Significance
The role of B-cell lymphoma-2 (BCL-2) ovarian killer (BOK) in apoptosis regulation has been a long-standing enigma. Despite the homology to BAX and BAK, BOK has yet to be linked to a definitive physiologic function in the classical apoptotic pathway. Here, we report a selective role for BOK in promoting mitochondrial apoptosis in response to endoplasmic reticulum stress.
Keywords: BOK, apoptosis, BCL-2 family, endoplasmic reticulum stress, unfolded protein response
Abstract
B-cell lymphoma 2 (BCL-2) ovarian killer (BOK) is a BCL-2 family protein with high homology to the multidomain proapoptotic proteins BAX and BAK, yet Bok−/− and even Bax−/−Bok−/− and Bak−/−Bok−/− mice were reported to have no overt phenotype or apoptotic defects in response to a host of classical stress stimuli. These surprising findings were interpreted to reflect functional compensation among the BAX, BAK, and BOK proteins. However, BOK cannot compensate for the severe apoptotic defects of Bax−/−Bak−/− mice despite its widespread expression. Here, we independently developed Bok−/− mice and found that Bok−/− cells are selectively defective in their response to endoplasmic reticulum (ER) stress stimuli, consistent with the predominant subcellular localization of BOK at the ER. Whereas Bok−/− mouse embryonic fibroblasts exposed to thapsigargin, A23187, brefeldin A, DTT, geldanamycin, or bortezomib manifested reduced activation of the mitochondrial apoptotic pathway, the death response to other stimuli such as etoposide, staurosporine, or UV remained fully intact. Multiple organs in Bok−/− mice exhibited resistance to thapsigargin-induced apoptosis in vivo. Although the ER stress agents activated the unfolded protein response, both ATF4 and CHOP activation were diminished in Bok−/− cells and mice. Importantly, BAX and BAK were unable to compensate for the defective apoptotic response to ER stress observed in SV40-transformed and primary Bok−/− cells, and in vivo. These findings support a selective and distinguishing role for BOK in regulating the apoptotic response to ER stress, revealing—to our knowledge—the first bona fide apoptotic defect linked to Bok deletion.
BAX and BAK are essential B-cell lymphoma-2 (BCL-2) family proteins that porate the mitochondrial outer membrane in response to a broad range of apoptotic stimuli (1). BCL-2 ovarian killer (BOK) exhibits ∼70–80% sequence homology to BAX and BAK, sharing BH1-3 domains and a carboxy-terminal transmembrane domain (2). Like BAX and BAK, BOK is widely expressed and induces cell death upon transient overexpression (2–9). In the context of BOK overexpression, BOK-induced cell death manifests classical apoptotic features, including the release of cytochrome c, activation of caspase-3, and nuclear and DNA fragmentation (6, 9–11). In addition, overexpression of antiapoptotic MCL-1, BFL-1/A1, and BHRF-1 inhibit BOK-mediated cell death (2, 7, 12, 13). Based on these similarities, it has been suggested that BOK acts in a redundant fashion to BAX and BAK and may be responsible for the apparently normal development of numerous organs in Bax−/−Bak−/− mice (14).
Despite these initial observations, the physiologic role of BOK in responding to cell stress has remained enigmatic. Indeed, the development and characterization of Bok-deficient mice revealed little to no phenotype (3). Moreover, leukocyte subsets from Bok−/− mice showed no alteration in their death response to classical proapoptotic agents, such as etoposide, dexamethasone, Fas ligand, ABT-737, or cytokine deprivation (3). Even Bok−/−Bax−/− and Bok−/−Bak−/− doubly deficient mice are mostly normal, and cells derived from these mice are equally susceptible to death stimuli (15), in stark contrast to the severe apoptotic blockade of Bax−/−Bak−/− cells. Also intriguing, a functional role for Bok as a tumor suppressor was suggested by its genetic location in 1 of the 20 most deleted regions in all human cancers (16).
To investigate a role for BOK in the apoptotic pathway, we generated Bok−/− mice and cells. Based on the recent localization of BOK at the endoplasmic reticulum (ER) (4), we focused our inquiry on ER stress pathways, including the unfolded protein response (UPR). We find that Bok−/− cells manifest reduced activation of the classical mitochondrial apoptotic pathway in response to a battery of ER stress stimuli. Moreover, the apoptotic deficiency is selective, with no observed differences in response to other inducers of apoptosis. From a mechanistic standpoint, we find that BOK requires downstream BAX/BAK activation to induce mitochondrial apoptosis, as BOK expression can rescue the defective response of Bok−/− cells, but not Bax−/−Bak−/− cells. These findings support a selective role for BOK in regulating the apoptotic response to ER stress, a functional activity that corresponds to its recent subcellular localization at the ER.
Results
Targeted Disruption of the Murine Bok Gene Results in Normal Development.
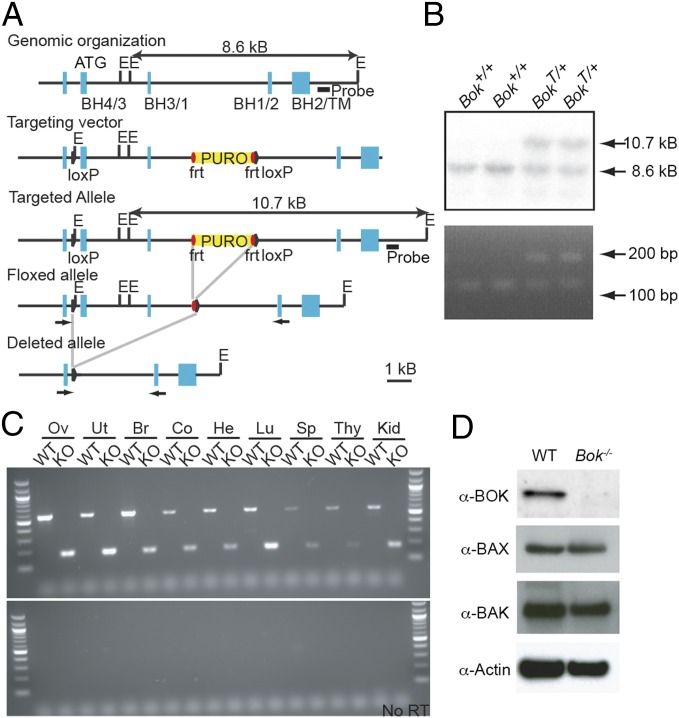
To disrupt the Bok gene, we electroporated RW4 ES cells with a targeting vector that introduces LoxP sites in the introns between exons 1 and 2, and exons 3 and 4 (Fig. 1A). Properly targeted ES cells with both the 5′ LoxP site and the puromycin cassette (Fig. 1B) were injected into host blastocysts to generate chimeric mice. Targeted mice were bred to mice ubiquitously expressing Cre recombinase to generate an allele that deletes exons 2 and 3, which includes the ATG start site as well as the BH4, BH3, and one-half of the BH1 BCL-2 homology (BH) regions. Heterozygous Bok+/− mice were interbred to generate viable Bok−/− mice. Analysis of Bok−/− mice revealed minimal differences in embryonic histology, complete blood count, and serum chemistries (Fig. S1). Although a shortened transcript was detected in Bok−/− mice by RT-PCR (Fig. 1C), Western blot revealed the absence of BOK protein (Fig. 1D). RT-PCR analysis confirmed that BOK exhibits a wide distribution of expression.
Fig. 1.
Targeted disruption of the mouse Bok gene. (A) Partial map of the murine Bok locus (Top), Bok targeting vector (Top Middle), targeted homologous recombination (Middle), removal of the puromycin resistance (puro) cassette (Bottom Middle), and complete deletion of the locus (Bottom). Bok exons are shown as blue boxes with positions of BCL-2 homology (BH) and transmembrane (TM) domains indicated below. Solid triangles represent LoxP sites, and red ovals indicate FRT sites. The probe used in Southern blotting is shown as a black line. Arrows represent primers used for PCR genotyping in B and RT-PCR in C. E, EcoRI. (B) Southern blot analysis of ES cell DNA showing the presence of WT (+/+) and heterozygous (+/Targeted) mutant genotypes (Top). PCR analysis of ES cell DNA showing the presence of the 5′ LoxP site in targeted clones (Bottom). (C) RT-PCR of Bok in multiple tissues from WT and Bok−/− mice: ovary (Ov), uterus (Ut), brain (Br), colon (Co), heart (He), lung (Lu), spleen (Sp), thymus (Thy), and kidney (Kid). (D) Western blot for BOK, BAX, BAK, and Actin from WT and Bok−/− MEFs.
Loss of BOK Partially Protects Cells from ER Stress-Induced Cell Death.
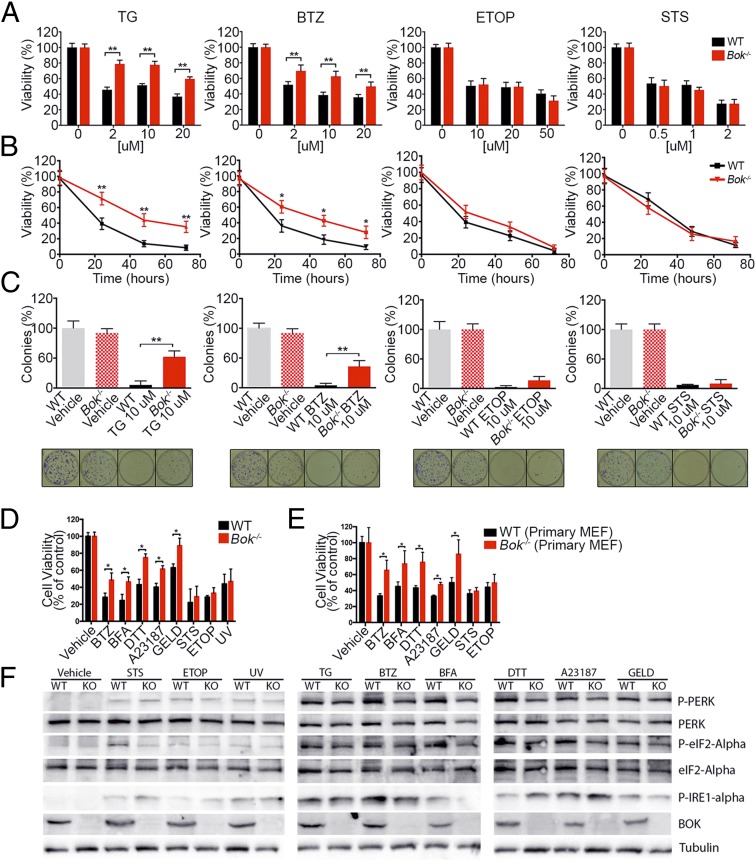
A series of viability assays were performed to determine whether BOK has a proapoptotic function. Wild-type (WT) and Bok−/− murine embryonic fibroblasts (MEFs) were generated by SV40 immortalization of cells isolated from embryonic day 13.5 liver. Bok−/− MEFs grew similarly to WT MEFs as measured by PrestoBlue (Fig. S2A). Whereas the growth of WT MEFs was impaired by treatment with the ER stressors thapsigargin (TG) (a SERCA pump inhibitor) (10 μM) and bortezomib (BTZ) (a proteasome inhibitor) (10 μM), Bok−/− MEFs continued to grow normally (Fig. S2A). In contrast, both WT and Bok−/− MEFs exhibited decreased growth upon exposure to the DNA-damaging agent etoposide (ETOP) and the promiscuous protein kinase inhibitor staurosporine (STS) (Fig. S2A). In agreement with these data, loss of BOK partially protected the cells from treatment with TG or BTZ, but not STS or ETOP, in a dose- and time-dependent fashion, compared with WT cells in an XTT assay (Fig. 2 A and B). Clonogenic rescue analysis further documented that Bok−/− cells treated for 48 h with 10 μM TG or 10 μM BTZ remained ∼10-fold more viable than WT cells (Fig. 2C). To further validate these findings, we tested additional ER stress agents including DTT (reducing agent), geldanamycin (GELD) (Hsp90 inhibitor), brefeldin A (BFA) (Golgi/ER transport inhibitor), and A23187 (calcium ionophore), and again found that loss of BOK partially protected cells from these ER stress stimuli but not 5 min of 100 J/m2 UV irradiation (Fig. 2D and Fig. S2B). We confirmed that the observed selectivity in apoptotic defect was also present in primary Bok−/− MEFs (Fig. 2E and Fig. S2C). To interrogate whether the blunted ER stress response derived from a defect in afferent stress sensing, we performed Western blot analysis of PERK, phospho-PERK, eIF2-α, phospho–eIF2-α, and phospho–IRE1-α. For each of the six ER stress stimuli tested, both WT and Bok−/− cells manifested intact, upstream signaling pathway responses as assessed by appropriate phosphorylation of PERK and eIF2-α (Fig. 2F and Fig. S2D). Phosphorylation of IRE1-α was detected for both WT and Bok−/− cells treated with TG, BTZ, and A23187, but there was decreased phosphorylation in Bok−/− cells treated with BFA (Fig. 2F and Fig. S2D). Of note, based on densitometry from three independent experiments, there were no statistically significant changes in BOK expression in WT cells as a result of drug treatment except for mildly decreased levels upon BFA treatment (Fig. 2F and Fig. S2D). Taken together, these results demonstrate that Bok−/− cells are selectively deficient in the apoptotic response to ER stress but not STS (kinase inhibition), ETOP (DNA damage), or UV irradiation. Strikingly, the presence of BAX and BAK does not compensate for the loss of BOK in this context.
Fig. 2.
Loss of Bok protects cells from ER stress-induced death. (A and B) Cell viability of SV40-immortalized WT and Bok−/− MEFs as measured by the XTT assay at 24 h using the indicated concentrations of thapsigargin (TG), bortezomib (BTZ), etoposide (ETOP), or staurosporine (STS) (A) and at multiple time points with 10 μM TG, 10 μM BTZ, 10 μM ETOP, or 1 μM STS (B). Bars and lines represent percent cell viability with respect to untreated cells. (C) Number of colonies formed when SV40-immortalized WT and Bok−/− cells were plated at clonal densities after 48 h of treatment with 10 μM TG, 10 μM BTZ, 10 μM ETOP, or 1 μM STS, as compared to untreated cells. Representative wells are shown below. (D and E) Cell viability was measured at 24 h by XTT of SV40-immortalized MEFs (D) and primary WT and Bok−/− MEFs (n = 5) (E) at 1 μM BTZ, 2 μM brefeldin A (BFA), 1 μM DTT, 2 μM A23187, 1 μM geldanamycin (GELD), 1 μM STS, 1 μM ETOP, and after 5 min of 100 J/m2 UV (UV light; SV40-immortalized MEFs only). All experiments were performed at least in triplicate. Error bars represent SD. Significance was calculated using an ANOVA test (*P < 0.05, **P < 0.005). (F) Representative Western blot analysis for ER stress response pathway in WT and Bok−/− (KO) MEFs 6 h after treatment with vehicle, 1 μM STS, 1 μM ETOP, 5 min of 100 J/m2 UV, 2 μM TG, 1 μM BTZ, 2 μM BFA, 1 μM DTT, 2 μM A23187, and 1 μM GELD.
BOK Mediates ER Stress-Induced Caspase-3/7 Activation and Annexin V Exposure.
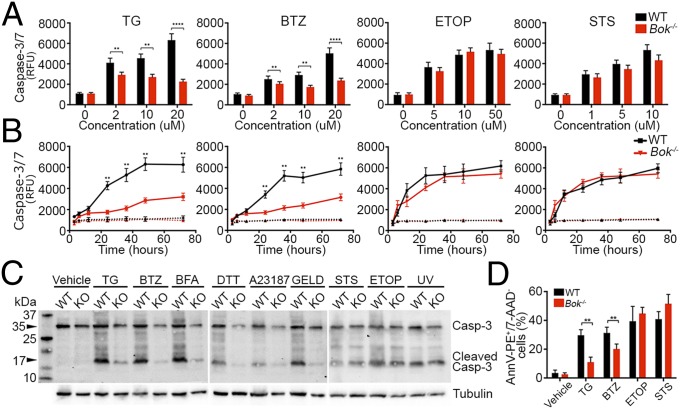
To determine whether the observed apoptotic deficiency in Bok−/− cells places BOK upstream of caspase activation, we monitored caspase-3/7 activation in response to ER stress in Bok−/− vs. WT MEFs. Loss of BOK prevented both the time- and dose-dependent increase in caspase-3/7 activation observed in WT cells exposed to TG or BTZ (Fig. 3 A and B). As a measure of specificity, Bok−/− cells treated with STS or ETOP maintained normal levels of caspase-3/7 activation (Fig. 3A). Similar results were obtained in primary MEFs (Fig. S3A) and for each of the ER stress agents, as evaluated by Western blot for cleaved caspase-3 (Fig. 3C). Correspondingly, Bok−/− MEFs treated with TG or BTZ manifested reduced annexin V positivity compared with WT MEFs, as measured by FACS analysis; however, no differences were observed in response to treatment with ETOP or STS (Fig. 3D). Primary Bok−/− MEFs treated with TG, but not STS, also showed reduced annexin V positivity compared with WT MEFs (Fig. S3B). Thus, upon exposure to ER stress stimuli, the presence of BOK correlates with canonical activation of the apoptotic cascade, as reflected by caspase-3/7 and annexin V positivity.
Fig. 3.
Loss of Bok protects cells from ER stress-induced caspase-3/7 activation and annexin V exposure. (A and B) Caspase-3/7 activation in SV40-immortalized WT and Bok−/− cells at 24 h in response to the indicated concentrations of thapsigargin (TG), bortezomib (BTZ), etoposide (ETOP), or staurosporine (STS) (A), and at multiple time points with 10 μM TG, 10 μM BTZ, 10 μM ETOP, or 1 μM STS (B). Bars and lines represent relative fluorescence units (RFU). Dashed lines, vehicle; solid lines, treated. (C) Representative Western blot analysis for activated caspase-3 in SV40-immortalized WT and Bok−/− (KO) MEFs 16 h after treatment with vehicle, 2 μM TG, 1 μM BTZ, 2 μM BFA, 1 μM DTT, 2 μM A23187, 1 μM GELD, 1 μM STS, 1 μM ETOP, and 5 min of 100 J/m2 UV. (D) Percent annexin V-positive/7AAD-negative SV40-immortalized WT and Bok−/− cells treated for 12 h with vehicle, 10 μM TG, 10 μM BTZ, 10 μM ETOP, or 1 μM STS. All experiments were performed at least in triplicate. Error bars represent (A and B) SD or (D) SEM. Significance was calculated using an ANOVA test (**P < 0.005, ****P < 0.0001).
BOK Engages the Intrinsic Mitochondrial Pathway of Apoptosis.
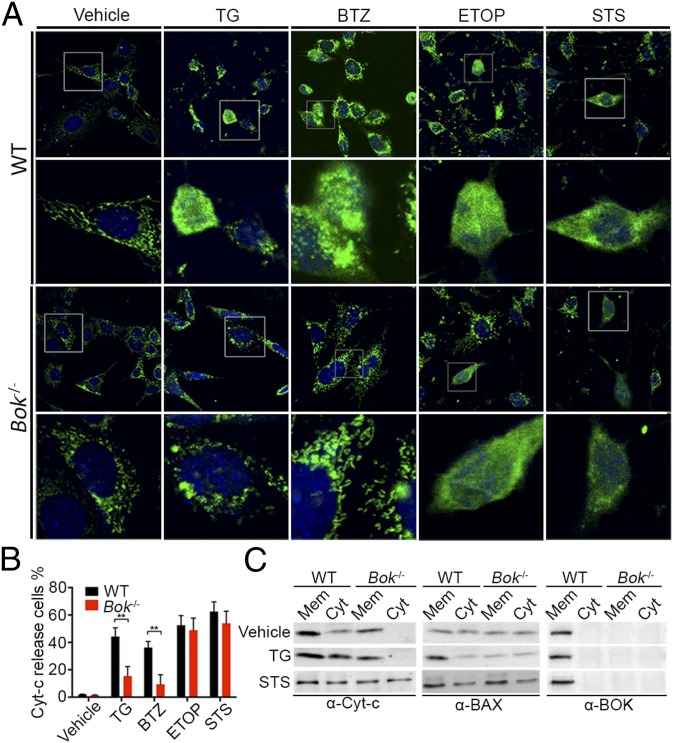
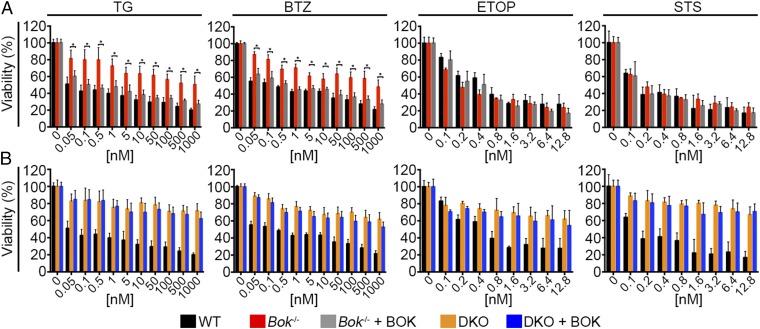
Previous reports indicate that, although BOK is a multidomain proapoptotic BCL-2 family member like BAX and BAK, it is predominantly localized to the ER (4). This raised the question of whether BOK-mediated cell death involves the mitochondria. Indeed, we found that ER stress-induced cytochrome c release was blunted in Bok−/− cells compared with WT cells, as monitored by anti-cytochrome c immunofluorescence (Fig. 4 A and B). Diminished cytochrome c release was further demonstrated by Western analysis of membrane and cytosolic fractions from Bok−/− cells treated with 10 μM TG (Fig. 4C and Fig. S4). Whereas WT cells demonstrated BAX translocation in response to ER stress, the Bok−/− cells failed to show an increase in BAX translocation (Fig. 4C and Fig. S4). Moreover, BOK did not show any change in localization from the membrane fraction, which contained ER and mitochondria (Fig. S4), in response to TG or STS (Fig. 4C). No differences in BAX translocation or cytochrome c release were observed in WT and Bok−/− cells treated with STS (Fig. 4C and Fig. S4), confirming that the canonical proapoptotic machinery in Bok−/− cells is otherwise intact. Importantly, overexpression of BOK restored the capacity of Bok−/− cells to fully respond to ER stress stimuli, as reflected by annexin V positivity and loss of viability responses (Fig. 5A and Fig. S5). However, added BOK was unable to alter the apoptotic resistance of Bax−/−Bak−/− cells, highlighting the downstream requirement of BAX/BAK for BOK’s proapoptotic activity (Fig. 5B and Fig. S5). Although we do not detect a specific interaction between overexpressed FLAG-BOK with native BAX or BAK by coimmunoprecipitation from untreated cells or cells exposed to TG or STS (Fig. S6), we cannot rule out the role of a transient proapoptotic interaction or intramembrane engagement that is disrupted by cell lysis. Nevertheless, we find that BOK selectively regulates the mitochondrial apoptotic response to ER stress upstream of BAX/BAK.
Fig. 4.
BOK promotes cytochrome c release and BAX translocation in response to ER stress. (A) Immunofluorescence detection of cytochrome c release by SV40-immortalized WT and Bok−/− cells subjected to vehicle or 24-h exposure to 10 μM TG, 10 μM BTZ, 10 μM ETOP, or 1 μM STS. Cytochrome c release and cell nuclei were examined by confocal immunofluorescence microscopy using anti-cytochrome c antibody (green) and TO-PRO3 (blue) staining. (B) Quantitative analysis of cytochrome c release from A. For each condition, 100 cells were analyzed in each of five randomly chosen fields. Three independent experiments were performed, and the means ± SEM were plotted. Significance was calculated using an ANOVA test (**P < 0.005). (C) Representative Western analyses of cytosolic (Cyt) and membrane (Mem) fractions of WT and Bok−/− cells subjected to vehicle or 24-h treatment with 10 μM TG or 1 μM STS to monitor cytochrome c release (Left), and BAX (Middle) and BOK (Right) translocation. The presence of mitochondria and ER in the membrane preparation is further documented by Western analyses for COX IV and calnexin, respectively (Fig. S4).
Fig. 5.
BOK rescues the ER stress-induced death response of Bok−/−, but not Bax−/−Bak−/−, cells. (A and B) Cell viability was measured at 24 h by XTT for WT (A and B), Bok−/− (A), and Bax−/−Bak−/− [double knockout (DKO)]. (B) MEFs were either not transfected or transfected with a BOK expression vector, and after 24 h were treated with a broad dose range of TG, BTZ, ETOP, and STS for an additional 24 h. All experiments were performed at least in triplicate. Error bars represent SD. Significance was calculated using an ANOVA test (*P < 0.05).
BOK Is Required for Activation of ATF4 and CHOP.
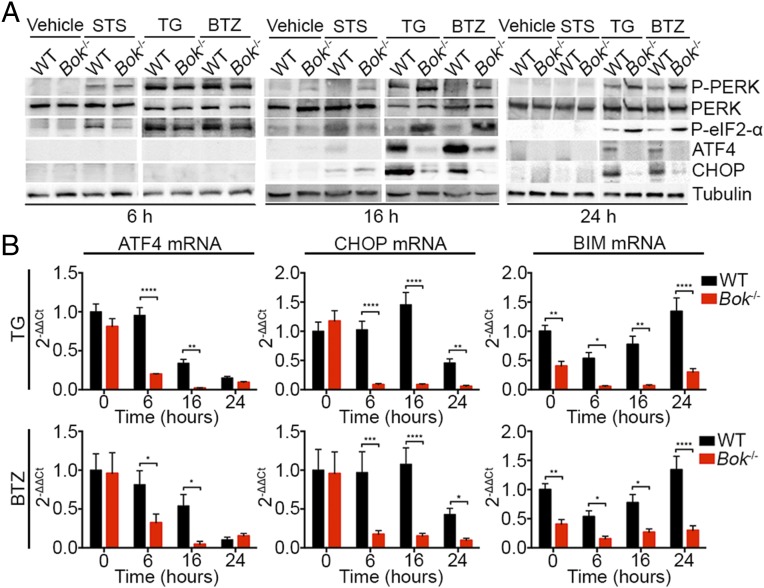
To characterize the signaling defect between ER stress and mitochondrial apoptosis in Bok−/− cells, we first interrogated the UPR. By 6 h after initiation of ER stress with TG or BTZ, the UPR is activated in both WT and Bok−/− cells as reflected by the phosphorylation of PERK and eIF2-α (Figs. 2F and 6A and Fig. S7). At this early time point, eIF2-α has not yet stimulated the transcription of ATF4, which subsequently activates CHOP (Fig. 6A and Fig. S7). However, by 16 and 24 h after induction, WT cells exhibited decreased PERK and eIF2-α phosphorylation and increased expression of ATF4 and CHOP, whereas Bok−/− cells have markedly increased PERK and eIF2-α phosphorylation and blunted expression of ATF4 and CHOP (Fig. 6A and Fig. S7). WT and Bok−/− cells treated with STS showed no differences in the expression of UPR components. Decreased expression of ATF4 and CHOP mRNA in Bok−/− cells compared to WT cells treated with TG or BTZ, further suggests that BOK’s regulatory role occurs between eIF2-α phosphorylation and ATF4 transcription (Fig. 6B). In agreement with the reported activation of BIM by CHOP (17), the Bok−/− cells, which have diminished CHOP expression, display concomitant loss of BIM expression (Fig. 6B). Thus, we find that loss of BOK results in decreased UPR-induced expression of ATF4, CHOP, and BIM.
Fig. 6.
ATF4 and CHOP expression is diminished in Bok−/− cells. (A) Representative Western blot analysis for the ER stress response pathway and (B) quantitative RT-PCR analysis for ATF4, CHOP, and BIM in WT and Bok−/− MEFs 6, 16, and 24 h after treatment with vehicle, 1 μM STS, 2 μM TG, and 1 μM BTZ. RNA expression levels are fold change of the target gene normalized to β-actin and expressed relative to WT cells at time 0. Error bars represent SD. Significance was calculated using an ANOVA test (*P < 0.05, **P < 0.005, ****P < 0.0001).
Bok−/− Mice Display Decreased Activation of CHOP and Apoptosis in Response to ER Stress.
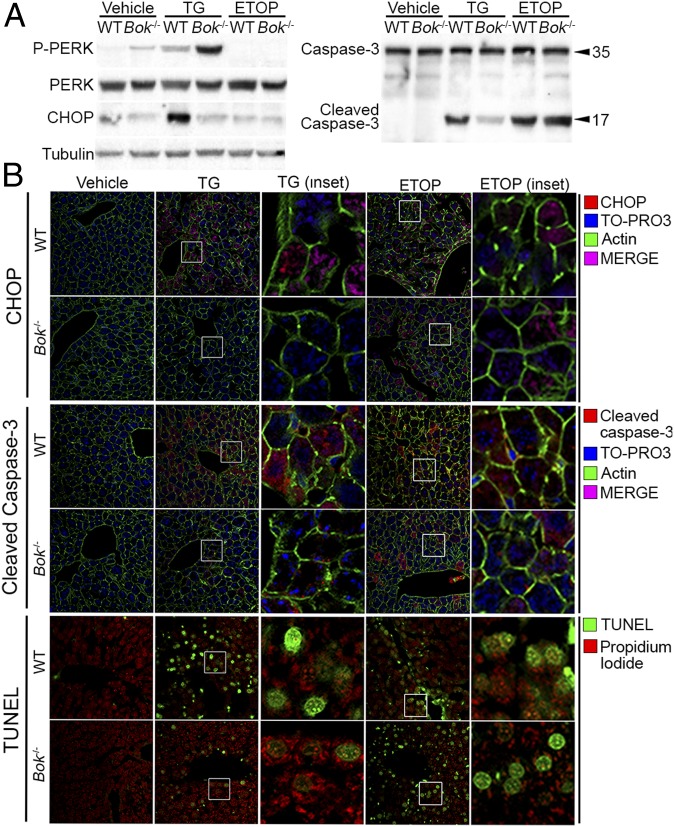
To determine whether the apoptotic defect observed in Bok−/− MEFs is functionally relevant in an in vivo context, WT and Bok−/− mice were i.p. injected with vehicle (150 mM dextrose), TG (1 mg/kg), or ETOP (10 mg/kg). Mice were euthanized at 24 h, and full necropsies were performed. Interestingly, a subset of WT mice treated with TG, but none of the Bok−/− mice, died before the 24 h end time point. Western blot of liver extracts revealed activation of the UPR as evidenced by phosphorylation of PERK in both WT and Bok−/− mice treated with TG, but not vehicle or ETOP (Fig. 7A and Fig. S8). However, Bok−/− mice treated with TG showed diminished expression of CHOP and activated caspase-3 in the liver by Western blot (Fig. 7A and Fig. S8). Equivalent activated caspase-3 levels were detected in ETOP-treated mice. Immunofluorescence of the liver, kidney, and lung further confirmed diminished expression of CHOP and activated caspase-3, and decreased TUNEL positivity (Fig. 7B and Fig. S9). Taken together, these findings support a physiologic role for BOK in mediating ER stress-induced UPR activation and apoptosis in vivo.
Fig. 7.
Loss of Bok protects mice from ER stress-induced apoptosis. (A) Representative Western blot analysis (n = 3) for the ER stress response pathway and activated caspase-3. (B) Immunofluorescence detection of CHOP, activated caspase-3, and TUNEL in WT and Bok−/− livers (n = 3) from a representative mouse treated with vehicle, 1 mg/kg TG, or 10 mg/kg ETOP. Merge is between red (CHOP in rows 1 and 2; cleaved caspase-3 in rows 3 and 4) and blue (TO-PRO3).
Discussion
BOK was identified as a BAX/BAK homolog nearly two decades ago, but its physiologic role has remained enigmatic. Although BOK, BAX, and BAK are widely expressed, BOK does not compensate for the severe apoptotic defects that contribute to the embryonic lethality of Bax−/−Bak−/− mice (18). Likewise, cells deficient in both BAX and BAK are highly resistant to a wide variety of apoptotic agonists despite the presence of BOK (19–21). In striking contrast, the intimate functional relationship between BAX and BAK is underscored by the fact that cells singly deficient for BAX or BAK exhibit little to no apoptotic resistance to death stimuli (20, 21). Given the normal growth and development of visceral tissues, such as liver, heart, and lung, in Bax−/−Bak−/− mice, we and others hypothesized that perhaps BOK plays a major, independent apoptotic role in these tissues. Nevertheless, Bok−/− mice show no overt defects in these tissues either (3, 14, 15). Thus, we explored whether BOK could subserve a specific apoptotic function independent from BAX and BAK at the ER, its site of predominant intracellular localization (4).
A previous study reported no difference in annexin V positivity between WT and independently derived Bok−/− MEFs treated with TG, and even an increased sensitivity of Bok−/− cells to BFA exposure (4). In contrast, here we observe apoptotic resistance in SV40-transformed Bok−/− MEFs exposed to six different ER stress agents, including TG and BFA, at various time points and over a broad dose range, as analyzed by XTT, caspase-3/7, clonogenic, and annexin V assays. Importantly, we observe a similar profile of selective apoptotic resistance to ER stress stimuli in primary Bok−/− MEFs. Although Ke et al. (3) deleted one-half of exon 1 and all of exon 2, and we deleted exons 2 and 3, both studies document loss of BOK by Western blot and RT-PCR, so the source of the discrepancy is unclear. However, we demonstrate that reexpression of BOK fully rescues the capacity of Bok−/− cells to respond to the ER stress agents, confirming the dependence of the apoptotic defect on BOK. Furthermore, we observe the selective apoptotic defect to ER stress in multiple tissues of our Bok−/− mice. Indeed, protection from ER stress-induced apoptosis upon loss of BOK is consistent with its proposed proapoptotic functionality, as observed upon overexpression (2, 5–8, 10, 12).
It is especially striking that BOK confers at least partial apoptotic resistance independent of BAX and BAK, whereas only the combination of BAX and BAK deficiency can protect against ER stress (20). This suggests that at least one of BOK’s functions involves a distinct step in the ER stress-induced apoptotic pathway, and this function cannot be compensated for by BAX or BAK. The absence of cytochrome c release and mitochondrial translocation of BAX in the stimulated Bok−/− cells, and the inability of overexpressed BOK to kill stimulated Bax−/−Bak−/− cells, suggests that BOK functions upstream of BAX and BAK. Although it is plausible that BOK could directly and/or indirectly activate BAX/BAK in an analogous fashion to a BH3-only protein via its conserved BH3 domain, we did not detect a BAX/BAK interaction by coimmunoprecipitation.
To determine how BOK connects ER stress signaling to the core apoptotic machinery, we further dissected the UPR. Whereas upstream UPR sensing was intact in Bok−/− cells, as measured by PERK and eIF2-α phosphorylation at 6 h, downstream effector signaling of the UPR was defective. In particular, by 16 h, ATF4 and CHOP transcriptional induction is greatly diminished in the Bok−/− cells and eIF2-α phosphorylation is markedly increased. Importantly, the defects in downstream UPR signaling identified in MEFs were also found in the liver, kidneys, and lungs of Bok−/− mice treated with TG. Because CHOP regulates BIM (17), and BIM levels are diminished in the ER-stressed Bok−/− cells, the observed reduction in classical apoptotic hallmarks, such as cytochrome c release, caspase-3/7 activation, and annexin V positivity, may actually be due to BOK’s regulation of CHOP. Thus, the requirement of BAX/BAK may result from BOK induction of BIM through CHOP and subsequent indirect and direct activation of BAX and BAK by BIM.
Additional afferent roles of BOK may operate at the level of the ER, the mitochondria, or both subcellular locations. Because BOK and BAX/BAK independently resist TG-induced death despite the other protein(s) being present, there are likely multiple BCL-2 family-regulated control points for the ER stress pathway. Indeed, in addition to their roles at the mitochondria, BAX and BAK have proximal functions at the ER in both regulating calcium homeostasis and the UPR (22, 23). As BOK binds inositol 1,4,5-trisphosphate receptors (24), it too might function in discrete homeostatic processes of the ER, and thereby contribute to the complex interplay of BCL-2 family functions at the intersection of ER and mitochondrial physiology. Our in vitro and in vivo loss-of-function studies demonstrate that BOK has a bona fide proapoptotic function in regulating ER stress-induced apoptosis, linking apoptotic sensing at the ER to apoptotic execution at the mitochondria.
Experimental Procedures
Cell Survival and Apoptosis Assays.
XTT (Cell Signaling), PrestoBlue (Life Technologies), caspase-3/7 (Life Technologies), and annexin V (BD Pharmingen) assays were performed according to manufacturers’ instructions and measured on a Wallac 1420 VICTOR2 multilabel counter or a FACSCanto II (annexin V). Full details of these procedures and the clonogenic cell survival assay may be found in SI Experimental Procedures.
Molecular Biology.
Immunoblot analysis, cellular fractionation, and quantitative RT-PCR were performed using standard techniques. A full description of buffers, antibodies, and primers may be found in SI Experimental Procedures.
Animal Work.
Generation and handling of C57BL/6 backcrossed Bok−/− mice, including generation of MEFs and treatment with proapoptotic agents, were approved by the institutional animal care and use committees of the Dana–Farber Cancer Institute and Yale University. These details as well as tissue fixative protocols, TUNEL (Invitrogen), antibodies, and a description of the imaging analysis may be found in SI Experimental Procedures.
Supplementary Material
Acknowledgments
This work was supported by NIH Grant 5R01CA050239, a Leukemia and Lymphoma Society (LLS) Marshall A. Lichtman Specialized Center of Research project grant, and LLS Scholar Award (to L.D.W.), and NIH Grant 5K08HL103847 (to S.G.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1421063112/-/DCSupplemental.
References
- 1.Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell. 2010;37(3):299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu SY, Kaipia A, McGee E, Lomeli M, Hsueh AJ. Bok is a pro-apoptotic Bcl-2 protein with restricted expression in reproductive tissues and heterodimerizes with selective anti-apoptotic Bcl-2 family members. Proc Natl Acad Sci USA. 1997;94(23):12401–12406. doi: 10.1073/pnas.94.23.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ke F, et al. BCL-2 family member BOK is widely expressed but its loss has only minimal impact in mice. Cell Death Differ. 2012;19(6):915–925. doi: 10.1038/cdd.2011.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Echeverry N, et al. Intracellular localization of the BCL-2 family member BOK and functional implications. Cell Death Differ. 2013;20(6):785–799. doi: 10.1038/cdd.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ha SH, et al. The expression of Bok is regulated by serum in HC11 mammary epithelial cells. Mol Cells. 2001;12(3):368–371. [PubMed] [Google Scholar]
- 6.Igaki T, et al. Drob-1, a Drosophila member of the Bcl-2/CED-9 family that promotes cell death. Proc Natl Acad Sci USA. 2000;97(2):662–667. doi: 10.1073/pnas.97.2.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inohara N, et al. Mtd, a novel Bcl-2 family member activates apoptosis in the absence of heterodimerization with Bcl-2 and Bcl-XL. J Biol Chem. 1998;273(15):8705–8710. doi: 10.1074/jbc.273.15.8705. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez JM, Glozak MA, Ma Y, Cress WD. Bok, Bcl-2-related ovarian killer, is cell cycle-regulated and sensitizes to stress-induced apoptosis. J Biol Chem. 2006;281(32):22729–22735. doi: 10.1074/jbc.M604705200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yakovlev AG, et al. BOK and NOXA are essential mediators of p53-dependent apoptosis. J Biol Chem. 2004;279(27):28367–28374. doi: 10.1074/jbc.M313526200. [DOI] [PubMed] [Google Scholar]
- 10.Bartholomeusz G, et al. Nuclear translocation of the pro-apoptotic Bcl-2 family member Bok induces apoptosis. Mol Carcinog. 2006;45(2):73–83. doi: 10.1002/mc.20156. [DOI] [PubMed] [Google Scholar]
- 11.Zhang H, Holzgreve W, De Geyter C. Evolutionarily conserved Bok proteins in the Bcl-2 family. FEBS Lett. 2000;480(2-3):311–313. doi: 10.1016/s0014-5793(00)01921-9. [DOI] [PubMed] [Google Scholar]
- 12.Soleymanlou N, et al. Hypoxic switch in mitochondrial myeloid cell leukemia factor-1/Mtd apoptotic rheostat contributes to human trophoblast cell death in preeclampsia. Am J Pathol. 2007;171(2):496–506. doi: 10.2353/ajpath.2007.070094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, et al. Drosophila pro-apoptotic Bcl-2/Bax homologue reveals evolutionary conservation of cell death mechanisms. J Biol Chem. 2000;275(35):27303–27306. doi: 10.1074/jbc.M002846200. [DOI] [PubMed] [Google Scholar]
- 14.Youle RJ, Strasser A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9(1):47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 15.Ke F, et al. Consequences of the combined loss of BOK and BAK or BOK and BAX. Cell Death Dis. 2013;4:e650. doi: 10.1038/cddis.2013.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beroukhim R, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463(7283):899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puthalakath H, et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129(7):1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 18.Lindsten T, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6(6):1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shroff EH, et al. BH3 peptides induce mitochondrial fission and cell death independent of BAX/BAK. PLoS One. 2009;4(5):e5646. doi: 10.1371/journal.pone.0005646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei MC, et al. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science. 2001;292(5517):727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zong WX, Lindsten T, Ross AJ, MacGregor GR, Thompson CB. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 2001;15(12):1481–1486. doi: 10.1101/gad.897601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hetz C, et al. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science. 2006;312(5773):572–576. doi: 10.1126/science.1123480. [DOI] [PubMed] [Google Scholar]
- 23.Scorrano L, et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: A control point for apoptosis. Science. 2003;300(5616):135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 24.Schulman JJ, Wright FA, Kaufmann T, Wojcikiewicz RJ. The Bcl-2 protein family member Bok binds to the coupling domain of inositol 1,4,5-trisphosphate receptors and protects them from proteolytic cleavage. J Biol Chem. 2013;288(35):25340–25349. doi: 10.1074/jbc.M113.496570. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.