Abstract
Objective:
In this study four tea samples Gumero black, Wushwush black and Wushwush green from Agri- Ceft Plc and East Africa black tea leaves from East African Agribusiness Plc were investigated for total polyphenols, caffeine, catechin and L-theanine content.
Materials and Methods:
The aqueous extracts were investigated for their antioxidant and antileishmanial property and effect on amphotericin B, miltefocine and sodium stibogluconate, the commonly used antileishmanial drugs. Antileishmanial studies were conducted on L. aethiopica.
Results:
Wushwush green tea had the highest content of polyphenol (19.98 ± 1.15 mg gallic acid equivalent /100 g dry leaf weight), catechin (37.06 mg/g) and L-theanine (48.54 mg/g but the lowest caffeine content). It exhibited the highest antioxidant activity. The highest antioxidant effect of Wushwush green tea may be attributed to the highest polyphenol content. East African black tea had the lowest L-theanine (20.72 mg/g) and antioxidant activity but the highest caffeine (16.60 mg/g) content.
Conclusion:
Wushwush green tea showed slight inhibitory effect on L. aethiopica while the lack tea extracts (Gumero, East Africa and Wushwush) exhibited no antileishmanial activity. Wushwush green tea did not show any synergistic or antagonistic effect on the antileishmanial drugs used in this study while Gumero, East Africa and Wushwush black tea extracts exhibited dose dependant inhibitory activity to the commonly used antileishmanial drugs included in this study.
Keywords: Caffeine, Camellia sinensis, Catechin, L-theanine, Polyphenols
INTRODUCTION
Tea is one of the most commonly consumed beverages throughout the world. Beside the attractive aroma and specific taste, its potential health-promoting properties are attracting more interest and popularity in tea products. Tea infusions, consumed by two-thirds of the world's population are obtained from the leaves of the plant Camellia sinensis.[1] For the most part of the world, tea is simply considered a tasteful drink, but the scientific community has recently re-discovered the therapeutic potential of this beverage.[2,3]
Based on the processing techniques, tea leaves are classified into green tea, black tea, oolong tea and white tea.
Green tea is a nonoxidized tea produced by drying and steaming the fresh leaves to inactivate the enzyme polyphenol oxidase, which oxidize tea polyphenols and cause the formation of the brown or black color.
Black tea, which is the most commonly consumed tea in the world is the one processed in the largest amount among the four types. Black teas usually undergo full oxidation leading to the development of the characteristic dark brown and black colors of the leaves. Oolong tea is partially oxidized tea before drying to preserve the natural flavors.[4,5] White tea is produced by picking the tea leaves and buds just before they open fully, when the buds are still covered in fine, white hairs. That, of course, is why it is called “white” tea.[6] The leaves and buds are then steamed thoroughly to inactivate the enzymes responsible for tea discoloration, and the product is subsequently dried either in the dryer or in the sun.[7,8]
Chemical composition of tea varies with climate, season, variety, horticultural practices and the age of the leaf[9] which suggests that various levels of the bioactive compounds could be expected from tea that is grown in different parts of the world. The most important compounds present in tea, which are of considerable pharmacological significance, are the polyphenols and caffeine.
Various reports indicate that tea extracts have biological and pharmacological activities such as antioxidant,[10] antiviral,[11] antibacterial,[12] anticarcinogenic,[13,14] antimutagenic,[15] anti-inflammatory,[16] anti-aging,[17] anti-diabetic,[18,19] body weight control,[20,21] reducing stress and anxiety, improving learning and concentration[22,23] and anti-HIV effect.[24,25]
Leishmaniasis is a vector born disease caused by protozoan parasites of the genus Leishmania.(L) Motile form of the parasite (promastigote) is transmitted to human by the bite of infected hematophagous female sandflies (subfamily Phlebotominae). Human leishmaniasis is found in South America, North America, Asia, Europe and Africa and is endemic in the tropical and sub-tropical regions. Visceral leishmaniasis is caused by two L species; L. donovani or L. infantum. L. infantum infects mostly children and immuno-suppressed individuals while L. donovani infects people of all age groups. Cutaneous leishmaniasis (CL) is caused by L. major, L. tropica or L. aethiopica.
In Ethiopia, CL manifests in three forms: Localized, diffused and mucosal. The diffuse and mucosal forms are often continuation of the self-healing CL caused by L. aethiopica.[26]
Compounds available in tea may contribute negatively or positively to the activity of medicinal agents.
Animal studies showed that addition of tea or coffee (50 mg/mL) to equal volumes of solutions of chlorpromazine, fluphenazine hydrochloride and thioridazine hydrochloride (4 mg/mL) at 37°C produced heavy precipitates in all cases.[27] Black tea extracts are observed to cause precipitation of amitriptyline, fluphenazine, haloperidol and imipramine in vitro.[28,29] Xanthine derivatives (e.g., caffeine, theophylline) from tea are also reported to diminish effects of coronary vasodilator drugs like dipyridamole. Thus, it is recommended that dipyridamole should not be taken concurrently with xanthine derivatives.[30]
Tea is indicated to cause a possible synergistic effect when taken with sulindac and/or tamoxifen and may reduce their adverse effects.[31] Synergistic activity between various teas and antibiotics such as the carbapenem, β-lactam, ciprofloxacin, gentamicin, methicillin and nalidixic acid has been also reported.[32]
Methanolic extract of green tea in vitro at doses of 150, 300, 450, 600 and 750 µg/mL inhibited the multiplication of L. parasite.[33]
Based on the fact that tea may have synergetic or inhibitory effect on activity of drugs, the present study was conducted to investigate the in vitro effect of aqueous tea extract on currently used antileishmanial drugs, to determine the total polyphenol content, antioxidant activity and to quantify caffeine, catechin and L-theanine content.
RESULTS
Total polyphenol content
The gallic acid calibration plot was obtained by plotting the absorbance against concentration of gallic acid (mg/mL). Table 1 summarizes the preparation of the reaction mixture for phenol content determination.
Table 1.
UV absorbance of standard gallic acid at different concentrations
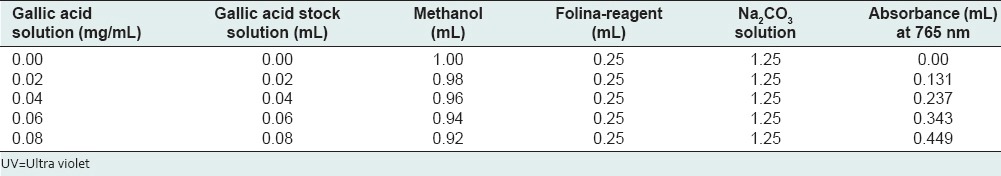
The concentration of total phenol compounds in tea leaf extracts was determined as milligrams of gallic acid equivalent (GAE) using an equation obtained from a standard gallic acid graph whose concentration ranging from 0.02 to 0.08 mg/mL.
As shown in Table 2, the total polyphenol content in Wushwush green tea (WGT) was found to be 19.98 ± 1.15 mg/100 g dry leaf weight and was the highest among the tested samples. The total polyphenol content in Wushwush black tea (WBT), Gumero black tea (GBT) and East Africa black tea (EABT) leaves were found to be 16.24 ± 0.9, 17.90 ± 0.93 and 16.17 ± 0.33 mg/100 g dry leaf weight, respectively.
Table 2.
Total polyphenol contents for tea extracts
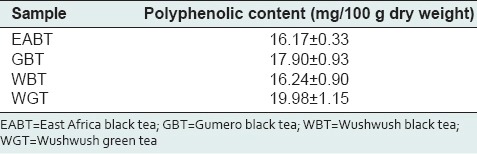
No significant difference in polyphenol content was found between WBT and EABT (16.24 ± 0.9 mg/100 g vs. 16.17 ± 0.33 mg/100 g, P = 0.8623), EABT and GBT (16.17 ± 0.33 mg/100 g vs. 17.90 ± 0.93 mg/100 g, P = 0.098) and WBT and GBT (16.24 ± 0.9 mg/100 g vs. 17.90 ± 0.93 mg/100 g, P = 0.6352). A significant difference in polyphenol content was observed between WBT and WGT (16.24 ± 0.9 mg/100 g vs. 19.98 ± 1.15 mg/100 g), EABT and WGT (16.17 ± 0.33 mg/100 g vs. 19.98 ± 1.15 mg/100 g) and GBT and WGT (17.90 ± 0.93 mg/100 g vs. 19.98 ± 1.15 mg/100 g) as it is determined from one-way ANOVA and Student- Newman–Keuls’ tests P < 0.05.
The polyphenol content of GBT (17.90 ± 0.93 mg/100 g) was higher than that obtained for WBT and EABT.
The gallic acid calibration plot was obtained by plotting the absorbance against concentration of gallic acid (mg/mL). Table 1 summarizes the preparation of the reaction mixture for phenol content determination. The concentration of total phenol compounds in tea leaf extracts was determined as milligrams of gallic acid equivalent (GAE) using an equation obtained from a standard gallic acid graph whose concentration ranging from 0.02 to 0.08 mg/mL.
2,2-diphenylpicrylhydrazyl scavenging activities
The results for percent 2,2-diphenylpicrylhydrazyl (DPPH) scavenging activities of the four tea extracts are summarized in Table 3.
Table 3.
DPPH radical scavenging activities of tea extract at different concentrations
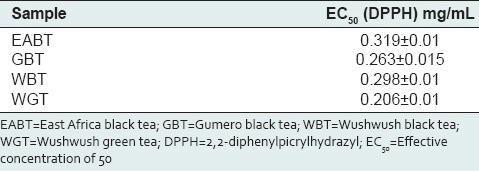
The scavenging activity of the tea extracts decreased in the order of WGT > GBT > WBT > EABT [Table 3].
From the results of black teas, GBT had greater DPPH scavenging activity than WBT and EABT.
The radical scavenging activity of all of the four tea extracts as shown in Table 3 was observed to be concentration dependent.
Wushwush green tea needed the lowest concentration (0.206 ± 0.01 mg/mL) to give 50% of DPPH inhibition in comparison with GBT (0.263 ± 0.015 mg/mL), WBT (0.298 ± 0.01 mg/mL) and EABT (0.319 ± 0.01 mg/mL) tea extracts.
Caffeine, Catechin and L-theanine contents of tea extract
Catechin contents in WBT (17.90 mg/g dry extract), GBT (21.70 mg/g dry extract) and EABT (19.80 mg/g dry extract) were in agreement with the reported results, but WBT (37.06 mg/g dry extract) has lower catechin content.[34,35]
Caffeine content of the tea extracts in this study varied from 7.96 to 16.6 mg/g dry extract; EABT had the highest caffeine content compared to the other samples investigated [Table 5].
Table 5.
Levels of caffeine, catechin and L-theanine in hot water tea extracts (mg/g of dried extract)
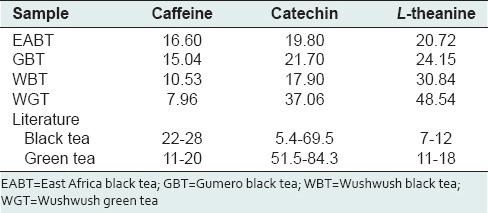
Wushwush green tea (48.54) had the highest L-theanine content, followed by WBT (30.84) and GBT (24.15) while EABT (20.72) had the lowest [Table 5] and these values were higher than previously reported values for green (11–18 mg/g) and black teas (7–12 mg/g).[36,37]
Effect of tea extracts on Leishmania parasite (Leishmania aethiopica)
Wushwush green tea had shown some inhibitory effect on the L. parasite unlike WBT, GBT and EABT as its concentration increased [Figure 1]. There was no any synergistic effect between WGT and the standard drugs: Amphotericin B (AmB), miltefosine and sodium stibogluconate (SSG).
Figure 1.
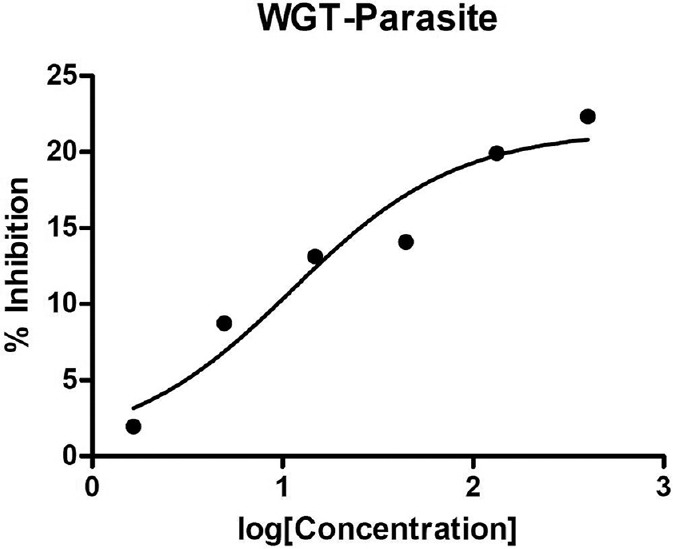
Effect of Wushwush green tea extract on Leishmania parasite
There is a direct relation between the concentration of WGT extract and it's percent inhibition. The inhibitory activity of WGT may be attributed to the presence of major catechins such as epicatechin (EC), epigallocatechin (EGC), epigallocatechin gallate (EGCG) and epicatechin gallate (ECG), which are very important in determining health potential and the chemical properties of tea. There are a significant differences between WGT and the standard drugs as it was determined from one-way ANOVA and Student-Newman–Keuls’ tests P < 0.05.
Effect of tea extracts on antileishmanial drugs activity
To investigate the effects of the tea extracts on most commonly used antileishmanial drugs all of the four tea samples (EABT, GBT, WBT and WGT) were studied. As the concentrations of WGT extract added to a constant concentration of standard drugs the antileishmanial effect of all the standard drugs used was not significantly affected (P > 0.05).
Increasing the concentration of WBT, GBT and EABT extracts decreases the activity of AmB, miltefosine and SSG showing that WBT, GBT and EABT extracts have an inhibitory effect on these available standard drugs used in this study [Figures 2–4], respectively.
Figure 2.
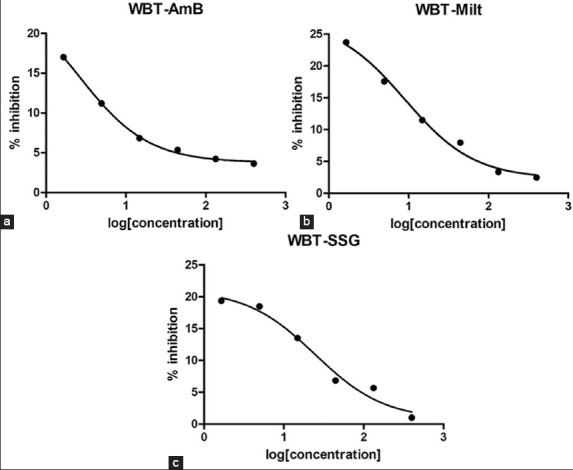
(a-c) Effect of Wushwush black tea (WBT) extract on antileishmanial activity of standard drugs. Effect of WBT extract on antileishmanial activity of standard drugs. Effect of WBT extract on antileishmanial activity of standard drugs. Effect of WBT extract on antileishmanial activity of standard drugs
Figure 4.
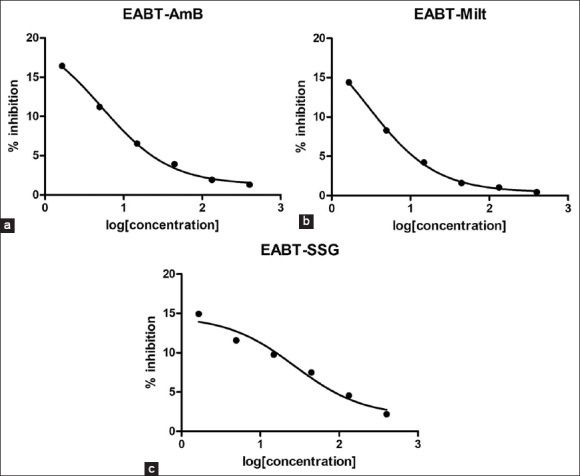
(a-c) Effect of East Africa black tea (EABT) extract on antileishmanial activity of standard drugs. EABT extract on antileishmanial activity of standard drugs. EABT extract on antileishmanial activity of standard drugs
Figure 3.
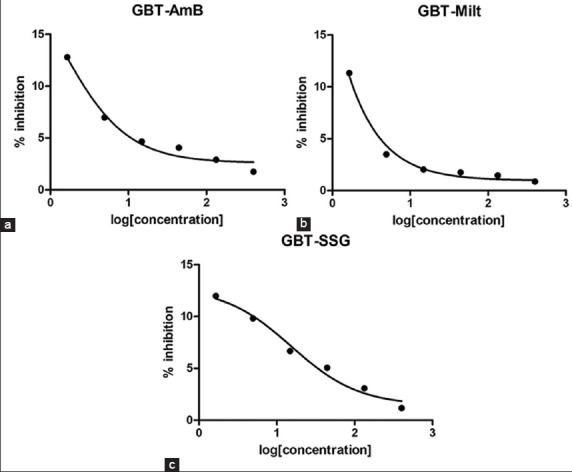
(a-c) Effect of Gumero black tea (GBT) extract on antileishmanial activity of standard drugs. Effect of GBT extract on antileishmanial activity of standard drugs. Effect of GBT extract on antileishmanial activity of standard drugs
DISCUSSION
Total polyphenol content of four tea samples: One green and three black teas cultivated and processed in Ethiopia was analyzed following the method developed by Folin–Ciocalteu.[38] The high polyphenol content of WGT extracts could be due to the fact that such teas do not undergo oxidation process that will decrease the total polyphenol content. These differences in polyphenol content found between the extracts could be due to genetic makeup, rainfall, nutrient availability and a postmaturation process where black tea continues to oxidize.[39] The polyphenol levels observed in this work for WGT, WBT, GBT and EABT were relatively higher than those reported for different brands commercialized in Malaysia (19.13 ± 0.37 and 11.37 ± 1.48 for green tea and 8.49 ± 0.80 and 6.06 ± 0.54 for black tea extract)[40] but lower than those reported for samples in Argentina (% GAE values of 21.02 ± 1.54 and 17.62 ± 0.42 for green and black tea respectively).[41]
This slight difference from our results might be due to the difference in plant variety and also due to different environmental conditions. The violet color of DPPH was reduced to a pale yellow color due to the abstraction of the hydrogen atom from antioxidant compounds found in tea extracts, and the absorbance was measured spectrophotometrically at 517 nm.
The higher the antioxidant activities of the sample, the more the DPPH reduction will occur.[42] The antioxidant activity trend observed in this study between green and black teas was in agreement with data reported for some other studies.[43]
The degree of oxidation of polyphenols in tea would greatly contribute to the difference in percentage inhibition and antioxidant scavenging activity between green and black tea extracts.
The gallocatechins, that is, (+)-EGC and (+)-EGCG, which are potent antioxidants[44,45] are thefirst to be oxidized by polyphenol oxidases in the leaves because of their high oxidation potential in green tea. They are oxidized to form thearubigens and theaflavins, the major phenolic compounds of black tea,[46] which are less effective antioxidants compared with the flavanols.[45] This change in phenol composition explains the lower inhibition of the DPPH radical by black tea. Effective concentration of 50 (EC50) value was determined from the plotted graph of scavenging activity against the concentration of tea extracts.
The EC50 values for the aqueous tea extracts are given in Table 4. The lowest EC50 indicates the strongest ability of the extracts to act as DPPH scavengers.
Table 4.
EC50 values of the tea extracts for antioxidant activity

The results [Table 5] show that the level of catechin was lower in all the three black teas compared to the green tea included in this study.
This lower content of catechin in black teas can be attributed to the oxidation process that generates the EC polymers such as theaflavins and thearubigins and their gallate derivatives. GBT has the highest catechin content among the black teas, followed by EABT. WBT has the lowest catechin content.
Catechin content of black tea from a variety of origins was reported to range from 5.6 to 47.5 mg/g and that of green teas varied from 51.5 to 84.3 mg/g dry extract.[34,35] The contents of caffeine in this study were observed to be lower than what is reported for black (22–28 mg/g) and green (11–20 mg/g) teas.[34]
Unlike the caffeine and catechin contents, the studied tea samples had higher L-theanine contents compared to previous reports. The three black tea extracts WBT, GBT and EABT at different concentrations had shown no effect on the L. parasite when they were added to the parasite containing 96-well cell culture plates without standard drugs as it was observed fluorometrically. This may be due to the oxidation of catechins to form thearubigens and theaflavins, the major phenolic compounds of black teas, which are less effective in health benefits than EC, EGC, EGCG and ECG.
Wushwush green tea has no effect on the antileishmanial activity of standard drugs: AmB, miltefosine and SSG. However, the black teas have an inhibitory effect on the standard drugs.
Amphotericin B inhibits the L. parasite by forming complexes with 24-substituted sterols such as ergosterol in the cell membrane, thus causing pores, which alter ion balance and result in cell death.[47] Components of WBT, GBT, and EABT extracts may block this activity of amphotericin to show the inhibitory effect.
The mechanism of action of miltefosine is not fully understood. However, experimental results suggest the drug to have effects on mammalian protein kinase C, phosphatidylcholine biosynthesis, lipid metabolism, cell signaling and calcium.[47] WBT, GBT, and EABT extracts may block any one or more of these activities of the drug or may cause the precipitation of the drug to have an inhibitory effect.
Sodium stibogluconate inhibits trypanothione reductase in vitro, inducing the loss of intracellular thiols and a lethal imbalance in thiol homeostasis, leading to accumulation of reactive oxygen species.[47] Components of WBT, GBT and EABT extracts may block one or more of these activities or may cause the precipitation of the drug to have an inhibitory effect.
CONCLUSION
In this paper four tea samples were studied to determine total polyphenols, caffeine, catechin, L-theanine content, antioxidant effect and their effect on commonly used antileishmanial drugs. WGT showed the highest antioxidant activity, catechin, theanine, polyphenol content and antileishmanial effect but no activity on the antileishmanial drugs tested. L-theanine and polyphenols that were found in higher quantity in WGT are very important compounds for health promotion. For example, L-theanine is important compound in improving learning and concentration, heightening mental acuity, supporting the immune system, lowering cholesterol, reducing stress and anxiety and reducing the negative side effects of caffeine; polyphenols are effective in antioxidant, anticancer, anti-inflammatory activities and many other health related properties. Therefore, we will recommend that drinking green tea is more safe and important to patients than drinking black tea.
Therefore, future research needs to define the actual magnitude of health benefits, establish the safe range of WGT consumption associated with these benefits and elucidate potential mechanisms of action.
Wushwush, Gumero and EABT extracts decrease the activity of the standard antileishmanial drugs: AmB, miltefocine (Milt) and SSG, but they had no effect on the L. parasite. As the concentration of black tea added to respective constant concentration of drugs, the antileishmanial effect of all standard drugs used was decreased. Therefore, patients taking AmB, miltefosine and SSG for the treatment of leishmaniasis have to avoid taking or drinking Wushwush, Gumero and EABTs. Because all black tea samples used in this study were shown to have an inhibitory effect on the drugs activity.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Li YH, Gu W, Ye S. Expression and location of caffeine synthase in tea plants. Russ J Plant Physiol. 2007;54:698–701. [Google Scholar]
- 2.Karakaya S, Nehir El S. Total phenols and antioxidant activities of some herbal teas and in vitro bioavailability of black tea polyphenols. Ege University, Faculty of Engineering, Department of Food Engineering. GOȠ Ziraat Fak Derg. 2006;l23:1–8. [Google Scholar]
- 3.Luczaj W, Skrzydlewska E. Antioxidative properties of black tea. Prev Med. 2005;40:910–8. doi: 10.1016/j.ypmed.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Lin LZ, Chen P, Harnly JM. New phenolic components and chromatographic profiles of green and fermented teas. J Agric Food Chem. 2008;56:8130–40. doi: 10.1021/jf800986s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alcázar A, Ballesteros O, Jurado JM, Pablos F, Martín MJ, Vilches JL, et al. Differentiation of green, white, black, Oolong, and Pu-erh teas according to their free amino acids content. J Agric Food Chem. 2007;55:5960–5. doi: 10.1021/jf070601a. [DOI] [PubMed] [Google Scholar]
- 6.Teixeira LG, Lages PC, Jascolca TL, Aguilar EC, Soares FLP, Pereira SS, et al. White tea (Camellia sinensis) extract reduces oxidative stress and triacylglycerols in obese mice. Department of Biochemistry and Immunology, Institute of Biological Science. Univ Fed Minas Gerais. 2012;32:733–41. [Google Scholar]
- 7.Dufresne CJ, Farnworth ER. A review of latest research findings on the health promotion properties of tea. J Nutr Biochem. 2001;12:404–21. doi: 10.1016/s0955-2863(01)00155-3. [DOI] [PubMed] [Google Scholar]
- 8.Santana-Rios G, Orner GA, Amantana A, Provost C, Wu SY, Dashwood RH. Potent antimutagenic activity of white tea in comparison with green tea in the Salmonella assay. Mutat Res. 2001;495:61–74. doi: 10.1016/s1383-5718(01)00200-5. [DOI] [PubMed] [Google Scholar]
- 9.Owuor PO, Obanda M, Nyirenda HE, Mandala WL. Influence of region of production on clonal black tea chemical characteristics. Food Chem. 2008;108:263–71. [Google Scholar]
- 10.Erba D, Riso P, Bordoni A, Foti P, Biagi PL, Testolin G. Effectiveness of moderate green tea consumption on antioxidative status and plasma lipid profile in humans. J Nutr Biochem. 2005;16:144–9. doi: 10.1016/j.jnutbio.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Xiao X, Yang ZQ, Shi LQ, Liu J, Chen W. Antiviral effect of epigallocatechin gallate (EGCG) on influenza A virus. Zhongguo Zhong Yao Za Zhi. 2008;33:2678–82. [PubMed] [Google Scholar]
- 12.Takabayashi F, Harada N, Yamada M, Murohisa B, Oguni I. Inhibitory effect of green tea catechins in combination with sucralfate on Helicobacter pylori infection in Mongolian gerbils. J Gastroenterol. 2004;39:61–3. doi: 10.1007/s00535-003-1246-0. [DOI] [PubMed] [Google Scholar]
- 13.Johnson JJ, Bailey HH, Mukhtar H. Green tea polyphenols for prostate cancer chemoprevention: 0 A translational perspective. Phytomedicine. 2010;17:3–13. doi: 10.1016/j.phymed.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laurie SA, Miller VA, Grant SC, Kris MG, Ng KK. Phase I study of green tea extract in patients with advanced lung cancer. Cancer Chemother Pharmacol. 2005;55:33–8. doi: 10.1007/s00280-004-0859-1. [DOI] [PubMed] [Google Scholar]
- 15.Cheng KW, Wong CC, Chao J, Lo C, Chen F, Chu IK, et al. Inhibition of mutagenic PhIP formation by epigallocatechin gallate via scavenging of phenylacetaldehyde. Mol Nutr Food Res. 2009;53:716–25. doi: 10.1002/mnfr.200800206. [DOI] [PubMed] [Google Scholar]
- 16.Koo MW, Cho CH. Pharmacological effects of green tea on the gastrointestinal system. Eur J Pharmacol. 2004;500:177–85. doi: 10.1016/j.ejphar.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 17.Esposito E, Rotilio D, Di Matteo V, Di Giulio C, Cacchio M, Algeri S. A review of specific dietary antioxidants and the effects on biochemical mechanisms related to neurodegenerative processes. Neurobiol Aging. 2002;23:719–35. doi: 10.1016/s0197-4580(02)00078-7. [DOI] [PubMed] [Google Scholar]
- 18.Iso H, Date C, Wakai K, Fukui M, Tamakoshi A JACC Study Group. The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann Intern Med. 2006;144:554–62. doi: 10.7326/0003-4819-144-8-200604180-00005. [DOI] [PubMed] [Google Scholar]
- 19.Zhang ZF, Li Q, Liang J, Dai XQ, Ding Y, Wang JB, et al. Epigallocatechin-3-O-gallate (EGCG) protects the insulin sensitivity in rat L6 muscle cells exposed to dexamethasone condition. Phytomedicine. 2010;17:14–8. doi: 10.1016/j.phymed.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Zheng G, Sayama K, Okubo T, Juneja LR, Oguni I. Anti-obesity effects of three major components of green tea, catechins, caffeine and theanine, in mice. In vivo. 2004;18:55–62. [PubMed] [Google Scholar]
- 21.Moon HS, Lee HG, Choi YJ, Kim TG, Cho CS. Proposed mechanisms of (-)-epigallocatechin-3-gallate for anti-obesity. Chem Biol Interact. 2007;167:85–98. doi: 10.1016/j.cbi.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Ito K, Nagato Y, Aoi N. Effects of L-theanine on the release of alpha-brain waves in human volunteers. Nippon Nogeikagaku Kaishi. 1998;72:153–7. [Google Scholar]
- 23.Juneja LR, Chu DC, Okubo T. L-theanine a unique amino acid of green tea and its relaxation effect in humans. Trends Food Sci Technol. 1999;10:199–204. [Google Scholar]
- 24.Williamson MP, McCormick TG, Nance CL, Shearer WT. Epigallocatechin gallate, the main polyphenol in green tea, binds to the T-cell receptor, CD4: Potential for HIV-1 therapy. J Allergy Clin Immunol. 2006;118:1369–74. doi: 10.1016/j.jaci.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 25.Hamza A, Zhan CG. How can (-)-epigallocatechin gallate from green tea prevent HIV-1 infection? Mechanistic insights from computational modeling and the implication for rational design of anti-HIV-1 entry inhibitors. J Phys Chem B. 2006;110:2910–7. doi: 10.1021/jp0550762. [DOI] [PubMed] [Google Scholar]
- 26.Hailu A, Gebre-Michael T, Berhe N, Meshesha B. 1st ed. Addis Ababa, Ethiopia: SA Books; 2006. The Epidemiology and Ecology of Health and Diseases in Ethiopia; pp. 615–34. [Google Scholar]
- 27.Cheeseman HJ, Neal MJ. Interaction of chlorpromazine with tea and coffee. Br J Clin Pharmacol. 1981;12:165–9. doi: 10.1111/j.1365-2125.1981.tb01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lasswell WL, Jr, Weber SS, Wilkins JM. In vitro interaction of neuroleptics and tricylic antidepressants with coffee, tea, and gallotannic acid. J Pharm Sci. 1984;73:1056–8. doi: 10.1002/jps.2600730809. [DOI] [PubMed] [Google Scholar]
- 29.Kulhanek F, Linde OK. Coffee and tea influence pharmacokinetics of antipsychotic drugs. Lancet. 1981;2:359–60. doi: 10.1016/s0140-6736(81)90666-8. [DOI] [PubMed] [Google Scholar]
- 30.Morant J, Ruppanner H. Basel, Switzerland: Documed AG; 2001. Arzneimittel-Kompendium der Schweiz® 2001. [Google Scholar]
- 31.Suganuma M, Okabe S, Kai Y, Sueoka N, Sueoka E, Fujiki H. Synergistic effects of (--)-epigallocatechin gallate with (--)-epicatechin, sulindac, or tamoxifen on cancer-preventive activity in the human lung cancer cell line PC-9. Cancer Res. 1999;59:44–7. [PubMed] [Google Scholar]
- 32.Friedman M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol Nutr Food Res. 2007;51:116–34. doi: 10.1002/mnfr.200600173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehdi S, Hajar Z, Mohammad A, Ahmad D, Akbar E, Masoumeh R. Effect of methanolic extracts of Artemisia aucheri and Camellia sinensis on Leishmania major (In vitro) Turk J Med Sci. 2006;36:365–9. [Google Scholar]
- 34.Khokhar S, Magnusdottir SG. Total phenol, catechin, and caffeine contents of teas commonly consumed in the United Kingdom. J Agric Food Chem. 2002;50:565–70. doi: 10.1021/jf010153l. [DOI] [PubMed] [Google Scholar]
- 35.Friedman M, Carol EL, Suk HC, Seung U, Nobuyuki K. HPLC analysis of catechins, theaflavins and alkaloids in commercial teas and green tea dietary supplements: Comparison of water and 80% ethanol/water extracts. J Food Sci. 2006;71:330–4. [Google Scholar]
- 36.Helena C, Michal V, Jana M, Frantisek K. Authenticity evaluation of tea based products. Czech J Food Sci. 2008;26:259–61. [Google Scholar]
- 37.Friedman M, Levin CE, Choi SH, Lee SU, Kozukue N. Changes in the composition of raw tea leaves from the Korean Yabukida plant during high-temperature processing to pan-fried Kamairi-cha green tea. J Food Sci. 2009;74:C406–12. doi: 10.1111/j.1750-3841.2009.01185.x. [DOI] [PubMed] [Google Scholar]
- 38.Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method Enzymol. 1999;299:152–78. [Google Scholar]
- 39.Cloughley JB. Storage deterioration in central African tea: The effect of some production variables on theaflavin degradation. J Sci Food Agric. 1981;32:1229–34. [Google Scholar]
- 40.Chan EW, Lim YY, Chew YL. Antioxidant activity of Camellia sinensis leaves and tea from a lowland plantation in Malaysia. Food Chem. 2007;102:1214–22. [Google Scholar]
- 41.Anesini C, Ferraro GE, Filip R. Total polyphenol content and antioxidant capacity of commercially available tea (Camellia sinensis) in Argentina. J Agric Food Chem. 2008;56:9225–9. doi: 10.1021/jf8022782. [DOI] [PubMed] [Google Scholar]
- 42.Blois MS. Antioxidants determination by the use of a stable free radical. Nature. 1958;181:1199–200. [Google Scholar]
- 43.Von Gadow A, Joubert E, Hansmann CF. Comparison of the antioxidant activity of rooibos tea (Aspalathus linearis) with green, oolong, and black tea. Food Chem. 1997;60:73–7. [Google Scholar]
- 44.Lunder TL, Huang MT, Ho CT, Lee CY. Washington: American Chemical Society; 1992. Catechins of green tea: I. Antioxidant activity in phenolic compounds in food and their effects on health. II. Antioxidants and cancer prevention; pp. 114–20. [Google Scholar]
- 45.Xie B, Shi H, Chen Q, Ho CT. Antioxidant properties of fractions and polyphenol constituents from green, oolong and black teas. Proc Natl Sci Counc Repub China B. 1993;17:77–84. [PubMed] [Google Scholar]
- 46.Robertson A. The chemistry and biochemistry of black tea production-the non volatiles. In: Willson KC, Clifford MN, editors. Tea Cultivation to Consumption. London: Champan and Hall; 1992. pp. 555–602. [Google Scholar]
- 47.Yinebeb T. Addis Ababa, Ethiopia: Master Thesis Addis Ababa University; 2008. In vitro efficacy study of some selected medicinal plants against Leishmania specious. [Google Scholar]


