Abstract
The purpose of this retrospective case series was to assess the outcome of an autologous vaccination procedure on single and multiple sarcoid lesions, determine complication rate, and report owner satisfaction. Medical records (18 cases) from January 2009 through May 2014 were evaluated to identify horses undergoing the procedure. Signalment, number, size, anatomic location of lesions, and type of historical treatment were recorded. Follow-up was obtained via standardized owner survey, veterinary examination, and digital images. Data recorded and analyzed included ancillary therapies post-procedure, decrease in number and/or size of sarcoid lesions, sarcoid regrowth, complications, and owner satisfaction. There was a decrease in number of lesions observed by owners in 75% of cases and a decrease in size of sarcoids in 93.8% of cases. Clinical regression observed by owners was noted in 68.8% of cases. There were complications in 43.8% of cases and owner satisfaction in 75% of cases.
Résumé
Vaccination autologue pour le traitement des sarcoïdes équins : 18 cas (2009–2014). Le but de cette série de cas rétrospectifs était d’évaluer le résultat d’une procédure de vaccination autologue sur des lésions de sarcoïdes simples et multiples, de déterminer le taux de complication et de signaler la satisfaction des propriétaires. Les dossiers médicaux (18 cas) datant de janvier 2009 à mai 2014 ont été évalués afin d’identifier les chevaux subissant l’intervention. Le signalement, le nombre, la taille, l’emplacement anatomique des lésions et le type de traitement historique ont été consignés. Le suivi a été obtenu par un sondage standard auprès des propriétaires, un examen vétérinaire et des images numériques. Les données consignées et analysées incluaient des traitements auxiliaires après l’intervention, une baisse du nombre et/ou de la taille des lésions des sarcoïdes, la repousse des sarcoïdes, les complications et la satisfaction des propriétaires. Il s’est produit une baisse du nombre des lésions observées par les propriétaires et de la taille des sarcoïdes dans 93,8 % des cas. La régression clinique observée par les propriétaires a été consignée dans 68,8 % des cas. Il y a eu des complications dans 43,8 % des cas et les propriétaires étaient satisfaits dans 75 % des cas.
(Traduit par Isabelle Vallières)
Introduction
Sarcoids are the most common skin tumor in the equid population (1,2). Most tumors are benign, locally invasive, and fibroblastic; however, tumors can become large, ulcerate, and prevent proper use of equipment during athletic activity. Rarely, in one form of the disease, the tumor cells can migrate along chains of lymph nodes (1,2). Sarcoids can be classified based on their clinical appearance and behavior. Whether deemed occult, verrucous, nodular, fibroblastic, mixed or malignant, no significant distinctions have been noted histopathologically between the classifications (3). Sarcoids often require treatment due to their location and discomfort to the animal. Multiple modalities are described in the literature with no one treatment accepted as the gold standard (4).
The pathogenesis of equine sarcoids has not been completely elucidated. Bovine papilloma virus (BPV), however, has been strongly linked with equine sarcoids through the demonstration of viral DNA in 73% to 100% of sarcoid samples analyzed, but has not been detected in other types of equine skin tumors or equine papillomas (5). Typically, papilloma viruses are species-specific and the identification of BPV in equine sarcoids represents an unusual case of inter-species infection (6). In 1 study, BPV gene expression revealed the presence of the E5 protein in 23 sarcoid samples analyzed and its absence from all non-sarcoid samples (7). The E5 protein is a transforming protein with multiple modes of action. It promotes the down regulation of MHC class I molecules, thus allowing host immunosurveillance to overlook infected cells (6,7). This protein is also thought to aid in isolation of the infected cell and disruption of cell protein processing (6).
Transmission of BPV in equids is unclear. No intact virions have been detected within equine sarcoids (6) and equids were traditionally considered dead-end hosts. Recently, BPV DNA was detected in both the dermis and epidermis of equine skin, suggesting that equid to equid transfer may occur (8). Bovine papilloma virus DNA has also been found in face flies removed from horses with sarcoid lesions (9). Stable management practices may also contribute to the spread of the virus through contaminated tack or pastures (5).
There is a genetic predisposition, with Thoroughbreds, Quarter Horses, Arabians, and Appaloosas having an increased risk for sarcoid development (10,11). The presence of specific major histocompatibility (MHC) class II molecules has been noted to increase the likelihood of sarcoid lesions in the Thoroughbred population (10). Greater than 70% of sarcoids develop in horses < 4 y old (1). Sarcoids may be found anywhere along the body, most commonly on the head, ears, and limbs (1,4,11). Sites of previous injury are reportedly predisposed to sarcoid development (1,2,11).
Treatment modalities include surgical removal, cryotherapy, chemotherapy, interstitial brachytherapy, electrochemotherapy, topical antiviral treatment, and topical herbal preparations (1,2,4,12–19). Immunotherapy has also been proposed with the use of Bacillus Calmette-Guerin (BCG) cell wall extract as an intralesional injection to induce an immunological response (16,17). An autologous vaccine that is based on the polymerization of antigenic tumor markers was reported to induce complete remission in 19 of 21 horses after varying lengths of treatment (20). In 2008, Epsy (21) reported on an autologous vaccination procedure performed in the field utilizing sarcoid tissue frozen in liquid nitrogen and subsequently implanted into subcutaneous pockets in the neck. Complete tumor regression was noted in 12 of 15 cases; however, no further results or larger case series have been published (21).
The objective of this retrospective study was to assess the effect of an autologous vaccine procedure on single and multiple sarcoid lesions, determine complication rate, and report owner satisfaction. Favorable results would potentially provide veterinarians an adjunctive modality to treat equine sarcoids, specifically in cases with multiple lesions in anatomically challenging regions of the body where satisfactory surgical margins are not possible.
Materials and methods
Case selection
The medical records of all horses undergoing an autologous vaccination procedure at the George D. Widener Hospital for Large Animals and the William H. Boucher Field Service section at the University of Pennsylvania’s New Bolton Center, from January 2009 to May 2014 were reviewed. Prior to implantation, all horses were required to have a biopsy with histopathology confirming the diagnosis of sarcoid. If multiple lesions were present, only 1 was chosen for biopsy and histopathology.
Medical records review
The gender and breed distribution of all equine patients at the George D. Widener Hospital from January 2009 through May 2014 were obtained. Data for the patients undergoing the autologous vaccination procedure included age, breed, gender, number and size of lesions, anatomic location of lesions, and type of historical treatment. Of the 18 horses undergoing this procedure, follow-up data were obtained for 16 patients.
Follow-up was done by telephone conversation with the owner using a consistent, standardized survey by a single author. Owners were asked to subjectively evaluate the number and size of current lesions. Where applicable, owners were asked to estimate the size of current lesions in centimeters. Complications associated with the procedure and sarcoid regrowth at the time of follow-up were recorded. Owner satisfaction was also recorded as positive, negative, or indifferent. When possible, follow-up measurements were obtained by a veterinarian through physical examination. Digital images of the lesions were obtained at the time of follow-up depicting the pre- and post-procedural appearance when possible.
Autologous vaccination procedure
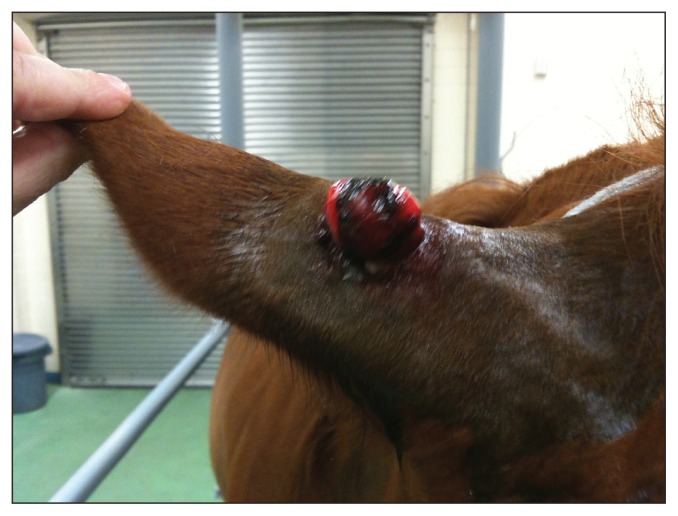
The procedure was performed according to the technique described by Epsy (21). The patient was restrained and sedated using a combination of detomidine hydrochloride (Dormosedan; Pfizer Animal Health, New York, New York, USA), 0.01 mg/kg body weight (BW), IV, and butorphanol tartrate (Torbugesic; Fort Dodge Animal Health, Fort Dodge, Iowa, USA), 0.01 mg/kg BW, IV. However in 2 cases, the patient was placed under general anesthesia to facilitate sarcoid debulking in a difficult anatomic location (medial thigh and ventral abdomen). The sarcoid to be removed and surrounding skin were prepared by cleaning with 4% chlorhexidine gluconate (Betasept; Purdue Pharma, Stamford, Connecticut, USA) and rinsing with clean water. Using sharp dissection, the lesion was then debulked to approximately skin level, leaving the base in situ (Figure 1). If mild to moderate hemorrhage ensued, sterile 4 × 4 gauze squares were used for compression at the site. The removed sarcoid was sectioned into multiple small tissue cubes, measuring about 0.5 × 0.5 × 0.5 cm. Any superficial areas of gross necrotic tissue were removed from the cubes. The pieces were placed in sterile gauze and submerged in liquid nitrogen for 7 to 10 min. An area just ventral to the nuchal ligament, 10 cm long and 5 cm wide, was clipped and aseptically prepared. Approximately 2 to 3 mL of 2% mepivicaine hydrochloride (Carbocaine-V; Pfizer Animal Health) was infused subcutaneously in 2 to 3 locations. Stab incisions were created through the skin and subcutis using a number 10 scalpel blade. Hemostat forceps were used to aid in the creation of a pocket large enough to hold 1 to 3 cubes of sarcoid tissue. These were then placed subcutaneously in each stab incision. The skin was closed over the implanted sarcoid with 1 simple interrupted suture with a non-absorbable suture material. Phenylbutazone 20% (Phenylbutazone 20% Injection; Vet One, MWI Veterinary Supply, Boise, Idaho, USA), 2.2 mg/kg BW, was administered intravenously or orally after surgery in all cases for 1 to 2 d. No antimicrobials were used.
Figure 1.
Pre-implantation lesion located at the base of the ear. This was debulked and the autologus procedure was performed.
Signalment, number and size of lesions, anatomic location of lesions, type of historical treatment, and follow-up data were evaluated descriptively. Continuous variables were examined for normality using the Shapiro-Wilk test. As most parameters were not normally distributed, data are presented as medians and interquartile ranges (IQR, 25th and 75th percentiles). Percentages were used to describe discrete data. The presence of multiple initial sarcoids, the initial tumor area (cm2), the type of historical treatment, and the use of topical historical treatments were analysed by logistic regression, in order to identify association with tumor regrowth or a decrease in tumor size. All analyses were performed using STATA ICV 12.1 (StataCorp, College Station, Texas, USA). Values of P ≤ 0.05 were considered significant.
Results
Medical records review
The autologous vaccination procedure was performed on 15 geldings and 3 mares. The median age was 11 y (n = 15, IQR: 8 to 14). A variety of breeds were represented including Warmbloods [n = 8 (44.4%)], Quarter Horses [n = 4 (22.2%)], Thoroughbreds [n = 3 (16.7%)], Arabians [n = 2 (11.1%)], and mixed breed [n =1 (5.6%)]. At this institution, during the same time frame, 41.0% of patients were mares, 41.0% were geldings, and 18% were stallions. Warmbloods represented 20.6% of the population, Quarter Horses 6.8%, Thoroughbreds 34.2%, and Arabians 3.2%. Two horses were vaccinated in the field and 16 were vaccinated in hospital.
Median number of lesions at presentation was 1.5 (n = 18, IQR: 1 to 3). Nine of the 18 horses had a solitary lesion and 9 had 2 or more sarcoids. In 16 of 18 (88.9%) cases, 3 or fewer lesions were recorded. Median area of the presenting lesions was 13.75 cm2 (n = 17, IQR: 6.25 to 16).
Sarcoids were located on the head in 7 of 18 horses (38.9%), the limbs in 5 of 18 (27.8%) and the body and trunk in 4 of 18 (22.2%). Two horses (11.1%) had lesions in multiple anatomic regions. Ten of 17 horses (58.8%) were treated historically prior to the autologous implantation procedure including topical bloodroot extract [Xxterra; Larson Laboratories, Fort Collins, Colorado, USA, n = 3 (30%)], topical Imiquimod [n = 1 (10%)], surgical debulking with intralesional cisplatin [n = 1 (10%)], and multiple modalities [n = 5 (50%)].
Long-term follow-up
The median time to follow-up from the autologous implantation was 10.5 mo (n = 15, IQR: 3 to 17.5). As reported by the owners, 12 of 16 (75%) horses showed an overall decrease in the number of sarcoids with 15 of 16 (93.8%) having a decrease in size of sarcoids present. Complete regression was noted in 11 of 16 (68.8%) cases (Figure 2). Follow-up physical examination confirming owner findings was performed by a veterinarian in 5 of 18 cases. Digital images were provided to the author by owners for an additional 3 cases. Therefore, 8 of 16 (50%) cases available for follow-up had results confirmed secondarily to owner reporting.
Figure 2.
Six month follow-up from the owner demonstrating complete regression with no ancillary therapy.
The cases available for follow-up were divided into groups based upon whether other post-procedural treatments were instituted or not. In 11 of 16 cases (68.8%) there was no other post-operative sarcoid treatment (Table 1). Veterinary examination or digital images provided confirmation of owner-reported results in 5 of 11 cases belonging to this cohort. Of these horses, 6 of 11 (54.5%) had single lesions. Five of 6 (83.3%) regressed completely following autologous vaccination and the remaining horse had a decrease in size. The remaining 5 horses had > 1 lesion. Three of 5 (60%) horses had complete regression of all lesions while 1 of 5 (20%) had a decrease in size and number (Table 1). Overall, in this cohort, 8 horses had complete regression with no further treatment following the autologous vaccination procedure.
Table 1.
Horses treated with the autologous vaccine procedure with no ancillary therapies
| Breed | Age | Gender | Number of lesions | Total tumor area (cm2) | Historical treatment | Complete regression | Decrease in number | Decrease in size | Time to follow-up (mo) | Veterinary confirmation of results |
|---|---|---|---|---|---|---|---|---|---|---|
| ARAB | 12 | MC | 1 | 15 | YES | NO | NO | YES | 8 | NO |
| QH | 13 | MC | 3 | 8 | NO | NO | YES | YES | 28 | NO |
| WB | 4 | F | 9 | 25 | NO | NO | NO | NO | 2 | YES |
| QH | 22 | MC | 1 | 6.25 | NO | YES | — | — | 3 | YES |
| QH | 8 | MC | 1 | 2 | NO | YES | — | — | 12 | YES |
| TB | 14 | MC | 1 | 50 | YES | YES | — | — | 16 | YES |
| QH | 14 | MC | 1 | 12.5 | YES | YES | — | — | 17 | NO |
| ARAB | 11 | MC | 1 | 12 | YES | YES | — | — | 18 | NO |
| TB | 13 | MC | 3 | 1.5 | YES | YES | — | — | 39 | YES |
| WB | 5 | MC | 3 | 16 | YES | YES | — | — | 1 | NO |
| TB | 20 | MC | 5 | 8 | NO | YES | — | — | 12 | NO |
ARAB — Arabian; QH — Quarter Horse; WB — Warmblood; TB — Thoroughbred; MC — male castrated; F — female.
Post-procedural treatment was instituted in 5 of 16 (31.3%) cases (Table 2). These included imiquimod (n = 4) and cisplatin (n = 1). Of these, 3 (60%) had complete regression of all sarcoid lesions while all 5 (100%) had decreases in size (Table 2).
Table 2.
Horses treated with the autologous vaccine procedure and ancillary therapies
| Breed | Age | Gender | Number of lesions | Total tumor area (cm2) | Historical treatment | Complete regression | Decrease in number | Decrease in size | Time to follow-up (mo) | Veterinary confirmation of results |
|---|---|---|---|---|---|---|---|---|---|---|
| WB | 7 | MC | 1 | 25 | YES | NO | NO | YES | 5 | NO |
| WB | 9 | MC | 2 | 16 | YES | NO | NO | YES | 30 | NO |
| WB | 19 | MC | 1 | 16 | NO | YES | — | — | 2 | YES |
| WB | 11 | MC | 1 | 32 | YES | YES | — | — | 3 | YES |
| WB | 18 | F | 2 | 6 | NO | YES | — | — | 4 | YES |
WB — Warmblood; MC — male castrated; F — female.
Results were also examined based on the presence of absence of historical treatment. Nine of 16 (56.3%) horses were treated historically prior to implantation procedure. In these cases, 6 of 9 (66.7%) completely regressed, and the remaining 3 horses had decreases in overall size of sarcoids. At the time of follow-up, 3 of 16 horses (18.8%) had regrowth of 1 or more sarcoid lesions. All 3 cases belonged to the cohort of horses with historically treated lesions.
No statistical significance was found when analyzing the associations between the presence of multiple initial sarcoids, the initial tumor area in cm2, the type of historical treatment, and the use of topical historical treatments with tumor regrowth or a decrease in tumor size.
Mild complications were noted in 7 of 16 cases (43.8%). The most common complication reported was swelling at the site of implantation [n = 5 (71.4%)] followed by fever and a single abscess at the implantation site. All complications resolved with a short course of anti-inflammatory therapy. Overall, 12 of 16 (75%) owners were reportedly satisfied with the autologous vaccine procedure. The remaining 4 owners were indifferent to the procedure and outcome but were dissatisfied with the complications.
Discussion
The results of this retrospective case series are promising as 75% of horses had a decrease in number of sarcoids, 93.8% had a decrease in size of sarcoids, and 69% of horses had complete regression. Of the 11 horses in which the autologous vaccine procedure was performed with no post-procedural treatment, 8 horses had complete regression of all sarcoids. Three of the five patients with multiple lesions had complete regression of all lesions without any other direct treatment. These findings demonstrate the ability of the autologous vaccine to cause a decrease in the number and size of sarcoids in multiple areas of the body with no other treatment. The 2 remaining cases of multiple lesions with no further post-procedural treatment were followed up at just 30 and 60 d post-procedure which may not be long enough to note significant change.
Many of the current treatment protocols for sarcoids rely on a multimodal approach. It is also clear that biopsy or removal with incomplete margins often causes aggressive regrowth of lesions (1,11). Reports of surgical excision alone vary dramatically in success rate, ranging from 52% to 82% (1,4,11). Surgical debulking followed by topical treatment with acyclovir has resulted in complete regression in both nodular and mixed sarcoids (14). In the present case series, 9 horses had single lesions. With surgical debulking and the autologous vaccine procedure, 7 of 9 (77.8%) had complete remission thereby demonstrating a benefit in utilizing the autologous preparation as an adjunct therapy. Of the 9 cases unresponsive to historical treatment, all 9 had decreases in size with 7 (77.8%) showing decreases in number of lesions following the autologous procedure.
Treatment failure and regrowth of equine sarcoids are common (4,12,14). The choice of initial treatment is often based on location of the lesion, the presence of multiple lesions, and cost/availability. It is noted anecdotally that sarcoids that do not respond to therapy can become problematic and unresponsive to ancillary therapies. While 3 horses in this series had regrowth, all 3 had received historical treatment and thus most likely belong to a cohort of horses with more persistent lesions. In these cases, while regrowth of the original lesion had occurred, no new lesions were reported.
Complications were noted in 43.8% of cases but mostly consisted of mild swelling at the site of implantation. Mild complications are often associated with a variety of sarcoid treatments (12,14,16,18). One horse developed an abscess at the site of implantation. This was treated by the animal’s referring veterinarian and resulted in a small scar along the neck. No inciting cause could be identified but the patient went on to have complete remission of 2 lesions.
In the present study, Quarter Horses, Warmbloods, and Thoroughbreds represented the majority of cases. These findings reflect the genetic predisposition for sarcoids discussed in the literature (1,4,11), as Quarter Horses do not constitute a high percentage of the horses seen at this institution (6.8% of the patient caseload). Geldings were overrepresented in this study, as this institution recorded approximately equal numbers of mares and geldings in the same time frame. The median age at presentation (11 y) is likely related to a referral population bias, as the initial onset of sarcoid lesions is widely accepted to occur in younger animals ranging from 1 to 7 y old (1,2,6). The average age in this study is older than in most; therefore, these cases may represent more aggressive lesions requiring referral or lesions located in difficult anatomic locations where benign neglect is not a valid option. However, the anatomic distribution of lesions in this case series reflects the most common areas of sarcoid occurrence in the general population. Multiple lesions are common in patients with sarcoids, ranging from 14% to 84% of cases (11) which is reflected in this report as 46.7% of horses had 2 or more sarcoids.
All initial follow-up was performed through conversation with the owner; however, 5 horses were examined by a veterinarian for other medical reasons and the presence or absence of sarcoid lesions was noted. All owners were asked to submit digital before and after images during the standardized survey. Owner reported findings were confirmed in half of the cases available for follow-up by either veterinary examination or digital images. The use of an owner survey has several limitations including placebo effect and subjectivity and a prospective, case-controlled study with bi-weekly tumor measurements would provide more accurate documentation for the regression of tumor lesions. However, due to the extended geographic area this institution serves and the retrospective nature of the study, owner reporting was necessary in order to obtain long-term follow-up data.
While the exact mechanism of the autologous preparation is unknown, we suspect the implanted tissue is acting as an immunomodulatory agent to stimulate a host response against the debulked lesion and other lesions. The innate response consists of primarily non-specific phagocytic cells that both engulf antigen and release granules in an attempt to remove an antigen. Soluble factors such as complement, acute phase proteins, and cytokines aid in cell signaling and phagocytosis (22). While the innate response has no antigenic memory, the acquired response consists of T-cells and memory B-cells that are primed to recognize specific antigens. Vaccine technology relies on creating a product that does not cause clinical disease but stimulates the production of memory B- and T-cells to prevent future infection (22).
There have been recent studies on the development of vaccines to both prevent and aid in the treatment of cervical neoplasia, a disease in which 99.7% of tumors are positive for specific strains of human papilloma virus DNA (23,24). Papillomaviruses are unique in that development of cancerous lesions depends on negative regulation of cell cycle control as well as immune evasion (24). A variety of therapeutic vaccines including protein based products, plant-derived products, DNA-based vaccines as well as bacterial and viral vectors have been investigated with varying degrees of success. Combination therapy including traditional radiation therapy and a therapeutic vaccine was effective in a pre-clinical model (24) suggesting that a therapeutic vaccine is useful as an adjuvant to traditional therapy. Other work on a vaccine for HPV 16 demonstrated a strong immune response in the form of titers and cytokine production, but limited regression of tumors (23). These studies were mimicked in work on sarcoid-bearing donkeys which were vaccinated with a virus-like particle containing the L1 and E7 proteins; while a strong immunologic response was elicited, tumor regression was noted in only about half the donkeys (25).
Immuno-modulation differs from traditional vaccine therapy in that it aims to stimulate a non-specific enhancement of the innate or acquired response (26). In the equine population, this has traditionally consisted of a variety of crude products of bacterial, viral or plant origin (26). The proposed mechanism relies on macrophage activation and subsequent cytokine release that increases phagocytic activity, antibody production, and lymphocyte cytotoxicity. In equids, immune-modulation has centered on the treatment of equine respiratory disease complex. A recent systematic literature review examined studies on the use of Parapoxvirus ovis and Propionibacterium acnes as immuno-modulators for equine respiratory disease; overall, favorable results in both the in vitro and in vivo work were noted with patients receiving these therapies showing improvement in recovery time (27).
The mechanism of action of these immuno-modulatory agents is largely unknown; however, clear success has been demonstrated in equids suffering from chronic virus-associated respiratory disease (27). In other species, in-depth in vitro work has identified interleukin (IL)-16, a proinflammatory cytokine secreted by activated T-cells, as a potential immuno-modulator in viral disease (28). In caprines, IL-16 may decrease the integration of caprine arthritis-encephalitis viral DNA into peripheral white blood cells (29).
Immuno-modulation represents a new front in both human and animal medicine as a targeted means to treat both chronic viral disease and a variety of neoplasms. Future in vitro work is needed to support the use of the autologous sarcoid preparation utilized in this report. Larger case series or a prospective case-controlled report may provide stronger evidence for the efficacy of this procedure. However, the clinical results, in combination with owner satisfaction and mild complications, make this an attractive therapy to aid in sarcoid treatment. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Rees CA. Disorders of the skin. In: Reed SE, Bayly WM, Sellon DC, editors. Equine Internal Medicine. St Louis, Missouri: Saunders Elsevier; 2010. pp. 710–713. [Google Scholar]
- 2.Pascoe RRR, Knottenbelt DC. Manual of Equine Dermatology. Philadelphia, Pennsylvania: WB Saunders; 1999. [Google Scholar]
- 3.Martens A, De Moor A, Demeulemeester J, Ducatelle R. Histopathological characteristics of five clinical types of equine sarcoid. Res Vet Sci. 2000;69:295–300. doi: 10.1053/rvsc.2000.0432. [DOI] [PubMed] [Google Scholar]
- 4.Taylor S, Haldorson G. A review of equine sarcoid. Equine Vet Educ. 2013;25:210–216. [Google Scholar]
- 5.Chambers G, Ellsmore VA, O’Brien PM, et al. Association of bovine papillomavirus with the equine sarcoid. J Gen Virol. 2003;84:1055–1062. doi: 10.1099/vir.0.18947-0. [DOI] [PubMed] [Google Scholar]
- 6.Nasir L, Campo MS. Bovine papillomoviruses: Their role in the aetiology of cutaneous tumours of bovids and equids. Vet Dermtatol. 2008;19:243–254. doi: 10.1111/j.1365-3164.2008.00683.x. [DOI] [PubMed] [Google Scholar]
- 7.Carr EA, Théon AP, Madewell BR, Hitchcock ME, Schlegel R, Schiller JT. Expression of a transforming gene (E5) of bovine papilloma virus in sarcoids obtained from horses. Am J Vet Res. 2001;62:1212–1217. doi: 10.2460/ajvr.2001.62.1212. [DOI] [PubMed] [Google Scholar]
- 8.Brandt S, Tober R, Corteggio A, et al. BVP-1 infection is not confined to the dermis but also involves the epidermis of equine sarcoids. Vet Microbiol. 2011;150:35–40. doi: 10.1016/j.vetmic.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 9.Finlay M, Yuan ZQ, Burden F, et al. The detection of bovine papillomavirus type 1 DNA in flies. Virus Res. 2009;144:315–317. doi: 10.1016/j.virusres.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Meredith D, Elser AH, Wolf B, Soma LR, Donawick WJ, Lazary S. Equine leukocyte antigens; relationships with sarcoid tumors and laminitis in two pure breeds. Immunogenetics. 1986;23:221–225. doi: 10.1007/BF00373016. [DOI] [PubMed] [Google Scholar]
- 11.Bergvall KE. Sarcoids. Vet Clin Equine. 2013;29:657–671. doi: 10.1016/j.cveq.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Théon AP, Pascoe JR, Carlson GP, Krag DN. Intratumoral chemotherapy with cisplatin in oily emulsion in horses. J Am Vet Med Assoc. 1993;202:261–267. [PubMed] [Google Scholar]
- 13.Théon AP, Pascoe JR. Iridium-192 interstitial brachytherapy for equine periocular tumours: Treatment results and prognostic factors in 115 horses. Equine Vet J. 1995;27:117–121. doi: 10.1111/j.2042-3306.1995.tb03046.x. [DOI] [PubMed] [Google Scholar]
- 14.Stadler S, Kainzbauer C, Haralambus R, Brehm W, Hainisch E, Brandt S. Successful treatment of equine sarcoids by topical aciclovir application. Vet Rec. 2001;168:187–190. doi: 10.1136/vr.c5430. [DOI] [PubMed] [Google Scholar]
- 15.Christen-Clottu O, Klocke P, Burger D, Straub R, Gerber V. Treatment of clinically diagnosed equine sarcoid with a mistletoe extract (Viscum album austriacus) J Vet Intern Med. 2010;24:1483–1489. doi: 10.1111/j.1939-1676.2010.0597.x. [DOI] [PubMed] [Google Scholar]
- 16.Lavach JD, Sullins KE, Roberts SM, Severin GA, Wheeler C, Lueker DC. BCG treatment of periocular sarcoid. Equine Vet J. 1985;17:445–448. doi: 10.1111/j.2042-3306.1985.tb02552.x. [DOI] [PubMed] [Google Scholar]
- 17.Vanselow BA, Abetz I, Jackson AR. BCG emulsion immunotherapy of equine sarcoid. Equine Vet J. 1988;20:444–447. doi: 10.1111/j.2042-3306.1988.tb01571.x. [DOI] [PubMed] [Google Scholar]
- 18.Nogueira SA, Torres SM, Malone SD, Diaz SF, Jessen C, Gilbert S. Efficacy of imiquimod 5% cream in the treatment of equine sarcoids: A pilot study. Vet Dermatol. 2006;17:259–265. doi: 10.1111/j.1365-3164.2006.00526.x. [DOI] [PubMed] [Google Scholar]
- 19.Théon AP, Wilson WD, Magdesian KG. Long-term outcome associated with intratumoral chemotherapy with cisplatin for cutaneous tumors in equidae: 573 cases (1995–2004) J Am Vet Med Assoc. 2007;230:1506–1513. doi: 10.2460/javma.230.10.1506. [DOI] [PubMed] [Google Scholar]
- 20.Kinnunen RE, Tallberg T, Stenbäck H, Sama S. Equine sarcoid tumour treated by autogenous tumour vaccine. Anticancer Res. 1999;19:3367–3374. [PubMed] [Google Scholar]
- 21.Epsy BK. How to treat equine sarcoids by autologous implantation. Proc Am Assoc Equine Pract. 2008;54:68–73. [Google Scholar]
- 22.Delves PJ, Roitt IM. The immune system. First of two parts. N Engl J Med. 2000;343:37–49. doi: 10.1056/NEJM200007063430107. [DOI] [PubMed] [Google Scholar]
- 23.Van Poelgeest ME, Welters MJ, van Esch EM, et al. HPV16 synthetic long peptide (HPV16-SLP) vaccination therapy of patients with advanced or recurrent HPV-16 induced gynecological carcinoma, a phase II trial. J Transl Med. 2013;11:88–102. doi: 10.1186/1479-5876-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vici P, Mariani L, Pizzuti L, et al. Immunologic treatments for pre-cancerous lesions and uterine cervical cancer. J Exp Clin Cancer Res. 2014;33:29–44. doi: 10.1186/1756-9966-33-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashrafi GH, Piujo K, Burden F, et al. Vaccination of sarcoid-bearing donkeys with chimeric virus-like particles of bovine papillomavirus type 1. J Gen Virol. 2008;89:148–157. doi: 10.1099/vir.0.83267-0. [DOI] [PubMed] [Google Scholar]
- 26.Rush BR, Flaminio JB. Immunomodulation in horses. Vet Clin North Am Equine Pract. 2000;16:183–197. doi: 10.1016/s0749-0739(17)30126-8. [DOI] [PubMed] [Google Scholar]
- 27.Paillot R. A systematic review of the immune-modulators Parapoxvirus ovis and Propionibacterium acnes for the prevention of respiratory disease and other infections in the horse. Vet Immunol Immunopathol. 2013;153:1–9. doi: 10.1016/j.vetimm.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Baier M, Werner A, Bannert N, Metzner K, Kurth R. HIV suppression by interleukin-16. Nature. 1995;378:563. doi: 10.1038/378563a0. [DOI] [PubMed] [Google Scholar]
- 29.Nimmanapalli R, Sharmilla C, Reddy PG. Immunomodulation of caprine lentiviral infection by interleukin–16. Comp Immunol Microbiol Infect Dis. 2010;33:529–536. doi: 10.1016/j.cimid.2009.09.003. [DOI] [PubMed] [Google Scholar]