Significance
The role of nerves in regulating stem cells is largely unknown. Here, we use the touch dome epithelium in skin as a model to study neural regulation of adult stem cells. We find that sensory nerves trophically maintain the touch dome epithelium by signaling with Sonic hedgehog (Shh) to lineage-specific stem cells. This novel aspect of touch dome innervation demonstrates retrograde paracrine signaling to sensory epithelium progenitors by afferent sensory neurons. Indeed, neural Shh is a key regulatory factor in the perineural niche required for long-term renewal of touch dome stem cells. We further demonstrate that Hedgehog upregulation alone is not sufficient to drive malignant expansion of mouse Merkel cells, despite reports of active Hedgehog signaling in Merkel cell carcinoma.
Keywords: stem cell, perineural niche, touch dome, Merkel cell, Hedgehog
Abstract
The touch dome is a highly patterned mechanosensory structure in the epidermis composed of specialized keratinocytes in juxtaposition with innervated Merkel cells. The touch dome epithelium is maintained by tissue-specific stem cells, but the signals that regulate the touch dome are not known. We identify touch dome stem cells that are unique among epidermal cells in their activated Hedgehog signaling and ability to maintain the touch dome as a distinct lineage compartment. Skin denervation reveals that renewal of touch dome stem cells requires a perineural microenvironment, and deleting Sonic hedgehog (Shh) in neurons or Smoothened in the epidermis demonstrates that Shh is an essential niche factor that maintains touch dome stem cells. Up-regulation of Hedgehog signaling results in neoplastic expansion of touch dome keratinocytes but no Merkel cell neoplasia. These findings demonstrate that nerve-derived Shh is a critical regulator of lineage-specific stem cells that maintain specialized sensory compartments in the epidermis.
The skin is one of several tissues in which the epithelium is organized into specialized regions, and each compartment is maintained by lineage-specific stem cells (1). Epithelial–mesenchymal interactions are classically regarded as critical signals in establishing and maintaining epidermal lineage compartments (2). The importance of adjacent dermal mesenchyme in regulating epidermal lineages has been demonstrated in the cycling hair follicle (3) and the interscale epidermis in mouse tail (4). However, other tissues influence epidermal stem cell biology, including subcutaneous fat (5), differentiated keratinocytes (6), and nerves (7). The importance of sensory nerves in sustaining epithelia is suggested by the fact that nerve damage can result in degeneration of the innervated tissue, including the corneal epithelium (8), lingual taste buds (9), and cutaneous Merkel cells (10). In this study, we investigate how neural signals maintain Merkel cell progenitors and sustain the mammalian touch dome lineage in skin.
The touch dome (Haarscheibe) is a specialized epidermal structure that mediates slow-adapting touch sensation (11). In mice, touch domes form in association with guard hair follicles and are found adjacent to adult guard hairs (12). The touch dome is thicker than the surrounding epidermis and has morphologically distinct columnar keratinocytes in the basal layer. Arrayed among the touch dome basal keratinocytes are neuroendocrine Merkel cells that form synapse-like associations with myelinated sensory nerve endings (13). Merkel cells are epithelial cells that express keratin 8 (K8) (14), and originate from keratin 14 (K14)-positive progenitors in the epidermis (15). Touch dome keratinocytes have a distinct molecular profile compared with the surrounding epidermis, including expression of keratin 17 (K17) in basal cells (16). There is ongoing cellular turnover in touch domes, with constant production of stratified epithelial keratinocytes and cyclical expansion of terminally differentiated Merkel cells that coincides with anagen regeneration in the hair cycle (17). Genetic fate mapping has shown that the progenitors maintaining these two cell types are found among the K17+ cells in the touch dome (18), with unipotent atonal homolog 1 (Atoh1)-positive progenitors maintaining the Merkel cells (19). However, the signaling pathways involved in touch dome and Merkel cell homeostasis remain unclear.
Here we use genetic fate mapping to confirm the touch dome is a distinct lineage within the epidermis and show that Gli1+ stem cells maintain touch dome Merkel cells and keratinocytes. Surgical denervation demonstrates that the entire touch dome lineage is dependent on a perineural microenvironment. Notably, a specific requirement for nerve-derived Sonic hedgehog (Shh) in long-term touch dome maintenance is shown via conditional knockout of Shh from dorsal root ganglion (DRG) neurons or of Smoothened (Smo) from the epidermis. Although Shh is necessary for touch dome stem cell renewal, overactivation of Hedgehog (Hh) signaling fails to induce proliferation of Merkel cells. Thus, by signaling to lineage-specific touch dome stem cells with Shh, neurons maintain the specialized sensory epithelium they innervate and sustain functional regionalization of the skin epithelium.
Results
The Touch Dome Epithelium Is Composed of Hh-Responding Cells.
In many adult tissues Hh signaling regulates stem cell populations. Hh ligand binds Patched-1 receptor to disinhibit Smo, allowing nuclear translocation of full-length Gli2 and Gli3 transcription factors to drive the transcription of Hh target genes (20). Gli1 expression is induced by activator forms of Gli2 and Gli3 and is a marker of active Hh signaling. Using juvenile and adult Gli1LacZ reporter mice (n = 8), we determined that only the touch dome contains Hh-responding cells within the interfollicular epidermis (Fig. 1 A and A′). In the touch dome, only rare (<2%) K8+ Merkel cells were Gli1+ (Fig. 1B and Fig. S1 A–C), whereas basal K17+ keratinocytes were prominently Gli1+ (Fig. 1C and Fig. S1 A–D). Scattered Gli1+ dermal cells seen beneath the touch dome were likely S100+ Schwann cells (Fig. S2C). Gli2LacZ/+ reporter mice (n = 4) were used to show that adult touch domes also expressed Gli2 (Fig. S1E), an obligate effector of Hh signaling. In contrast to the touch dome, the remainder of the interfollicular epidermis was negative for Gli1 and Gli2, indicating an absence of Hh signaling. Hh signaling was restricted to the touch dome within the interfollicular epidermis regardless of the phase of the hair cycle. Using K5-tTA;TRE-cre;Smoflox/flox;Gli1LacZ/+ mice (n = 3), we deleted Smo from the entire adult epidermis. Within 7 wk of doxycycline (dox) withdrawal, Gli1 expression was completely absent from the touch dome epithelium (Fig. 1G and Fig. S1F), indicating that touch dome progenitors express keratin 5 (K5) and that touch dome Gli1 expression reflects canonical Hh signaling. Thus, active, Smo-dependent Hh signaling in touch dome keratinocytes and rare Merkel cells distinguishes the touch dome from the surrounding epidermis.
Fig. 1.
Gli1+, Hh-responding stem cells maintain the touch dome in mouse skin. (A and A′) X-Gal whole-mount and section staining in skin from the back of a P59 adult Gli1LacZ/+ mouse. Arrowheads indicate touch domes. (B) β-Galactosidase (β-gal) and K8 staining in skin from the back of an adult Gli1LacZ/+ mouse. (C) X-Gal (false-colored red) with K17 staining in skin from the back of an adult Gli1LacZ/+ mouse. (D) K8 and GFP staining in skin from a Gli1-GIFM (Gli1CreER/+;R26YFP/+) mouse 9 d after tamoxifen induction at P23. Asterisks in B and D indicate nonspecific staining. Yellow arrows in D and E indicate labeled Merkel cells. The red arrows in D indicate unlabeled Merkel cells. (E) K8, K17, and GFP staining in skin from an adult Gli1-GIFM (Gli1CreER/+;R26YFP/+) mouse 3 mo after depilation and tamoxifen induction. (F and F′) X-Gal whole-mount and section staining in skin from the back of a Gli1-GIFM (Gli1CreER/+;R26LacZ/+) mouse 4.5 mo after tamoxifen induction. (G) X-Gal whole-mount staining in skin from adult control and K5-tTA;TRE-cre;Smoflox/flox;Gli1LacZ/+ mice 7 wk after dox withdrawal. (Scale bars, 50 μm for sections; 0.5 mm for whole-mount skin.)
Gli1+ Touch Dome Cells Are Long-Lived Stem Cells for Both K17+ Keratinocytes and K8+ Merkel Cells.
To determine the fate of Hh-responding cells in the touch dome, we used genetic inducible fate mapping (GIFM) with adult Gli1CreER/+;R26LacZ/+ mice (n = 9). After induction with tamoxifen, labeled basal touch dome cells were observed at day 4 (Fig. S2A). These cells had expanded and differentiated to replenish the entire suprabasal squamous epithelium of the touch dome by 13 d after tamoxifen administration. Because new Merkel cells are generated during the anagen phase of the hair cycle (21), we examined Gli1CreER/+;R26YFP/+ Gli1-GIFM mice (n = 5) induced with tamoxifen in early anagen [postnatal day (P)23∼P26]. By 9 d after induction, <10% of K8+ Merkel cells were labeled (Fig. 1D and Fig. S2B), indicating that new Merkel cells arise from Gli1+ progenitors. Interestingly, most of the newly labeled Merkel cells expressed low levels of K8 (Fig. S2B), similar to the cells observed after short-term fate mapping with Atoh1CreER (19), suggesting that both Atoh1 and Gli1 may mark unipotent Merkel cell progenitors in the touch dome. Approximately the same percent of Merkel cells remained labeled 2 mo after induction, because the animals had not yet reached the next anagen phase. Labeled dermal cells beneath the touch dome are likely Schwann cells, based on morphology and S100+ staining (Fig. S2C). We also evaluated Gli1-GIFM mice (n = 6) that were depilated and given tamoxifen to induce anagen at 2 or 4 mo of age (22). By 3 mo after depilation, the animals had undergone two anagen expansions, and >90% of K8+ Merkel cells were labeled (Fig. 1E and Fig. S2D). In both non-depilated and depilated mice, labeled cells were retained in the K17+ basal layer of the touch dome and continued to produce keratinocytes and Merkel cells for up to 2 y (Fig. 1 F and F′ and see Fig. 3B), indicating that self-renewing Gli1+ stem cells maintain both touch dome keratinocytes and Merkel cells. The restriction of labeled cells to the K17+ touch dome epithelium (Fig. 1E and Fig. S2D) demonstrates that Gli1+ touch dome stem cells are lineage restricted and are distinct from the adjacent interfollicular epidermis.
Fig. 3.
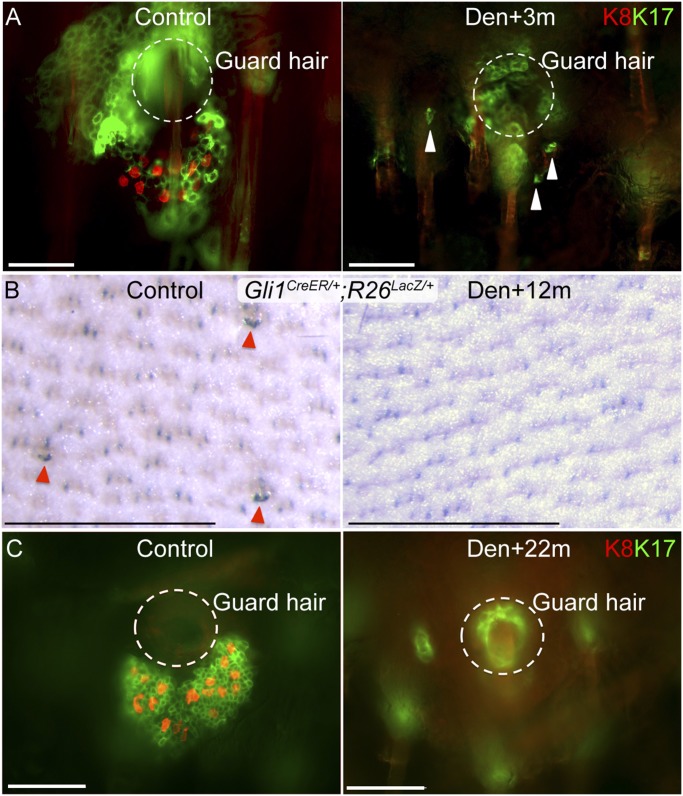
Surgical denervation causes gradual loss of touch dome. (A) K8 and K17 whole-mount staining in mouse skin 3 mo after denervation (Den). The arrowhead indicates residual K17+ touch dome cells. (B) X-Gal whole-mount staining in skin from the back of a Gli1-GIFM (Gli1CreER/+;R26LacZ/+) mouse 12 mo after surgical denervation. Denervation was performed at 2 wk after tamoxifen induction. Arrowheads indicate touch domes. (C) K8 and K17 whole-mount staining in mouse skin 22 mo after denervation. Circles show the location of the guard follicle based on visualization in a deeper focal plane. (Scale bars, 50 μm for immunofluorescent staining; 0.5 mm for whole-mount X-Gal staining.)
Shh-Producing Nerves Are the Source of Hh Signaling to Touch Domes.
We previously identified a subset of cutaneous sensory nerves that express Shh and signal to hair follicles (7). Taking advantage of neuronal Tau expression, we used adult ShhCreGFP/+;TauLacZ-mGFP mice (n = 3) to express a Cre-inducible membrane-bound GFP reporter in Shh-expressing neurons. In these mice, GFP was detected in the touch dome’s Merkel cell–neurite complex (Fig. 2A), but no signal was present in the cutaneous nerves of ShhCreGFP/+ control mice. Because touch dome keratinocytes also contact the nerve terminals that innervate Merkel cells (23), we hypothesized a neural source for Shh signaling to the Gli1+ touch dome stem cells. Indeed, surgical denervation of the dorsal cutaneous nerves completely abrogated Gli1 expression from touch domes in adult Gli1LacZ/+ mice (n = 7) within 2 wk (Fig. 2 B and C). The absence of Gli1 expression was not caused by cell loss, because touch dome morphology and K17+ keratinocyte and K8+ Merkel cell immunostaining remained intact 2 wk after denervation (Fig. S3A). These results demonstrate that Shh-expressing nerves are the source of Hh signaling to the touch dome.
Fig. 2.
Shh from sensory nerves is the source of Hh signaling to touch dome stem cells. (A) GFP and K8 staining at the Merkel cell–neurite complex in skin from an adult ShhCreGFP/+;TauLacZ-mGFP/+ mouse. Asterisks indicate nonspecific staining. (B and C) X-Gal whole-mount staining (B) and section staining (C) in denervated (Den) skin from a Gli1LacZ/+ mouse 2 wk after denervation (Right) and in control skin (Left). Dermal pigment and adjacent guard hairs mark touch dome locations. (Insets) Magnified guard hair follicle opening (circles) and adjacent touch dome (arrowheads). (D) X-Gal whole-mount staining in denervated (Right) and control (Left) skin from a Gli1-GIFM (Gli1CreER/+;R26LacZ/+) mouse 4 wk after denervation. (Scale bars, 50 μm for sections; 0.5 mm for whole-mount skin.)
Neural Shh Is Necessary for Long-Term Homeostasis of the Touch Dome and Its Merkel Cells.
To test the requirement for neural regulation of the touch dome, we first induced adult Gli1-GIFM mice (n = 18) to label the touch dome lineage and then surgically denervated half of the dorsal skin. Labeled cells persisted in the touch dome for more than 4 wk after denervation (Fig. 2D), indicating that touch dome progenitors continued to replenish the lineage for weeks in the absence of neural signals. However, by 6 wk after denervation we noted changes in touch dome morphology and reduced detection of K8 and K17 immunostaining (Fig. S3A), suggesting impaired maintenance of the linage. Within 3 mo of denervation, differentiated K8 and K17 cells of the touch dome were diminished or absent (Fig. 3A), with many guard hairs having no adjacent Merkel cells (mean Merkel cells per touch dome in remaining touch domes = 8.8 versus 20.21 in control skin; P < 0.0001). By 6 and 12 mo after denervation, there were no labeled cells remaining in the epidermis of the Gli1-GIFM mice induced 2 wk before denervation (Fig. 3B), and at 22 mo after denervation no K8+ Merkel cells or K17+ touch dome keratinocytes were detected adjacent to guard hairs (Fig. 3C). These findings demonstrate that the perineural niche microenvironment is necessary for the long-term maintenance of touch dome stem cells.
Because touch domes are closely associated with hair follicles, and nerves regulate a subpopulation of hair follicle stem cells, it is possible that denervation impacts touch dome homeostasis by altering hair follicle biology. To assess the long-term impact of nerve loss on hair follicles and the hair cycle, we assessed Gli1LacZ/+ mice (n = 6) 9 mo after denervation. Persistent absence of Gli1 in the upper bulge region of hair follicles confirmed that nerve regeneration had not occurred (Fig. S3B). In the denervated skin, there was no effect on hair follicle morphology, on the ability of follicles to cycle spontaneously (Fig. S3B), or on the response to depilation. We also crossed an Atoh1 reporter allele to Hairless (Hr) mice to visualize Merkel cells in skin in which hair follicles undergo cystic degeneration and do not cycle at all. Merkel cells formed normal touch domes in Hrhr/hr;Atoh1LacZ/+ mice (n = 2), and innervated K8+ Merkel cells and K17+ keratinocytes were present in touch domes of 9-mo-old animals (Fig. S3C). Touch dome staining in skin from Hrhr/hr;Gli1LacZ/+ mice (n = 3) at 5 mo was indistinguishable from staining in skin from control mice (Fig. S3D), further demonstrating that touch dome maintenance does not require cycling hair follicles. Therefore, the disappearance of the touch dome lineage after denervation is not likely to be caused by unobservable changes in hair follicle biology and most likely is a direct effect of the loss of the perineural microenvironment in the touch dome itself.
Because nerves potentially signal via many mechanisms, we sought to test the specific requirement for neural Shh in touch dome maintenance. We deleted Shh in DRG neurons using Wnt1-Cre;Shhflox/flox mice (n = 11). These mice developed ataxia, likely because of the importance of Shh in cerebellar development, and were smaller than littermate controls. Despite the loss of DRG Shh (Fig. 4D and Fig. S4D), touch dome innervation remained intact (Fig. S4A). At P0 and P4, touch dome Merkel cell number and distribution did not differ in DRG mutant and WT control mice (Fig. 4A), suggesting that normal touch dome development occurs in these mutant mice. However, by P13 reductions in touch dome size and Merkel cell number were evident by whole-mount immunostaining (Fig. S4B). By P18, touch dome size and Merkel cell number were significantly smaller in mutant mice than in WT littermate controls (Fig. 4 B and C). We then evaluated mutant mice whose coats had completed a postnatal anagen phase (P78) and found a continued reduction in Merkel cell number (Fig. 4C), with some guard hairs completely lacking Merkel cells or having only a single adjacent Merkel cell. When these mice had aged to 7 mo, the mean number of Merkel cells in the remaining touch domes was 5.66 (control mean = 28.12; P < 0.0001) (Fig. S4C), and many guard hairs had no adjacent Merkel cells observable by immunostaining. Similar results were observed in AdvillinCre/+;Shhflox/flox mice (n = 8) (Fig. S4 E–G), in which Shh is deleted in peripheral nerves but there is no ataxia or growth defect. Taken together, these results demonstrate that neural Shh is essential for touch dome maintenance after birth and is a critical component of the requisite perineural niche.
Fig. 4.
Nerve-derived Shh signaling is essential for touch dome maintenance. (A) K8 whole-mount staining in P0 WT and Wnt1-Cre;Shhflox/flox mice. (B) K8 and K17 whole-mount staining in P18 WT and Wnt1-Cre;Shhflox/flox mice. (C) The number of K8+ Merkel cells per touch dome (mean ± SEM) in skin from WT and mutant (Mut;, Wnt1-Cre;Shhflox/flox) mice at P18 and P78. (D) Relative expression of Shh mRNA in P16 WT and mutant DRG neurons. (E) The experimental scheme for epidermal deletion of Smo by dox withdrawal. (F) The number of K8+ Merkel cells per touch dome (mean ± SEM) in skin from WT and mutant (Mut; K5-tTA;TRE-cre;Smoflox/flox) mice after Smo deletion at P0 or P21. ***P < 0.0001. (Scale bars, 50 μm.)
To confirm the requirement for Hh signaling to touch dome stem cells, we removed their ability to receive Hh signaling by conditionally deleting Smo in the epidermis using K5-tTA;TRE-cre;Smoflox/flox mice. When dox was withdrawn at P0 (n = 8), touch dome Merkel cell reduction was evident by P18–P23 (Fig. 4 E and F and Fig. S4I), recapitulating the postnatal reduction in touch dome Merkel cells seen in mice lacking Shh in their DRG neurons. To test the effect of epidermal Smo deletion before a postnatal anagen cycle, we withdrew dox at P21 (n = 5), resulting in a reduction of Merkel cells by P70 (Fig. 4 E and F). This phenotype resembles the more protracted Merkel cell loss observed after surgical denervation. By 3.5 mo after dox withdrawal at P0 (n = 3) or 4.5 mo after withdrawal at P21 (n = 2), touch domes often were absent from whole-mount stained skin, with a significant Merkel cell reduction in remaining touch domes (Fig. S4H). Thus, deletion of Hh responsiveness in touch dome progenitors abrogates their ability to maintain the touch dome lineage, further supporting an essential requirement for Shh signaling in the touch dome’s perineural stem cell niche. At the same time, the residual small touch domes seen adjacent to some guard hairs months after deletion of Shh in DRG neurons or Smo in the epidermis contrasts with the almost complete loss of the Gli1 touch dome lineage observed months after skin denervation, suggesting that other perineural factors also may support the touch dome lineage.
Activation of Shh Signaling Is Not Sufficient to Establish or Expand Touch Dome Merkel Cells.
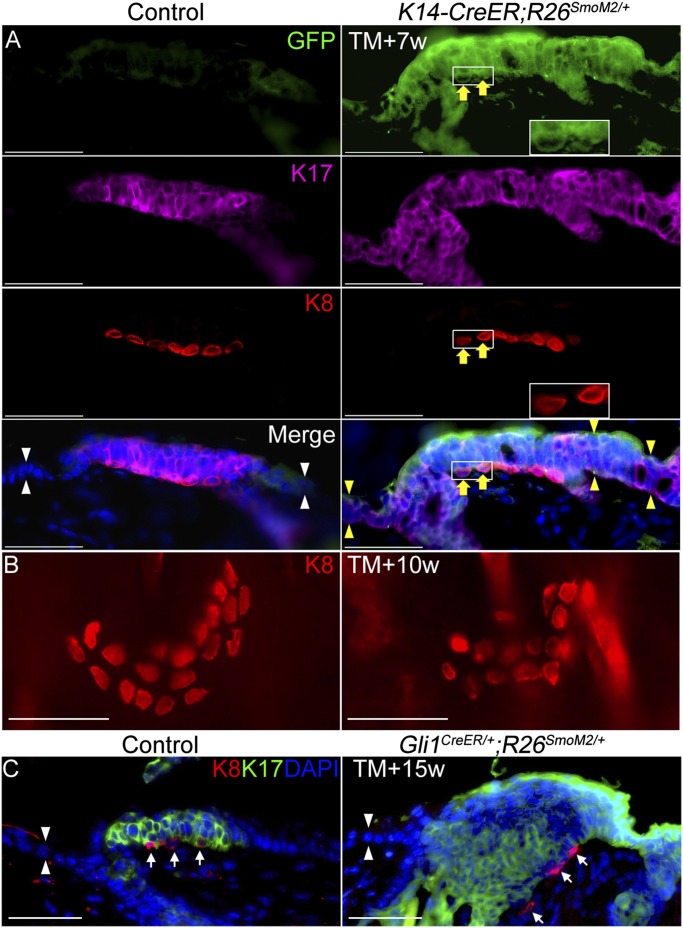
K17 is a known target of Hh signaling (24), and expressing the constitutively active SmoM2 allele to activate Hh signaling in the epidermis causes K17 expression and epidermal thickening (25), recapitulating aspects of the touch dome microenvironment. To test if Hh activation is sufficient to drive Merkel cell production or expansion in the epidermis, we used adult K14-CreER;R26SmoM2/+ mice (n = 8) to activate the Hh signaling pathway in epidermal keratinocytes. Within 7 wk after tamoxifen induction, the SmoM2-YFP fusion gene was expressed in the epidermis, including the touch dome, and in ∼10% of K8+ Merkel cells (Fig. 5A). We also observed K17 expression throughout the thickened neoplastic epidermis (Fig. 5A). However, there was no production of K8+ Merkel cells outside the existing touch domes adjacent to guard hairs (Fig. 5A). By 10 wk, the induced skin began to form K17+ basal cell carcinoma-like growths (26), but there still were no detectable K8-expressing cells outside existing touch domes (Fig. 5B). At 7 and 10 wk after induction, there was hyperplastic expansion of keratinocytes within touch domes, but no increase in Merkel cell number was observed (mean Merkel cells per touch dome = 15.90 at 10 wk after induction for mutant mice versus 14.79 for controls; P = 0.31). By 8 mo after induction, there were marked neoplastic changes in the skin, and the number of Merkel cells per touch dome was significantly reduced (mean Merkel cells per touch dome = 10.00 versus 16.61 for controls, P < 0.0001), suggesting that hyperactivation of Hh signaling and/or the neoplastic microenvironment also are detrimental to long-term Merkel cell maintenance. Using adult Gli1CreER/+;R26SmoM2/+ mice (n = 3), we induced Hh activation in the touch dome while sparing the rest of the epidermis. By 15 wk after tamoxifen induction, we saw neoplastic expansion of touch dome keratinocytes with no obvious change in Merkel cell number (Fig. 5C). In aggregate, these data suggest that inducing two markers of Merkel cell stem cells—Hh signaling and K17 expression—in epidermal progenitors is not sufficient to produce Merkel cell differentiation. Moreover, autonomous activation of Hh signaling in touch dome progenitors can drive neoplastic expansion of touch dome keratinocytes but is not sufficient to cause hyperproliferation of Merkel cells.
Fig. 5.
Autonomous activation of Hh signaling in epidermis induces keratinocyte expansion but not Merkel cell hyperplasia. (A) K8, K17, and GFP staining in vehicle-treated (control) and tamoxifen-treated K14-CreER;R26SmoM2/+ mice 7 wk after tamoxifen induction. The white arrowheads indicate normal epidermis. Yellow arrowheads indicate neoplastic epidermis. (Insets) SmoM2-YFP expressing K8+ Merkel cells. Yellow arrows indicate a GFP-labeled Merkel cell. (B) K8 whole-mount staining in control and tamoxifen-treated K14-CreER;R26SmoM2/+ mice 10 wk after tamoxifen induction. (C) K8 and K17 staining in control and Gli1CreER/+;R26SmoM2/+ mice 15 wk after tamoxifen induction. White arrowheads indicate normal epidermis. White arrows indicate K8+ Merkel cells. (Scale bars, 50 μm.)
Discussion
We found that afferent sensory neurons signal via Shh to stem cells that maintain touch dome keratinocytes and Merkel cells as a distinct lineage compartment in the skin. Furthermore, the perineural microenvironment is necessary for the ongoing regeneration of touch domes, and neural Shh is specifically required for the long-term self-renewal of touch dome stem cells. These results demonstrate that neurons can trophically maintain the specialized sensory epithelia they associate with by signaling directly to lineage-specific epithelial stem cells.
Prior observations showed that damaging cutaneous nerves results in decreased numbers of touch dome Merkel cells in the skin (10, 27). Our results demonstrate that this loss of Merkel cells can be attributed, in part, to a loss of nerve-derived Shh signaling to touch dome stem cells. The gradual loss of the differentiated touch dome lineage over weeks suggests that neural signals are important in the self-renewal of touch dome stem cells but are dispensable for differentiation of existing stem cells. That microenvironmental signals can prevent the exhaustion of somatic stem cells has been observed in other organs with respect to aging (28–30). Our results show a similar failure of stem cell maintenance when neural Shh is removed from the touch dome microenvironment.
There is mounting evidence that the perineural microenvironment is important in regulating stem cell biology. In lower vertebrates, intact nerves are required for blastema-mediated limb regeneration (30). Mammalian wound healing also is impaired by nerve loss (31, 32); however, this impairment may be caused by patterning defects rather than stem cell dysregulation (33). In mice, autonomic innervation is critical for the proper establishment and maintenance of salivary gland stem cells during development (34). In adult bone marrow, nonmyelinating Schwann cells, associated with autonomic nerves, regulate hematopoietic stem cells via TGF-β signaling (35). Shh-producing nerves in particular have been shown to regulate mesenchymal stem cells in the mouse incisor (36) and epithelial stem cells in the upper bulge of the hair follicle (7). In both the tooth and the hair follicle, denervation impacted the function of target stem cells, demonstrating the importance of the perineural microenvironment. However, neither study specifically tested the requirement of neural Shh. It is interesting that Shh-producing nerves regulate stem cells in two epidermal compartments. However, we have shown that the cycling hair follicle does not require neural signals for its ongoing maintenance. In contrast, our conditional Shh and Smo knockout results indicate that stem cell renewal in the touch dome lineage absolutely requires a perineural microenvironment containing Shh signaling.
Our results suggest two populations of Gli1+ touch dome stem cells may maintain K17+ keratinocytes and K8+ Merkel cells independently. If so, neural Shh may be directly necessary for renewal of only one of the populations, and the loss of the other population is caused by secondary changes in the touch dome microenvironment. Although an indirect requirement for Hh signaling is a possibility, the net effect is that nerve-derived Shh is a necessary trophic factor for the maintenance of the entire touch dome lineage compartment.
Patterning and specification of the developing touch dome occurs concomitantly with that of guard hair follicles and requires Eda/Edar signaling in the developing guard hair follicles (12). However, our findings in Hairless mice show that the cycling guard hair is dispensable in the maintenance of adult touch domes. We have shown that neural Shh is critical for supporting adult touch domes. However, the loss of Shh in developing DRG neurons did not impact touch dome development. The concept that neural regulation of the touch dome is exclusively postnatal is consistent with prior studies in which cutaneous innervation was decreased by deletion of Neurotrophin-3 or increased by expression of Neurotrophin-3 in the epidermis. In the mice with fewer nerves, touch dome Merkel cells decreased in number only after birth (37). In mice with more neurons, touch domes grew larger only after birth (38, 39). Thus, it appears touch dome Merkel cells depend on guard follicle signals, but not on nerves, during development and on nerves, but not on follicles, after birth. This scenario contrasts with the hair follicle lineage in which a single adjacent structure, the dermal papilla, is critical in regulating both development and adult maintenance.
Nerve regeneration after injury results in partial restoration of Merkel cell numbers in rat touch domes (40). This restoration suggests that reestablishing neural Shh can expand the degenerated touch domes and restore touch dome homeostasis. However, using SmoM2 to activate Hh signaling aberrantly in the epidermis did not result in an expansion of Merkel cell numbers. In mice and humans, keratinocyte tumors form as a result of Hh activation, and expression of Hh signaling molecules has been reported in some cases of Merkel cell carcinoma (41). Nonetheless, activation of Hh signaling was not sufficient to induce a Merkel cell tumor when SmoM2 was expressed in mouse Merkel cells and their progenitors. Interestingly, the neoplastic growths arising from SmoM2-expressing touch domes resemble trichoblastomas or trichoepitheliomas, benign epidermal tumors that can express K17 (42, 43), can have up-regulation of Gli1 (44), and often are accompanied by scattered Merkel cells (45, 46). Although these similarities are intriguing, we currently have no direct evidence to show that these tumors originate from touch domes in humans.
Overall, our results demonstrate that Shh and the perineural niche are essential for maintaining Gli1+ touch dome stem cells and sustaining the touch dome lineage compartment in the epidermis. Although it has been suggested that nerve-derived Shh can regulate stem cell populations (7, 36), by specifically knocking out Shh from nerves and Smo from the epidermis, we definitively show that Shh has an essential role in driving the renewal of touch dome stem cells. This novel aspect of the Merkel cell neurite complex demonstrates that, in addition to afferent sensory signaling, there is retrograde paracrine signaling to touch dome progenitors. By understanding the microenvironmental requirements for Merkel cell production in the touch dome, we gain insight into how specialized sensory structures in the peripheral nervous system are maintained and how sensory nerves can be critical regulators of stem cells and lineage organization within epithelia. We anticipate that further insights into this neuroendocrine stem cell niche will facilitate efforts to culture Merkel cells for in vitro studies and will advance our understanding of Merkel cell carcinoma formation and maintenance.
Materials and Methods
Animals.
Mice were housed and bred on an outcrossed Swiss Webster background in a pathogen-free facility at the National Cancer Institute (NCI), Bethesda, MD. All experiments were performed in accordance with institutional guidelines according to protocols approved by the National Cancer Institute’s Institutional Animal Care and Use Committee.
Quantification of Merkel Cells.
The numbers of Merkel cells per touch dome were assessed by direct visualization of immunofluorescently stained K8+ Merkel cells in whole-mount tissue based on counting >200 Merkel cells. Double-stained Merkel cells in sectioned tissues are reported as estimates based on counting >50 Merkel cells.
Statistical Analyses.
Population datasets are shown as the mean value; error bars represent the SEM. For comparisons between sets, an unpaired, two-tailed t test was applied.
Details about animals, animal treatments, tissue processing, immunostaining, surgical denervation, and Merkel cell quantification are given in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Alexandra Joyner (Memorial Sloan Kettering Cancer Center) for supplying mice and for extensive scientific discussions, Dr. Sunny Wong (University of Michigan) and Dr. Mark Udey (NCI) for scientific discussions and critical reading of the manuscript, Dr. Amy Coxon and Mr. Eric Wang for technical assistance, and the Center of Cancer Research (CCR) Confocal Microscopy Core Facility for assistance in confocal imaging. This work was supported by the NIH Intramural Research Program, CCR, NCI.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1504177112/-/DCSupplemental.
References
- 1.Page ME, Lombard P, Ng F, Göttgens B, Jensen KB. The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell. 2013;13(4):471–482. doi: 10.1016/j.stem.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu S, Zhang H, Duan E. Epidermal development in mammals: Key regulators, signals from beneath, and stem cells. Int J Mol Sci. 2013;14(6):10869–10895. doi: 10.3390/ijms140610869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan BA. 2014. The dermal papilla: An instructive niche for epithelial stem and progenitor cells in development and regeneration of the hair follicle. Cold Spring Harb Perspect Med 4(7):a015180.
- 4.Gomez C, et al. The interfollicular epidermis of adult mouse tail comprises two distinct cell lineages that are differentially regulated by Wnt, Edaradd, and Lrig1. Stem Cell Reports. 2013;1(1):19–27. doi: 10.1016/j.stemcr.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Festa E, et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146(5):761–771. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu YC, Pasolli HA, Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell. 2011;144(1):92–105. doi: 10.1016/j.cell.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brownell I, Guevara E, Bai CB, Loomis CA, Joyner AL. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell. 2011;8(5):552–565. doi: 10.1016/j.stem.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ueno H, et al. Dependence of corneal stem/progenitor cells on ocular surface innervation. Invest Ophthalmol Vis Sci. 2012;53(2):867–872. doi: 10.1167/iovs.11-8438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeda M, Suzuki Y, Obara N, Nagai Y. Apoptosis in mouse taste buds after denervation. Cell Tissue Res. 1996;286(1):55–62. doi: 10.1007/s004410050674. [DOI] [PubMed] [Google Scholar]
- 10.Nurse CA, Macintyre L, Diamond J. A quantitative study of the time course of the reduction in Merkel cell number within denervated rat touch domes. Neuroscience. 1984;11(2):521–533. doi: 10.1016/0306-4522(84)90042-3. [DOI] [PubMed] [Google Scholar]
- 11.Smith KR., Jr The structure and function of the Haarscheibe. J Comp Neurol. 1967;131(4):459–474. doi: 10.1002/cne.901310406. [DOI] [PubMed] [Google Scholar]
- 12.Vielkind U, Sebzda MK, Gibson IR, Hardy MH. Dynamics of Merkel cell patterns in developing hair follicles in the dorsal skin of mice, demonstrated by a monoclonal antibody to mouse keratin 8. Acta Anat (Basel) 1995;152(2):93–109. doi: 10.1159/000147688. [DOI] [PubMed] [Google Scholar]
- 13.Maksimovic S, et al. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature. 2014;509(7502):617–621. doi: 10.1038/nature13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maricich SM, et al. Merkel cells are essential for light-touch responses. Science. 2009;324(5934):1580–1582. doi: 10.1126/science.1172890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Keymeulen A, et al. Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis. J Cell Biol. 2009;187(1):91–100. doi: 10.1083/jcb.200907080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woo SH, Stumpfova M, Jensen UB, Lumpkin EA, Owens DM. Identification of epidermal progenitors for the Merkel cell lineage. Development. 2010;137(23):3965–3971. doi: 10.1242/dev.055970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakafusa J, et al. Changes in the number of Merkel cells with the hair cycle in hair discs on rat back skin. Br J Dermatol. 2006;155(5):883–889. doi: 10.1111/j.1365-2133.2006.07441.x. [DOI] [PubMed] [Google Scholar]
- 18.Doucet YS, Woo SH, Ruiz ME, Owens DM. The touch dome defines an epidermal niche specialized for mechanosensory signaling. Cell Reports. 2013;3(6):1759–1765. doi: 10.1016/j.celrep.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright MC, et al. Unipotent, Atoh1+ progenitors maintain the Merkel cell population in embryonic and adult mice. J Cell Biol. 2015;208(3):367–379. doi: 10.1083/jcb.201407101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merchant AA, Matsui W. Targeting Hedgehog—a cancer stem cell pathway. Clin Cancer Res. 2010;16(12):3130–3140. doi: 10.1158/1078-0432.CCR-09-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moll I, Paus R, Moll R. Merkel cells in mouse skin: Intermediate filament pattern, localization, and hair cycle-dependent density. J Invest Dermatol. 1996;106(2):281–286. doi: 10.1111/1523-1747.ep12340714. [DOI] [PubMed] [Google Scholar]
- 22.Plikus MV, et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451(7176):340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iggo A, Muir AR. The structure and function of a slowly adapting touch corpuscle in hairy skin. J Physiol. 1969;200(3):763–796. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Callahan CA, et al. MIM/BEG4, a Sonic hedgehog-responsive gene that potentiates Gli-dependent transcription. Genes Dev. 2004;18(22):2724–2729. doi: 10.1101/gad.1221804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grachtchouk V, et al. The magnitude of hedgehog signaling activity defines skin tumor phenotype. EMBO J. 2003;22(11):2741–2751. doi: 10.1093/emboj/cdg271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Youssef KK, et al. Identification of the cell lineage at the origin of basal cell carcinoma. Nat Cell Biol. 2010;12(3):299–305. doi: 10.1038/ncb2031. [DOI] [PubMed] [Google Scholar]
- 27.English KB, Kavka-Van Norman D, Horch K. Effects of chronic denervation in type I cutaneous mechanoreceptors (Haarscheiben) Anat Rec. 1983;207(1):79–88. doi: 10.1002/ar.1092070109. [DOI] [PubMed] [Google Scholar]
- 28.Conboy IM, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433(7027):760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 29.Ryu BY, Orwig KE, Oatley JM, Avarbock MR, Brinster RL. Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells. 2006;24(6):1505–1511. doi: 10.1634/stemcells.2005-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voog J, Jones DL. Stem cells and the niche: A dynamic duo. Cell Stem Cell. 2010;6(2):103–115. doi: 10.1016/j.stem.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohammad KS, Neufeld DA. Denervation retards but does not prevent toetip regeneration. Wound Repair Regen. 2000;8(4):277–281. doi: 10.1046/j.1524-475x.2000.00277.x. [DOI] [PubMed] [Google Scholar]
- 32.Barker AR, Rosson GD, Dellon AL. Wound healing in denervated tissue. Ann Plast Surg. 2006;57(3):339–342. doi: 10.1097/01.sap.0000221465.69826.b7. [DOI] [PubMed] [Google Scholar]
- 33.Rinkevich Y, et al. Clonal analysis reveals nerve-dependent and independent roles on mammalian hind limb tissue maintenance and regeneration. Proc Natl Acad Sci USA. 2014;111(27):9846–9851. doi: 10.1073/pnas.1410097111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knox SM, et al. Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science. 2010;329(5999):1645–1647. doi: 10.1126/science.1192046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamazaki S, et al. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147(5):1146–1158. doi: 10.1016/j.cell.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 36.Zhao H, et al. Secretion of shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell. 2014;14(2):160–173. doi: 10.1016/j.stem.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Airaksinen MS, et al. Specific subtypes of cutaneous mechanoreceptors require neurotrophin-3 following peripheral target innervation. Neuron. 1996;16(2):287–295. doi: 10.1016/s0896-6273(00)80047-1. [DOI] [PubMed] [Google Scholar]
- 38.Albers KM, et al. Cutaneous overexpression of NT-3 increases sensory and sympathetic neuron number and enhances touch dome and hair follicle innervation. J Cell Biol. 1996;134(2):487–497. doi: 10.1083/jcb.134.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krimm RF, Davis BM, Woodbury CJ, Albers KM. NT3 expressed in skin causes enhancement of SA1 sensory neurons that leads to postnatal enhancement of Merkel cells. J Comp Neurol. 2004;471(3):352–360. doi: 10.1002/cne.20041. [DOI] [PubMed] [Google Scholar]
- 40.Nurse CA, Macintyre L, Diamond J. Reinnervation of the rat touch dome restores the Merkel cell population reduced after denervation. Neuroscience. 1984;13(2):563–571. doi: 10.1016/0306-4522(84)90249-5. [DOI] [PubMed] [Google Scholar]
- 41.Brunner M, et al. Expression of hedgehog signaling molecules in Merkel cell carcinoma. Head Neck. 2010;32(3):333–340. doi: 10.1002/hed.21191. [DOI] [PubMed] [Google Scholar]
- 42.Anderson-Dockter H, et al. Diagnostic utility of cytokeratin 17 immunostaining in morpheaform basal cell carcinoma and for facilitating the detection of tumor cells at the surgical margins. Dermatolc Surg. 2012;38(8):1357–1366. doi: 10.1111/j.1524-4725.2012.02417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Battistella M, Peltre B, Cribier B. 2013 PHLDA1, a follicular stem cell marker, differentiates clear-cell/granular-cell trichoblastoma and clear-cell/granular cell basal cell carcinoma: A case-control study, with first description of granular-cell trichoblastoma. Am J Dermatopathol 36(8):643–650. [Google Scholar]
- 44.Hatta N, et al. Molecular diagnosis of basal cell carcinoma and other basaloid cell neoplasms of the skin by the quantification of Gli1 transcript levels. J Cutan Pathol. 2005;32(2):131–136. doi: 10.1111/j.0303-6987.2005.00264.x. [DOI] [PubMed] [Google Scholar]
- 45.Schulz T, Hartschuh W. Merkel cells are absent in basal cell carcinomas but frequently found in trichoblastomas. An immunohistochemical study. J Cutan Pathol. 1997;24(1):14–24. doi: 10.1111/j.1600-0560.1997.tb00780.x. [DOI] [PubMed] [Google Scholar]
- 46.Hartschuh W, Schulz T. Merkel cells are integral constituents of desmoplastic trichoepithelioma: An immunohistochemical and electron microscopic study. J Cutan Pathol. 1995;22(5):413–421. doi: 10.1111/j.1600-0560.1995.tb00756.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.