Significance
CD4+ regulatory T (Treg) cells expressing CD25 and the transcription factor forkhead box P3 (FOXP3) play indispensable roles for immunological self-tolerance and homeostasis. Because human FOXP3+CD25+CD4+ T cells are heterogeneous in function and differentiation status, their analysis and manipulation for treating immunological diseases remains a challenge. Here we show that CD15s (sialyl Lewis x) is specifically expressed by activated, terminally differentiated, and most suppressive FOXP3high Treg cells, allowing their separation from nonsuppressive FOXP3+CD4+ T cells secreting inflammatory cytokines. Removal of CD15s+CD4+ T cells from human blood is indeed sufficient to enhance in vitro antitumor and antiviral antigen responses. CD15s is therefore useful for phenotypic as well as functional analysis of human Treg subpopulations and for targeting them to control immune responses.
Keywords: regulatory T cells, FOXP3, CD15s, autoimmunity, tumor immunity
Abstract
CD4+ regulatory T (Treg) cells expressing CD25 and the transcription factor forkhead box P3 (FOXP3) are indispensable for immunological self-tolerance and homeostasis. FOXP3+CD25+CD4+ T cells in humans, however, are heterogeneous in function and differentiation status, including suppressive or nonsuppressive cells as well as resting or activated Treg cells. We have searched for cell surface markers specific for suppression-competent Treg cells by using a panel of currently available monoclonal antibodies reactive with human T cells. We found that CD15s (sialyl Lewis x) was highly specific for activated, terminally differentiated, and most suppressive FOXP3high effector Treg (eTreg) cells and able to differentiate them in various clinical settings from nonsuppressive FOXP3+ T cells secreting inflammatory cytokines. For example, CD15s+FOXP3+ eTreg cells were increased in sarcoidosis, whereas it was nonsuppressive CD15s−FOXP3+ T cells that were expanded in lupus flares. FOXP3+ cells induced from conventional CD4+ T cells by T-cell receptor stimulation hardly expressed CD15s. CD15s+CD4+ T-cell depletion was sufficient to evoke and enhance in vitro immune responses against tumor or viral antigens. Collectively, we have identified CD15s as a biomarker instrumental in both phenotypic and functional analysis of FOXP3+CD4+ T-cell subpopulations in health and disease. It allows specific targeting of eTreg cells, rather than whole FOXP3+CD4+ T cells, in controlling immune responses.
Regulatory T (Treg) cells expressing the transcription factor forkhead box P3 (FOXP3) play essential roles for the maintenance of immune self-tolerance and homeostasis (1). They are also involved in the suppression of effective immune responses against autologous cancer cells as well as invading microbes. Whereas the majority of FoxP3-expressing CD4+ T cells are considered to be suppression-competent Treg cells in mice, there is accumulating evidence that FOXP3+CD4+ T cells in humans are heterogeneous in phenotype and function (2). For example, any human conventional CD4+ T cell can transiently up-regulate FOXP3 upon activation; such FOXP3+ T cells are hardly suppressive (3). In addition, not all FOXP3-expressing CD4+ T cells in peripheral blood mononuclear cells (PBMCs) possess suppressive function (4). It is therefore imperative to determine how functionally and developmentally distinct human FOXP3+ subpopulations can be reliably delineated to better understand the roles of Treg cells in immunological diseases and to specifically target them for the control of pathological and physiological immune responses.
Efforts have been made for a decade to divide human FOXP3+ T cells into functionally or developmentally distinct subpopulations based on the expression of various cell surface or intracellular molecules, for example, CD25, CD127, CD45RA (or CD45RO), ICOS, HLA-DR, and Helios (5–8). We have found that FOXP3+CD25+CD4+ T cells can be divided into naïve/resting Treg cells as CD25+CD45RA+FOXP3low (nTreg), effector/activated Treg cells as CD25highCD45RA−FOXP3high (eTreg), and nonsuppressive CD4+ T cells as CD25+CD45RA−FOXP3low (FOXP3+ non-Treg) (4). Upon antigenic stimulation, nTreg cells differentiate into eTreg cells, the majority of which die by apoptosis after exerting suppression. In contrast, CD45RA−FOXP3low non-Treg cells are capable of secreting inflammatory cytokines. This functional and phenotypic delineation of FOXP3-expressing CD4+ T-cell subpopulations is useful in assessing Treg cell development, function, and their status in immunological diseases and aging. It remains challenging, however, how these two populations with opposite functions, i.e., the highly suppressive FOXP3high eTreg population and the nonsuppressive and cytokine-secreting FOXP3low non-Treg population, can be more discretely and reliably differentiated by specific molecular markers, especially, those expressed on the cell surface. This is critically important in analyzing the possible contribution of Treg cells to the dynamics of immune responses and preparing a pure suppression-competent Treg cell preparation for cell therapy of immunological diseases.
In this report, we have searched for cell surface markers specific for FOXP3+ T-cell subpopulations, in particular, suppression-competent eTreg cells, by evaluating a panel of currently available monoclonal antibodies reactive with human T cells. We show that the expression of CD15s (sialyl Lewis x) is able to accurately delineate eTreg cells from other FOXP3+ T cells, enabling assessment of Treg-mediated suppression in various immunological conditions and targeting them to control immune responses.
Results
Cell Surface Molecules Specific for FOXP3+ Subsets.
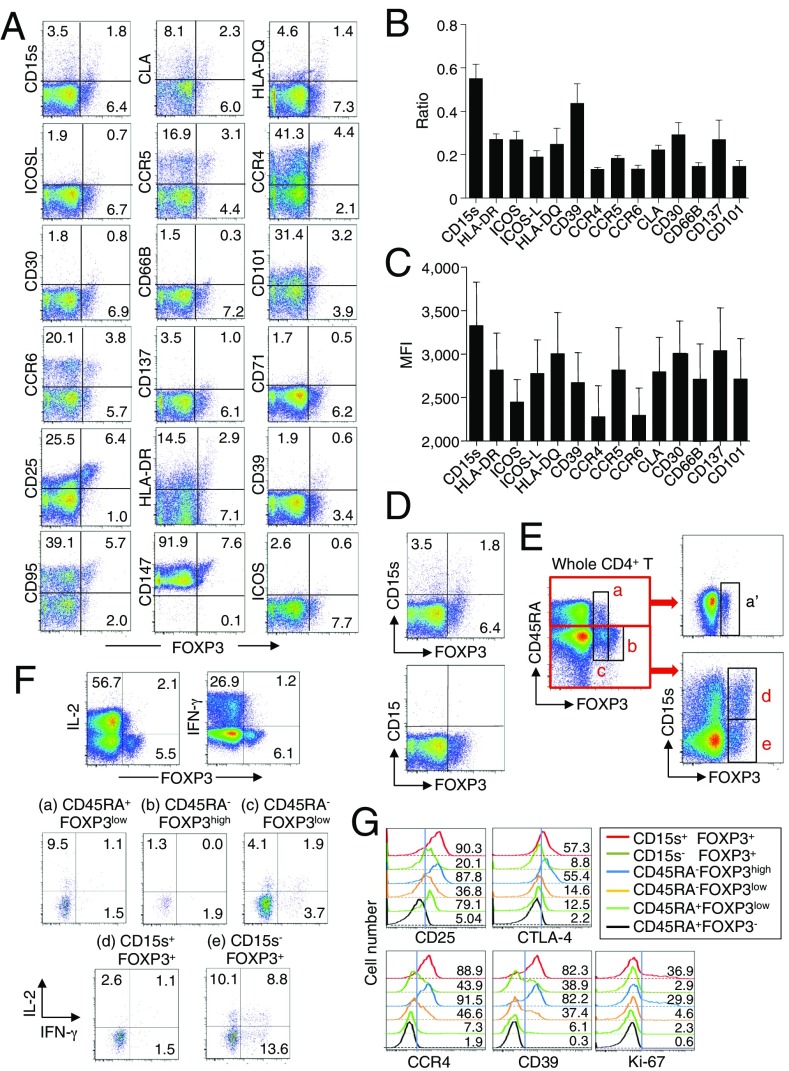
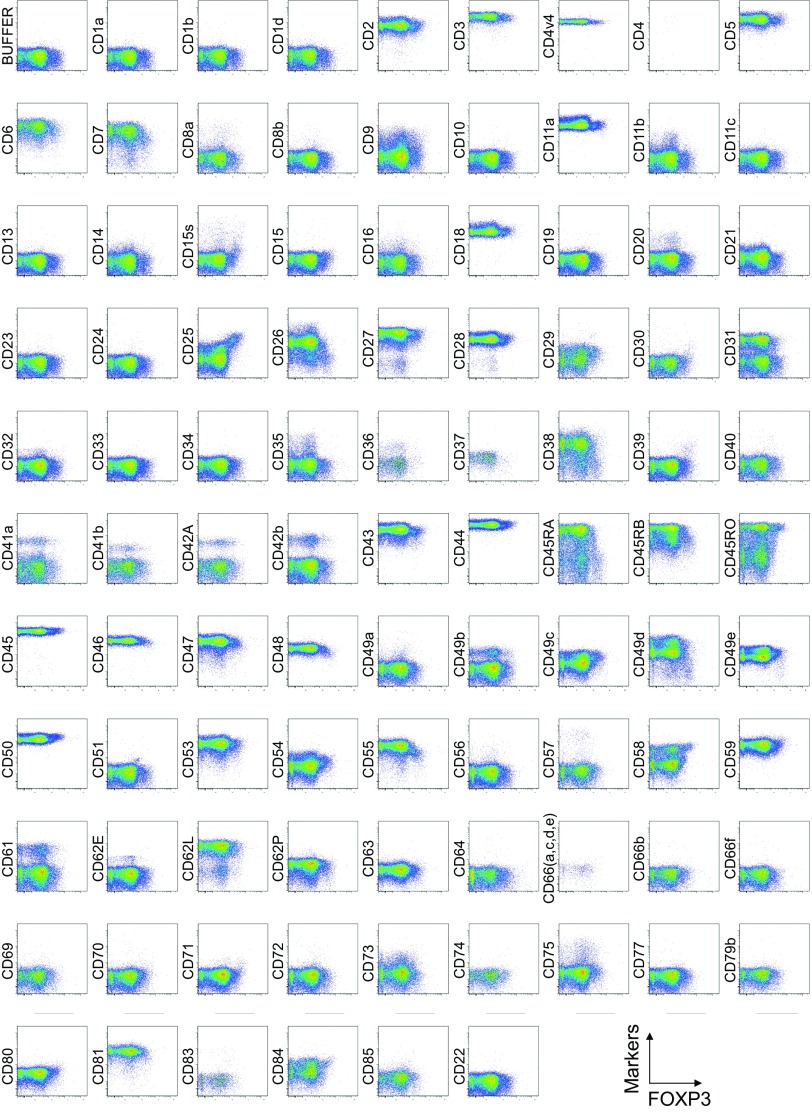
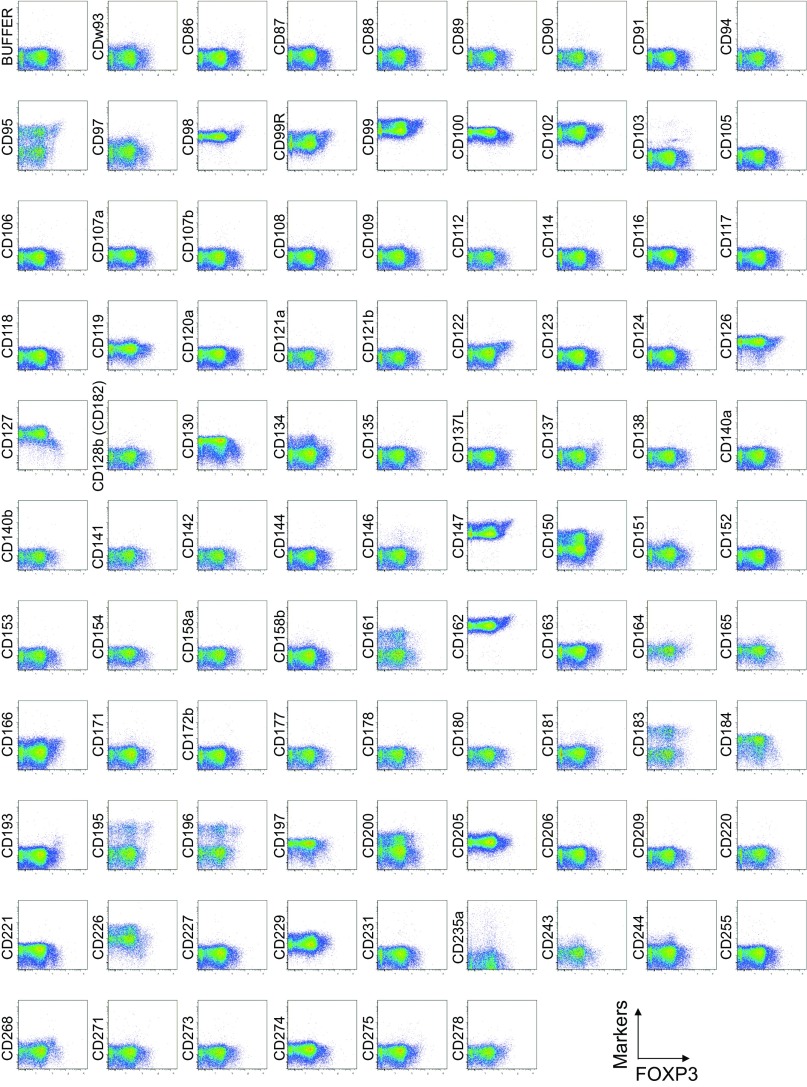
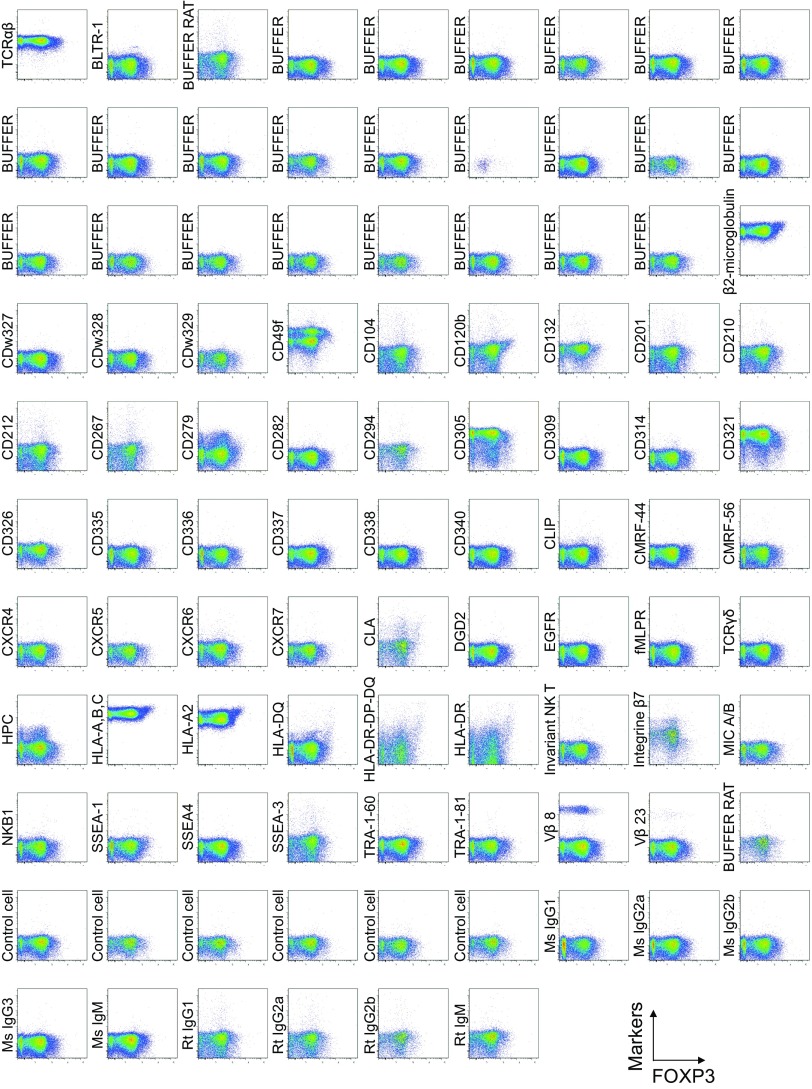
To search for specific surface markers that are able to differentiate functional subsets among FOXP3-expressing CD4+ T cells, we conducted a multiparameter flow cytofluorometric analysis of human CD4+ T cells by using the currently available 323 monoclonal antibodies reactive with human T cells. We compared each putative marker with known Treg markers, such as CD25, CD45RA, ICOS (5), HLA-DR (6), Ki-67, Helios (8), and FOXP3. The analysis revealed that 18 surface markers were highly expressed on FOXP3high eTreg cells compared with FOXP3low non-Treg cells (4). They included 12 molecules [CD15s, CLA, HLA-DQ, CD30, CD66B, CD101, CD275 (ICOS-L), CCR5, CCR6, CCR4, CD137, and CD71] newly reported here and 6 markers [CD25 (2), HLA-DR (6), CD39 (7, 9), CD95 (10), CD147 (11), and CD278, i.e., ICOS (5)] previously reported (Fig. 1A and Figs. S1–S3).
Fig. 1.
Cell surface markers for FOXP3+CD4+ T-cell subpopulations. (A) Expression of intracellular FOXP3 and each indicated surface marker by CD4+ T cells in a healthy donor. (B) Ratios obtained as the proportion of marker+ FOXP3+ cells among CD4+ T cells divided by the proportion of marker+FOXP3− cells among CD4+ T cells. (C) MFI of FOXP3 expression by marker+FOXP3+ cells. (D) CD4+ T-cell expression of FOXP3 and CD15s or CD15. (E) FOXP3+ subpopulations (a–c) depicted by staining of CD4+ T cells for FOXP3 and CD45RA expression were further divided into three populations (a′, d, and e) by the level of CD15s expression. A–E are representative of six independent experiments. (F) IL-2 and IFN-γ production in CD4+ T cells (Top) and gated CD4+ T-cell subsets as defined in E, after stimulation with PMA and ionomycin for 5 h. Percentages of cytokine-secreting cells are shown. (G) Analysis of CD25, intracellular CTLA-4, CCR4, CD39, and Ki-67 expression by indicated populations. F and G are representative of three independent experiments.
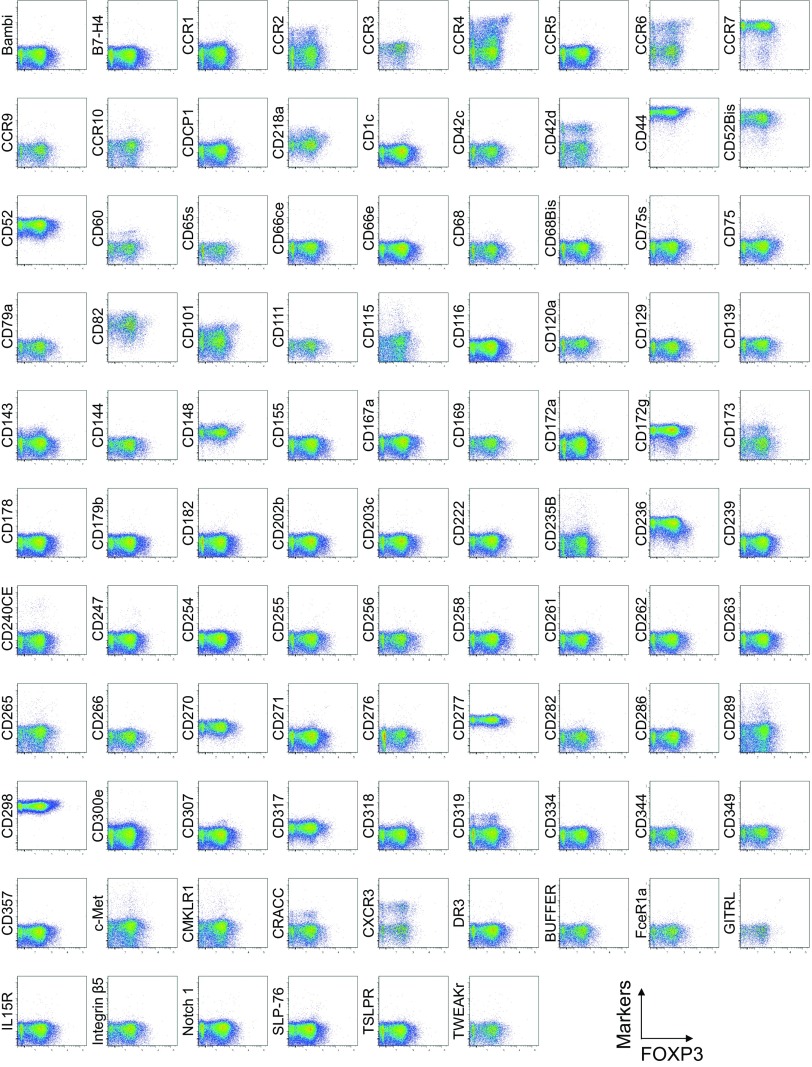
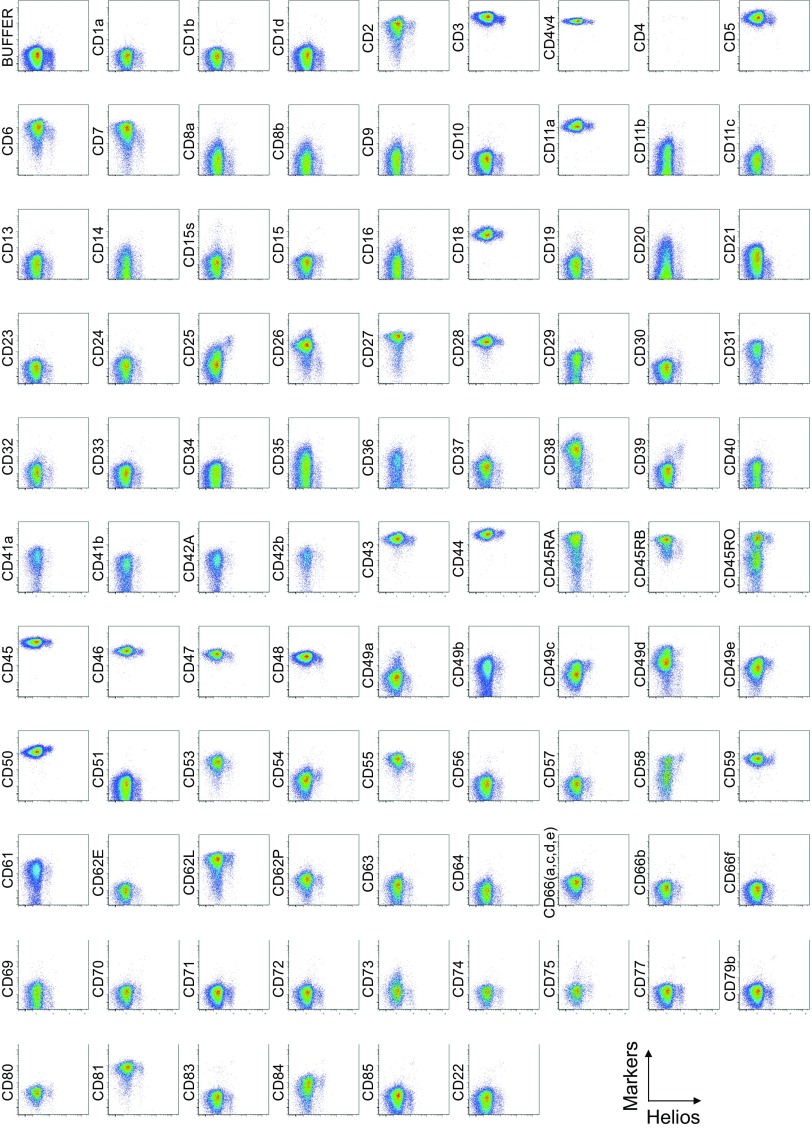
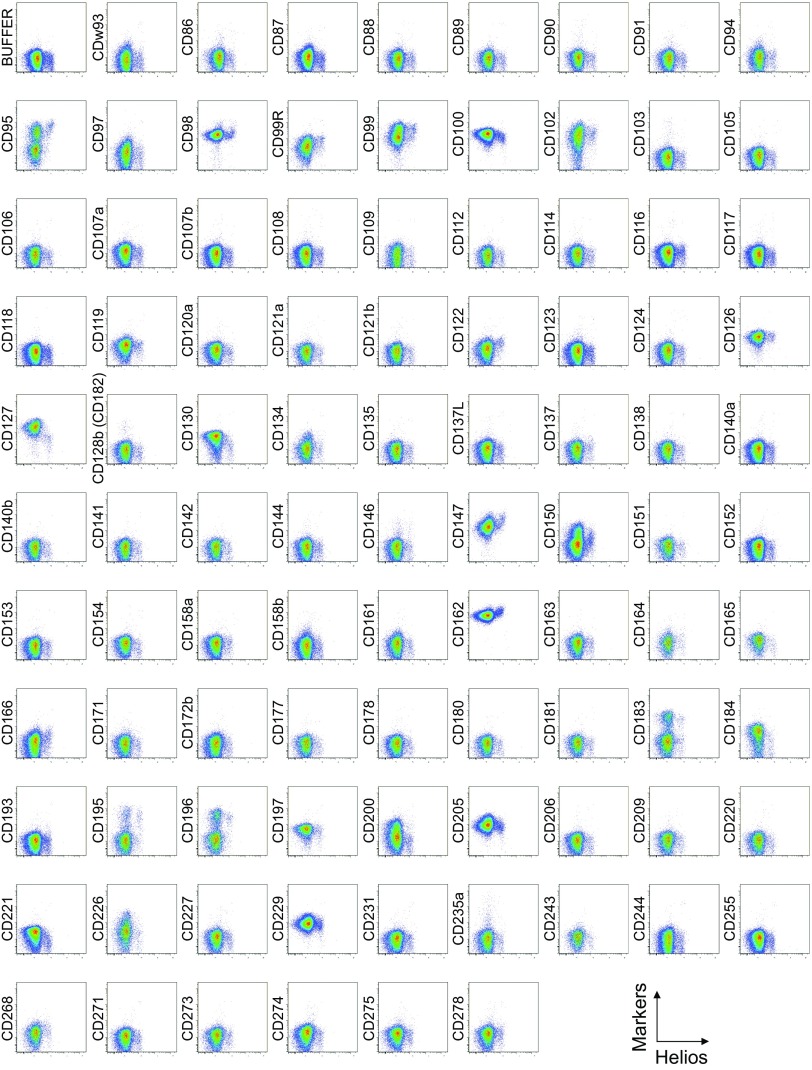
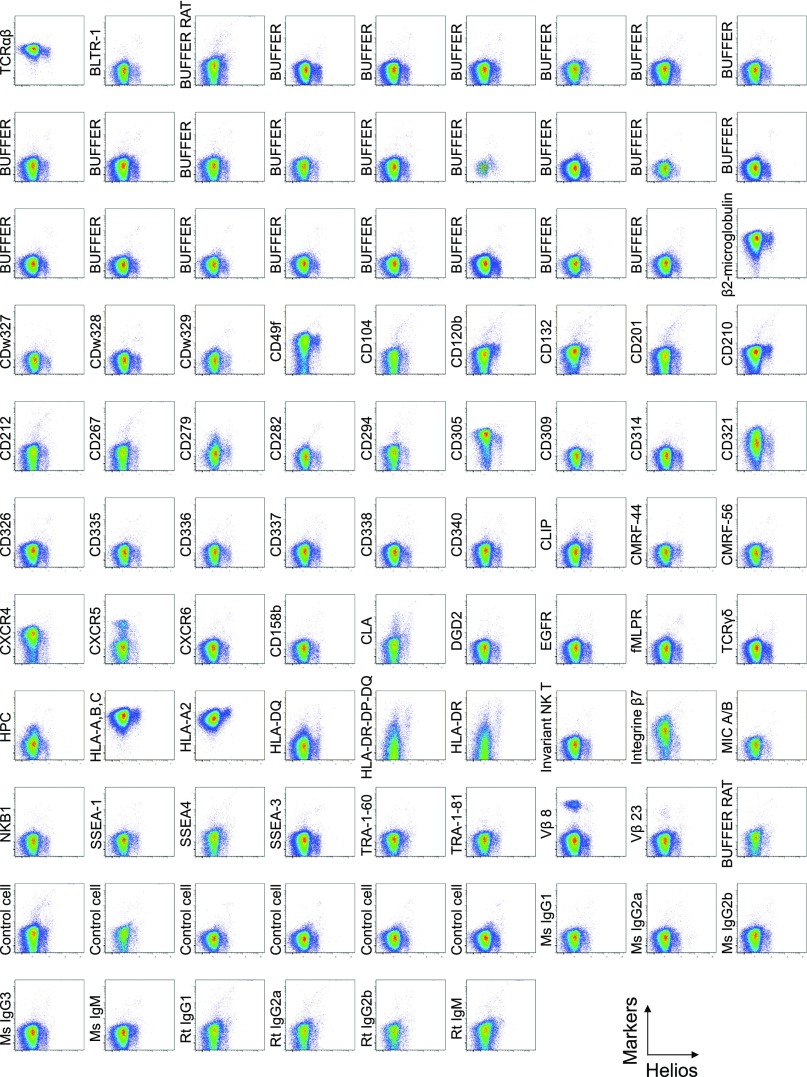
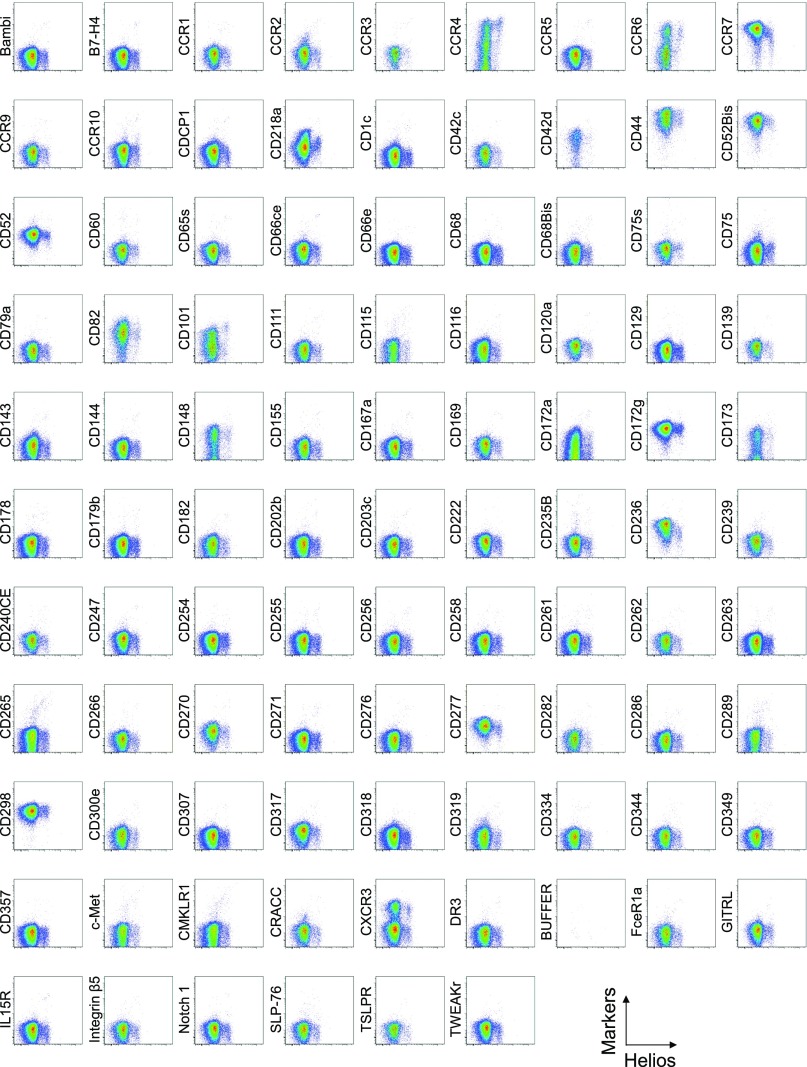
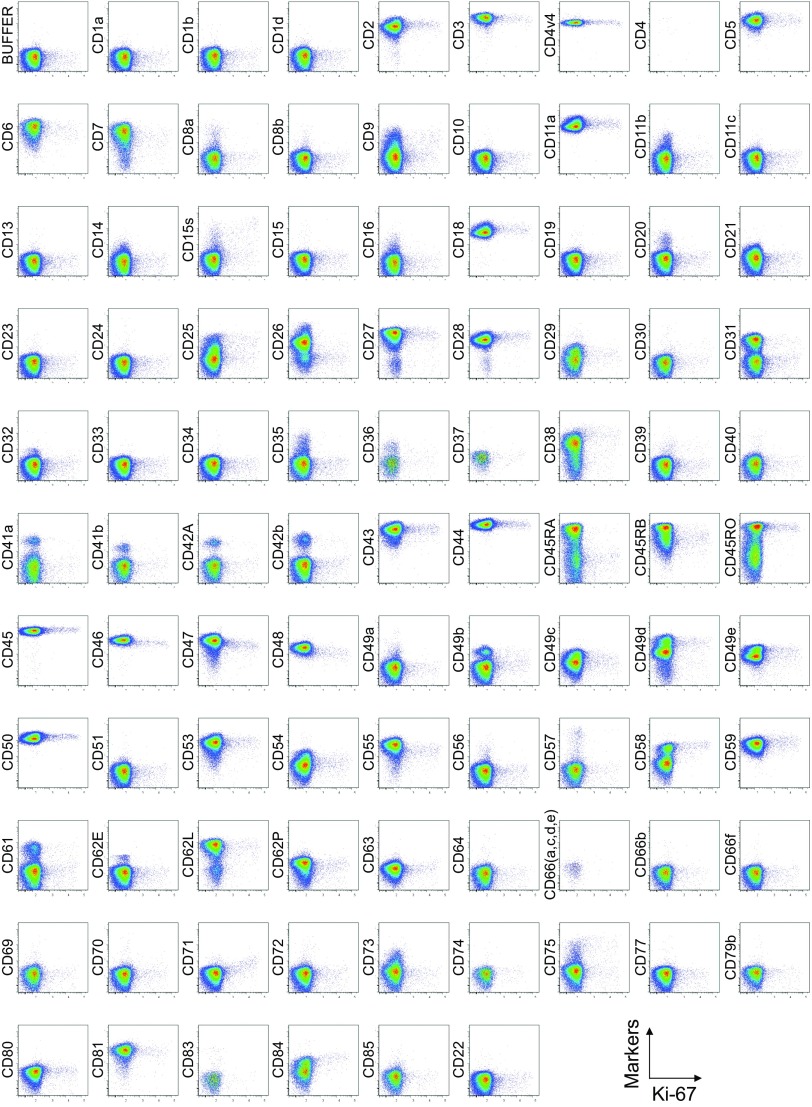
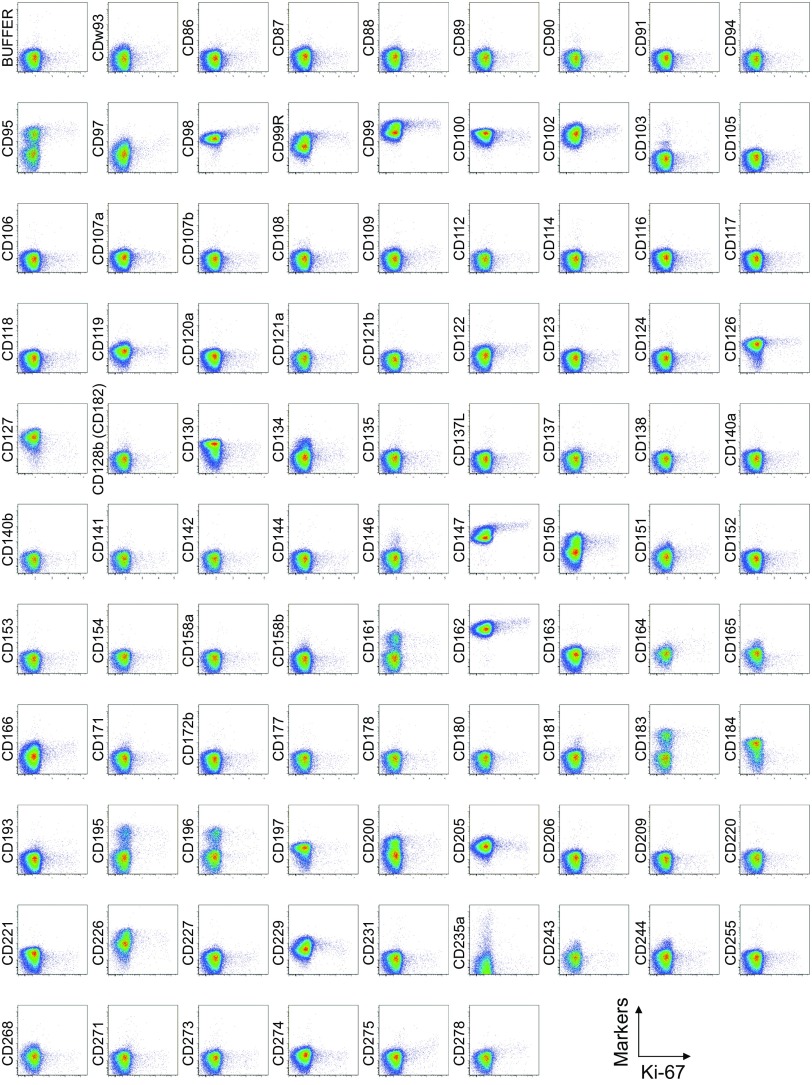
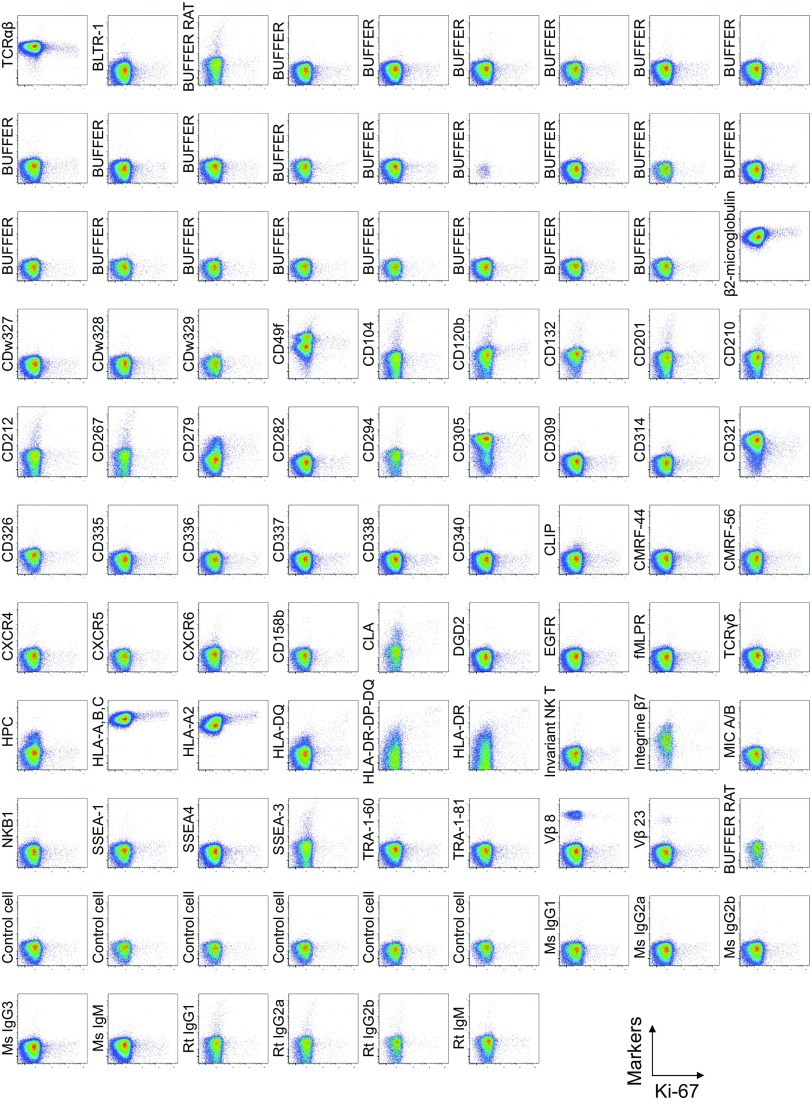
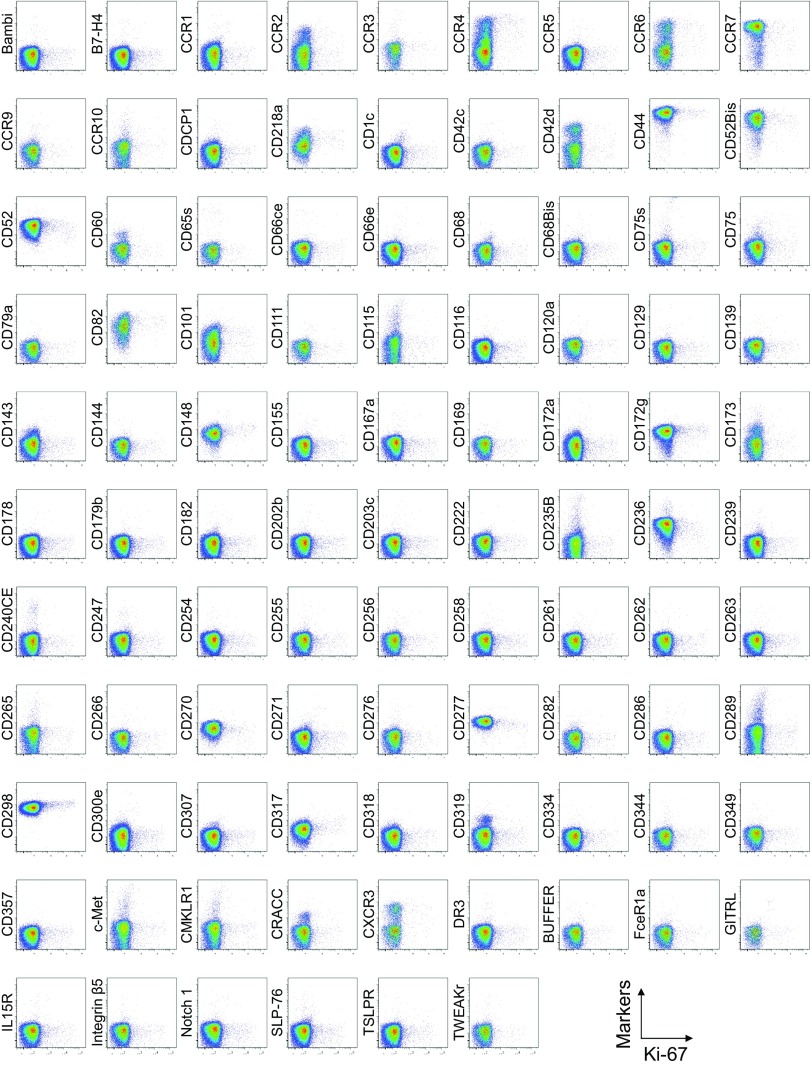
Fig. S1.
Cell surface marker expression by FOXP3-expressing CD4+ T cells. Expression of intracellular FOXP3 and each indicated surface marker assessed by flow cytometry of PBMCs gated on CD4+ T cells. Data are representative of 6 healthy donors. PBMCs were first incubated with unconjugated antibodies, then with dye-conjugated antibodies (CD3, CD8, CD4, CD45RA, CD25, HLA-DR, ICOS, CD31, FOXP3, Ki-67, and Helios). In CD4 panels, because unconjugated and dye-conjugated anti-CD4 mAb was of the same clone (RPA-T4), the dye-conjugated anti-CD4 mAb failed to stain after incubating with the unconjugated anti-CD4 mAb.
Fig. S3.
Cell surface marker expression by Helios-expressing CD4+ T cells. Expression of intracellular Helios and each indicated surface marker assessed by flow cytometry of PBMCs gated on CD4+ T cells. Data are representative of 6 healthy donors. PBMCs were first incubated with unconjugated antibodies, then with dye-conjugated antibodies (CD3, CD8, CD4, CD45RA, CD25, HLA-DR, ICOS, CD31, FOXP3, Ki-67, and Helios). In CD4 panels, because unconjugated and dye-conjugated anti-CD4 mAb was of the same clone (RPA-T4), the dye-conjugated anti-CD4 mAb failed to stain after incubating with the unconjugated anti-CD4 mAb.
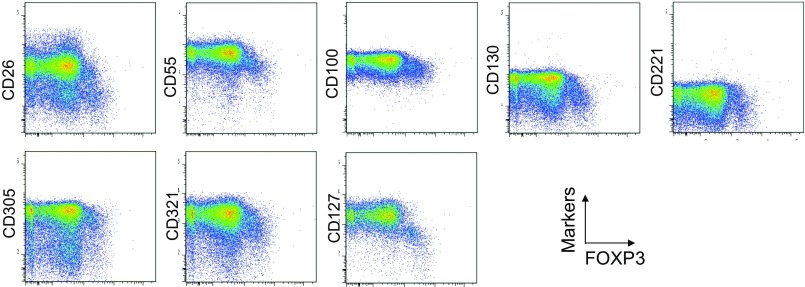
To assess the specificities of these molecules for FOXP3high eTreg cells, we calculated the percentage of Marker+ cells among FOXP3+ or FOXP3−CD4+ T cells and the ratio of the former to the latter (Fig. 1B), with a high ratio indicative of more specific expression of the marker by FOXP3+CD4+ T cells. We also compared FOXP3 mean fluorescence intensities (MFIs) of Marker+FOXP3+CD4+ T cells (Fig. 1C). These analyses revealed that CD15s was the marker most specifically expressed by FOXP3+ cells, in particular by CD45RA− cells with the highest FOXP3 MFI. In addition, in contrast with CD15s, FOXP3+ T cells scarcely expressed nonsialylated CD15 (Fig. 1D). Our search for markers that were preferentially down-regulated by eTreg cells, compared with conventional CD4+ T cells, identified seven markers, i.e., CD26, CD55, CD100, CD130, CD221, CD305, and CD321; none of them was, however, as discriminative as CD127 (12, 13) (Figs. S1 and S4).
Fig. S4.
Cell surface markers down-regulated in FOXP3high eTreg cells. Eight surface markers were down-regulated in FOXP3high eTreg cells detected by analyzing 323 mAbs as shown in Fig. S1. Expression of intracellular FOXP3 and each indicated surface marker was analyzed by flow cytometry of PBMCs gated on CD4+ T cells. Data are representative of six healthy donors.
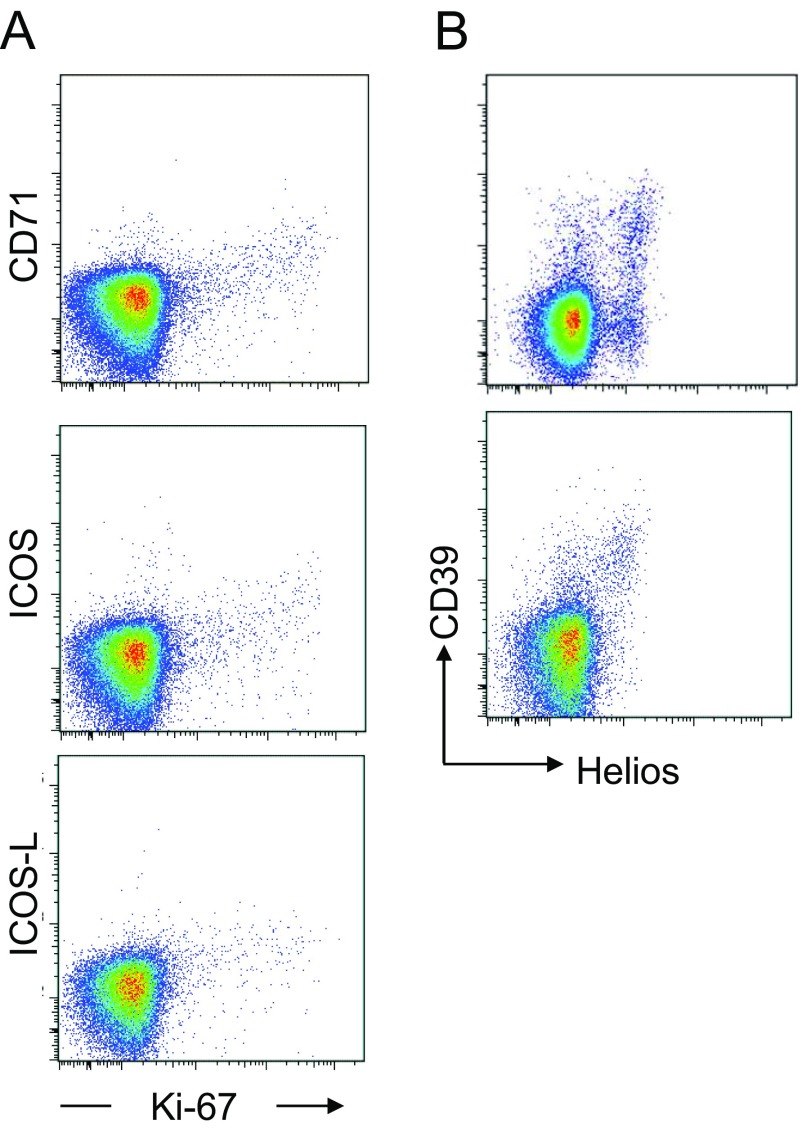
Comparison of surface markers with intracellular expression of the proliferation marker Ki-67 or the transcription factor Helios revealed a correlation between Ki-67 and CD71, ICOS, or ICOS-L, and between Helios and CD39 in some, but not all, healthy donors (Figs. S2, S3, and S5).
Fig. S2.
Cell surface marker expression by Ki-67–expressing CD4+ T cells. Expression of intracellular Ki-67 and each indicated surface marker assessed by flow cytometry of PBMCs gated on CD4+ T cells. Data are representative of 6 healthy donors. PBMCs were first incubated with unconjugated antibodies, then with dye-conjugated antibodies (CD3, CD8, CD4, CD45RA, CD25, HLA-DR, ICOS, CD31, FOXP3, Ki-67, and Helios). In CD4 panels, because unconjugated and dye-conjugated anti-CD4 mAb was of the same clone (RPA-T4), the dye-conjugated anti-CD4 mAb failed to stain after incubating with the unconjugated anti-CD4 mAb.
Fig. S5.
Surface markers correlated with Ki-67 and Helios expression. (A) Expression of Ki-67 correlated with surface expression of CD71, ICOS, and ICOS-L. Representative dot plots depicting CD4+ T-cell expression of intranuclear Ki-67 and the surface markers CD71, ICOS, and ICOS-L. Data are representative of six healthy blood donors. (B) Expression of CD39 correlated with intracellular expression of Helios. Representative dot plots depicting the expression by CD4+ T cells of intracellular Helios and CD39. The expression of CD39 corresponded to intracellular expression of Helios in some donors (Bottom) but not in others (Top).
Use of CD15s and CD45RA indeed enabled us to dissect FOXP3+CD4+ T cells into three discrete fractions; that is, CD15s−CD45RA+ cells (which were FOXP3low), CD15s+CD45RA− cells (FOXP3high), and CD15s−CD45RA− cells (FOXP3low) (Fig. 1E). CD15s was also expressed by some CD45RA−FOXP3− memory or activated conventional T cells but not by CD45RA+FOXP3− naïve T cells. Functionally, in accordance with the production of inflammatory cytokines such as IL-2 and IFN-γ by some FOXP3low cells, in particular by CD45RA−FOXP3low cells (4), CD45RA−CD15s−FOXP3+ cells in healthy individuals actively produced IFN-γ and IL-2, whereas CD15s+CD45RA− or CD15s−CD45RA+ cells did not (Fig. 1F).
Finally, CD15s+CD45RA−FOXP3+ Treg cells displayed equivalent or higher levels of various markers formerly described as expressed by human Treg cells (e.g., CD39, CCR4, intracellular CTLA-4, CD25, and Ki-67) compared with FOXP3highCD45RA− Treg cells defined by CD45RA and FOXP3 expression levels (4) (Fig. 1G).
Taken together, within the FOXP3+ population, CD15s represents a specific cell surface marker for eTreg cells, which are CD15s+CD45RA−, and differentiate them from cytokine-secreting FOXP3+ non-Treg cells as CD15s−CD45RA− and nTreg cells as CD15s−CD45RA+.
CD15s Defines Suppression-Competent FOXP3high Treg Cells.
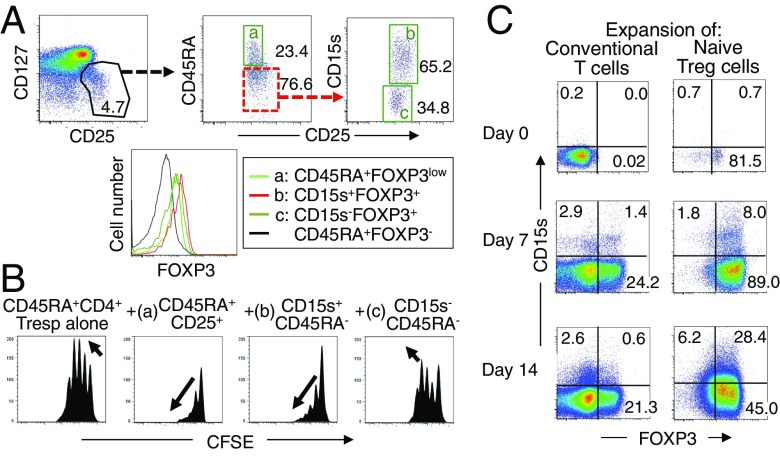
To confirm that CD15s expression is indeed a marker that is able to distinguish functional eTreg cells from FOXP3+ non-Treg cells, we prepared each population as live cells by sorting CD15s+CD45RA−CD127lowCD25+CD4+ T cells and CD15s−CD45RA−CD127lowCD25+CD4+ T cells (Fig. 2A). The CD15s+ population expressed the highest level of FOXP3 (Fig. 2A) and was highly suppressive, whereas the CD15s− population exhibited the lowest level of FOXP3 expression and lacked significant suppressive function (Fig. 2B and Fig. S6).
Fig. 2.
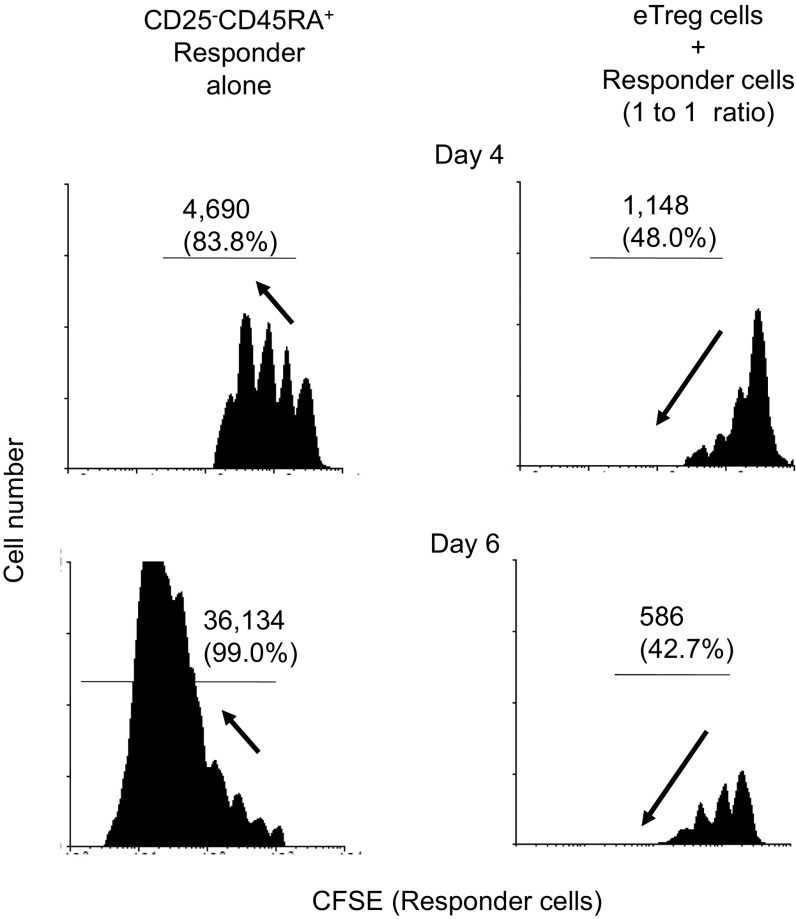
CD15s is a marker for functional FOXP3+ Treg cells. (A) CD25+CD127lowCD4+ T cells are dissected as live cells into CD45RA+CD25+ cells (a) and CD15s+CD45RA−CD25+ (b) or CD15s−CD45RA−CD25+ cells (c). Expression of FOXP3 by each population is also shown. (B) Suppressive activity of FOXP3+ T-cell subpopulations prepared as shown in A. CFSE dilution by 104 CFSE-labeled CD25−CD45RA+CD4+ responder T cells assessed after 84–90 h coculture with indicated cell populations at 1–1 ratio in the presence of anti-CD3 stimulation. As shown by the arrows, complete suppression is characterized by fewer proliferation cycles and decreasing amplitudes in consecutive cycle peaks, whereas ongoing proliferation is characterized by increasing amplitudes in consecutive cycle peaks (see further experimental explanations in Fig. S4). Data are representative of three independent experiments. (C) Expanding nTreg cells up-regulate CD15s in vitro. CD25−CD45RA+CD4+ conventional T cells and nTreg cells were FACS isolated and cultured for 0, 7, or 14 d in the presence of anti-CD3/CD28 beads, IL-2, and rapamycin. Cells were then analyzed for the expression of CD15s and FOXP3. Data are representative of three independent experiments.
Fig. S6.
Complete suppression of effector T cell proliferation by eTreg cells. Flow cytometric analysis of CFSE dilution by human CFSE-labeled responder cells after 4 d (Top) or 6 d (Bottom) coculture with (Right) or without eTregs isolated by the previously described protocol (4) (Left). Number (and percentage) of cycling cell is indicated. In coculture of human eTregs with responder cells, proliferating responder cells could be detected on day 4, yet only a minority of them entered the third cycle of division, whereas most responder cells cultured alone had entered the third and fourth cycle. Furthermore, on day 6, most dividing responder cells cultured alone had entered the sixth or the seventh cycle of cell division. Treg-cocultured responder cells still remained halted at the third cycle, whereas decreasing amplitudes are observed in successive peaks of proliferation. This pattern of CFSE dilution in suppressed responder cells indicates that the few responder cells that have proliferated in the first few days of coculture have arrested their proliferation. Thus, human Treg cells can potently arrest the proliferation of responder cells.
We also examined in vitro whether CD15s expression could be induced in naïve Treg cells and/or FOXP3+ T cells derived from conventional T cells, by T-cell receptor (TCR) stimulation in the presence of high dose IL-2 and rapamycin (14, 15). Whereas few expanding nTreg cells expressed CD15s after 7 d of culture, a significant proportion of expanding cells up-regulated CD15s by day 14, whereas CD15s was only weakly expressed on expanding conventional CD4+ T cells (Fig. 2D).
These results indicate that highly suppression-competent eTreg cells and nonsuppressive FOXP3+ T cells in PBMCs can be purified as CD15s+ or CD15s−CD45RA−CD127lowCD25+CD4+ live cells, respectively, by flow cytometry-based cell separation. In addition, CD15s can differentiate between FOXP3+ natural Treg cells and FOXP3+ cells induced from conventional T cells by TCR stimulation.
CD15s Expression in Developing Treg Cells in the Thymus.
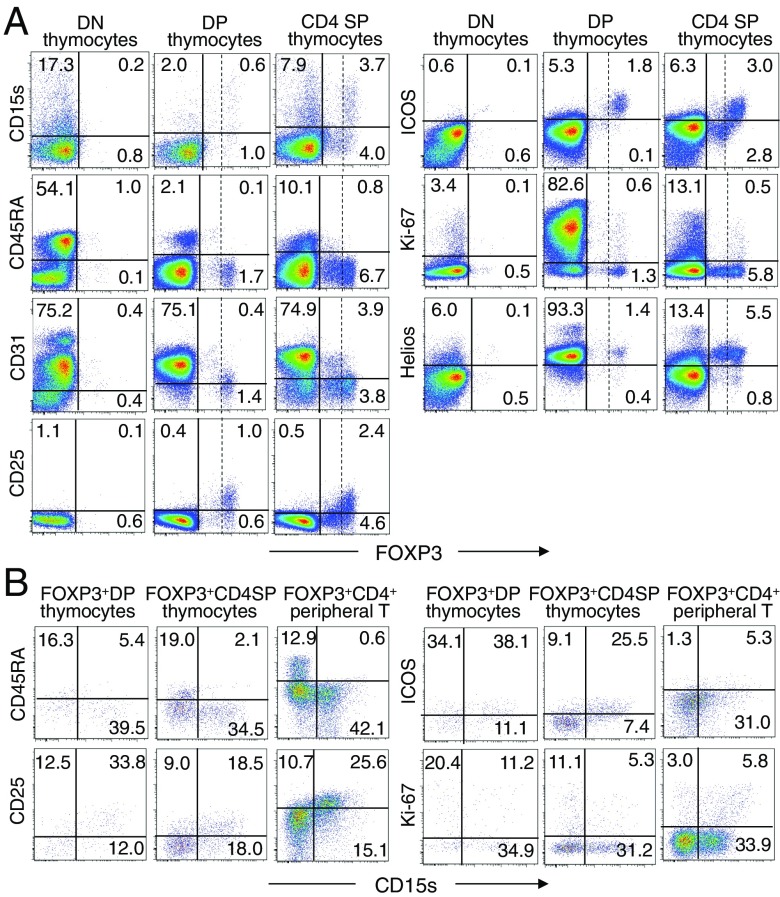
In the thymus, ∼15% of CD4/CD8 double-negative (DN) thymocytes, 1∼2% of double-positive (DP) thymocytes, and ∼10% of CD4 single-positive (SP) thymocytes including FOXP3+ and FOXP3− cells expressed CD15s (Fig. 3A). Approximately half of FOXP3+ CD4 SP thymocytes and FOXP3+ DP thymocytes, which constituted 1∼2% of DP thymocytes (16), were CD15s+ (Fig. 3B). At the CD4 SP stage, FOXP3high CD4 SP thymocytes, which were largely CD45RA−, CD31−, ICOS+, Helios+, and CD25+, included CD15s+ cells, whereas FOXP3lowCD4 SP thymocytes did not. Such CD15s+FOXP3high cells included Ki-67+ cells. In contrast, FOXP3low CD4 SP thymocytes, which were Helios+, expressed little or no CD15s, Ki-67, or ICOS, and only low levels of CD25; some of them were CD31+ and CD45RA+. Overall, FOXP3high cells’ expression patterns of cell surface markers, including CD15s, were largely similar among FOXP3+ DP thymocytes, CD4 SP thymocytes, and peripheral FOXP3+CD4+ T cells. FOXP3low thymocytes at DP stage, which included some CD45RA+ and/or CD31+ cells, were also similar to FOXP3low CD4 SP thymocytes and CD15s−CD45RA+FOXP3+ naïve Treg cells in the periphery. Because peripheral eTreg cells appear to be mainly derived from naïve Treg cells (4), the cell fate of FOXP3high CD4SP cells remains to be determined, in particular, whether they might down-regulate FOXP3 expression together with other activation markers including CD15s to become FOXP3lowCD45RA+CD31+ cells, constitute a part of peripheral FOXP3high eTreg cells, or die by apoptosis upon activation in the thymus.
Fig. 3.
CD15s expression by FOXP3-expressing thymocytes. (A) Flow cytometry analysis of FOXP3 and the indicated markers by DN, DP, and CD4 SP thymocytes. Vertical dashed lines separate FOXP3+ cells into FOXP3high and FOXP3low cells. (B) Expression of CD15s and indicated markers by FOXP3-expressing DP or CD4 SP thymocytes and peripheral FOXP3+CD4+ T cells. Data are representative of three independent experiments.
CD15s+ T-Cell Depletion Induces Tumor Antigen-Specific CD4+ T-Cell Responses and Enhances Antiviral CD8+ T-Cell Responses in Vitro.
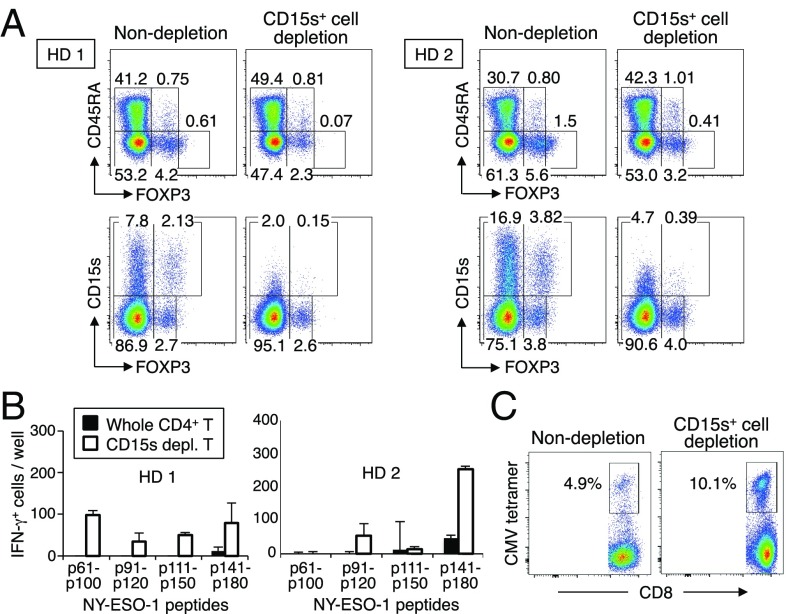
We next attempted to determine whether CD15s was able to define Treg cells suppressing antitumor or antiviral responses. We depleted CD15s+ cells from healthy donor CD4+ T cells and stimulated the remaining cells for 20 d with autologous X-irradiated T-cell depleted PBMCs pulsed with peptides from the NY-ESO-1 protein, an antigen commonly expressed by human germ-line cells and cancer cells (17). CD15s-expressing cell depletion markedly enhanced the induction of IFNγ-producing CD4+ T cells measured by ELISPOT assay (Fig. 4 A and B). In addition, similar CD15s+ cell depletion and subsequent antigen stimulation enhanced in vitro immune responses of an HLA-A2+ individual against an HLA–A2-restricted cytomegalovirus (CMV) antigen as illustrated by a marked increase in the proportion of CMV–HLA–A2 tetramer positive cells among CD8+ T cells (Fig. 4C). These results collectively indicate that CD15s is a specific marker for suppression-competent eTreg cells and can be useful for boosting antitumor or antiviral T-cell responses.
Fig. 4.
Induction of in vitro T-cell immune responses by depletion of CD15s-expressing T cells. (A) Flow cytometry analysis of CD15s and CD45RA expression by subpopulations of FOXP3+CD4+ T cells from two healthy donors before or after in vitro depletion of CD15s+ cells. (B) CD4+ T-cell responses to NY-ESO-1 peptides after CD15s+ cell depletion. CD15s+ cell-depleted or nondepleted CD4+ T cells isolated from two healthy donors as shown in A were cultured with T-cell–depleted autologous PBMCs pulsed with overlapping NY-ESO-1 peptides covering the entire sequence of NY-ESO-1 protein. IFN-γ–secreting CD4+ T-cell counts were measured by ELISpot assay. (C) CD8+ T-cell responses by the same donors to CMV peptides after CD15s+ cell depletion. PBMCs from HLA-A2+ individuals were CD15s+ cell depleted or nondepleted and cultured in the presence of 10 µM CMV 495–503 HLA-A*0201–restricted peptide for 7 d, and analyzed for the percentages of CMV tetramer-positive CD8+ T cells. Data are representative of two independent experiments.
CD15s+ Treg Cells in Human Immunological Diseases.
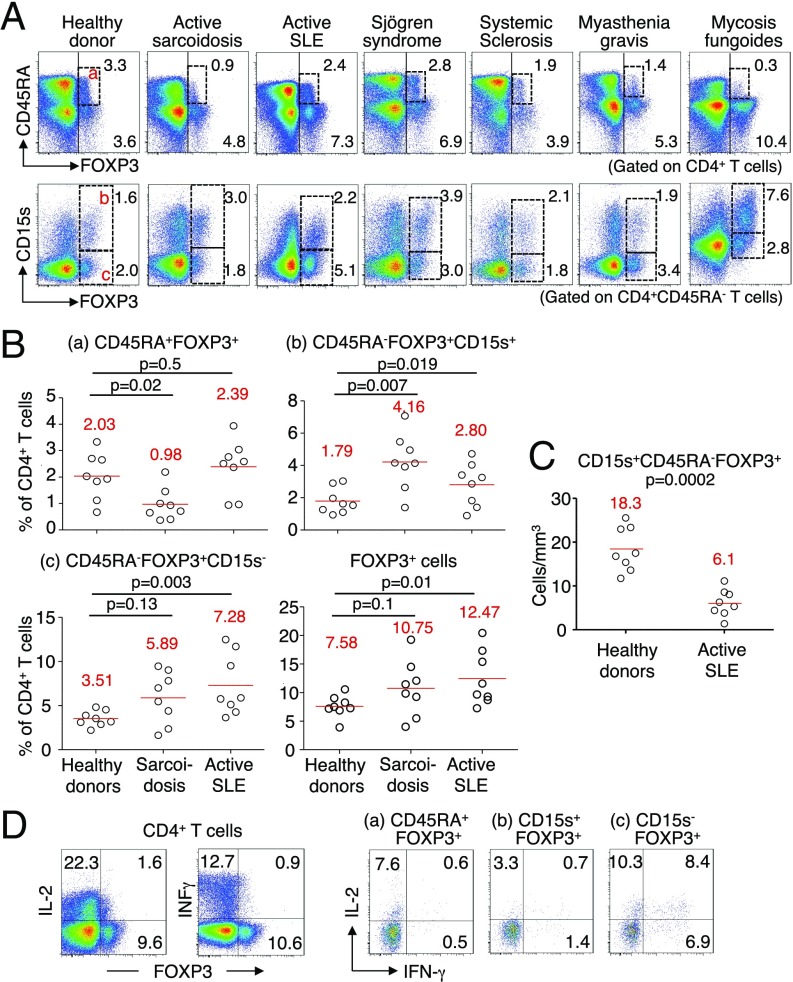
We also examined possible relevance of CD15s in analyzing the dynamics of Treg cell subset composition in various immunological diseases, including active systemic lupus erythematosus (SLE), active sarcoidosis, Sjögren syndrome, systemic sclerosis, myasthenia, and mycosis fungoides (MF) (Fig. 5A). CD15s expression indeed revealed disease-specific changes in the proportions of three FOXP3+ subpopulations (i.e., CD15s−CD45RA+FOXP3low nTreg cells, CD15s+CD45RA−FOXP3high eTreg cells, and CD15s−CD45RA−FOXP3low non-Treg cells), with general increase of FOXP3+ cells among CD4+ T cells in these inflammatory diseases. For example, CD15s+FOXP3+ cells increased in ratio among CD4+ T cells in sarcoidosis, to a lesser extent in active SLE, and notably in an untreated patient with MF. The latter observation is in accordance with a previous report showing circulating Treg cell expansion in MF (18). CD45RA−FOXP3high eTreg cells increase in sarcoidosis accompanied a decrease in CD45RA+FOXP3+ nTreg cells, whereas CD45RA−FOXP3low non-Treg cells were increased in active SLE (4). These alterations, in particular the significant increase of CD15s+FOXP3+ eTreg cells in sarcoidosis and that of CD15s−FOXP3+ non-Treg cells in active SLE, were confirmed with individual samples obtained from healthy donors (n = 8) and patients with sarcoidosis (n = 8) or SLE (n = 8) (Fig. 5B). Notably, although the ratio of CD15s+ eTreg cells significantly increased, their absolute numbers were significantly lower in SLE patients compared with healthy donors, a result consistent with previous observations (4, 19, 20) (Fig. 5C). Functionally, FOXP3low cells, in particular CD15s−CD45RA−FOXP3+CD4+ T cells from individuals with SLE flares actively produced IL-2 and IFN-γ as observed in healthy donors (Fig. 1F), whereas CD15s+FOXP3+ cells did not (Fig. 5D).
Fig. 5.
CD15s+ eTreg cells in patients with immunological diseases. (A) Flow cytometry analysis of PBMCs gated on CD4+ T cells of a representative healthy donor and of patients with active sarcoidosis, active SLE, Sjögren syndrome, systemic sclerosis, myasthenia gravis, or untreated mycosis fungoides. Expression of CD45RA and FOXP3 on whole CD4+ T cells (Top) and of CD15s and FOXP3 on CD45RA−CD4+ T cells (Bottom). Numbers indicate percentage of respective populations among whole CD4+ T cells. (B) Proportions of FOXP3+ subsets among CD4+ T cells in eight healthy donors, eight patients with active sarcoidosis, and eight patients with active SLE. Mean values are in red. For comparisons and to establish statistical significance, a nonparametric Mann–Whitney u test was performed with P < 0.05 as significant. (C) Absolute counts of CD15s+ eTreg cells in eight healthy donors and the eight active SLE patients shown in B are compared using a nonparametric Mann–Whitney u test. Mean values are shown in red with P < 0.05 as significant. (D) Flow cytometry of the production of IL-2 and IFN-γ by CD4+ T cells from an active SLE patient and FOXP3+ subpopulations gated as shown after stimulation with PMA and ionomycin for 5 h. Percentages of cytokine-secreting cells are shown. Data are representative of three independent experiments.
Altogether, these data demonstrate that CD15s expression allows a clear segregation between eTreg and non-Treg cells, even in conditions associated with FOXP3+CD4+ T-cell expansions.
Discussion
We have shown in this report that CD15s is highly specifically expressed by suppression-competent FOXP3+ eTreg cells but not by nonsuppressive FOXP3+ non-Treg T cells. CD15s is therefore useful for assessing dynamic changes in the FOXP3+ regulatory/nonregulatory T-cell balance in physiological and disease states. In addition, finer tuning of immune responses can be achieved by targeting CD15s-expressing FOXP3+ eTreg cells, rather than whole FOXP3+ cells.
CD15s (sialyl Lewis x), a tetrasaccharide carbohydrate, is the α2-3 sialylated form of lacto-N-fucopentaose III (CD15) (21). In addition to eTreg cells and some memory and/or activated T cells, CD15s is highly expressed on monocytes, neutrophils, and some myeloid precursors (22). The carbohydrate nature of this antigen explains why previous transcriptome analyses of Treg-specific markers failed to identify the CD15s molecule (4, 23). In addition, eTreg cells do not express CD15, which is nonsialylated. CD15s and CD15 synthesis is mediated by fucosyltransferase 7 (FUT7) and fucosyltransferase 9 (FUT9), respectively (24–26). Our previous transcriptome analysis of FOXP3-expressing CD4+ T cells indeed showed that FUT7 was highly expressed by eTreg cells, compared with other FOXP3+ or FOXP3− subsets, whereas FUT9 was not expressed by CD4+ T cells (4). This indicates that CD15 sialylation is highly specific for eTreg cells. Functionally, CD15s is a ligand for selectins and is involved in the cellular interaction with endothelial cells, promoting the migration of CD15s+ lymphocytes from the peripheral blood into the tissues (27). Murine CD4+CD25+ Treg cells isolated from FUT7−/− mice were unable to prevent a delayed type hypersensitivity reaction (28). CD15s does not appear to play a significant role in Treg-mediated suppression because eTreg cells coated with anti-CD15s mAb for their isolation, hence blocked in their interaction with selectin, exhibited an equivalent in vitro suppressive activity as eTreg cells isolated by CD45RA and CD25 expression. Taken together, CD15s is likely involved in the transmigration of eTreg cells toward target tissues to suppress tissue-localized inflammation.
We have previously shown that FOXP3-expressing CD4+ T cells in humans could be separated into three subsets based on their expression levels of intracellular FOXP3 and of CD45RA (4). CD45RA+FOXP3lowCD4+ nTreg cells, which correspond to thymus-derived FoxP3+ Treg cells in mice raised in a specific pathogen-free environment, are easily and distinctly distinguished from other T cells. They can also be reliably isolated from human peripheral blood as live CD45RA+CD25+CD4+ T cells (4). In contrast, the CD45RA−FOXP3+ population is functionally and phenotypically heterogeneous: FOXP3highCD4+ T cells, which are completely demethylated at the Treg-specific demethylated region of the FOXP3 gene, are highly suppressive, whereas FOXP3lowCD4+ T cells, which were hardly demethylated at the region, failed to show suppressive activity. The latter appear to correspond to, or at least include, those conventional T cells with an activation-induced FOXP3 expression, which is not high enough to induce a suppressive function (2). FOXP3highCD4+ T cells include Treg cells expressing HLA-DR and/or ICOS. HLA-DR expression reportedly characterizes highly and rapidly suppressive Treg cells (6), whereas ICOS+ cells appear to be more prone to produce IL-10 (5). ICOS expression was found to be highly correlated with Ki-67 expression in the present study, indicating that ICOS expression may primarily be a proliferation marker rather than a marker defining a specific eTreg population. In addition, it was proposed that the transcription factor Helios could represent a marker for thymus-derived Treg cells (8), although conventional CD4+ T cells with induced expression of FOXP3 and other activated CD4+ T cells can also express Helios (29–31). We have failed to find a specific surface marker associated with Helios expression, although CD39 showed a correlation in some healthy donors but not in others. A recent study reported TIGIT as a specific marker for highly suppressive Treg cells in both mice and humans (32). Human TIGIT+FOXP3+CD4+ T cells were shown to completely suppress T-cell proliferation, whereas TIGIT−FOXP3+CD4+ T cells were poorly suppressive. It remains to be determined whether TIGIT+FOXP3+CD4+ T cells, which were CD45RO+ (i.e., CD45RA−), also contain FOXP3low non-Treg cells and to what extent TIGIT+FOXP3+CD4+ T cells are similar to CD15s+ eTreg cells. Collectively, compared with these Treg markers, an important advantage of CD15s is in its ability to discriminate between suppressive eTreg cells and cytokine-secreting non-Treg cells.
Naïve or resting Treg cells differentiate into CD45RA−FOXP3high eTreg cells upon antigenic stimulation (4). Although the majority of such CD15s+ eTreg cells appear to be derived in vivo from CD15s−CD45RA+ nTreg cells (4), our finding that in vitro TCR stimulation in the presence of IL-2 and rapamycin enhanced FOXP3 expression but failed to efficiently up-regulate CD15s in nTreg cells suggests that longer or additional stimulations might be required for CD15s up-regulation and possibly for final eTreg maturation. It is therefore required, especially in vitro, to use CD15s in combination with other markers such as CD25, CD127, and CD45RA for the analysis and purification of FOXP3+ subpopulations.
CD15s also enables the analysis of the behavior and dynamics of Treg subsets in disease states more accurately and clearly than before. For example, using CD15s as a specific marker for eTreg cells, our analysis has shown that previous estimates of eTreg cell numbers (based on the expression of FOXP3, CD45RA, and Ki-67) are largely similar in healthy donors to the current estimates, but slightly overestimated in sarcoidosis (33) and slightly underestimated in SLE (20). Application of this new definition of Treg subpopulations also revealed an increase in CD15s+FOXP3high eTreg cells as a distinct population in PBMCs of a patient with mild untreated MF. Further analysis of the dynamics of Treg subpopulations using the CD15s marker is under way for other physiological and disease states.
Finally, CD15s can be a good target to control immune responses via Treg cells, in particular by depleting eTreg cells. For example, depletion of CD15s+ cells from CD4+ T cells isolated from healthy donor PBMCs indeed evoked and enhanced in vitro anti–NY-ESO-1 or anti-CMV immune responses in a similar manner as Treg cell depletion by other markers such as CD25 or CCR4 (17). These functional results further support the relevance of CD15s as a marker for phenotypic and functional study of Treg cells in various clinical settings.
Methods
Flow Cytometry Analysis of FOXP3-, Ki-67–, and Helios-Expressing CD4+ T Cells.
Blood samples were obtained from young healthy adult volunteers and patients with active sarcoidosis, active SLE, Sjögren syndrome, systemic sclerosis, mycosis fungoides, or myasthenia gravis. The study was done according to the Helsinki declaration with the approval from the local human ethics committee (Comité Consultatif de Protection des Personnes dans la Recherche Biomédicale of Pitié-Salpétrière Hospital, Paris). For the analysis of thymocytes, the approval by the Biomedecine Agency (PFS13-007) was obtained. Cytometry methods and mAbs are detailed in Dataset S1 and SI Methods.
Suppression Assay.
PBMCs were isolated through Ficoll gradient separation from freshly drawn blood. CD4+ T cells were first magnetically isolated using a CD4+ T-cell separation kit (Miltenyi Biotec) and subsequently surface stained using a combination of flurochrome-conjugated mAbs: anti–CD4-PErCP 5.5, –CD25-PE, –CD127-Pacific blue (Human Regulatory T-Cell Mixture, BD Biosciences), –CD45RA-PECy7, and –CD15s-AF647 obtained from BD Biosciences. CD127+CD25−CD45RA+CD4+, CD127lowCD25+ naïve FOXP3lowCD45RA+CD4+, CD127lowCD25+ effector FOXP3highCD45RA−CD4+ Treg cells, and CD127lowCD25+CD45RA−CD4+ T cells were sorted according to the gating strategy we validated previously (4) using a FACSAria (BD Biosciences) and CD127lowCD25+CD15s−FOXP3+CD4+ T cells and CD127lowCD25+FOXP3+CD15s+CD4+ T cells according to the gating strategy depicted in Fig. 2A. CFSE labeling and suppression assays were performed as described in SI Methods. Complete suppression is characterized by fewer proliferation cycles and decreasing amplitudes in consecutive cycle peaks, whereas ongoing proliferation or absence of suppression is characterized by increasing amplitudes in consecutive cycle peaks.
nTreg Cell Expansion.
The 3 × 104 FACS sorted CD45RA+CD127low/−CD25+FOXP3lowCD4+ nTregs were immediately distributed into a U-bottom well for culture. Cells were cultured in X-Vivo 15 media (Lonza) with 50 g/L AB serum (Invitrogen Lifetech), and supplemented with 2 mM l-glutamine, 1 mM sodium pyruvate, 1% nonessential amino acid MEM, 100 units/mL penicillin, 100 µg/mL streptomycin and amphotericin B (all from Gibco), and anti-CD3/anti-CD28–coated Treg expander beads (Invitrogen Lifetech), in the presence of 300 IU/mL IL-2 (Miltenyi Biotec) in culture media alone or in the presence of rapamycin (Sigma-Aldrich) diluted in culture medium (1 µg/mL). Cultures were replenished with 300–1,000 IU/mL IL-2 every 3–4 d.
In Vitro Sensitization of NY-ESO-1–Specific CD4+ T Cells and in Vitro Sensitization of CMV-Specific CD8+ T Cells.
Methods are detailed in SI Methods.
Statistical Analysis.
To compare the percentage of cell subsets in healthy donors versus patients, a nonparametric Mann–Whitney u test was used. P < 0.05 was considered significant.
SI Methods
Diagnosis of Human Diseases.
Diagnosis for active sarcoidosis, active SLE, Sjögren syndrome, systemic sclerosis, mycosis fungoides, or myasthenia gravis were made according to previously described criteria (20, 33–36).
Cytometry.
Human peripheral blood mononuclear cells (PBMCs) and human thymocytes were prepared by Ficoll gradient centrifugation and stained with anti-hCD3, anti-hCD8, anti–hCD4-PerCP-Cy5.5 or –APC, anti–hCD25-PE, anti–hCD45RA-PE-Cy7, anti–ICOS-, anti–HLA-DR-PE (from BD Biosciences), anti-CD31 (-APC from eBioscience), anti-hCD127 (-Pacific blue). Intracellular detection of FOXP3 with anti-hFOXP3 (PE or Alexa Fluor 647, clone 259D/A7, BD Biosciences) and of Ki-67 antigen with Ki-67 antibody (FITC or PE from BD Biosciences) was performed on fixed and permeabilized cells using Intracellular Fixation and Permeabilization Buffer Set (eBioscience). Most mAbs used for the study were obtained from the Lyoplate system (BD Biosciences). All mAbs for the cell surface marker screening were unconjugated and secondary stained. Clones and species for mAbs are described in Dataset S1. For subsequent cytometry analysis, Alexa Fluor 647-conjugated anti-CD15s mAbs (BD) were used. For the analysis of cytokine production, PMBCs were stimulated for 5 h with PMA and ionomycin. Data acquired by LSR-Fortessa or FACSCanto-II were analyzed with FlowJo software.
Treg Suppression Assays.
The 1 × 104 CFSE (1 µM, Invitrogen)-labeled responder CD25−CD45RA+CD4+ T cells were cocultured with 1 × 104 unlabeled cells assessed for their suppressive capacity together with 1 × 105 irradiated autologous accessory cells containing B cells and monocytes. Cells were stimulated with 0.5 μg/mL plate-bound anti-CD3 (OKT3 mAb) in 96-well round-bottom plate in RPMI medium supplemented with 100 mL/L FBS (Bio West), 2 mM l-glutamine, 1 mM sodium pyruvate, 1% nonessential amino acid MEM, 100 units/mL penicillin, 100 µg/mL streptomycin and amphotericin B (all from Gibco). Proliferation of CFSE-labeled cells was assessed by flow cytometry after 84–90 h of culture.
In Vitro Sensitization of NY-ESO-1–Specific CD4+ T Cells.
CD8+ T cells were depleted from PBMCs with CD8 Microbeads (Miltenyi Biotec). The remaining cells were subjected to negative selection of CD4+ T cells with CD4+ T Cell Isolation Kit (Miltenyi Biotec). CD4+ T cells were treated with biotin-anti-CD15s mAb for 15 min at 4 °C. Subsequently, anti-Biotin MicroBeads (Miltenyi Biotec) were added as described in the manufacturer’s protocol, then washed using PBS containing 20 mL/L FCS. CD15s− cells were separated on autoMACS Pro Separator (Miltenyi Biotec). CD4−CD8− cells were used as antigen-presenting cells (APCs) after pulsing with pooled peptides (10 µM) overnight at 37 °C as previously described (17). After irradiation (35 Gy), 3∼5 × 105 APCs were added to cultures containing 1∼3 × 105 CD4+ T cells, and were fed with IL-2 (10 units/mL; Roche Diagnostics) and IL-7 (20 ng/mL; R&D Systems) in round-bottom 96-well plates (Thermo Fisher Scientic). Subsequently, one-half of the medium was replaced by fresh medium containing IL-2 (20 units/mL) and IL-7 (40 ng/mL) twice per week.
Synthetic Peptides of NY-ESO-1.
Peptides 1–20 (MQAEGRGTGGSTGDADGPGG), NY-ESO-111–30 (STGDADGPGGPGIPDGPGGN), NY-ESO-121–40 (PGIPDGPGGNAGGPGEAGAT), NY-ESO-131–50 (AGGPGEAGATGGRGPRGAGA), NY-ESO-141–60 (GGRGPRGAGAARASGPGGGA), NY-ESO-151–70 (ARASGPGGGAPRGPHGGAAS), NY-ESO-161–80 (PRGPHGGAASGLNGCCRCGA), NY-ESO-171–90 (GLNGCCRCGARGPESRLLEF), NY-ESO-181–100 (RGPESRLLEFYLAMPFATPM), NY-ESO-191–110 (YLAMPFATPMEAELARRSLA), NY-ESO-1101–120 (EAELARRSLAQDAPPLPVPG), NY-ESO-1111–130 (QDAPPLPVPGVLLKEFTVSG), NY-ESO-1119–143 (PGVLLKEFTVSGNILTIRLTAADHR), NY-ESO-1131–150 (NILTIRLTAADHRQLQLSIS), NY-ESO-1139–160 (AADHRQLQLSISSCLQQLSLLM), NY-ESO-1151–170 (SCLQQLSLLMWITQCFLPVF), and NY-ESO-1161–180 (WITQCFLPVFLAQPPSGQRR) were obtained from Invitrogen.
In Vitro Sensitization of CMV-Specific CD8+ T Cells.
For in vitro sensitization of CMV-specific CD8+ T cells, 0.5∼1 × 106 PBMCs were cultured with CMV peptides (CMV 495–503 for HLA-A*0201 restricted, 10 µM) in a round-bottom 96-well plate. After 8 h, one-half of the medium was replaced by fresh medium containing IL-2 (20 units/mL) and IL-7 (40 ng/mL) and repeated twice per week. Presensitized CD8+ T cells were stained after 7 d culture with PE-labeled HLA-A*0201/ tetramer for 10 min at 37 °C before additional staining with cell surface markers for 15 min at 4 °C.
Enzyme-Linked Immunospot Assay.
Flat-bottomed, 96-well nitrocellulose plates (MAHAS4510; Millipore) were coated with anti–IFN-γ mAb (4 µg/mL, 1-D1K; MABTECH) and incubated overnight at 4 °C and washed and blocked with RPMI with 100 mL/L AB serum. Presensitized 2∼5 × 104 CD4+ T cells and 5 × 104 target cells (peptide-pulsed autologous activated T-cell APCs) were added to each well and incubated for 20–22 h at 37 °C. Spots were developed using biotinylated anti–IFN-γ mAb (0.2 µg/mL, 7-B6-1-biotin; MABTECH), alkaline phosphatase conjugated streptavidin (Roche Diagnostics), and 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium (Sigma) and counted with a CTL ImmunoSpot S5 Micro Analyzer (Cellular Technologies).
Supplementary Material
Acknowledgments
We thank J. B. Wing and D. Adeegbe for critically reading the manuscript; the patients, their families, the nurses, and the healthy donors; and the members of the Flow Cytometry Core CyPS facility (Université Pierre et Marie Curie, Paris) for their expertise and assistance. The study was supported by Assistance Publique - Hôpitaux de Paris (Programme Hospitalier de Recherche Clinique AOR09-085), Centre d'Investigation Biologique Pitié-Salpêtrière, Société Nationale Française de Médecine Interne, and Fondation Arthritis. S.S. is the recipient of Grants-in-Aid for Specially Promoted Research (20002007) and for Scientific Research (A) (26253030) from the Ministry of Education, Culture, Sports, Science and Technology of Japan and Core Research for Evolutional Science and Technology from the Japan Science and Technology Agency.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1508224112/-/DCSupplemental.
References
- 1.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10(7):490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 3.Allan SE, et al. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19(4):345–354. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 4.Miyara M, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30(6):899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Ito T, et al. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity. 2008;28(6):870–880. doi: 10.1016/j.immuni.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baecher-Allan C, Wolf E, Hafler DA. MHC class II expression identifies functionally distinct human regulatory T cells. J Immunol. 2006;176(8):4622–4631. doi: 10.4049/jimmunol.176.8.4622. [DOI] [PubMed] [Google Scholar]
- 7.Borsellino G, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: Hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110(4):1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 8.Thornton AM, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184(7):3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deaglio S, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204(6):1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fritzsching B, et al. In contrast to effector T cells, CD4+CD25+FoxP3+ regulatory T cells are highly susceptible to CD95 ligand- but not to TCR-mediated cell death. J Immunol. 2005;175(1):32–36. doi: 10.4049/jimmunol.175.1.32. [DOI] [PubMed] [Google Scholar]
- 11.Solstad T, et al. CD147 (Basigin/Emmprin) identifies FoxP3+CD45RO+CTLA4+-activated human regulatory T cells. Blood. 2011;118(19):5141–5151. doi: 10.1182/blood-2011-02-339242. [DOI] [PubMed] [Google Scholar]
- 12.Liu W, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203(7):1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seddiki N, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203(7):1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann P, et al. Only the CD45RA+ subpopulation of CD4+CD25high T cells gives rise to homogeneous regulatory T-cell lines upon in vitro expansion. Blood. 2006;108(13):4260–4267. doi: 10.1182/blood-2006-06-027409. [DOI] [PubMed] [Google Scholar]
- 15.Battaglia M, et al. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177(12):8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 16.Nunes-Cabaço H, Caramalho I, Sepúlveda N, Sousa AE. Differentiation of human thymic regulatory T cells at the double positive stage. Eur J Immunol. 2011;41(12):3604–3614. doi: 10.1002/eji.201141614. [DOI] [PubMed] [Google Scholar]
- 17.Sugiyama D, et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci USA. 2013;110(44):17945–17950. doi: 10.1073/pnas.1316796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hallermann C, Niermann C, Schulze HJ. Regulatory T-cell phenotype in association with large cell transformation of mycosis fungoides. Eur J Haematol. 2007;78(3):260–263. doi: 10.1111/j.1600-0609.2006.00809.x. [DOI] [PubMed] [Google Scholar]
- 19.Miyara M, Amoura Z, Gorochov G. Human lupus, fewer Treg cells indeed: Comment on the article by Venigalla et al. Arthritis Rheum. 2009;60(2):630. doi: 10.1002/art.24287. [DOI] [PubMed] [Google Scholar]
- 20.Miyara M, et al. Global natural regulatory T cell depletion in active systemic lupus erythematosus. J Immunol. 2005;175(12):8392–8400. doi: 10.4049/jimmunol.175.12.8392. [DOI] [PubMed] [Google Scholar]
- 21.Munro JM, et al. Expression of sialyl-Lewis X, an E-selectin ligand, in inflammation, immune processes, and lymphoid tissues. Am J Pathol. 1992;141(6):1397–1408. [PMC free article] [PubMed] [Google Scholar]
- 22.Easton EW, Schiphorst WE, van Drunen E, van der Schoot CE, van den Eijnden DH. Human myeloid alpha 3-fucosyltransferase is involved in the expression of the sialyl-Lewis(x) determinant, a ligand for E- and P-selectin. Blood. 1993;81(11):2978–2986. [PubMed] [Google Scholar]
- 23.Sugimoto N, et al. Foxp3-dependent and -independent molecules specific for CD25+CD4+ natural regulatory T cells revealed by DNA microarray analysis. Int Immunol. 2006;18(8):1197–1209. doi: 10.1093/intimm/dxl060. [DOI] [PubMed] [Google Scholar]
- 24.Natsuka S, Gersten KM, Zenita K, Kannagi R, Lowe JB. Molecular cloning of a cDNA encoding a novel human leukocyte alpha-1,3-fucosyltransferase capable of synthesizing the sialyl Lewis x determinant. J Biol Chem. 1994;269(24):16789–16794. [PubMed] [Google Scholar]
- 25.Sasaki K, et al. Expression cloning of a novel alpha 1,3-fucosyltransferase that is involved in biosynthesis of the sialyl Lewis x carbohydrate determinants in leukocytes. J Biol Chem. 1994;269(20):14730–14737. [PubMed] [Google Scholar]
- 26.de Vries T, Knegtel RM, Holmes EH, Macher BA. Fucosyltransferases: Structure/function studies. Glycobiology. 2001;11(10):119R–128R. doi: 10.1093/glycob/11.10.119r. [DOI] [PubMed] [Google Scholar]
- 27.Polley MJ, et al. CD62 and endothelial cell-leukocyte adhesion molecule 1 (ELAM-1) recognize the same carbohydrate ligand, sialyl-Lewis x. Proc Natl Acad Sci USA. 1991;88(14):6224–6228. doi: 10.1073/pnas.88.14.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siegmund K, et al. Migration matters: Regulatory T-cell compartmentalization determines suppressive activity in vivo. Blood. 2005;106(9):3097–3104. doi: 10.1182/blood-2005-05-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gottschalk RA, Corse E, Allison JP. Expression of Helios in peripherally induced Foxp3+ regulatory T cells. J Immunol. 2012;188(3):976–980. doi: 10.4049/jimmunol.1102964. [DOI] [PubMed] [Google Scholar]
- 30.Himmel ME, MacDonald KG, Garcia RV, Steiner TS, Levings MK. Helios+ and Helios- cells coexist within the natural FOXP3+ T regulatory cell subset in humans. J Immunol. 2013;190(5):2001–2008. doi: 10.4049/jimmunol.1201379. [DOI] [PubMed] [Google Scholar]
- 31.Serre K, et al. Helios is associated with CD4 T cells differentiating to T helper 2 and follicular helper T cells in vivo independently of Foxp3 expression. PLoS ONE. 2011;6(6):e20731. doi: 10.1371/journal.pone.0020731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joller N, et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity. 2014;40(4):569–581. doi: 10.1016/j.immuni.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyara M, et al. The immune paradox of sarcoidosis and regulatory T cells. J Exp Med. 2006;203(2):359–370. doi: 10.1084/jem.20050648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van den Hoogen F, et al. 2013 classification criteria for systemic sclerosis: An American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis. 2013;72(11):1747–1755. doi: 10.1136/annrheumdis-2013-204424. [DOI] [PubMed] [Google Scholar]
- 35.Olsen EA, et al. International Society for Cutaneous Lymphomas United States Cutaneous Lymphoma Consortium Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: A consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29(18):2598–2607. doi: 10.1200/JCO.2010.32.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaretzki A, 3rd, et al. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America Myasthenia gravis: Recommendations for clinical research standards. Ann Thorac Surg. 2000;70(1):327–334. doi: 10.1016/s0003-4975(00)01595-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.