses of archaeological human skeletal samples. In: Katzenburg MA, Saunders SR, editors. Biological Anthropology of the Human Skeleton. 2nd Ed. John Wiley and Sons, Inc.; New York: 2008. pp. 183–206. [Google Scholar]
- 30.Shaw CN, Stock JT. Intensity, repetitiveness, and directionality of habitual adolescent mobility patterns influence the tibial diaphysis morphology of athletes. Am J Phys Anthropol. 2009;140(1):149–159. doi: 10.1002/ajpa.21064. [DOI] [PubMed] [Google Scholar]
- 31.Shaw CN, Stock JT. Habitual throwing and swimming correspond with upper limb diaphyseal strength and shape in modern human athletes. Am J Phys Anthropol. 2009;140(1):160–172. doi: 10.1002/ajpa.21063. [DOI] [PubMed] [Google Scholar]
- 32.Carter DR. Anisotropic analysis of strain rosette information from cortical bone. J Biomech. 1978;11(4):199–202. doi: 10.1016/0021-9290(78)90013-1. [DOI] [PubMed] [Google Scholar]
- 33.Burr DB, et al. In vivo measurement of human tibial strains during vigorous activity. Bone. 1996;18(5):405–410. doi: 10.1016/8756-3282(96)00028-2. [DOI] [PubMed] [Google Scholar]
- 34.Macdonald HM, Cooper DM, McKay HA. Anterior-posterior bending strength at the tibial shaft increases with physical activity in boys: Evidence for non-uniform geometric adaptation. Osteoporos Int. 2009;20(1):61–70. doi: 10.1007/s00198-008-0636-9. [DOI] [PubMed] [Google Scholar]
- 35.Rantalainen T, Nikander R, Heinonen A, Suominen H, Sievänen H. Direction-specific diaphyseal geometry and mineral mass distribution of tibia and fibula: A pQCT study of female athletes representing different exercise loading types. Calcif Tissue Int. 2010;86(6):447–454. doi: 10.1007/s00223-010-9358-z. [DOI] [PubMed] [Google Scholar]
- 36.Ruff CB. Sexual dimorphism in human lower limb bone structure: Relationship to subsistence strategy and sexual division of labor. J Hum Evol. 1987;16(5):391–416. [Google Scholar]
- 37.Stock J, Pfeiffer S. Linking structural variability in long bone diaphyses to habitual behaviors: Foragers from the southern African Later Stone Age and the Andaman Islands. Am J Phys Anthropol. 2001;115(4):337–348. doi: 10.1002/ajpa.1090. [DOI] [PubMed] [Google Scholar]
- 38.Stock JT. Hunter-gatherer postcranial robusticity relative to patterns of mobility, climatic adaptation, and selection for tissue economy. Am J Phys Anthropol. 2006;131(2):194–204. doi: 10.1002/ajpa.20398. [DOI] [PubMed] [Google Scholar]
- 39.Holt BM. Mobility in Upper Paleolithic and Mesolithic Europe: Evidence from the lower limb. Am J Phys Anthropol. 2003;122(3):200–215. doi: 10.1002/ajpa.10256. [DOI] [PubMed] [Google Scholar]
- 40.Ruff CB, et al. Body size, body proportions, and mobility in the Tyrolean “Iceman”. J Hum Evol. 2006;51(1):91–101. doi: 10.1016/j.jhevol.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Marchi D, Sparacello V, Shaw C. Mobility and lower limb robusticity of a pastoralist Neolithic population from north-western Italy. In: Pinhasi R, Stock JT, editors. Human Bioarchaeology of the Transition to Agriculture. Wiley-Blackwell; New York: 2011. pp. 317–346. [Google Scholar]
- 42.Macintosh AA, Pinhasi R, Stock JT. Lower limb skeletal biomechanics track long-term decline in mobility across ∼6150 years of agriculture in Central Europe. J Arch Sci. 2014;52:376–390. [Google Scholar]
- 43.Wallace IJ, et al. Exercise-induced bone formation is poorly linked to local strain magnitude in the sheep tibia. PLoS ONE. 2014;9(6):e99108. doi: 10.1371/journal.pone.0099108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holliday TW. Body proportions in Late Pleistocene Europe and modern human origins. J Hum Evol. 1997;32(5):423–448. doi: 10.1006/jhev.1996.0111. [DOI] [PubMed] [Google Scholar]
- 45.Shaw CN, Stock JT. The influence of body proportions on femoral and tibial midshaft shape in hunter-gatherers. Am J Phys Anthropol. 2011;144(1):22–29. doi: 10.1002/ajpa.21363. [DOI] [PubMed] [Google Scholar]
- 46.Weaver TD. The shape of the Neandertal femur is primarily the consequence of a hyperpolar body form. Proc Natl Acad Sci USA. 2003;100(12):6926–6929. doi: 10.1073/pnas.1232340100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davies TG, Stock JT. The influence of relative body breadth on the diaphyseal morphology of the human lower limb. Am J Hum Biol. 2014;26(6):822–835. doi: 10.1002/ajhb.22606. [DOI] [PubMed] [Google Scholar]
- 48.Ruff CB. Biomechanics of the hip and birth in early Homo. Am J Phys Anthropol. 1995;98(4):527–574. doi: 10.1002/ajpa.1330980412. [DOI] [PubMed] [Google Scholar]
- 49.Brandt G, et al. Genographic Consortium Ancient DNA reveals key stages in the formation of central European mitochondrial genetic diversity. Science. 2013;342(6155):257–261. doi: 10.1126/science.1241844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haak W, et al. Members of the Genographic Consortium Ancient DNA from European early neolithic farmers reveals their near eastern affinities. PLoS Biol. 2010;8(11):e1000536. doi: 10.1371/journal.pbio.1000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brewster C, Meiklejohn C, von Cramon-Taubadel N, Pinhasi R. Craniometric analysis of European Upper Palaeolithic and Mesolithic samples supports discontinuity at the Last Glacial Maximum. Nat Commun. 2014;5:4094. doi: 10.1038/ncomms5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.von Cramon-Taubadel N, Stock JT, Pinhasi R. Skull and limb morphology differentially track population history and environmental factors in the transition to agriculture in Europe. Proc R Soc Lond B Biol Sci. 2013;280(1767):20131337. doi: 10.1098/rspb.2013.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seguin-Orlando A, et al. Paleogenomics. Genomic structure in Europeans dating back at least 36,200 years. Science. 2014;346(6213):1113–1118. doi: 10.1126/science.aaa0114. [DOI] [PubMed] [Google Scholar]
- 54.Whittle A. Europe in the Neolithic; The Creation of New Worlds. Cambridge Univ Press; Cambridge, UK: 1996. [Google Scholar]
- 55.Milisauskas S. Early Neolithic, The first farmers in Europe. In: Milisauskas S, editor. European Prehistory: A Survey. Kluwer Academic/Plenum; New York: 2002. pp. 143–192. [Google Scholar]
- 56.Harding AF. The Bronze Age. In: Milisauskas S, editor. European Prehistory: A Survey. Kluwer Academic/Plenum; New York: 2002. pp. 271–334. [Google Scholar]
- 57.Wells P. The Iron Age. In: Milisauskas S, editor. European Prehistory: A Survey. Kluwer Academic/Plenum; New York: 2002. pp. 335–383. [Google Scholar]
- 58.Bakker JA, Kruk J, Lanting AE, Milisauskas S. The earliest evidence of wheeled vehicles in Europe and the Near East. Antiquity. 1999;73(282):778–790. [Google Scholar]
- 59.Ludwig A, et al. Coat color variation at the beginning of horse domestication. Science. 2009;324(5926):485. doi: 10.1126/science.1172750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vilà C, et al. Widespread origins of domestic horse lineages. Science. 2001;291(5503):474–477. doi: 10.1126/science.291.5503.474. [DOI] [PubMed] [Google Scholar]
- 61.Ruff CB, Larsen CS. Long bone structural analyses and reconstruction of past mobility: A historical review. In: Carlson K, Marchi D, editors. Mobility: Interpreting Behavior from Skeletal Adaptations and Environmental Interactions. Springer; New York: 2014. pp. 13–29. [Google Scholar]
- 62.Holt BM, et al. Temporal and geographic variation in robusticity. In: Ruff CB, editor. Skeletal Variation and Adaptation in Europeans: Upper Paleolithic to the Twentieth Century. Wiley-Blackwell; New York: in press. [Google Scholar]
- 63.Mosekilde L. Osteoporosis and exercise. Bone. 1995;17(3):193–195. doi: 10.1016/8756-3282(95)00256-d. [DOI] [PubMed] [Google Scholar]
- 64.Lees B, Molleson T, Arnett TR, Stevenson JC. Differences in proximal femur bone density over two centuries. Lancet. 1993;341(8846):673–675. doi: 10.1016/0140-6736(93)90433-h. [DOI] [PubMed] [Google Scholar]
- 65.Ekenman I, Eriksson SA, Lindgren JU. Bone density in medieval skeletons. Calcif Tissue Int. 1995;56(5):355–358. doi: 10.1007/BF00301601. [DOI] [PubMed] [Google Scholar]
- 66.Poulsen LW, Qvesel D, Brixen K, Vesterby A, Boldsen JL. Low bone mineral density in the femoral neck of medieval women: A result of multiparity? Bone. 2001;28(4):454–458. doi: 10.1016/s8756-3282(01)00407-0. [DOI] [PubMed] [Google Scholar]
- 67.Mays S, Lees B, Stevenson JC. Age-dependent bone loss in the femur in a medieval population. Int J Osteoarchaeol. 1998;8:97–106. [Google Scholar]
- 68.Mays SA. Age-related cortical bone loss in women from a 3rd-4th century AD population from England. Am J Phys Anthropol. 2006;129(4):518–528. doi: 10.1002/ajpa.20365. [DOI] [PubMed] [Google Scholar]
- 69.McEwan JM, Mays S, Blake GM. Measurements of bone mineral density of the radius in a medieval population. Calcif Tissue Int. 2004;74(2):157–161. doi: 10.1007/s00223-002-1047-0. [DOI] [PubMed] [Google Scholar]
- 70.Roberts C, Cox M. Health and Disease in Britain: From Prehistory to the Present Day. Sutton Publishing; Stroud, UK: 2003. [Google Scholar]
- 71.Buikstra JE, Ubelaker DH. Standards for Data Collection from Human Skeletal Remains. Arkansas Archaeological Survey; Fayetteville, AR: 1994. [Google Scholar]
- 72.Ruff CB. Long bone articular and diaphyseal structure in old world monkeys and apes. I: Locomotor effects. Am J Phys Anthropol. 2002;119(4):305–342. doi: 10.1002/ajpa.10117. [DOI] [PubMed] [Google Scholar]
- 73.Sylvester AD, Garofalo E, Ruff C. Technical note: An R program for automating bone cross section reconstruction. Am J Phys Anthropol. 2010;142(4):665–669. doi: 10.1002/ajpa.21299. [DOI] [PubMed] [Google Scholar]
- 74.Ruff CB. 2006 MomentMacro. Available at: www.hopkinsmedicine.org/fae/mmacro.htm. Accessed April 30, 2015.
- 75.Ruff CB, et al. Stature and body mass estimation from skeletal remains in the European Holocene. Am J Phys Anthropol. 2012;148(4):601–617. doi: 10.1002/ajpa.22087. [DOI] [PubMed] [Google Scholar]
- 76.Raxter MH, Auerbach BM, Ruff CB. Revision of the Fully technique for estimating statures. Am J Phys Anthropol. 2006;130(3):374–384. doi: 10.1002/ajpa.20361. [DOI] [PubMed] [Google Scholar]
- 77.Ruff CB. Body size, body shape, and long bone strength in modern humans. J Hum Evol. 2000;38(2):269–290. doi: 10.1006/jhev.1999.0322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.