Abstract
Background:
Teucrium hyrcanicum belonging to the Lamiaceae family is a native plant in Iran; it is called Maryam nokhodi-e-jangali in Farsi.
Objective:
The aim of this study is to evaluate acetylcholinesterase inhibition (AChEI), antioxidant activity and flavonoids content of T. hyrcanicum methanol extract.
Materials and Methods:
The air-dried and the ground aerial parts of T. hyrcanicum were extracted by percolation method with methanol. Antioxidant activity of the extract was investigated by using 2,2'-diphenyl-1-picrylhydrazyl (DPPH) and ferric reducing antioxidant power assay (FRAP) methods. In addition, AChEI and flavonoid content of T. hyrcanicum methanol extract were measured.
Results:
The results showed that total flavonoid content of T. hyrcanicum in reference to the standard curve for quercetin was 20.70 ± 0.05 mg quercetin equivalents/g of extract. In the FRAP method, the antioxidant activity of T. hyrcanicum extract and butyl hydroxyanisole (BHA) (as a positive control) were 657.5 ± 0.04 and 880 ± 0.06 mmol Fe II/1 g dried extract. According to results of DPPH assay, half maximal inhibitory concentration (IC50) value for DPPH radical-scavenging activities of T. hyrcanicum methanol extract, vitamin E and BHA were 74.6, 14.12 and 7.8 μg/mL, respectively. IC50 value for AChEI of T. hyrcanicum and donepezil as a positive control were 2.12 mg/mL and 0.013 mg/mL.
Conclusion:
The results of the present study showed T. hyrcanicum is a natural antioxidant that the flavonoid content can be responsible for extract effects.
Keywords: Acetylcholinesterase inhibitory, antioxidant activity, flavonoid contents, Teucrium hyrcanicum
INTRODUCTION
Alzheimer's disease (AD) is the important degenerative disease of the brain among the elderly.[1,2] Studies suggest that AD is caused by reduced levels of acetylcholine.[3] Thus, the use of acetylcholinesterase enzyme inhibitors (AChEIs) is a major treatment option for AD.[4] Some synthetic AD drugs exhibit several side-effects, and many efforts have been made to find natural AChEIs from plants with less adverse effects.[5] Free radicals such as the superoxide anion and hydroxyl and peroxyl radicals, generated from activated neutrophils and macrophages may cause serious diseases including neurodegenerative disorders, cancer and atherosclerosis.[6] Oxidative stress is responsible for cellular damage especially in organs such as the brain.[7,8] Some studies suggest that the brain of an Alzheimer's patient is under oxidative stress resulting from an imbalance of calcium ions within their neurons and mitochondria.[9,10] Siddhuraju (2007) showed herbal products are the natural antioxidants that able to reduce oxidative damage.[11] Some previous studies on the antioxidant activity in plants have focused on phenolic compounds.[12]
Teucrium is a genus belonging to the Lamiaceae family and consists 340 species in the world Among them 12 species exist in Iran and three species are endemic.[13] These species have been used in traditional medicine for their diuretic, diaphoretic, tonic and anti-spasmodic effects.[14] In Iranian and Arab traditional medicine, Teucrium polium and Teucrium persicum have been used for the treatment of diabetes, gastric inflammation and convulsion.[14] In the genus Teucrium, flavonoids are important active compounds that attribute to many of the biological effects observed in these species. The quantity of flavonoids can be influenced by various factors. For example, different seasons as well as the locations of plant growth are important to consider in the evaluation of flavonoid content.[15] In one study, T. polium subsp. polium methanol extract showed antioxidant activity.[16] In another study, flavonoids content of T. polium has been reported as 47.80 ± 0.44 mg of rutin equivalent/g of extract.[17]
Teucrium hyrcanicum L. (called Maryam nokhodi-e-jangali in Farsi) is a native plant in Iran which wildly grows in north and northwest of the country.[18] In previous studies, anti-nociceptive and anti-inflammatory activities of T. hyrcanicum were investigated.[19] The aim of this study was to evaluate AChEI, antioxidant activity and flavonoids content of T. hyrcanicum methanol extract.
MATERIALS AND METHODS
Plant material
The aerial parts of T. hyrcanicum were collected from Ardebil province in North-West of Iran, in June 2012. The plant was identified by Dr. Yousef Ajani and given herbarium specimen number (M. Khanavi 1446). The voucher specimen was deposited in Herbarium of Institute of Medicinal Plant (ACECR), Tehran, Iran.
Extraction
The aerial parts of T. hyrcanicum (500 g) were dried in the shade and powdered. The air-dried and the ground aerial parts of T. hyrcanicum were extracted by percolation method with methanol. The extract was dried using a rotary evaporator to give 52.5 g solid residues. The extract was stored in a refrigerator until required.
Total flavonoid content
Total flavonoid content was determined as described by Saeidnia and Gohari.[20] Five microliter of aluminum trichloride (AlCl3) (2% in methanol) was added to 5 mL of extract (0.4 mg/mL). After 10 min, the absorbance of the mixture was measured at 415 nm. Blank sample consists of 5 mL extract and 5 mL methanol without AlCl3. Total flavonoid content was measured by using a standard curve with quercetin (0–100 mg/L). Total flavonoid content was expressed as mg of quercetin as equivalents (QE)/g of extract. Because quercetin has reported from Teucrium species, previously such as Teucrium arduini L.[21]
Evaluation of antioxidant activity using ferric reducing antioxidant power assay method
The antioxidant activity of extracts were measured by the ferric reducing antioxidant power assay (FRAP) method based on an established protocol.[22] The principle of this method is the reduction of ferric tripyridyl triazine (Fe [III]-TPTZ) complex to the ferrous tripyridyl triazine (Fe [II]-TPTZ), to its colored ferrous form in the presence of antioxidants. The FRAP reagent contained 5 mL of TPTZ (2,4,6-tripyridyl-s-triazine) solution (10 mmol/L) in HCl (40 mmol/L) plus 5 mL of FeCl3 solution (20 mmol/L) and 50 mL of a 0.3 mol/L acetate buffer solution (pH 3.6) that was prepared freshly and warmed at 37°C. Aliquots of 50 μL sample were mixed with 1.5 mL FRAP reagent then were incubated at 37°C for 10 min. The absorbance of the reaction mixture was measured at 593 nm. For the construction of the calibration curve, five concentrations of FeSO4.7H2O (1000, 750, 500, 250, 125 μmol/L) were used, and the absorbance values were measured as sample solutions. Butyl hydroxyanisole (BHA) was used as a positive control. The antioxidant activity of T. hyrcanicum methanol extract was expressed as the concentration of antioxidants having a ferric reducing ability equivalent to that of 1 mmol/L FeSO4. [23]
2,2’ -diphenyl-1-picrylhydrazyl radical-scavenging activity assay
The antioxidant activities of samples were determined by the 2,2’ -diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging.[24] Different concentrations of sample solutions (1 mL) of methanol were added to DPPH methanol solution (2 ml, 40 μg/mL). After 30 min, the absorbance was measured at 517 nm. Vitamin E and BHA were used as positive controls. Percentage of radical scavenging activity of samples was calculated by using the equation: Inhibition% = [(A0–As)/A0] × 100 that A0 is the absorbance of the control and As is the absorbance of the sample. Half maximal inhibitory concentration (IC50) values (indicate the concentration of the sample (mg/mL), required to scavenge 50% of DPPH) were calculated from graph-plotted against scavenging percentage and extract concentration.
Acetylcholinesterase inhibition
The enzymatic activity was measured as described by Aazza et al.[25] with minor modifications. Fifty microliter of buffer 0.1 M (pH = 8) and 25 μL of test compound (or Donepezil as a positive control) were dissolved in methanol at different concentrations and 25 μL of AChE enzyme (0.22 U/ml) (Sigma-Aldrich Chemie, Germany) was added and mixed. After incubation at 37°C for 15 min, 25 μL of 15 mM acetylthiocholine iodide (Sigma-Aldrich Chemie, Germany) and 125 μL of 3 mM 5,5’-dithiobis (2-nitrobenzoic acid) (Sigma-Aldrich Chemie, Germany) were added and the resulting mixture incubated at room temperature for 30 min. Absorbance of the mixture was measured at 405 nm using a microplate reader (ELX808, BioTek, USA). The inhibitory effect of test compound was calculated by comparison to the negative control: % = [(A0–A1)/A0] × 100 where A0 was the absorbance of the blank sample and A1 was the absorbance of the test sample. Each test was replicated three times. The inhibition of enzyme activity was expressed as IC50 (the concentration of the sample, required to inhibit 50% of the enzyme).[26]
Statistical analyses
All data was expressed as mean ± standard deviation. Statistical analysis was performed with One-way analysis of variance, followed by Tukey post-hoc test for multiple comparisons. P < 0.05 was considered as significant.
RESULTS AND DISCUSSION
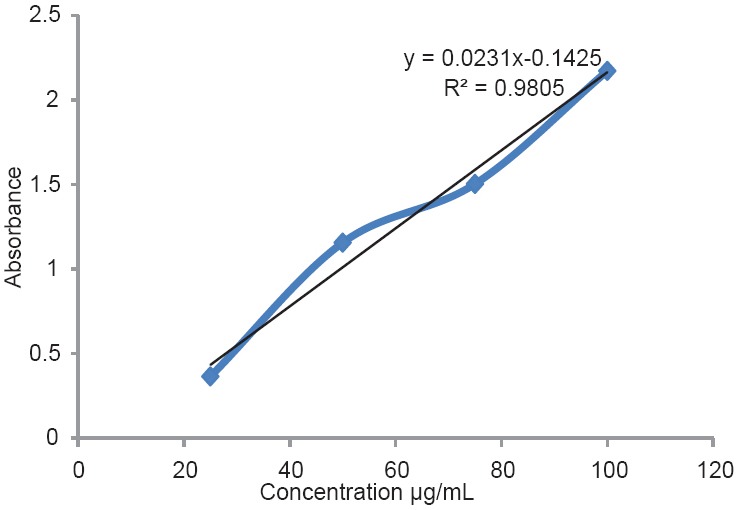
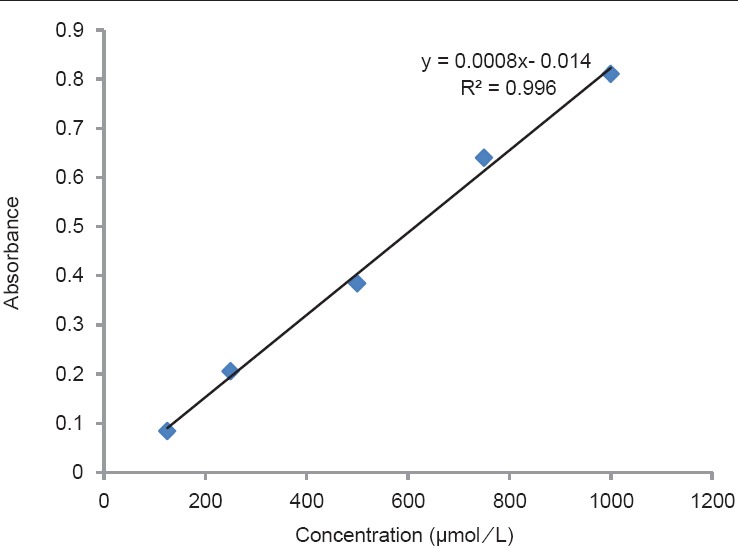
In this study, total flavonoid contents, antioxidant activity and AChEI of methanol extract of T. hyrcanicum were determined. The extract yield of the plant was determined as 10.5%. The results of antioxidant activity and flavonoid contents of T. hyrcanicum are shown in Table 1. The total flavonoid content of T. hyrcanicum in reference to the standard curve for quercetin (y = 0.0231x − 0.1425, R2 = 0.9805) was 20.70 ± 0.05 mg QE/1 g of extract. The standard curve for quercetin is shown in Figure 1. The obtained results from antioxidant activity using FRAP method expressed as FRAP value [Figure 2]. These values represented mmol. Fe II/1 g dried extract. The antioxidant activity of the T. hyrcanicum extract and BHA (as a positive control) were 657.5 ± 0.04 and 880 ± 0.06 mmol Fe II/1 g dried extract, respectively. As this result, in FRAP method, T. hyrcanicum extract showed better antioxidant activity than BHA. According to results of DPPH assay, IC50 value for DPPH radical-scavenging activities of T. hyrcanicum methanol extract, vitamin E and BHA were 74.6, 14.12 and 7.8 μg/mL, respectively. The radical scavenging activity of T. hyrcanicum at 400 μg/mL was comparable with vitamin E (40 μg/mL) and with BHA (100 μg/mL) (P > 0.05). AchEI activity of methanol extract of T. hyrcanicum was studied for the first time. IC50 value was calculated from graph-plotted inhibition percentage against extract concentration [Figure 3]. The results showed that IC50 value for AchEI of T. hyrcanicum and donepezil as a positive control were 2.12 mg/mL and 0.013 mg/mL, respectively.
Table 1.
Antioxidant activity and flavonoid contents of Teucrium hyrcanicum methanol extract
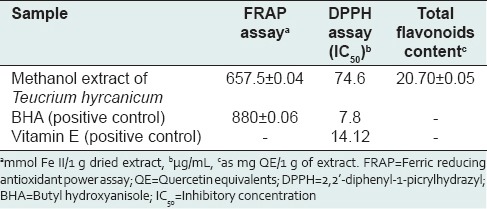
Figure 1.
The standard curve of quercetin for measuring total flavonoid content
Figure 2.
Calibration curve in ferric reducing anti-oxidant power assay method. Five concentrations of FeSO4.7H2O [1000, 750, 500, 250, 125 µmol/L] were used
Figure 3.
Acetylcholinesterase inhibition activity of methanol extract of Teucrium hyrcanicum
There are reports about flavonoid content and antioxidant activity of other Teucrium species like Teucrium stocksianum and T. polium.[14,27] The previous studies indicated that methanol extract of T. polium and two isolated flavonoids (rutin and apigenin) had radical scavenging activity with IC50 = 20.1 ± 1.7, 23.7 ± 1.9 and 30.3 ± 2.1 μg/ml, respectively.[17] The results of another study showed that total flavonoid content of T. polium in reference to the standard curve for rutin was 47.80 ± 0.44 mg of rutin equivalent/g of extract).[17] In this investigation, T. hyrcanicum showed the antioxidant effect and total flavonoid content less than T. polium. The phenolic content and other compounds can be responsible for the antioxidant activity of T. hyrcanicum extract.
In previous study, the ethanolic extract of T. arduini, Teucrium chamaedrys, Teucrium montanum and T. polium showed remarkable AChEI activity above 50% inhibition rate at 1 mg/mL.[28] In another study, AChEI activity of methanol extract of T. polium was 39.10% with inhibition rate at 1 mg/L. Inhibitory activity of methanol extract of T. hyrcanicum was 40.50% with inhibition rate at 1 mg/mL. The results of previous study indicated that Lamiaceae species containing phenolic and terpenic compounds can affect AChE activity and oxidative stress. Lamiaceae species may be effective in the prevention and therapy of AD and other neurodegenerative disorders.[28]
CONCLUSION
The results of the present study indicated that T. hyrcanicum collected from North-West of Iran is a natural antioxidant that able to reduce oxidative damage. The flavonoid content can be responsible for the antioxidant activity of T. hyrcanicum extract.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Shen Q, Peng Q, Shao J, Liu X, Huang Z, Pu X, et al. Synthesis and biological evaluation of functionalized coumarins as acetylcholinesterase inhibitors. Eur J Med Chem. 2005;40:1307–15. doi: 10.1016/j.ejmech.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Zhou X, Wang XB, Wang T, Kong LY. Design, synthesis, and acetylcholinesterase inhibitory activity of novel coumarin analogues. Bioorg Med Chem. 2008;16:8011–21. doi: 10.1016/j.bmc.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 3.Weinstock M. Possible role of the cholinergic system and disease models. J Neural Transm Suppl. 1997;49:93–102. doi: 10.1007/978-3-7091-6844-8_10. [DOI] [PubMed] [Google Scholar]
- 4.Grutzendler J, Morris JC. Cholinesterase inhibitors for Alzheimer's disease. Drugs. 2001;61:41–52. doi: 10.2165/00003495-200161010-00005. [DOI] [PubMed] [Google Scholar]
- 5.Karimi GH, Iranshahi M, Hosseinalizadeh F, Riahi B, Sahebkar A. Screening of acetylcholinesterase inhibitory activity of terpenoid and coumarin derivatives from the genus Ferula. Pharmacologyonline. 2010;1:566–74. [Google Scholar]
- 6.Conforti F, Sosa S, Marrelli M, Menichini F, Statti GA, Uzunov D, et al. In vivo anti-inflammatory and in vitro antioxidant activities of Mediterranean dietary plants. J Ethnopharmacol. 2008;116:144–51. doi: 10.1016/j.jep.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 7.Halliwell B, Gutteridge JM. Oxygen radicals in the nervous system. Trends Neurosci. 1985;8:22–6. [Google Scholar]
- 8.Floyd RA, Hensley K. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol Aging. 2002;23:795–807. doi: 10.1016/s0197-4580(02)00019-2. [DOI] [PubMed] [Google Scholar]
- 9.Emilien G, Beyreuther K, Master CL, Maloteaux JM. Prospects for pharmacological intervention in Alzheimer's disease. Arch Neurol. 2000;57:454–9. doi: 10.1001/archneur.57.4.454. [DOI] [PubMed] [Google Scholar]
- 10.Tabet N. Acetylcholinesterase inhibitors for Alzheimer's disease: Anti-inflammatories in acetylcholine clothing! Age Ageing. 2006;35:336–8. doi: 10.1093/ageing/afl027. [DOI] [PubMed] [Google Scholar]
- 11.Siddhuraju P, Becker K. The antioxidant and free radical scavenging activities of processed cowpea (Vigna unguiculata (L.) Walp.)seed extracts. Food Chem. 2007;101:10–9. [Google Scholar]
- 12.Jafari S, Moradi A, Salaritabar A, Hajiakhoondi A, khanavi M. Determination of total phenolic and flavonoid contents of Leonurus cardiac L. in compare with antioxidant activity. Res J Biol Sci. 2010;5:484–7. [Google Scholar]
- 13.Kazemizadeh Z, Habibi B, Moradi A. Chemical composition of the essential oils of two populations Teucrium hyrcanicum L. in two different localities. J Med Plant. 2008;7:87–93. [Google Scholar]
- 14.Hasani-Ranjbar SH, Nayebi N, Larijani B, Abdollahi M. A systematic review of efficacy and safty of Teucrium species; from anti-oxidant to anti diabetic effects. Int J Pharm. 2010;6:315–25. [Google Scholar]
- 15.Stefkov G, Karapandzova M, Stefova M, Kulevanova S. Seasonal variation of flavonoids in Teucrium polium L.(Lamiaceae) Macedon Pharm Bull. 2009;55:33–40. [Google Scholar]
- 16.Stankovic MS, Niciforovic N, Mihailovic V, Topuzovic M, Solujic S. Antioxidant activity, total phenolic content and flavonoid concentrations of different plant parts of Teucrium polium L. subsp. polium. Acta Soc Bot Pol. 2012;81:117–22. [Google Scholar]
- 17.Sharififar F, Dehghn-Nudeh GH, Mitrajaldini M. Major flavonoids with antioxidant activity from Teucrium polium L. Food chem. 2009;112:885–8. [Google Scholar]
- 18.Rechinger KH. Vol. 150. Flora Iranica: Akademische Druck-u Verlagsanstalt; 1982. Flora Iranica. [Google Scholar]
- 19.Farshchi A, Ghiasi G, Abdollahi Asl A. Antinociceptive and antiinflammatory effects of Teucrium hyrcanicum aqueous extract in male mice and rats. Physiol Pharmacol. 2010;14:78–84. [Google Scholar]
- 20.Saeidnia S, Gohari AR. Saarbrücken. Germany: Lambert Academic Publishing; 2012. Pharmacognosy and Molecular Pharmacognosy in Practice: A Laboratory Desk Reference of Pharmacognosy for Researchers and Students. [Google Scholar]
- 21.Kremer D, Kosir IJ, Kosalec I, Koncic MZ, Potocnik T, Cerenak A, et al. Investigation of chemical compounds, antioxidant and antimicrobial properties of Teucrium arduini L.(Lamiaceae) Curr Drug Targets. 2013;14:1006–14. doi: 10.2174/1389450111314090009. [DOI] [PubMed] [Google Scholar]
- 22.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 23.Yousefbeyk F, Gohari AR, Hashemighahderijani Z, Ostad SN, Salehi Sourmaghi MH, Amini M, et al. Bioactive terpenoids and flavonoids from daucus littoralis smith subsp. hyrcanicus Rech. f, an endemic species of Iran. Daru. 2014;22:12. doi: 10.1186/2008-2231-22-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yokozawa T, Chen CP, Dong E, Tanaka T, Nonaka GI, Nishioka I. Study on the inhibitory effect of tannins and flavonoids against the 1,1-diphenyl-2 picrylhydrazyl radical. Biochem Pharmacol. 1998;56:213–22. doi: 10.1016/s0006-2952(98)00128-2. [DOI] [PubMed] [Google Scholar]
- 25.Aazza S, Lyoussi B, Miguel MG. Antioxidant and antiacetylcholinesterase activities of some commercial essential oils and their major compounds. Molecules. 2011;16:7672–90. doi: 10.3390/molecules16097672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golfakhrabadi F, Khanavi M, Ostad SN, Saeidnia S, Vatandoost H, Abai MR, et al. Biological activities and composition of Ferulago carduchorum essential Oil. J Arthropod Borne Dis. 2015;9:104–15. [PMC free article] [PubMed] [Google Scholar]
- 27.Iriadam M, Musa D, Gumushan H, Baba F. Effects of two Turkish medicinal plants Artemisia herba-alba and Teucrium polium on blood glucose levels and other biochemical parameters in rabbits. J Cell Mol Biol. 2006;5:19–24. [Google Scholar]
- 28.Vladimir-Knežević S, Blažeković B, Kindl M, Vladić J, Lower-Nedza AD, Brantner AH. Acetylcholinesterase inhibitory, antioxidant and phytochemical properties of selected medicinal plants of the Lamiaceae Family. Molecules. 2014;19:767–82. doi: 10.3390/molecules19010767. [DOI] [PMC free article] [PubMed] [Google Scholar]


