Significance
Knowledge of the molecules that guide retinal interneuron formation is incomplete. We showed that PRDI-BF1 and RIZ homology domain containing 8 (PRDM8) is required for the development of rod bipolar cells and OFF-cone bipolar subtypes as well as amacrine cell identity. Although bipolar cells were specified in Prdm8-null mice, rod bipolar cell differentiation was impaired, leading to their death and near absence from adult retina. This defect disrupts postphotoreceptor signal transduction, as shown by nonprogressive b-wave deficits in electroretinograms. Our findings suggest PRDM8 as a candidate gene for human congenital stationary night blindness. They also establish PRDM8 as a component of the regulatory network governing bipolar cell development and amacrine cell diversity, aiding efforts to generate these essential interneurons in vitro.
Keywords: retina, bipolar cell, amacrine cell, genetics, development
Abstract
Retinal bipolar (BP) cells mediate the earliest steps in image processing in the visual system, but the genetic pathways that regulate their development and function are incompletely known. We identified PRDI-BF1 and RIZ homology domain containing 8 (PRDM8) as a highly conserved transcription factor that is abundantly expressed in mouse retina. During development and in maturity, PRDM8 is expressed strongly in BP cells and a fraction of amacrine and ganglion cells. To determine whether Prdm8 is essential to BP cell development or physiology, we targeted the gene in mice. Prdm8EGFP/EGFP mice showed nonprogressive b-wave deficits on electroretinograms, consistent with compromised BP cell function or circuitry resembling the incomplete form of human congenital stationary night blindness (CSNB). BP cell specification was normal in Prdm8EGFP/EGFP retina as determined by VSX2+ cell numbers and retinal morphology at postnatal day 6. BP subtype differentiation was impaired, however, as indicated by absent or diminished expression of BP subtype-specific markers, including the putative PRDM8 regulatory target PKCα (Prkca) and its protein. By adulthood, rod bipolar (RB) and type 2 OFF-cone bipolar (CB) cells were nearly absent from Prdm8-null mice. Although no change was detected in total amacrine cell (AC) numbers, increased PRKCA+ and cholinergic ACs and decreased GABAergic ACs were seen, suggesting an alteration in amacrine subtype identity. These findings establish that PRDM8 is required for RB and type 2 OFF-CB cell survival and amacrine subtype identity, and they present PRDM8 as a candidate gene for human CSNB.
Retinal bipolar (BP) cells are the first interneurons in the mammalian visual signaling pathway, connecting photoreceptors (PRs) to ganglion cells and then, through the optic nerve, to the brain. In mouse retina, 13 BP subtypes are distinguished by their (i) predominant presynaptic (rod or cone) input, (ii) morphology (axon length and terminal field width), (iii) functional response to increased illumination (depolarizing or ON-BP cells and hyperpolarizing or OFF-BP cells), and (iv) molecular markers (1). Mouse retina has only one type of rod BP (RB) cell, primarily postsynaptic to rod PRs, which extends its axon to the innermost sublamina of the inner plexiform layer (IPL) and depolarizes in response to increments in illumination. The axons of cone BP (CB) cells ramify throughout the IPL, but those that terminate in the outer sublaminae are functionally OFF-CB cells that hyperpolarize to light increments, whereas those that terminate in the inner sublaminae are ON-CB cells (1). Moreover, ON- and OFF-BP cells make direct and indirect synaptic connections with corresponding ON- and OFF-retinal ganglion cells (RGCs) in the IPL. The ON and OFF properties of BP cells result from differences in glutamate receptor activity on BP cell dendrites (1). Hence, the early integration of visual signals in the mammalian retina is determined by the specific synaptic connectivity and physiology of BP cells.
The specification and differentiation of BP cells and their subtypes depend on the coordinated expression of several transcription factors (TFs) (2–7). The homeodomain TF VSX2 is first expressed by retinal progenitor cells, later becoming restricted to BP cells and Müller glia in the inner nuclear layer (INL) of the postnatal retina (8). Vsx2-null alleles cause microphthalmia and a complete absence of BP cells (9, 10), whereas the overexpression of Vsx2 in retinal progenitor cells leads to an increase in the number of immature BP cells and Müller glia in the INL (10, 11). Like Vsx2 mutants, mice lacking both basic helix–loop–helix (bHLH) TFs MATH3 and MASH1 also lack BP cells (11). Conversely, the combined overexpression of Vsx2 with either Mash1 or Math3 promotes the generation of mature PKCα+ (PRKCA+) BP cells (11). Altogether, these data suggest that VSX2 is required for the specification of BP cells and INL cell identity, at least in part through the repression of rod PR development (10), but that VSX2 alone is not sufficient for BP cell differentiation.
Another group of TFs, including BHLHB4 and VSX1, is not essential for the specification of BP cells or their subtypes but is required for their survival or function. Thus, BP cell genesis is normal in both Bhlhb4 and Vsx1 loss of function mutants, but in Bhlhb4−/− retinas, RB cells do not survive, resulting in the near absence of RB cells in the mature Bhlhb4-null retina (3, 7). In the Vsx1−/− adult retina, in contrast, BP cells are morphologically normal, but CB cells fail to differentiate fully, resulting in defects in ON- and OFF-CB cell-mediated visual signaling (3, 12). Thus, the development of BP cells into subtypes of different morphology, physiology, and synaptic connectivity is transcriptionally regulated at each step of their genesis, differentiation, and maintenance.
Amacrine cells (ACs) are the primary inhibitory interneurons of the mammalian retina, modulating the output of BP cells onto RGCs (1). Approximately 40 morphologically-defined amacrine subtypes have been identified (1), but the molecular mechanisms that regulate amacrine diversity in the mammalian retina are largely unknown. The current model is that the homeodomain TFs PAX6 and SIX3 combined with the bHLH TFs MATH3 and NEUROD together specify a pan-AC identity, with PAX6 and SIX3 conveying positional identity in the INL (13). Other TFs are required for the specification and differentiation of amacrine subtypes, including ISLET1 (ISL1; cholinergic ACs) (4), NR4A2 (GABAergic ACs) (14), EBF family members (glycinergic ACs) (15), NEUROD6 (glycinergic ACs and non-GABAergic nonglycinergic ACs) (16), and BHLHB5 (GABAergic, glycinergic, dopaminergic, and cholinergic ACs) (5, 17).
We identified PRDI-BF1 and RIZ homology domain containing 8 (PRDM8) as an abundantly expressed gene in human retina encoding a highly conserved member of the PRDM family (18). Prdm8 is expressed regionally in the developing and adult vertebrate CNS, including retina (19). PR domains are 20–30% identical to the su(var)3–9, enhancer-of-zeste, and trithorax domain, a histone methyltransferase domain (18). Some PRDM proteins have intrinsic histone methyltransferase activity, whereas others modify chromatin indirectly through the recruitment of other polypeptides (18). Whether PRDM8 functions directly or indirectly to methylate histones is uncertain (20, 21). Nevertheless, nearly all of the PRDM proteins studied to date have been shown to regulate cell proliferation in development or cancer, and several are key cell fate determinants in model systems (18). The abundant expression of PRDM8 in retina and its relationship to a family of TFs critical to cell proliferation and cell fate in model systems suggested that PRDM8 was also likely to be an important regulator of neuronal development in the mammalian retina.
To define the role of Prdm8 in neural development, we generated mice carrying Prdm8-null alleles (Prdm8EGFP/EGFP mice) and investigated the morphological and physiological consequences of the loss of Prdm8 function. We reported previously that Prdm8EGFP/EGFP mice exhibit a spectrum of neurological phenotypes (22); here, we focus on the visual phenotype (namely, profound b-wave deficits in retinal electrophysiology), resulting from the near-complete absence of RB cells and type 2 OFF-CB cells. We discovered that Prdm8 is not required for BP cell specification but is essential for RB and CB subtype differentiation and that, in the absence of Prdm8, RB and type 2 CB cells are virtually absent from the adult retina. We also showed a change in the distribution of GABAergic, cholinergic, and PRKCA+ ACs in the absence of Prdm8, suggesting altered AC identity.
Results
PRDM8 Is Expressed by Differentiating Neurons in the Developing Mammalian Retina.
We identified PRDM8 as an abundantly expressed clone from a human retinal cDNA library screen for (CAG)n trinucleotide repeat sequences. To investigate its role in mammals, we first examined its expression pattern in adult mouse tissues. Consistent with other reports, we detected a single ∼3.4-kb Prdm8 mRNA transcript that was most abundant in the brain, with smaller transcripts in testis on RNA blots (Fig. S1A) (19, 20). We found that the expression of Prdm8 in the CNS was highest in the retina and hippocampus and moderate in the cortex and cerebellum (Fig. S1A).
Others reported expression of the PRDM8 protein during mouse retinal development at embryonic day 18.5 (E18.5) but not earlier (19). To begin to define the role of PRDM8 in retinal formation, we first examined its retinal expression during development and in adult mouse. We used antibodies to the N terminus of PRDM8 (this study and ref. 22) together with markers for major retinal cell classes. At E12.5, PRDM8 was expressed abundantly within many postmitotic BRN3B+ RGCs in the nascent ganglion cell layer (GCL) (Fig. 1A). Strong expression remained in a small fraction of RGCs throughout development and into adulthood, which was shown by occasional costaining with BRN3 (Fig. 1 H and L). At E18.5, PRDM8 expression was also detected in emerging ACs in the INL (Fig. 1B, AC[), the laminar position of which is demarcated by ISL1 staining, an early marker for differentiating cholinergic ACs and RGCs (4, 23); PRDM8 was not, however, expressed in ISL1+ ACs in the developing INL. In the GCL, a few ISL1+ PRDM8+ cells were present (Fig. 1B, arrows), indicating PRDM8 expression in a small RGC subset and/or displaced ACs. From E18.5 to postnatal day 6 (PN6), very low levels of PRDM8 staining were detected in newborn, migrating (oblong) cells in the outer neuroblast layer (ONBL) (Fig. 1 B–E, white arrowheads). We detected PRDM8 expression in immature BP cells as early as PN3 (Fig. 1D, ]BP and Fig. S1B). By PN6, PRDM8 was expressed widely among differentiating BP cells [as indicated by costaining with the pan-BP cell marker VSX2 (9)] (Fig. 1 E and F), in a substantial fraction of PAX6+ ACs in the INL (Fig. 1G, arrows), PAX6+ RGCs, and/or displaced ACs in the GCL (Fig. 1G, arrowheads), and in BRN3+ RGCs (Fig. 1H). From PN6 until maturity, PRDM8 expression was sustained at a relatively high level in the INL and GCL (Fig. S1B). In adult retina, PRDM8 was expressed in a large proportion of BP cells, a small number of ACs and RGCs, and PRs at a low level (Fig. 1 I–L). In conclusion, PRDM8 is expressed widely in the developing and adult mouse retina and in a broader range of cell types than identified previously (19).
Fig. 1.
PRDM8 is expressed in differentiating neurons in all layers of the developing neural retina. Representative confocal micrographs of sections from developing WT retina were costained with antibodies to PRDM8 and cell type-specific markers. (A) PRDM8 was expressed in postmitotic BRN3B+ RGCs at E12.5. (B) ISL1 is an early marker of cholinergic ACs and RGCs. At E18.5, Prdm8 was expressed in a subset of developing ISL1+ RGCs or displaced ACs in the GCL (arrows), but ISL1 and PRDM8 were expressed in different AC populations in the nascent INL (AC[). A few faintly PRDM8+ oblong cells, with the appearance of postmitotic migrating neurons, were present in the ONBL from E18.5 to PN6 (white arrowheads in B–E). (C) At PN1, the pattern of PRDM8 expression in RGCs and a band of ACs was similar to that at E18.5. (D) Low-intensity PRDM8 expression in BP cells was first observed at PN3 (yellow arrowheads and ]BP). (E and F) At PN6, PRDM8 was expressed widely among differentiating VSX2+ BP cells, (G) in PAX6+ ACs in the INL (arrows) and PAX6+ RGCs and/or displaced ACs in the GCL (arrowheads), and (H) in BRN3+ RGCs (arrowheads). (I–L) In adult retina, PRDM8 expression was maintained in all three nuclear layers but at a very low level in PRs [outer nuclear layer (ONL)]. (J) PRDM8 was coexpressed in a subset of VSX2+ BP cells, (K) a small fraction of PAX6+ ACs in the INL (arrows) and PAX6+ RGCs and/or displaced ACs in the GCL (arrowheads), and (L) some BRN3+ RGCs. le, lens.
PRDM8 Is Expressed by Bipolar and Amacrine Subtypes in the INL of the Adult Retina.
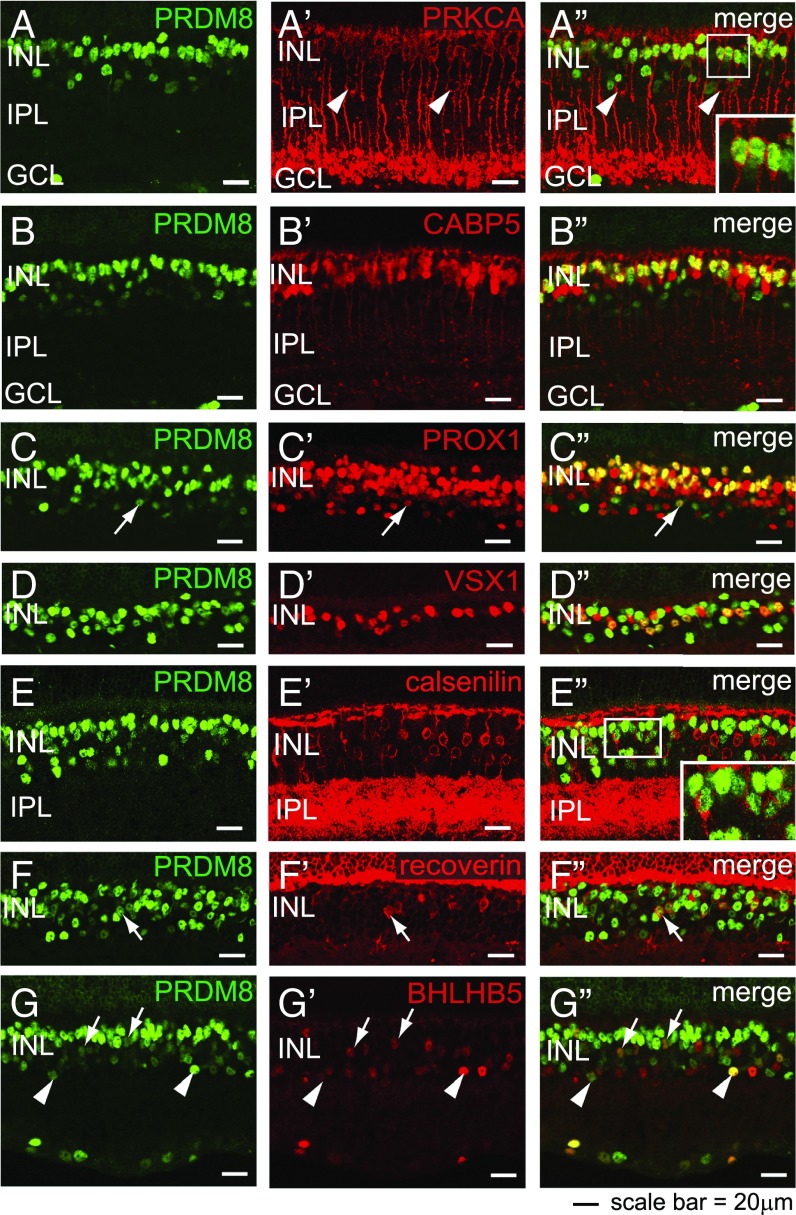
To establish the identity of the INL cells that express PRDM8 strongly in the adult retina, we costained retinas with PRDM8 antibodies and cell type-specific INL markers. Because PRDM8 staining in the INL colocalized with neither horizontal cells (calbindin+) (24) nor Müller glia (P27KIP1+) (25) (Fig. S1 C–D″), PRDM8 expression must be restricted to BP cells and ACs. To confirm PRDM8 expression in BP cells, which comprise 40% of the INL (24), we first costained retinas with PRDM8 antibodies and BP subtype-specific markers (1) (summarized in Table 1). PRKCA marks RB cells and a small population of ACs (1, 24). Using PRKCA antibodies, we found that all PRKCA+ RB cells colocalized with PRDM8-expressing cells at the outer aspect of the INL (Fig. 2 A–A″, Inset). PRDM8 also colocalized with a subset of CABP5+ cells (RB and types 3 and 5 CB cells) (1) that appeared to be mainly RB cells based on their INL location (Fig. 2 B–B″) as well as PROX1+ cells (RB cells, horizontal cells, undefined CB cells, and AII ACs) (26) (Fig. 2 C–C″). Thus, we have shown that PRDM8 is expressed by RB cells.
Table 1.
Summary of BP cell markers used in this study
| Marker | OFF-CB cells | ON-CB cells | RB cells | ||||||||||
| 1 | 2 | 3a | 3b | 4 | 5a | 5b | XBC | 6 | 7 | 8 | 9 | ||
| VSX2* | + | + | + | + | + | + | + | + | + | + | + | + | + |
| NK3R | + | + | |||||||||||
| VSX1 | + | + | + | ||||||||||
| SYT2 | + | + | |||||||||||
| BHLHB5† | + | ||||||||||||
| Recoverin | + | ||||||||||||
| HCN4 | + | ||||||||||||
| PKARIIβ | + | ||||||||||||
| Calsenilin | + | ||||||||||||
| CABP5 | + | + | + | + | + | ||||||||
| PRKCA† | + | ||||||||||||
| BHLHB4 | + | ||||||||||||
| Goα | + | + | + | + | + | + | + | + | |||||
| PROX1† | Undefined CB subtypes | + | |||||||||||
VSX2 single+ cells (i.e., counterstained for P27KIP1 to distinguish Müller glia).
Also recognizes amacrine subtypes; note that there are no specific antibody markers presently available for XBC, 8, and 9 ON-CB cells.
Fig. 2.
PRDM8 is expressed in adult BP cell subtypes. Representative confocal micrographs showing PRDM8 antibody costaining with BP subtype markers on WT retinal sections. (A–G) PRDM8 staining showed nuclear expression, which was most intense at the outer edge of the INL and of variable intensity in the rest of the INL. (A′) PRKCA stained intensely the perimeter of RB cell bodies at the outer edge of the INL, RB axons, dendrites, and a small number of ACs toward the inner edge of the INL (arrowheads). (A″) PRDM8+ nuclei were found within all PRKCA+ RB cell bodies (Inset shows an enlargement of the boxed area) but not within PRKCA+ ACs (arrowheads). (B′) CABP5 marks the nuclei and axons of types 3 and 5 CB and RB cells. (B″) PRDM8 colocalized with many CABP5+ nuclei at the upper edge of the INL but not with CABP5+ nuclei located mid-INL. (C′) The nuclear marker PROX1 stains RB cells, horizontal cells, undefined CB subtypes, and AII ACs (arrow). (C″) PRDM8 colocalized with many PROX1+ BP cells in the outer INL but only a rare subset of PROX1+ ACs in the basal INL (arrow) (Fig. S1 G–H). (D–D″) PRDM8 was expressed in many but not all VSX1+ cell nuclei (types 1, 2, and 7 CB cells). (E′) The cell perimeter and processes of type 4 OFF-CB cells were labeled with calsenilin. (E″) The great majority of PRDM8+ cells did not show any calsenilin+ staining, although infrequent PRDM8+calsenilin+ cells were observed (Inset shows an enlargement of the boxed area). (F′) Recoverin is a marker for all type 2 OFF-CB cell bodies. (F″) Most recoverin+ cell bodies colocalized with PRDM8 (arrow). (G′) BHLHB5 stains the nuclei of most type 2 OFF-CB cells (arrows) and subsets of GABAergic and glycinergic ACs (arrowheads). (G″) PRDM8 was present in many BHLHB5+ CB cells (arrows), but both markers were expressed at low relative intensity per cell. In comparison, the small subset of BHLHB5+PRDM8+ ACs showed varying levels of intensity for each marker (arrowheads).
To define the specific CB cell populations that express PRDM8, we colabeled retinal sections with antibodies to PRDM8 and six CB cell markers: VSX1 (types 1, 2, and 7), recoverin (type 2), HCN4 (type 3a), PKARIIβ (type 3b), calsenilin (type 4), and BHLHB5 (type 2), which also marks subsets of GABAergic and glycinergic ACs (Table 1) (1, 5, 17, 27). PRDM8 was expressed in many VSX1+ CB cells (Fig. 2 D–D″) and a small subset of calsenilin+ (type 4 OFF-CB) cells (Fig. 2 E–E″, Inset). Many recoverin+ cells also expressed PRDM8 (Fig. 2 F–F″, arrows). Similarly, many BHLHB5+ BP cells coexpressed PRDM8, although the signal intensities of both BHLHB5 and PRDM8 were low in such cells relative to other BP cells (Fig. 2 G–G″, arrows). In contrast, PRDM8+ BP cells did not express HCN4 (Fig. S1 E–E″) or PKARIIβ (Fig. S1 F–F″, arrowheads and F″, Inset). In summary, we have shown that PRDM8 is expressed in type 2 and a subset of type 4 OFF-CB cells but not type 3 OFF-CB cells.
To identify the subclasses of ACs that expressed PRDM8 in adult WT retina, we reanalyzed retinas for the markers PRKCA, PROX1, and BHLHB5 (Fig. 2 A–A″, C–C″, and G–G″) and costained for the neurotransmitter-related markers that define four subtypes of ACs: GAD65 for GABAergic cells, GLYT1 for glycinergic cells, CHAT for cholinergic cells, and TH for dopaminergic cells (24) (Fig. S1 G–L″). We found that PRDM8 was expressed in only two populations of mature ACs: a fraction of PROX1+PAX6+ ACs (Fig. 2 C–C″, arrows and Fig. S1 G–H, arrows) and a subset of BHLHB5+ ACs (Fig. 2 G–G″, arrowheads). In contrast, PRDM8 expression was not observed in any other AC subtype in adult WT retina, including PRKCA+ ACs at the inner edge of the INL (Fig. 2 A–A″, arrowheads). We cannot exclude the possibility, however, that PRDM8 is expressed in subsets of BHLHB5+ and PROX1+ ACs not identified by the amacrine neurotransmitter markers GAD65, GLYT1, CHAT, and TH. In summary, we have established that PRDM8 was expressed only in PROX1+ and BHLHB5+ subtypes of ACs in adult retina.
Loss of PRDM8 Function Leads to a Nonprogressive Electrophysiological Defect in Retinal Circuitry.
The robust expression of PRDM8 in developing and mature retinas and its relationship to the PRDM family of TFs, which is critical to the regulation of cell proliferation and cell fate in model systems, suggested that Prdm8 was likely to play a role in the development of the mouse retina and CNS. To determine whether Prdm8 is essential for neural development or physiology, we generated Prdm8EGFP/EGFP mice by homologous recombination, disrupting two exons with a nuclear-localized EGFP reporter gene (SI Materials and Methods and Fig. S2A). Heterozygous matings yielded WT, Prdm8+/EGFP, and Prdm8EGFP/EGFP mice in normal Mendelian ratios. Prdm8EGFP/EGFP mice appeared normal at birth and survived to adulthood, with neurological abnormalities reported previously (22). No PRDM8 protein was detected on retinal immunoblots or by immunofluorescence of adult retinas using PRDM8 N-terminal antibodies (Fig. S2 D–F), confirming that the Prdm8EGFP allele is a protein-null.
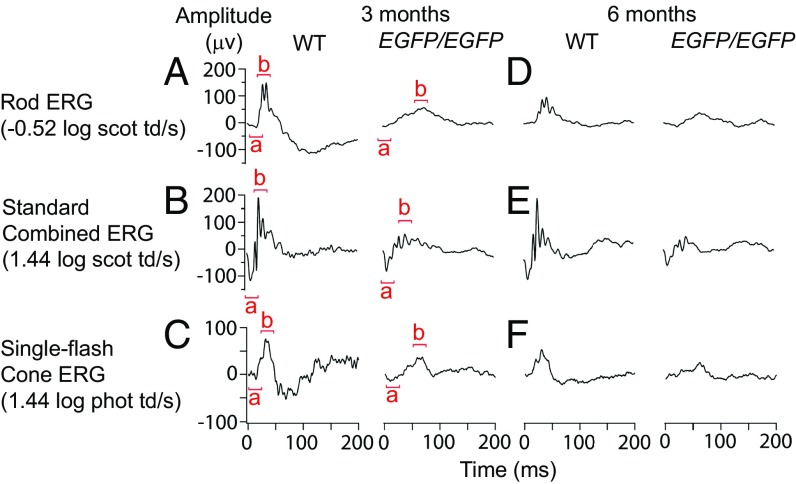
The abundant retinal expression of PRDM8 suggested that loss of PRDM8 function may disrupt visual physiology. We, therefore, recorded the retinal electrical responses [electroretinograms (ERGs)] to varying illuminations [−3.88 to 3.35 log scotopic trolands per second (scot td/s)] in Prdm8EGFP/EGFP and heterozygous and WT littermates. The ERG a wave almost exclusively represents the response from PRs, whereas the b wave reflects the response of retinal circuitry postsynaptic to PRs, primarily from the BP cell population. In photopic (daylight) conditions, the ERG is mainly cone-driven, whereas in scotopic (dim light) conditions after dark adaptation, the ERG is entirely rod-driven. At 3 mo of age, the mean scotopic b-wave amplitude of Prdm8EGFP/EGFP mice was significantly attenuated vs. WT mice (65.2 vs. 129.1 µV, respectively; P = 0.0002), and b-wave implicit time was significantly delayed (62.1 vs. 36.6 ms, respectively; P = 0.0005) (Fig. 3A). The photopic b-wave amplitude was also significantly reduced (43.2 vs. 70.9 µV, respectively; P = 0.01) (Fig. 3C). The ERGs of Prdm8+/EGFP mice were comparable with the WT. Notably, the a-wave amplitudes of Prdm8EGFP/EGFP and WT mice were comparable, suggesting that rod and cone PR functions were normal (Fig. 3 A–C). Moreover, rod PR parameters, maximal amplitude of rod PR component (RmP3) and rod PR sensitivity (S) were normal in Prdm8EGFP/EGFP mice (P > 0.3) (Fig. S3 A–C). We conclude that PR function is normal in Prdm8EGFP/EGFP mice but that the circuitry postsynaptic to both rod and cone PRs is disrupted.
Fig. 3.
Prdm8-null mice have a nonprogressive defect in retinal responses postsynaptic to PRs. ERG responses of 3- and 6-mo-old WT and Prdm8EGFP/EGFP mice. (A and D) The rod-driven b-wave response was clearly attenuated in Prdm8EGFP/EGFP mice at 3 mo of age compared with the WT and remained attenuated at 6 mo of age. (B and E) The standard combined b-wave response of Prdm8EGFP/EGFP mice at 3 and 6 mo was reduced compared with WT, whereas the a wave was normal. (C and F) Cone-derived ERG responses at 3 and 6 mo also showed a reduction in the b wave but not the a wave in Prdm8EGFP/EGFP mice compared with the WT.
To establish whether the ERG deficits of Prdm8EGFP/EGFP mice were stationary or progressive, we repeated the ERGs at 6 mo of age. We found that the ERG responses of Prdm8EGFP/EGFP and WT mice declined in amplitude at the same rate by 6 mo (Fig. 3 D–F and Fig. S3 D and E), indicating that the absence of PRDM8 causes stationary impairments in retinal function. This ERG phenotype, with a significantly reduced b:a-wave ratio (2.7 vs. 4.6 in Prdm8EGFP/EGFP vs. WT, respectively; P = 0.0002), resembles the negative ERG of humans with congenital stationary night blindness (CSNB) (28). In summary, we have found that Prdm8 loss of function leads to a nonprogressive electrophysiological defect in retinal circuitry postsynaptic to PRs and that this defect greatly impairs both cone- and rod-driven pathways in response to light.
Loss of PRDM8 Function Leads to the Absence of RB and Type 2 CB Cells in the Mature Retina.
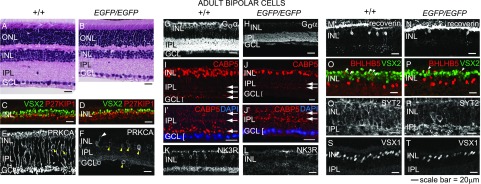
To determine the cellular basis of the ERG abnormalities, we first examined H&E-stained retinal sections from adult WT and Prdm8EGFP/EGFP littermates and quantified cells in each nuclear layer. We found that the INL and IPL were substantially thinner in the Prdm8EGFP/EGFP retina, whereas the lamination of the outer nuclear layer, GCL, and plexiform layers was normal (Fig. 4 A and B). The number of nuclei in the INL of Prdm8EGFP/EGFP retinas was reduced by 33% (P < 0.0005), but outer nuclear layer and GCL cell numbers were normal (Fig. S4A). The distribution of cone PRs and RGCs was also normal in the Prdm8EGFP/EGFP retina (Fig. S4 B–E and Table S1). We conclude that the adult Prdm8EGFP/EGFP retina was morphologically intact, except for the substantial reduction in INL cell number.
Fig. 4.
RB and type 2 OFF-cone BP cells are significantly reduced in the adult PRDM8-null retina. (A and B) H&E-stained retinal sections from adult WT and Prdm8EGFP/EGFP mice showed that Prdm8EGFP/EGFP mice have abnormally thin INL and IPL layers. (C–T) Representative confocal micrographs of retinal cryosections from adult Prdm8EGFP/EGFP mice and WT littermates (8–12 wk old) stained for RB and CB cell markers. (C and D) BP cell (VSX2+P27KIP1−) numbers were reduced in Prdm8EGFP/EGFP retina compared with WT, whereas numbers of Müller glia (VSX2+P27KIP1+ orange cells) were unchanged. (E and F) PRKCA expression in RB cells was nearly absent from the Prdm8EGFP/EGFP retina, but (F) a few PRKCA+ cells showing RB cell-like morphology persisted (white arrowhead), whereas the number of PRKCA+ ACs was significantly increased (yellow arrowheads). (G and H) Goα ON-BP staining of the INL and IPL was greatly reduced overall, with little staining in the INL and strongly diminished staining in the IPL. (I–J′) CABP5 staining (RB and types 3 and 5 CB cells) was reduced in Prdm8EGFP/EGFP retina. Furthermore, CABP5+ RB axonal projections to the innermost IPL were absent, such that only two bands of staining in the IPL were distinguishable in Prdm8EGFP/EGFP retina, whereas three were visible in the WT (arrows); the lowest band of axon terminals abutting the GCL in (I and I′) WT retina was absent in (J and J′) mutants. (I′ and J′) Enlargement of areas from I and J showing GCL nuclei counterstained with DAPI (blue). (K and L) Types 1 and 2 CB marker NK3R was dramatically reduced in the Prdm8EGFP/EGFP retina vs. WT. (M–P) The type 2 CB markers, recoverin and BHLHB5, were significantly reduced among BP cells in the Prdm8EGFP/EGFP retina. (O and P) Although there were fewer BHLHB5+VSX2+ BP cells in Prdm8EGFP/EGFP retina (arrowheads), the number of BHLHB5+VSX2− ACs was unchanged. (Q–T) The numbers of SYT2+ cells (types 2 and 6 CB cells) and VSX1+ nuclei (types 1, 2, and 7 CB cells) were reduced in the Prdm8EGFP/EGFP retina vs. WT. ONL, outer nuclear layer.
Because PRDM8 is expressed specifically in BP and AC subtypes in the adult WT INL, we reasoned that the absence of Prdm8 might disrupt these cell populations. Using VSX2 as a marker of both BP cells and Müller glia (8) and P27KIP1 (25) as a Müller glia marker, we determined that the number of BP cells (VSX2+P27KIP1− cells) was reduced by 56% (P = 0.008) in the Prdm8EGFP/EGFP retina, whereas the number of Müller glia (VSX2+P27KIP1+ cells) was unchanged (Fig. 4 C and D and Table S1). Similarly, the overall numbers of PAX6+ ACs (Fig. S4 F and G and Table S1) and calbindin+ horizontal cells (Fig. S4 H and I and Table S1) in the Prdm8EGFP/EGFP INL were also normal. We conclude that thinning of the INL in the Prdm8EGFP/EGFP retina is largely, if not entirely, because of the loss of BP cells.
Because PRDM8 was expressed predominantly in RB cells and some CB subtypes and because both rod- and cone-driven ERGs were abnormal in the Prdm8EGFP/EGFP retina, we used subtype-specific markers (Table 1) to identify the altered BP cell populations in the adult Prdm8 mutant. We found that RB cells were almost completely absent, which was indicated by the near absence of PRKCA staining (Fig. 4 E and F and Table S1). Residual PRKCA staining was evident in a small population of cells that resembled RB cells (Fig. 4F, white arrowhead). To confirm that the diminished PRKCA staining in BP cells was caused by the absence of RB cells rather than the absence of PRDM8-dependent Prkca expression in RB cells, we used two other markers: Goα, a G protein expressed by all ON-BP cells (i.e., RB and ON-CB cells) (24), and CABP5, expressed by RB cells, types 3 and 5 CB cell bodies, and their axonal projections (1). Goα staining of the Prdm8EGFP/EGFP retina was markedly reduced in the INL and the IPL (Fig. 4 G and H), consistent with the loss of RB cells, which comprise more than one-half of ON-BP cells in the mouse (29). The abundance of CABP5+ RB cell bodies in the INL was also greatly reduced (Fig. 4 I and J), and CABP5+ RB axonal projections to the IPL (the innermost band of CABP5 staining reaching the GCL in Fig. 4 I and I′) were absent from the mutant retina (Fig. 4 J and J′). Notably, the decrease in the mean number of CABP5+ cells [37 cells/(250 μm)2 field] in the Prdm8EGFP/EGFP retina closely matches the reduction in mean number of PRKCA+ RB cells [35 cells/(250 μm)2 field] (Table S1).
We next asked whether CB subtype populations were disrupted in the mature Prdm8EGFP/EGFP retina. To identify these populations, we used antibodies to nine different CB cell markers (Table S1). We found that NK3R+ staining (types 1 and 2 CB cells) was greatly reduced (by 90%) in the mutant retina (Fig. 4 K and L and Table S1). Type 2 (recoverin+) CB cells were also significantly decreased (by 74%) in the mutant (Fig. 4 M and N and Table S1), a result in accordance with the reduction in BHLHB5+VSX2+ cells, which also represent type 2 CB cells (Fig. 4 O and P and Table S1). Because NK3R is expressed in both types 1 and 2 CB cells, the dramatic reduction in NK3R staining suggested that type 1 CB cell numbers were also reduced in the Prdm8EGFP/EGFP retina. Alternatively, NK3R expression may be dependent on PRDM8, a distinction that could be investigated if specific markers of type 1 CB cells were available.
The three other CB cell subtypes with specific markers [type 3a, HCN4+ (Fig. S4 J and K); type 3b, PKARIIβ+ (Fig. S4 L and M); and type 4, calsenilin+ (Fig. S4 N and O and Table S1)] all had staining comparable with WT. In addition, type 5 CB cells also appeared to be present in normal abundance, which was deduced from (i) the presence of two residual bands of CABP5+ axon terminals of normal intensity in the outer (type 3) and mid-IPL (type 5) of the mutant retina (Fig. 4 I–J′, arrows), (ii) normal numbers of types 3a and 3b CB cells in mutant retina, and (iii) CABP5+ cell counts consistent with a loss of CABP5+ RB cells but not CABP5+ CB cells (Table S1). Similarly, there was no evidence of a reduction in type 6 CB cells: SYT2 marks CB types 2 and 6 cells, and the large loss of type 2 (recoverin+) cells is likely to account for most or all of the reduction in SYT2+ cells, so that the remaining 41% of SYT2+ cells are likely to be type 6 (Fig. 4 Q and R and Table S1). Although VSX1 (CB types 1, 2, and 7) staining was also reduced by 39% in the mutant retina (Fig. 4 S and T and Table S1), this reduction correlates with the substantial decrease in types 1 and 2 CB cell markers, suggesting that the abundance of type 7 CB cells is unaffected by the absence of PRDM8 activity. Because specific molecular markers for types XBC, 8, and 9 CB cells do not exist (1), we could not quantify these three cell populations. We conclude that the near-complete absence of RB and type 2 CB cells and the suggested reduction in type 1 OFF-CB cells in the Prdm8EGFP/EGFP retina show that Prdm8 is required for the formation or survival of these three classes of BP cells in the mammalian retina.
PRDM8 Is Required for the Survival of BP Cells.
We reasoned that the absence of BP cells from the Prdm8EGFP/EGFP retina resulted from (i) a defect in BP cell genesis; (ii) a defect in BP cell specification, resulting in a population of abnormal precursors that then die; (iii) the misspecification of BP cells to an alternate, inappropriate cell fate, resulting in cell death; or (iv) a defect in BP cell differentiation and survival. To distinguish among these alternatives, we examined retinal morphogenesis before and including the times of BP cell birth, differentiation, and developmental apoptotic pruning by quantifying cell numbers in the layers of the developing central retina between PN1 and PN14 in Prdm8EGFP/EGFP vs. WT mice.
At PN1, before the peak of BP cell birth (30), we found no difference between Prdm8EGFP/EGFP and WT mice in the total number of cells (i.e., ONBL plus GCL) in the central retina (Fig. S5 A–C). Moreover, after BP cell genesis had ended in the central retina (30), at PN7, we observed no difference from the WT in the number of cells in any cell layer (Fig. S5 D–F). By PN8, however, the number of cells in the INL of the central Prdm8EGFP/EGFP retina was 5.5% less than the WT (paired t test; P = 0.036), 15.7% less at PN10 (P = 0.003), and 16.1% less at PN12 (P = 0.042) (Table S2). By PN14, when retinal development is near completion, the number of cells in the INL was 30% less than the WT (P < 0.0002)—a decrease comparable with that of the mature retina at 2–3 mo, with no change in cell number in the other nuclear layers (Fig. S5 G–I).
To confirm that the decrease in INL cell number was caused by cell loss after PN7, we counted TUNEL+ cells in the retinas of Prdm8EGFP/EGFP and WT mice between PN8 and PN12, an interval when cell proliferation has ceased in the central retina and developmental apoptotic pruning occurs (30, 31). Although there were fewer total INL cells in the central Prdm8EGFP/EGFP retina than in the WT at all times from PN8 onward, we found significantly more TUNEL+ cells in the INL of Prdm8EGFP/EGFP vs. WT retinas at PN10 (P = 0.038) (Table S2). Altogether, the above studies establish that INL morphogenesis proceeds normally in the Prdm8EGFP/EGFP retina up to PN7 but that increased apoptosis subsequently reduces the number of cells in the adult INL.
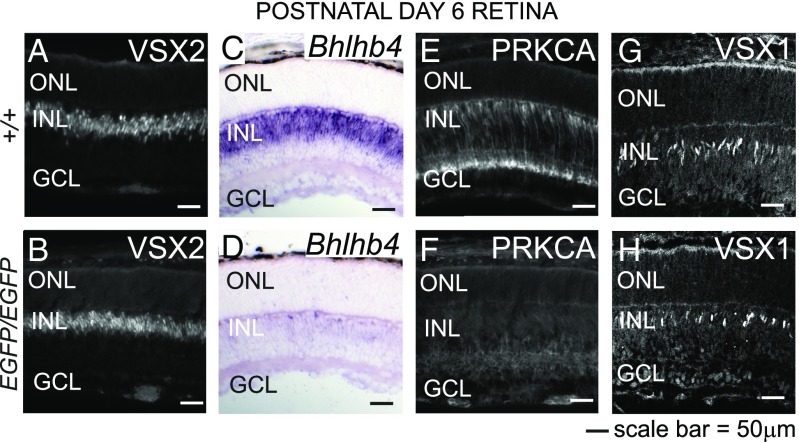
To determine whether BP cell specification and differentiation are impaired during the development of the Prdm8EGFP/EGFP retina, we identified specific BP cell populations by immunolabeling. At PN6, when the Prdm8EGFP/EGFP retina is morphologically normal, we detected no difference in the number of VSX2+ BP (or Müller glial) cells or the level of VSX2 expression in these cells (Fig. 5 A and B). Moreover, we found no evidence of misspecification of BP cells, because no ectopic VSX2 expression was found outside the INL from PN6 onward (Fig. S5 J, K, N, and O). These findings indicate that BP cell identity is established normally in the absence of PRDM8.
Fig. 5.
BP cells are specified but do not differentiate in Prdm8 mutants. (A and B) Immunofluorescence antibody staining for the BP cell marker, VSX2, was equivalent in WT and Prdm8EGFP/EGFP retina at PN6. (C–H) The expression of the BP cell markers Bhlhb4, PRKCA, and VSX1 is PRDM8-dependent. (C and D) By in situ hybridization, Bhlhb4 expression in developing BP cells was markedly reduced in the Prdm8EGFP/EGFP retina vs. WT. (E and F) PRKCA expression in RB cells is barely detectable in Prdm8EGFP/EGFP retina vs. WT retina. (G and H) VSX1+ BP cells were reduced in number in Prdm8EGFP/EGFP retina vs. WT. ONL, outer nuclear layer.
In contrast, using BP markers in the developing Prdm8EGFP/EGFP retina, we observed a near-complete absence at PN6 of cells expressing the RB markers Bhlhb4 (Fig. 5 C and D) and PRKCA (Fig. 5 E and F). Subsequently, at PN10, some PRKCA+ cells were present in both the inner and outer aspects of the INL (Fig. S5Q, yellow and white arrowheads), resembling the pattern observed in the adult Prdm8EGFP/EGFP retina. Consistent with the large decrease in these two RB markers, the intensity of Goα expression (which marks RB and ON-CB cells) (Table 1) in the IPL at PN8 was also greatly reduced (Fig. S5 R and S). The developmental expression of VSX1 in the developing CB cell population was also reduced at PN6, which is in accordance with the decrease seen in adult retina, although the intensity of VSX1 staining per cell was normal (Fig. 5 G and H). In contrast, BHLHB5 expression in BP cells, at PN6, was normal (Fig. S5 T and U, ]BP). Collectively, these results establish that PRDM8 is required for the developmental expression of genes that regulate (Bhlhb4) or define the differentiation (Prkca) of RB cells or in the case of Vsx1, several types of CB cells. Altogether, our studies indicate that PRDM8 is not required for BP cell specification but is required for the differentiation and survival of RB cells and some CB cell subtypes.
PRDM8 Is Required for the Development of Amacrine Subtype Identity.
Because PRDM8 was expressed in a significantly larger fraction of PAX6+ ACs in the developing vs. adult WT retina (Fig. 1 G and K), we asked whether the loss of PRDM8 function in the Prdm8EGFP/EGFP retina might disrupt AC as well as BP cell development or survival. We first examined AC populations in the adult Prdm8EGFP/EGFP retina. Although the total number of ACs in the mutant retina was comparable with the WT (Fig. S4 F and G and Table S1), we found using antibodies to eight additional markers (Table S1) a 2.5-fold increase in the fraction of PRKCA+ ACs (Figs. 4 E and F, yellow arrowheads, and 6 A′ and B′ and Fig. S6 A–B″), a 66% increase in a second expanded population (CHAT+ ACs) (Fig. 6 C and D), and a 36% decrease in GAD65+ ACs (Fig. 6 A and B and Fig. S6 C and D). These findings are particularly striking, because PRDM8 is not expressed in any of these amacrine subtypes in the WT adult retina, which was noted earlier (Fig. 2 A–A″ and Fig. S1 I–I″ and K–K″). In addition to decreasing the prevalence of GAD65+ ACs in the mutant retina, we also noted that the loss of PRDM8 function correlated with greatly reduced GAD65 expression in the expanded PRKCA+ AC population. Although we observed some PRKCA+GAD65+ cells in the Prdm8EGFP/EGFP retina (Fig. 6 B–B″, arrows), the majority of PRKCA+ ACs either failed to express GAD65 or expressed very little (Fig. 6 B–B″, arrowheads). We considered the possibility that this expanded population of PRKCA+ ACs in the Prdm8EGFP/EGFP retina might express another neurotransmitter marker instead of GAD65: either CHAT, which also labeled more ACs in the Prdm8 mutant (Fig. 6D), or GLYT1, because glycinergic and GABAergic ACs normally account for 85% of the AC population in mature retina (16) (Fig. S6 E–F″). However, PRKCA+ ACs did not coexpress either CHAT or GLYT1 in the PRDM8-null retina, suggesting that this mutant amacrine population represented a distinct subtype that was not defined by the common amacrine neurotransmitter markers (GAD65, GLYT1, or CHAT). No other alterations were seen in the abundance of other amacrine subtypes in the adult mutant retina (i.e., not in BHLHB5, TH, PROX1, or calretinin-positive cells) (Figs. 4 O and P and 6 E and F, Fig. S6 G–J, and Table S1). Overall, these findings suggest that PRDM8 normally favors the development of GAD65+ ACs but limits the formation of PRKCA+ and CHAT+ subtypes from progenitor cells.
Fig. 6.
Loss of PRDM8 alters AC-subtype identity. Confocal micrographs of retinal cryosections from adult Prdm8EGFP/EGFP mice and WT littermates stained for AC markers. (A and B) Fewer GAD65+ ACs in the INL and decreased GAD65 IPL staining were present in the Prdm8EGFP/EGFP retina, specifically of the middle and innermost GAD65+ bands of the IPL. (A–A″) PRKCA+ ACs colocalized with a subset of weakly positive GAD65+ cells in WT retina (arrows). (B–B″) In the Prdm8EGFP/EGFP retina, PRKCA+ AC numbers were significantly increased; some PRKCA+ cells were GAD65+ (arrows), whereas others expressed very low intensity or no GAD65 (arrowheads). (C) CHAT+ cholinergic AC bodies and their processes did not coexpress PRKCA in WT retina. (D) In Prdm8EGFP/EGFP retina, both CHAT+ and PRKCA+ ACs were increased in number, but the two populations remained distinct. (E–F′) VGLUT1 labels axon terminals of BP cells, and calretinin labels a subset of ACs, which together help to define the five sublaminae of the IPL. (E and F) Lamination was maintained in Prdm8EGFP/EGFP retina, but the density of VGLUT1 staining in each sublamina was reduced, and the layers appeared to be thinner. (E′ and F′) Enlargement of IPL areas described in E and F.
We considered that the absence of RB and CB cell projections to the IPL and the changes in the composition of the amacrine population might disrupt lamination of the IPL in the Prdm8EGFP/EGFP retina. The five strata of the mouse IPL are distinguished by alternating bands of immunohistochemical staining for VGLUT1, which stains BP cell axon termini, and calretinin, which marks a subset of ACs and their termini (1). In addition to normal numbers of calretinin+ cell bodies in the Prdm8EGFP/EGFP retina (Fig. 6 E and F and Table S1), we found that IPL lamination (indicated by the three broken lines in Fig. 6 E′ and F′ that show the position of calretinin+ termini) was unaffected by the loss of PRDM8 function. Conversely, the staining density of VGLUT1 within each sublamina was reduced, and each sublamina was thinner (Fig. 6 E–F′). These findings confirm that the lamination and position of residual AC and BP processes in the IPL of adult Prdm8EGFP/EGFP retina are normal but that there are significantly fewer BP axon termini, reflecting the reduction in the numbers of RB and type 2 CB cells.
To determine when the changes in the amacrine subtype populations in mature retina are present in the developing Prdm8EGFP/EGFP retina, we used subtype-specific markers to stain PN6 and PN8 WT and mutant retinas. We first established that several developing AC subtypes expressed PRDM8 in the developing postnatal WT retina, including subsets of PRKCA+ (Fig. S7 A–A″), TH+ (Fig. S7 C–C″), GAD65+ (Fig. S7 G–G″), PROX1+ (Fig. S7 I–I″), and BHLHB5+ (Fig. S7M) cells but not CHAT+ (Fig. S7 E–E″) or GLYT1+ cells (Fig. S7 K–K″). In the Prdm8EGFP/EGFP retina, the increased number of CHAT+ cells was evident at PN6 (Fig. S7 E′ and F), whereas the increase in PRKCA+ ACs in the INL (Fig. S5 L, M, P, and Q) and reduction in GAD65+ staining in the IPL of the Prdm8EGFP/EGFP retina were clearer at PN8 (Fig. S7 G′ and H). We also observed an increase in BHLHB5+ cells in the Prdm8EGFP/EGFP retina at PN6, appearing as a band of intensely stained cells in the proximal INL that was broader than in the WT retina (Fig. S5 T and U, ]AC). The increased population of BHLHB5+ cells, which became more apparent by PN8 (Fig. S7 N–P, ]AC), coexpressed the AC marker PAX6 and not the BP cell marker VSX2, indicating that they were, indeed, ACs. Although the prevalence of BHLHB5+ ACs in the adult PRDM8-null retina was normal, these data suggest that PRDM8 represses BHLHB5 expression in some populations of ACs during postnatal retinal development. In contrast, the populations of TH+ (Fig. S7D), PROX1+ (Fig. S7J), and GLYT1+ (Fig. S7L) amacrine subtypes were normal in postnatal Prdm8EGFP/EGFP retina. These results, together with the absence of PRDM8 expression in the PRKCA+, CHAT+, and GAD65+ amacrine subtypes in adult WT retina suggest that (i) PRDM8 acts early and transiently in development to define the fate of these amacrine subtypes, after which its expression is extinguished, and/or (ii) loss of PRDM8 function in the developing mutant retina disrupts the action of extrinsic regulators that participate in the formation of amacrine subtypes (for example, the development of CHAT+ cholinergic cells). In contrast to the role of PRDM8 in BP cell survival, ACs are maintained in normal total numbers in the PRDM8-null retina, but the prevalence of AC subtypes (PRKCA+, GAD65+, CHAT+, and during development, BHLHB5+) is altered.
PRDM8 Is Present at the Prkca Promoter.
The PRDM8-dependent expression of important RB and CB cell genes suggested that they may be directly or indirectly regulated by PRDM8. To determine whether any of these differentially expressed markers are direct targets of PRDM8 during retinal development, we performed ChIP studies of genomic DNA from PN6 WT mouse retina. ChIP real-time PCR primers were directed toward evolutionarily conserved noncoding sequences in the promoter region of candidate genes (Fig. 7A and Table S3). We found that PRDM8 was present at the Prkca promoter at PN6 but not our selected promoter regions of Bhlhb4, Bhlhb5, or Vsx1 (Fig. 7 A and B, C1 and C2 and Table S3). To examine whether PRDM8 might directly regulate Prkca expression, we cloned two overlapping Prkca promoter regions (PrkcaR1 and PrkcaR2) into a constitutively active luciferase reporter construct for heterologous cell expression analysis (Fig. 7C). Both regions included the 5′-most conserved noncoding sequence (−1,187 to −1,065 bp) upstream of the Prkca start codon with the greatest enrichment in ChIP assays and the highest conservation across species (Fig. 7 A–C, C1). Cotransfection into HEK293 cells of a mouse PRDM8 expression vector with either luciferase reporter construct led only to a nonspecific decrease in reporter activity compared with control cells not expressing PRDM8. The effect was nonspecific, because (i) a decrease in reporter activity was also demonstrable when PRDM8 was cotransfected with the empty pGL3 vector and (ii) the proportional change in reporter activity was not significantly different among the three constructs. Because we previously found that the sequence-specific targeting of PRDM8 to the dorsal telencephalon in vivo depends on another TF, BHLHB5, we interrogated our Prkca promoter sequences for the BHLHB5 consensus binding motif (22). Although we detected five examples of the classic E-box core enhancer sequence (CANNTG) (Fig. 7A, asterisks), we did not find the 8-nt consensus binding motif for BHLHB5 (CATATGNTNT) (22). Given that our studies of PRDM8 and BHLHB5 in the dorsal telencephalon have shown that they function within a transcriptional repressor complex (22), the simplest explanation for our results is that, in retina, PRDM8 also requires the cooperation of one or more other TFs that are absent from HEK293 cells to bind target promoters and/or mediate its influence on transcription; whether the outcome is the activation or repression of target genes may depend on the binding partner and the context.
Fig. 7.
PRDM8 binds at the Prkca promoter. (A) Schematic of mouse Prkca and its putative promoter region showing DNA sequence conservation among vertebrates (green peaks; PhastCons; genome.ucsc.edu) (39). The two most conserved noncoding sequences in the Prkca promoter region are located at −1,187 to −1,065 bp (C1; orange) and −680 to −568 bp (C2; blue) relative to the Prkca start codon. Asterisks represent the canonical E-box motif found at −1,414, −1,222, −855, −798, and −433 bp relative to the Prkca start codon. (B) ChIP PCR assays on WT PN6 retinal lysates showed that PRDM8 was present at the two target sequences, C1 (orange) and C2 (blue), with sixfold enrichment of C1 vs. the control sequence in exon 2 (+3 kb from the start codon). Enrichment scores were normalized to the negative antibody control. Error bars represent ±SEM. (C) Luciferase reporter constructs containing overlapping promoter regions PrkcaR1 and PrkcaR2 were cloned using primers F, R1, and R2 (arrows in A).
In conclusion, our results show that PRDM8 is required for the expression of genes that regulate RB and CB cell differentiation, maintenance, or survival and suggest that Prkca, a major RB marker and a marker of ACs that are increased in number in the PRDM8-null retina, may be a direct target of PRDM8.
Discussion
We have established that Prdm8 has an essential role in the development of the mammalian retina. In embryonic retina, PRDM8 is strongly expressed in RGCs and ACs and at lower levels, in the ONBL. The PRDM8-expressing cells in the ONBL may be retinal progenitors, newborn postmitotic neurons, or both. In the postnatal retina, PRDM8 expression in BP cells emerges at PN3, well after the onset of BP cell birth in the embryo (30), and is maintained at a high level in adult BP, amacrine, and ganglion cells. In adults, PRDM8 protein expression is maintained in all three nuclear layers, but highest in the INL, where it is present predominantly in RB cells as well as types 2 and 4 OFF-CB cells and a subset of ACs.
We determined that the INL of the adult Prdm8EGFP/EGFP retina is hypocellular because of a virtual absence of RB cells and substantially reduced type 2 OFF-CB cells, whereas other layers and the overall organization of the retina are normal. The absence of BP cells is not caused by a failure of BP cell specification, because the number of VSX2+ cells is normal at PN6, by which time the majority of BP cells have exited the cell cycle (30). Rather, both RB cell differentiation and survival are disrupted in the absence of PRDM8, which is reflected by the delayed expression of PRKCA in a small population of putative RB cells in the PN10 INL and the apoptotic death of the majority of RB cells by PN14. We suggest that type 2 OFF-CB cells are likely to have a comparable defect in cell survival, failing to differentiate and dying by apoptosis. The absence of these BP populations would account for the profound b-wave abnormalities in scotopic and photopic ERG traces of Prdm8EGFP/EGFP mice.
Within the network of genes that regulates the development of retinal BP cells, our findings suggest that Prdm8 is essential to the differentiation and survival of BP cell subtypes. The prevailing model of retinal BP cell development is that VSX2, MATH3, and MASH1 together specify a pan-BP cell identity, with VSX2 conveying positional identity (INL) and MATH3-MASH1 promoting neuronal (vs. glial) cell fate (10, 11). At PN6, VSX2+ BP cells are normal in abundance and distribution in Prdm8-null mice, signifying that Vsx2 expression is not dependent on PRDM8, which acts either downstream or independently of VSX2 (Fig. 8). The fact that PRDM8 is required for the survival of BP cell subtypes but not BP cell birth implies that it is critical during BP cell differentiation; the death of BP cells in the Prdm8EGFP/EGFP retina presumably reflects a failure to express one or more genes required for BP subtype survival. PRDM8 is similar to other TFs required for postmitotic BP subtype birth or survival, including BHLHB4 (RB cells) (7), BHLHB5 (type 2 OFF-CB cells) (5), and ISL1 (ON- and OFF-BP subtypes) (4) (Fig. 8). In contrast, another group of TFs is required for the differentiation but not the survival of BP subtypes, including IRX5 (2), IRX6 (32), and VSX1 (3).
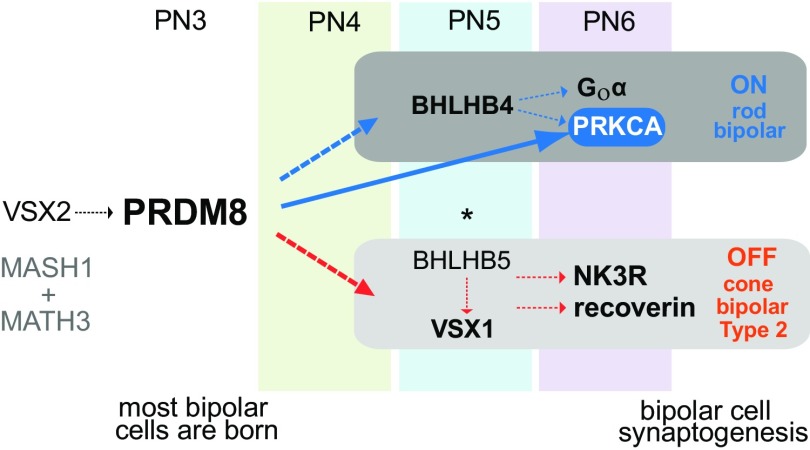
Fig. 8.
Model of the PRDM8 transcriptional regulatory network in BP cell development. VSX2, MASH1, and MATH3 specify BP cell fate. Normal VSX2 expression in the Prdm8EGFP/EGFP retina indicates that BP cell birth and identity are established in the absence of PRDM8 and that Vsx2 is not under the control of PRDM8. PRDM8 is expressed in BP cells at PN3 (before Bhlhb4 and Vsx1). BHLHB4 is essential for RB differentiation and survival (7), and BHLHB5 and VSX1 are essential for the genesis and complete differentiation of CB cells, respectively (3, 5, 6). PRDM8 binds at the promoter of Prkca and is required for the normal expression of RB markers PRKCA, Goα, and Bhlhb4 and the CB marker, VSX1. PRDM8 is not essential for the onset of BHLHB5 expression in type 2 OFF-CB cells; however, in the absence of PRDM8, the development of NK3R+ and recoverin+ type 2 OFF-CBs is impaired. PRDM8 represses BHLHB5 expression in a subset of ACs (this study). *PRDM8 represses BHLHB5 expression in developing CNS (22).
We showed that the expression of two TF genes required for normal BP cell differentiation, Bhlhb4 and Vsx1, is dependent on PRDM8. At PN6, before the developmental apoptotic pruning of BP cells, the expression of both TFs was reduced in the Prdm8-null retina. Bhlhb4 expression was significantly down-regulated in developing RB cells, and expression of the VSX1 protein in CB cells was reduced in the Prdm8EGFP/EGFP INL because of a reduction in the number of VSX1-expressing cells rather than a reduction in the level of VSX1 expression per cell. Our findings also suggest that PRDM8 shares a CB cell differentiation pathway with VSX1. Like Prdm8 mutants, Vsx1-null mutants have reduced expression of key CB cell differentiation markers (NK3R and recoverin), and they manifest CB cell-dependent deficits on ERG traces (3, 12).
BHLHB4 and BHLHB5 are members of the evolutionarily conserved OLIG subfamily of neuronal bHLH TFs, and within this subgroup, they are most closely related to each other (33). It is noteworthy, therefore, that the Prdm8-null phenotype has striking parallels with the Bhlhb4−/− and Bhlhb5−/− BP cell phenotypes, suggesting that PRDM8 and these bHLH TFs share developmental pathways in BP cells. First, PRDM8 and BHLHB4 are both expressed in RB cells, which are virtually absent from both adult Bhlhb4−/− (7) and Prdm8EGFP/EGFP retinas. The reduced Bhlhb4 expression that we observed in RB cells at PN6 in the Prdm8EGFP/EGFP retina suggests that PRDM8 may be required for Bhlhb4 expression during RB cell differentiation. Second, both adult Bhlhb4−/− and Prdm8-null mice lack RB cells and have an electronegative b wave on scotopic ERG responses (7). Third, PRDM8 and BHLHB5 are each found in type 2 OFF-CB cells: in Bhlhb5 mutants, VSX2+ BP cells are born but fail to assume a type 2 OFF-CB cell identity (5), an outcome comparable with the absence of type 2 OFF-CB cells in the Prdm8-null retina. Because BHLHB5 expression was normal in the developing BP population of the Prdm8EGFP/EGFP retina, however, we conclude that PRDM8 does not regulate Bhlhb5 expression in these cells. Rather, these findings are consistent with PRDM8 and BHLHB5 acting in parallel to control CB cell development.
In contrast to the requirement for PRDM8 function in the differentiation and survival of BP subtypes, AC survival is not affected by the lack of PRDM8 function. Our data indicate that PRDM8 is transiently expressed in several amacrine subtypes during postnatal development and contributes to the generation of AC diversity in the adult retina. Other transcription regulators of BP cell development are also implicated in the formation of ACs. For example, Math3 is transiently expressed by differentiating amacrine as well as BP cells (34). Although the overexpression of Math3 promotes the formation of rods rather than ACs, the combined overexpression of Pax6 with Math3 significantly increases the genesis of ACs (34). Thus, the combined action of MATH3 with the homeodomain TFs VSX2 or PAX6 determines the fate of BP cells or ACs, respectively. In contrast, the role of PRDM8 is more similar to the roles of ISL1 and BHLHB5, which are TFs expressed in developing and mature BP and AC subtypes that influence the generation of AC-subtype diversity (4, 5, 17, 35). ISL1-deficient retinas lack ON- and OFF-BP subtypes and cholinergic ACs (4), whereas in the Bhlhb5-null retina, type 2 OFF-CB cells are virtually absent, and the prevalence of GABAergic, glycinergic, dopaminergic, and cholinergic ACs is altered (5, 17).
We found that BHLHB5 expression in the Prdm8EGFP/EGFP retina was up-regulated in a subpopulation of ACs at PN6 and PN8. These BHLHB5+ cells may be ACs in which PRDM8 normally suppresses Bhlhb5 expression, cells that have been misspecified to an alternate amacrine subtype, or both. Because Prdm8 mutants have an increased number of BHLHB5+ cells in the maturing—but not the adult—AC population, PRDM8 and BHLHB5 may engage in a negative regulatory interaction in developing ACs, similar to their interaction in the dorsal telencephalon: Bhlhb5 itself is up-regulated in the developing telencephalon of Prdm8EGFP/EGFP mice, and Prdm8 is up-regulated in Bhlhb5−/− mutants (22). BHLHB5 and PRDM8 bind to the same target promoter elements in vivo, including the Bhlhb5 promoter, but whereas PRDM8 depends on BHLHB5 for sequence-specific DNA targeting, BHLHB5 can bind to the same sequences in the absence of PRDM8 (22). Furthermore, Prdm8 and Bhlhb5 mutants exhibit comparable (although not identical) changes in the prevalence of amacrine subtypes. First, GABAergic (GAD65+) ACs are significantly reduced in both Bhlhb5−/− and Prdm8EGFP/EGFP retina (17). Second, although neither BHLHB5 nor PRDM8 are expressed by cholinergic (CHAT+) ACs during retinal development, both BHLHB5 and PRDM8 seem to negatively regulate the differentiation of cholinergic ACs, presumably by noncell-autonomous mechanisms (17). Nevertheless, there are clear differences in the retinas of Prdm8 and Bhlhb5 mutants. In Bhlhb5 mutants, the prevalence of both dopaminergic and glycinergic ACs is reduced, and the abundance of calretinin+ ACs is increased, whereas none of these cell populations are abnormal in the Prdm8EGFP/EGFP retina (5, 17). Altogether, these findings suggest that PRDM8 and BHLHB5 may cooperate in the regulation of GABAergic and cholinergic AC development by cell- and noncell-autonomous mechanisms, respectively, but that BHLHB5 has a much wider role in the fate of other AC subtypes.
We showed that PRKCA expression in BP cells was greatly diminished in the Prdm8-null retina at PN6, a time when all BP cells were still present, suggesting that PRDM8 directly or indirectly regulates Prkca transcription. In contrast, the number of PRKCA-expressing ACs was increased in PN8, PN10, and adult Prdm8EGFP/EGFP retina, but total AC numbers were not altered, a change in the PRKCA+ amacrine population that, to date, seems to be unique to the Prdm8EGFP/EGFP retina. Both findings along with our demonstration that PRDM8 binds to the Prkca promoter are consistent with a role for PRDM8 in the regulation of Prkca expression. Our studies of PRDM8 in the dorsal telencephalon indicate that it acts as a transcriptional repressor (22). The fact that we observed a decrease in PRKCA expression in BP cells and an increase in PRKCA expression in ACs in the Prdm8-null retina suggest, however, that PRDM8 has a context-dependent influence on Prkca expression. Thus, PRDM8 may repress gene expression in some contexts (e.g., ACs) and activate it in others (e.g., RB cells). Indeed, PRDM16 can be both a repressor and an activator in the same cell depending on the target locus (18). Although PRKCA expression is a hallmark of RB cell identity, it is unlikely that the absence of PRKCA in the Prdm8EGFP/EGFP retina is responsible for BP cell loss, because the changes in retinal morphology and ERGs of Prkca−/− mutants are minor (36).
Using a heterologous cultured cell system, we were unable to show PRDM8-dependent modulation of Prkca transcription from reporter constructs. This result may reflect the fact that (i) PRDM8 may not bind the Prkca promoter directly but only through another cofactor not present in HEK293 cells, (ii) PRDM8-mediated expression of Prkca in retina may require the recruitment by PRDM8 of another transcriptional regulator not present in HEK293 cells, or (iii) the DNA sequence bound by PRDM8 in the ChIP experiments may be upstream of the PrkcaR1 and PrkcaR2 luciferase assay elements. We suggest that, as we observed in the dorsal telencephalon (22), PRDM8 requires the presence of another TF (perhaps another bHLH TF with an E-box binding motif) to regulate its binding to DNA and/or its transcriptional activity at the Prkca retinal promoter.
A role for other PRDM TFs in the developing vertebrate retina has been defined only for PRDM1 (BLIMP1) (18). Prdm1 mutants have fewer PRs and an increase in the number of BP cells, suggesting that PRDM1 acts as a molecular switch controlling PR vs. BP cell fate by repressing the BP cell program. In contrast, we found no evidence of altered BP cell fate in the Prdm8-null retina. However, we cannot exclude the possibility that the loss of PRDM8 function changes the fate of some BP subtypes, because specific markers are currently lacking for types 1 and 5–7 CBs and molecular markers are not widely available for types XBC, 8, and 9 CB cells (1, 29). A comprehensive analysis of BP cell ultrastructure and molecular phenotypes in the adult Prdm8EGFP/EGFP retina together with Prdm8 overexpression studies and lineage tracing analyses of Prdm8-expressing precursors may reveal additional roles for PRDM8 in controlling BP subtype identity.
In both mature Prdm8EGFP/EGFP and Bhlhb4−/− retinas, a residual population of PRKCA+ BP cells persists. The identity of these cells is uncertain, but they may account for the detectable (although greatly diminished) scotopic ERG b waves seen in these mice (7). Alternatively, residual CB cells may transduce some postsynaptic signals from PRs. This negative b-wave ERG phenotype [normal a wave (derived from PRs) and reduced or absent b wave (derived from postsynaptic circuitry)] is a distinct feature of human CSNB, which results from mutations in 12 known genes that are all involved in visual signal transduction to BP cells (37). Some CSNB patients have no detectable scotopic b wave (complete or CSNB type 1), whereas others have a minimal to moderate one (incomplete or CSNB type 2), with associated photopic abnormalities on ERGs. The Prdm8-null phenotype, thus, most closely mimics CSNB2 (28). The two genes linked to CSNB2 [CACNA1F, encoding a cation channel [Online Mendelian Inheritance in Man (OMIM) 300110], and CABP4, encoding a CACNA1F-associated Ca2+ binding protein (OMIM 608965)] have no known direct link to Prdm8. Mutations in TF genes that control BP cell differentiation and survival in animal models have not been described in human CSNB. Because Prdm8-null mice exhibit motor and sensory impairments outside the retina, patients carrying PRDM8 mutations may not present with CSNB but with or without night blindness as part of a complex neurological disorder. For instance, a Phe261Leu missense mutation of PRDM8 has been associated with early-onset Lafora disease, a severe myoclonic epilepsy syndrome (21).
In summary, our findings establish that PRDM8 is essential for the differentiation and survival of RB and type 2 OFF-CB cells in the developing and adult mouse retina. PRDM8 is required for the normal developmental expression of several TFs that govern BP and amacrine subtype identity and function, and binds at the promoter region of the classic RB marker gene Prkca. We suggest that mutations in human PRDM8 may cause human CSNB.
Materials and Methods
Animal Husbandry.
The use of animals was approved by the Toronto Centre for Phenogenomics Animal Care Committee and complied with United Kingdom Animals (Scientific Procedures) Act 1986 and associated guidelines. Gene targeting and genotyping are described in SI Materials and Methods. Mice were bred on a mixed genetic background (50% C57BL/6J, 25% 129S1/SviMJ, and 25% 129X1/SvJ).
Immunostaining and Histochemistry.
Immunostaining of mouse retinal sections was performed as described in SI Materials and Methods. Assays on adult mice were performed at 8–12 wk of age. Antibodies and modifications of the protocol for cell counting and TUNEL assays are presented in SI Materials and Methods.
Electroretinography.
Full-field ERGs were obtained in a custom Ganzfeld dome by using the techniques described previously (38). Comprehensive descriptions of all methods are available in SI Materials and Methods.
ChIP.
ChIP assays followed an established protocol (10) with modifications as described in SI Materials and Methods. Chromatin from cross-linked and sonicated PN6 WT mouse retinas was immunoprecipitated with polyclonal rabbit PRDM8 antibodies, subjected to real-time PCR for target sequences in the Prkca promoter, and normalized to a negative antibody control and nonspecific enrichment of a negative control sequence in exon 2. Primer sequences for quantitative PCR after ChIP assays are described in Table S3.
Supplementary Material
Acknowledgments
We thank R. L. Chow and Z. Ivakine for their assistance and T. M. Jessell, R. L. Chow, F. Haeseleer, A. Hirano, E. Levine, and F. Müller for the gifts of antibodies. This work was supported by a Foundation Fighting Blindness Canada Studentship (to C.C.J.), Hospital for Sick Children and University of Toronto Research Training Competition (Restracomp) Fellowships (to C.C.J. and D.N.), Wellcome Trust Postdoctoral Travel Award Fellowship WT084585 (to D.A.), National Eye Research Centre Grant RJ6042 (to D.A.), NIH Grants R01AR063772(to S.E.R.) and R21AR064445 (to S.E.R.), Foundation Fighting Blindness Grant C-CL-0812-0593-RFSW01 (to M.K. and D.G.B.), Canadian Institutes of Health Research Grants MOP-7315 and IOP-54037 (to R.R.M.), the Canadian Genetic Diseases Network (R.R.M.), and the Macula Vision Research Foundation (R.R.M.). R.R.M. is a Tier 1 Canada Research Chair in Neurogenetics.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1505870112/-/DCSupplemental.
References
- 1.Euler T, Haverkamp S, Schubert T, Baden T. Retinal bipolar cells: Elementary building blocks of vision. Nat Rev Neurosci. 2014;15(8):507–519. doi: 10.1038/nrn3783. [DOI] [PubMed] [Google Scholar]
- 2.Cheng CW, et al. The Iroquois homeobox gene, Irx5, is required for retinal cone bipolar cell development. Dev Biol. 2005;287(1):48–60. doi: 10.1016/j.ydbio.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 3.Chow RL, et al. Control of late off-center cone bipolar cell differentiation and visual signaling by the homeobox gene Vsx1. Proc Natl Acad Sci USA. 2004;101(6):1754–1759. doi: 10.1073/pnas.0306520101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elshatory Y, et al. Islet-1 controls the differentiation of retinal bipolar and cholinergic amacrine cells. J Neurosci. 2007;27(46):12707–12720. doi: 10.1523/JNEUROSCI.3951-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng L, et al. Requirement for Bhlhb5 in the specification of amacrine and cone bipolar subtypes in mouse retina. Development. 2006;133(24):4815–4825. doi: 10.1242/dev.02664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohtoshi A, et al. Regulation of retinal cone bipolar cell differentiation and photopic vision by the CVC homeobox gene Vsx1. Curr Biol. 2004;14(6):530–536. doi: 10.1016/j.cub.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 7.Bramblett DE, Pennesi ME, Wu SM, Tsai MJ. The transcription factor Bhlhb4 is required for rod bipolar cell maturation. Neuron. 2004;43(6):779–793. doi: 10.1016/j.neuron.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 8.Liu IS, et al. Developmental expression of a novel murine homeobox gene (Chx10): Evidence for roles in determination of the neuroretina and inner nuclear layer. Neuron. 1994;13(2):377–393. doi: 10.1016/0896-6273(94)90354-9. [DOI] [PubMed] [Google Scholar]
- 9.Burmeister M, et al. Ocular retardation mouse caused by Chx10 homeobox null allele: Impaired retinal progenitor proliferation and bipolar cell differentiation. Nat Genet. 1996;12(4):376–384. doi: 10.1038/ng0496-376. [DOI] [PubMed] [Google Scholar]
- 10.Livne-Bar I, et al. Chx10 is required to block photoreceptor differentiation but is dispensable for progenitor proliferation in the postnatal retina. Proc Natl Acad Sci USA. 2006;103(13):4988–4993. doi: 10.1073/pnas.0600083103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatakeyama J, Tomita K, Inoue T, Kageyama R. Roles of homeobox and bHLH genes in specification of a retinal cell type. Development. 2001;128(8):1313–1322. doi: 10.1242/dev.128.8.1313. [DOI] [PubMed] [Google Scholar]
- 12.Shi Z, et al. Vsx1 regulates terminal differentiation of type 7 ON bipolar cells. J Neurosci. 2011;31(37):13118–13127. doi: 10.1523/JNEUROSCI.2331-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatakeyama J, Kageyama R. Retinal cell fate determination and bHLH factors. Semin Cell Dev Biol. 2004;15(1):83–89. doi: 10.1016/j.semcdb.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Jiang H, Xiang M. Subtype specification of GABAergic amacrine cells by the orphan nuclear receptor Nr4a2/Nurr1. J Neurosci. 2009;29(33):10449–10459. doi: 10.1523/JNEUROSCI.3048-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin K, Jiang H, Mo Z, Xiang M. Early B-cell factors are required for specifying multiple retinal cell types and subtypes from postmitotic precursors. J Neurosci. 2010;30(36):11902–11916. doi: 10.1523/JNEUROSCI.2187-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kay JN, Voinescu PE, Chu MW, Sanes JR. Neurod6 expression defines new retinal amacrine cell subtypes and regulates their fate. Nat Neurosci. 2011;14(8):965–972. doi: 10.1038/nn.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang L, et al. Bhlhb5 is required for the subtype development of retinal amacrine and bipolar cells in mice. Dev Dyn. 2014;243(2):279–289. doi: 10.1002/dvdy.24067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hohenauer T, Moore AW. The Prdm family: Expanding roles in stem cells and development. Development. 2012;139(13):2267–2282. doi: 10.1242/dev.070110. [DOI] [PubMed] [Google Scholar]
- 19.Komai T, Iwanari H, Mochizuki Y, Hamakubo T, Shinkai Y. Expression of the mouse PR domain protein Prdm8 in the developing central nervous system. Gene Expr Patterns. 2009;9(7):503–514. doi: 10.1016/j.gep.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Eom GH, et al. Histone methyltransferase PRDM8 regulates mouse testis steroidogenesis. Biochem Biophys Res Commun. 2009;388(1):131–136. doi: 10.1016/j.bbrc.2009.07.134. [DOI] [PubMed] [Google Scholar]
- 21.Turnbull J, et al. Early-onset Lafora body disease. Brain. 2012;135(Pt 9):2684–2698. doi: 10.1093/brain/aws205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross SE, et al. Bhlhb5 and Prdm8 form a repressor complex involved in neuronal circuit assembly. Neuron. 2012;73(2):292–303. doi: 10.1016/j.neuron.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galli-Resta L, Resta G, Tan SS, Reese BE. Mosaics of islet-1-expressing amacrine cells assembled by short-range cellular interactions. J Neurosci. 1997;17(20):7831–7838. doi: 10.1523/JNEUROSCI.17-20-07831.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haverkamp S, Wässle H. Immunocytochemical analysis of the mouse retina. J Comp Neurol. 2000;424(1):1–23. [PubMed] [Google Scholar]
- 25.Dyer MA, Cepko CL. Control of Müller glial cell proliferation and activation following retinal injury. Nat Neurosci. 2000;3(9):873–880. doi: 10.1038/78774. [DOI] [PubMed] [Google Scholar]
- 26.Dyer MA, Livesey FJ, Cepko CL, Oliver G. Prox1 function controls progenitor cell proliferation and horizontal cell genesis in the mammalian retina. Nat Genet. 2003;34(1):53–58. doi: 10.1038/ng1144. [DOI] [PubMed] [Google Scholar]
- 27.Mataruga A, Kremmer E, Müller F. Type 3a and type 3b OFF cone bipolar cells provide for the alternative rod pathway in the mouse retina. J Comp Neurol. 2007;502(6):1123–1137. doi: 10.1002/cne.21367. [DOI] [PubMed] [Google Scholar]
- 28.Audo I, Robson AG, Holder GE, Moore AT. The negative ERG: Clinical phenotypes and disease mechanisms of inner retinal dysfunction. Surv Ophthalmol. 2008;53(1):16–40. doi: 10.1016/j.survophthal.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Wässle H, Puller C, Müller F, Haverkamp S. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J Neurosci. 2009;29(1):106–117. doi: 10.1523/JNEUROSCI.4442-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young RW. Cell differentiation in the retina of the mouse. Anat Rec. 1985;212(2):199–205. doi: 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]
- 31.Young RW. Cell death during differentiation of the retina in the mouse. J Comp Neurol. 1984;229(3):362–373. doi: 10.1002/cne.902290307. [DOI] [PubMed] [Google Scholar]
- 32.Star EN, et al. Regulation of retinal interneuron subtype identity by the Iroquois homeobox gene Irx6. Development. 2012;139(24):4644–4655. doi: 10.1242/dev.081729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. 2002;3(7):517–530. doi: 10.1038/nrn874. [DOI] [PubMed] [Google Scholar]
- 34.Inoue T, et al. Math3 and NeuroD regulate amacrine cell fate specification in the retina. Development. 2002;129(4):831–842. doi: 10.1242/dev.129.4.831. [DOI] [PubMed] [Google Scholar]
- 35.Elshatory Y, Deng M, Xie X, Gan L. Expression of the LIM-homeodomain protein Isl1 in the developing and mature mouse retina. J Comp Neurol. 2007;503(1):182–197. doi: 10.1002/cne.21390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruether K, et al. PKCalpha is essential for the proper activation and termination of rod bipolar cell response. Invest Ophthalmol Vis Sci. 2010;51(11):6051–6058. doi: 10.1167/iovs.09-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daiger SP, Rossiter BJF, Greenberg J, Christoffels A, Hide W. University of Texas-Houston Health Science Center; Houston: 1998. Data services and software for identifying genes and mutations causing retinal degeneration. Investigative Ophthalmology & Visual Science. [Google Scholar]
- 38.Clarke G, et al. Rom-1 is required for rod photoreceptor viability and the regulation of disk morphogenesis. Nat Genet. 2000;25(1):67–73. doi: 10.1038/75621. [DOI] [PubMed] [Google Scholar]
- 39.Waterston RH, et al. Mouse Genome Sequencing Consortium Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420(6915):520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.