Abstract
Background:
Swertia cordata and Swertia chirayita are temperate Himalayan medicinal plants used as potent herbal drugs in Indian traditional systems of medicine (Ayurvedic, Unani and Siddha).
Objective:
Assessment of Antioxidant, antibacterial, and antidiabetic potential of Swertia cordata and Swertia chirayita.
Materials and Methods:
Phytochemicals of methanolic and aqueous extracts of the two Swertia species were analyzed. The antioxidant potential of all the extracts was assessed by measuring total phenolic content, total flavonoid content and free radical scavenging potential was assessed by 1,1-diphenyl-2-picrilhydrazyl (DPPH) assay, antibacterial activity was assessed against various pathogenic and nonpathogenic bacteria in vitro by Kirby-Bauer agar well diffusion method and antidiabetic activity was assessed by α-amylase inhibition.
Results:
Methanolic leaf extracts of both the species of Swertia contain significant antibacterial as well as anti-diabetic potential, whereas methanolic root extracts of both species were found to have potential antioxidant activity. However, Swertia chirayita showed better activities than Swertia cordata although both species have good reputation in traditional Indian medicine.
Conclusion:
Both the species are having high medicinal potential in terms of their antioxidant, antibacterial and antidiabetic activities. Studies are required to further elucidate antioxidant, anti-diabetic and antibacterial potentials using various in-vitro, in-vivo biochemical and molecular biology techniques.
Keywords: Antibacterial activity, Anti-diabetic activity, Antioxidant, Chirata, Swertia chirayita, Swertia cordata
INTRODUCTION
Most of the developing countries have adopted traditional medical practice as an integral part of their culture. Historically, most medicinal preparations were derived from plants, whether in the simple form of raw plant materials or the refined form of crude extracts, mixtures, etc. In todays world, medicinal plants are gaining attention owing to the fact that the herbal drugs are cost-effective, easily available and with little or no side effects. Plant-based natural constituents can be obtained from any part of the plant like bark, leaves, flowers, fruits, roots, seeds, etc. Recently, the quest for the isolation of new compounds from medicinal plants has become an intriguing area of research. Plants with ethno pharmaceutical importance were being studied because of their healing properties[1] as well as for their efficient antimicrobial, antidiabetic and antioxidant properties.
All the living organisms use oxygen for metabolism and use food nutrients in order to get energy for survival. Thus, oxygen is an essential part of living organisms. Approximately, 5%–7% or more of the inhaled oxygen (O2) is converted to reactive oxygen species (ROS) such as O2 −, H2O2 and •HO.[2] Antioxidants remove these free radical intermediates inhibiting oxidation reactions. Antioxidants are those compounds that can delay or inhibit the oxidation of lipids, protein and nucleotides by suppressing the initiation of oxidative chain reactions, thus preventing damage done to the body cells by ROS.[3] These free radicals are capable of attacking the healthy cells of the body which may lead to diseases, disorders or cell damage. Cell damage caused by free radicals appear to be a major contributor to aging and diseases like cancer, heart disease, decline in brain function such as Alzheimer diseases, Parkinson diseases, and also decline in the immune function.[4] Swertia chirayita (S. chirayita) has shown to have antimicrobial activity against Gram positive and Gram negative bacteria. It is also used as a stringent tonic to the heart, liver, eyes, cough, scanty-urine, melancholia, dropsy, and skin diseases. The plant extracts are also used as a bitter tonic in gastrointestinal disorders, like dyspepsia and/or anorexia, it is used as a digestive, febrifuge, and laxative.[5] Swertia species contain xanthones as secondary metabolites which are used effectively to treat malarial fever and tuberculosis. Swertia species also contains amarogentin, a glycoside secondary metabolits that protects liver against carbon tetrachloride poisoning and its believed to be the bitterest component isolated till date.[6,7] Almost all species of Swertia possess the phytochemical active principles with varying degree of activities, but only S. chirayita as the major species has been extensively screened for anti-diabetic, antimicrobial and antioxidant active ingredient.
The plants under investigation are Swertia cordata and S. chirayita belonging to the family Gentianaceae. In this family, many plants are used as medicine. Both plants (S. cordata and S. chirayita) were selected for phytochemical and biological studies as they both have folkloric reputation.
MATERIALS AND METHODS
Chemicals and reagent
Chloroform, dimethyl sulfoxide (DMSO), sulfuric acid, dilute ammonia, ferric chloride, hydrogen chloride, deionized distilled water, Folin-Ciocalteau reagent, sodium carbonate, and gallic acid are used for phytochemical screening and antioxidant assay. Mueller-Hinton broth and agar media are used for antibacterial assay. All the reagent and media were purchased from Hi media. Starch and α-amylase enzyme are used for anti-diabetic assay.
Instrumentation
Grinder, water bath, glassware (conical flasks, beakers, test-tubs, petri plates), micropipette, gel puncher, digital balance, incubator, laminar flow hood, hot air oven, spectrophotometer, refrigerator, autoclave, and microwave oven.
Plant material
The plants S. cordata and S. chirayita were collected in December, 2013 from Garo hills at 1200 m to 1500 m altitude, Meghalaya. Identity of the plants was confirmed by a taxonomist from ICAR, Umiam, India. The plants were collected with roots as whole taken out from the soil and then cleaned in running tap water followed by distilled water. Then the plants were dried in the shade for 30 days. Each part (i.e., leaves, stem, and root) was then separated and ground in a clean mechanical grinder separately. The ground powder of root, stem, and leaves was kept in air-tight bag separately in room temperature.
Microorganisms used
Bacterial strains analyzed were obtained from Institute of Microbial Technology, Chandigarh (Bacillus megaterium Microbial Type Culture Collection (MTCC) 8510, Bacillus subtilis MTCC 441, Bacillus flexus MTCC 7024, Staphylococcus aureus MTCC 96, Clostridium perfringens MTCC 450, Lactobacillus rhamnnosus MTCC 1423, Pseudomonas oleovorans MTCC 617, Klebsiella pneumoniae MTCC 7028, Salmonella enteric MTCC 1164 and Escherichia coli MTCC 723).
Extraction of plant materials
The extraction of plant parts was carried out according to the method suggested by Fatope et al. 1993,[8] with minor modification. In this, 20 g powder of leaves; stem and root of both the species of Swertia were percolated at room temperature with 400 ml of methanol for methanolic extraction and with same volume of hot water (98°C initial temperature) for aqueous extraction, respectively. The conical were screw caped and placed in a shaker at room temperature for 24 h at 100 rpm. After 24 h, the extracts were filtered using a muslin cloth and then re-filtered using Whatman filter paper No. 1. The filtrates were concentrated using a water bath at 35–40°C. The extracts were labeled accordingly and preserved in the refrigerator at 4°C, till further work. Extract was dissolved in DMSO to made concentration of the stock solution of 0.5 g/ml.
Phytochemical screening
Phytochemical screening of root, stem, and leaves of S. chirayita and S. cordata was carried out according to the methods of Ayoola et al. 2008.[9] The different plant part extracts prepared were screened for terpenoids, flavonoids, saponins, tannins, and phenols, respectively.
Test for terpenoids (Salkowski test)
To 0.5 g of each of the plant part extracts were added with 2 ml of chloroform. Then into it 3 ml of concentrated sulfuric acid was carefully added, to form a layer. A reddish-brown coloration of the interface indicated the presence of terpenoids.
Test for flavonoids
In 0.5 ml of filtrate of each of the plant parts extracts, 5 ml of dilute ammonia was added, followed by addition of 1 ml of concentrated sulfuric acid to it. The presence of flavonoids was detected by yellow coloration of the solution that disappears on standing.
Test for saponins
To 0.5 g of each plant extracts, 5 ml of distilled water was added in test-tubes. The solution was shaken vigorously and observed for a stable persistent froth. The frothing was mixed with 3–4 drops of olive oil and again shaken vigorously after that it was observed for the formation of emulsions.
Test for tannins
About 0.5 g of each plant extracts was boiled in 10 ml of water in each test-tube and then filtered. Then few drops of 0.1% ferric chloride were added and observed for brownish-green or a blue-black color formation which indicate the presence of tannins.
Test for phenols (ferric chloride test)
Small quantities of alcoholic and aqueous extracts were dissolved in 2 ml of distilled water separately, and into it, few drops of 10% aqueous ferric chloride solution were added. A blue or green color was produced which indicating the presences of phenols.
DETERMINATION OF TOTAL FLAVONOID CONTENT
The flavonoids content of different plant part extracts of both Swertia spp. was measured based on methods described by Ebrahimzadeh et al. 2008.[10] According to this method, 0.5 ml of each extracts was mixed with 1.5 ml of methanol and then 0.1 ml of 10% aluminum chloride was added, followed by the addition of 0.1 ml of 1M potassium acetate and then 2.8 ml of distilled water was added to the total mixture. Then mixture was incubated for 30 min at room temperature. The absorbance of the reaction mixture was measured at 415 nm in a spectrophotometer. The total flavonoids content was expressed in mg/g of quercetin equivalent, as standard. All the determination was performed in triplicate.
DETERMINATION OF TOTAL PHENOLIC CONTENT
The total phenolic content of different plant part extracts of both Swertia spp. was evaluated using a method described by Kim et al. 2003.[11] According to this, 0.5 ml of extract was added to 4.5 ml of distilled water and then 0.5 ml of Folin Ciocalteu reagent was added to it. After mixing, the mixture was maintained at room temperature for 5 min followed by the addition of 5 ml of 7% sodium carbonate and 2 ml of distilled water into the mixture. After mixing, the solution was incubated for 90 min at 23°C. The absorbance was measured by spectrophotometer at 750 nm. The total phenolic content was expressed in mg/g of Gallic acid equivalent, as standard. Determination of total phenolic content was performed in triplicate.
DETERMINATION OF 1,1-DIPHENYL-2-PICRILHYDRAZYL RADICAL SCAVENGIN ACTIVITY
The DPPH radical scavenging activity of all the plant parts extracts for both Swertia spp. were determined using the method proposed by Von Gadow et al. 1997.[12] In this, 50 μl of samples of each plant extracts was placed in separate cuvettes, then into it, 2 ml of 6 × 10−5 M methanolic solution of DPPH radical was added immediately and absorbance was measured immediately at 517 nm. Then after 16 min of incubation decrease in absorbance was again measured for the entire samples. Methanolic solution of Ascorbic acid of 1 μl/mg concentration was used as a standard. All the determination was performed in triplicates. The DPPH radical scavenging activity was measured in percentage of reduction of the color. The percentage inhibition of the DPPH radical by the sample extracts was calculated by the formula suggested by Yen and Duh 1994.[13] Control was taken at time zero that mean before the antioxidant present in plant extracts starts its activities.
%inhibition = {(AC(0) − AA(t)))/AC(0)} × 100
Where,
AC(0) = Absorbance of control at time, t = 0 min
AA(t) = Absorbance of antioxidant at time, t = 16 min.
METAL CHELATING ACTIVITY
The metal (ferrous ion) chelating activity by methanolic and aqueous extracts of both the Swertia spp. were estimated by a method suggested by Dinis et al.[14] with minor modification. In this, about 100 μl the each plant part extracts samples were added to 50 μl solution of 2 mM FeCl2. Then the addition of 200 μl of 5 mM ferrozine started the reaction and then the mixture was shaken vigorously and left at room temperature for 10 min. Absorbance for reduction of coloration of the solution by each sample was measured at 562 nm in a spectrophotometer. Ethylenediaminetetraacetic acid solution in place of sample extracts was taken as a positive control and reagent mixture without sample was used as a negative control. The metal chelating activity is expressed in percentage reduction of color. All the determination was performed in triplicate. The metal chelating capacities of the extracts were evaluated using the following equation:
Metal chelating capacity (%) = [(A0 − A1)/A0] ×100
Where,
A0 = absorbance of the negative control
A1 = absorbance of the sample extracts.
ANTIMICROBIAL SCREENING
Well diffusion method
The antimicrobial activity of the extracts was determined by Kirby–Bauer method in agar well diffusion.[15] In this method, initially, the stock cultures of bacteria were revived by inoculating in broth media and grown at 37°C for 18 h in an incubator. Then agar plates of the Muller Hilton's Agar media were prepared. Each plate was inoculated by swabbing with bacterial suspension or with 18 h fresh culture (105–106 colony forming unit “CFU”/ml), which was swabbed evenly on the surface of solid agar media by the help of cotton swab. After 20 min, wells of 6 mm diameter were made in solid agar medium with the help of gel puncher and filled with 50 μl of various test samples of S. chirayita and S. cordata in different solvent extracts. The positive control wells were filled with Amikacin 30 (Standard drug). All the plates were incubated at 37°C for 24 h and then during observation after 24 h, the diameter of the zone of inhibition was measured in millimeter. All the equipment used like media, Petri plates, cotton swab etc., must be sterilized before performing the experiments. As well as proper labeling of the petri plates must be done before performing. All the determination of antibacterial activities of plant extracts were also done in triplicates.
ANTI-DIABETIC ACTIVITY ASSAY (IN VITRO)
Antidiabetic potential of S. chirayita and S. cordata was assay in vitro by a method proposed by Prabhakar et al. 2013.[16] The in vitro anti-diabetic activity was determined by assaying the inhibitory activity of the enzyme α-amylase which involves in the breakdown of starch to produce glucose. In this method, 1 ml of both the methanolic and aqueous extracts of both the Swertia spp. was tested separately and thus added to 1 ml of the enzyme α-amylase in a test-tube and incubated for 10 min at 37°C. Then 1 ml of 1% starch solution was added into it and again incubated for 15 min at 37°C. Then 2 ml 3,5-dinitrosalicylic acid reagent was added into it, in order to terminate the reaction. The reaction mixture was then incubated in boiling water bath for 5 min and then allows it to cool at room temperature. The absorbance of the reaction mixture was then measured at 546 nm in a spectrophotometer. The standard (control) of the reaction without the extract represents the 100% enzyme activity. The % age inhibition of enzyme activity of α-amylase was determined by:

Statistical analysis
The results are represented as mean of three replicates followed by the standard deviation, that is, mean ± standard deviation. The data were subjected to student t-test analysis, and the significance of the difference was determined by Graphpad. P < 0.05 were considered to be significant.
RESULTS AND DISCUSSION
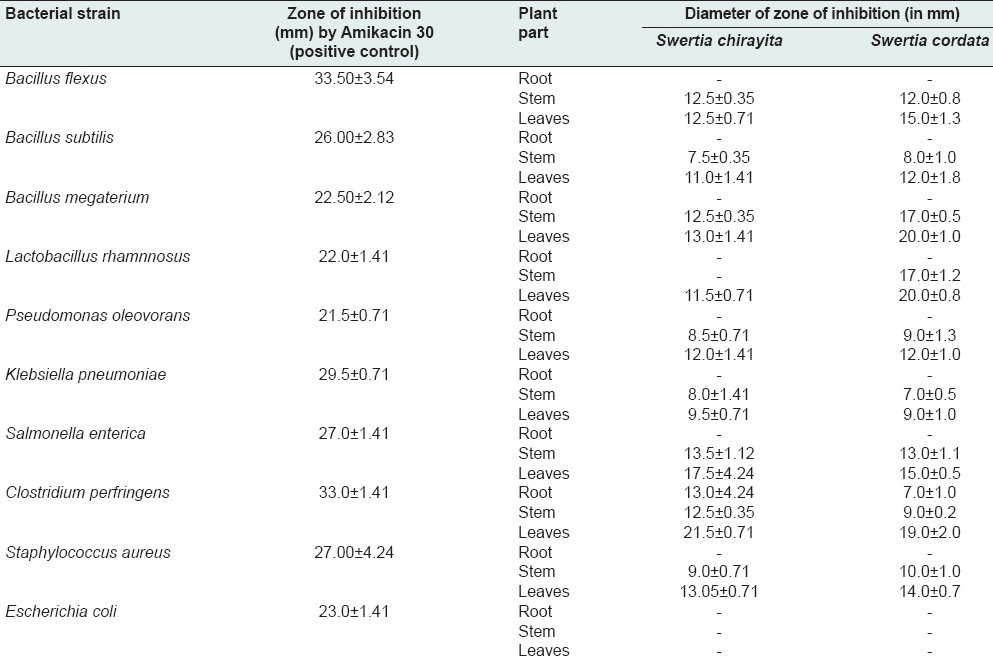
The different solvent extracts from various plant parts of both the species of S. chirayita and S. cordata exhibited varying degree of antioxidant activity in vitro. Only methanolic extract of stem and leaves of both the Swertia spp. showed antimicrobial activity.
Both the Swertia spp. plants showed significant [Tables 1–4.
Table 1.
Antioxidant activity in methanolic extracts of different part of Swertia spp.
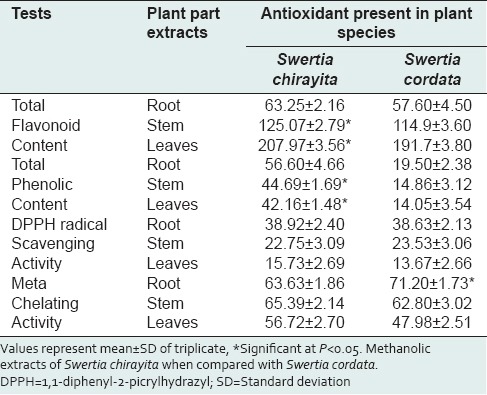
Table 4.
Anti-diabetic activity of Swertia chirayita and Swertia cordata assay in vitro with percentage of enzyme α-amylase inhibition activity
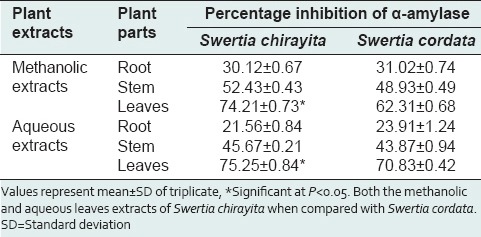
Table 2.
Antioxidant activity in an aqueous extract of different part of Swertia spp.
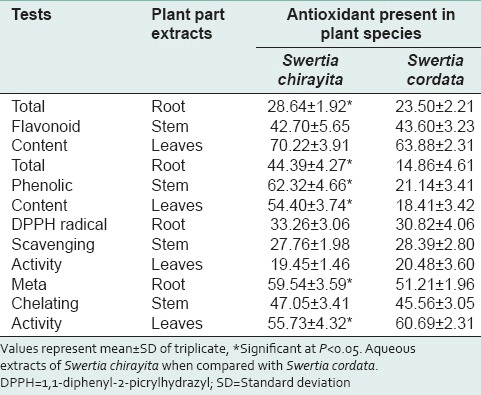
Table 3.
Antibacterial activity of Swertia chirayita and Swertia cordata with diameter of the zone of inhibition in millimeter
CONCLUSION
Antioxidant, antimicrobial and antidiabetic activity of Swertia cordata and Swertia chirayata plants were analyzed in this experiment. Among the different plant extracts analyzed methanolic leaf extracts, aqueous root extracts S chirayata and methanolic stem extract of S cordata showed most significant antioxidants potential. Very significant antidiabetic activity was observed in both methanolic and aqueous leaf extract followed by methanolic and aqueous stem extracts of both the Swertia species. Root extracts also showed minor antidiabetic activity.
Methanolic leaf extracts of both S chirayita and S cordata showed good antibacterial activity.
These results reveal significant antibacterial antioxidants and antidiabetic activities present in different plant parts of S. cordata and S chirayata. Local populations have already realized the medicinal importance of these rare plants and have been using them for treatment of medical ailments for past many centuries.
Further studies are required to elucidate and understand the antibacterial, antidiabetic and antioxidants potential of these both Swertia Spp. plants using various biochemical and molecular biology tools.
ACKNOWLEDGMENT
The authors are grateful to Lovely School of Biotechnology and Bioscience for providing the facilities for this research work.
Footnotes
Source of Support: Lovely Professional University
Conflict of Interest: None declared.
REFERENCES
- 1.Amrutha V, Mani P, Gireesha S, Supriya G, Pallavi R. Evaluation of immunostimulating activity in ethanol, ethyl acetate, methanol and chloroform extracts of Ocimum sanctum L. Int J Pharmacogn Phytochem Res. 2013;5:311–4. [Google Scholar]
- 2.Gupta K, Sharma K. Plant as natural antioxidants. Nat Prod Radiance. 2006;5:326–34. [Google Scholar]
- 3.Tachakittirungrod S, Okonogi S, Chowwanapoonpohn S. Study on antioxidant activity of certain plants in Thailand: Mechanism of antioxidant action of guava leaf extract. J Food Chem. 2007;103:381–8. [Google Scholar]
- 4.Joseph J, Cole G, Head E, Ingram D. Nutrition, brain aging, and neurodegeneration. J Neurosci. 2009;29:12795–801. doi: 10.1523/JNEUROSCI.3520-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar KPS, Bhowmik D, Chiranjib Swertia chirata: A traditional herb and its medicinal uses. J Chem Pharm Res. 2010;2:262–6. [Google Scholar]
- 6.Asthana PK, Sharma NK, Kulshreshtha DK, Chatterjee SK. A Xanthone from Swertia chirayita. Phytochemistry. 1991;30:1037–9. [Google Scholar]
- 7.Chakravarty AK, Mukhopadhayay S, Das B. Swertane terpenoids from Swertia chirata. Phytochemistry. 1991;30:4052–87. [Google Scholar]
- 8.Fatope AO, Ibrahim H, Takeda Y. Screening of higher plants reputed as pesticides using brine shrimp lethality bioassay. Int J Pharmacogn. 1993;31:250–6. [Google Scholar]
- 9.Ayoola GA, Coker HA, Adesegun SA, Adepoju-Bello AA, Obaweya K, Ezennia EC, et al. Phytochemical screening and anti-oxidant activities of some selected medicinal plants used for malaria therapy in south-western Nigeria. Trop J Pharm Res. 2008;7:1019–24. [Google Scholar]
- 10.Ebrahimzadeh MA, Pourmorad F, Bekhradnia AR. Iron chelating activity, phenol and flavonoid content of some medicinal plants from Iran. Afr J Biotechnol. 2008;7:3188–92. [Google Scholar]
- 11.Kim DO, Jeong SW, Lee CY. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003;81:321–6. [Google Scholar]
- 12.Von Gadow A, Joubert E, Hansmann CF. Comparison of antioxidant activity of aspalathin with that of other plant phenols of Rooibosed tea (Aspalathon linearis), a tocopherol, BHT, and BHA. J Agric Food Chem. 1997;45:632–8. [Google Scholar]
- 13.Yen GC, Duh PD. Scavenging effect of methanolic extracts of peanut hulls on free-radical and active oxygen species. J Agric Food Chem. 1994;42:629–32. [Google Scholar]
- 14.Dinis TC, Madeira VM, Almeida LM. Action of phenolic derivatives (acetoaminophen, salycilate and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315:161–9. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 15.Kirby MD, Bauer RW, Sherris JC, Turck M. Antibiotic susceptibility testing by standard single disc diffusion method. Am J Clin Pathol. 1996;45:493–6. [PubMed] [Google Scholar]
- 16.Prabhakar VK, Jaidka A, Singh R. In vitro study on alpha amylase inhibitory activity and phytochemical screening of few Indian medicinal plant having antidiabetic properties. Int J Sci Res Publ. 2013;3:2250–3153. [Google Scholar]


