Abstract
Background
Brucellosis is one of the most common zoonotic disease affecting humans and animals and is endemic in many parts of the world including the Gulf Cooperation Council region (GCC). The aim of this study was to identify the species and determine the antimicrobial susceptibility pattern of Brucella strains isolated from clinical specimens, from Qatar.
Results
We evaluated 231 Brucella isolates. All isolates were identified as B. melitensis. All the isolates were susceptible to doxycycline, tetracycline, streptomycin, gentamicin, trimethoprim / sulfamethoxazole and ciprofloxacin except rifampicin, where 48 % of the strains showed elevated MICs (>1 mg/L). The rifampicin-resistance related hotspots within the rpoB gene were amplified and sequenced using PCR and no rpoB mutations were found in strains with rifampicin MICs of >2 mg/L.
Conclusion
This study identified B. melitensis as the etiological agent of brucellosis in Qatar. No resistant isolates were detected among conventionally used antimicrobial agents.
Keywords: Brucella melitensis, Brucellosis, Antimicrobial susceptibility testing, Qatar
Background
Brucellosis is a worldwide zoonotic disease in both animals and humans with an estimated 500,000 new cases annually [1]. Although brucellosis is a notifiable disease in many countries, it is probably underreported and official numbers do not reflect the true incidence of this infection [2]. Thus the true incidence of human brucellosis is unknown and the estimated burden of the disease varies widely, from < 0.03 to >160 per 100,000 population. The highest recorded incidence of human brucellosis occurs in the Middle East and Central Asia [3]. Brucellosis is transmitted to humans by direct contact with infected animals or consumption of unpasteurized or inadequately cooked milk or milk products, inhalation of infected aerosolized particles and, to a lesser extent, meat derived from cattle, sheep, goats, pigs, camels, yaks, buffaloes or dogs. Four species of the genus Brucella are usually pathogenic for humans, which include B. melitensis (from sheep/goats and camel), B. abortus (from cattle and other bovine animals), B. suis (from pigs), and B. canis (from dogs) [4]. Routine susceptibility testing of Brucella species is not performed due to the need for containment level 3 facilities [4] and concerns of laboratory-acquired infections [5]. The main objective of this study was to determine the antimicrobial susceptibility pattern of human Brucella isolates from patients in Qatar collected over a 10 -year period.
Methods
Bacterial isolates
A total of 231 strains of Brucella isolates obtained from various clinical specimens (blood n = 225 and synovial fluid n = 6) collected between January 2005 and July 2014 from patients attending Hamad General Hospital and Al Khor Hospital, Qatar, were included in the study. All the isolates were collected as a part of standard patient care. Identification of isolates was based on colony morphology, Gram stain, oxidase, catalase, Vitek 2 compact (bioMerieux, Durham, USA) and Maldi-TOF MS (Bruker Daltonics, Bremen, Germany). For Maldi-TOF MS, cultures were processed using the ethanol/formic acid/acetonitrile protocol [6]. All cultures were processed in a Class II biological safety cabinet in a negative pressure room.
Antimicrobial susceptibility testing
Susceptibility of seven antibiotics (doxycycline, tetracycline, streptomycin, gentamicin, rifampicin, trimethoprim/sulfamethoxazole and ciprofloxacin) was determined with a gradient strip method (E-test strips, bioMerieux, Marcy L’Etoile, France). The antimicrobial strips were placed on Muller Hinton blood agar according to the manufacturer’s guidelines and read after 48 h incubation in ambient air at 37 °C. Minimum inhibitory concentrations (MIC) breakpoints of streptomycin, gentamicin, tetracycline, doxycycline and trimethoprim-sulfamethoxazole were used as recommended by CLSI [7]. As MIC breakpoints for Brucella against rifampicin and ciprofloxacin have not been established, guidelines for slow-growing bacteria (H.influenzae) were used [8, 9]. The MIC was interpreted as the value at which the inhibition zone intercepted the scale on the E-test strip and the MIC values were rounded up (to the next higher dilution) with the corresponding MIC of the microbroth dilution method. MIC50 and MIC90 levels were defined as the lowest concentration of the antibiotic at which 50 % and 90 % of the isolates were inhibited, respectively.
The reference strains- H. influenza ATCC 10211, E.coli ATCC 25922, S.pneumoniae ATCC 49619 and S.aureus ATCC 29213 were used as quality control.
PCR assay of rpoB gene
For isolates (n = 17) that showed a rifampicin MIC of > 2 mg/L, the rifampicin-resistance related hotspots within the rpoB gene were amplified and sequenced as described before [10]. The sequences were compared to rpoB sequences of wildtype and resistant isolates as provided by other studies [10, 11].
Results
All the isolates were identified by Vitek II compact and Maldi-TOF as B. melitensis. Table 1 represents MIC range; MIC50 and MIC90 of each antibiotic tested and MIC distributions are shown in Fig. 1.
Table 1.
Antimicrobial susceptibility of B.melitensis
| Range (mg/L) | MIC50 (mg/L) | MIC90 (mg/L) | Breakpoints for susceptibility (mg/L) | |
|---|---|---|---|---|
| Doxycycline | 0.032–1 | 0.125 | 0.25 | ≤1 [7] |
| Tetracycline | 0.032–0.5 | 0.125 | 0.25 | ≤ 1 [7] |
| Gentamicin | 0.064–2 | 0.25 | 0.5 | ≤ 4 [7] |
| Streptomycin | 0.125–4 | 0.5 | 2 | ≤ 8 [7] |
| Trimethoprim-Sulfamethoxazole | 0.008–2 | 0.064 | 0.25 | ≤ 2/38 [7] |
| Rifampicin | 0.008–4 | 1 | 2 | ≤ 1 [8] |
| Ciprofloxacin | 0.064–0.5 | 0.25 | 0.5 | ≤ 1 [8] |
Fig. 1.
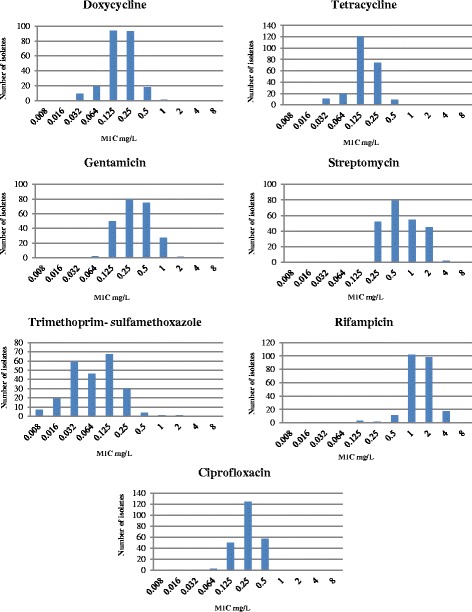
Distribution of MIC values in indicated antimicrobials of B. melitensis from Qatar
All the isolates were susceptible to doxycycline (MIC90, 0.25 mg/L), gentamicin (MIC90, 0.5 mg/L), streptomycin (MIC90, 2 mg/L), trimethoprim-sulfamethoxazole (MIC90, 0.25 mg/L) and tetracycline (MIC90, 0.25 mg/L). In addition, ciprofloxacin (MIC90, 0.5 mg/L) showed 100 % susceptibility when MIC breakpoint criteria for H.influenzae [8] were used. For rifampicin, the MIC values ranged from 0.008 to 4 mg/L and when compared with MIC breakpoint criteria for H.influenzae [8], resistance (MIC ≥ 2 mg/L) was demonstrated in 48 % strains. After sequencing of the rpoB gene, no mutations were found in isolates with MIC of > 2 mg/L (n = 17).
Discussion
Brucella melitensis is the commonest etiological agent causing human brucellosis worldwide. Intracellular localization of Brucella species within monocytes and macrophages of the reticulo-endothelial system limits the choices of antimicrobial agents for effective treatment of systemic and localized brucellosis. Although a variety of antimicrobial agents appear to be active in vitro, the results of susceptibility testing do not always correlate with clinical efficacy [4].
The recommended method for antimicrobial susceptibility testing of Brucella species is microbroth dilution using unsupplemented Brucella broth [7]. When the E-test method was compared with microbroth dilution, Gur et al. found no significant variations in obtained MIC values [12]. This method appear to be reliable, reproducible, easily performed, and produces similar results to those of conventional methods for Brucella on Muller Hinton blood agar [13, 14] and it is therefore that we used E-test for susceptibility testing.
Amongst tetracyclines, doxycycline is the most common antimicrobial drug used for treatment of uncomplicated brucellosis in adults and children of more than 8 years [4]. In the present study, MICs of tetracycline (range - 0.032–0.5 mg/L) and doxycycline (range - 0.032–1 mg/L) are found to be in the susceptible category and MIC50 and MIC90 values are consistent with previous reports [9, 13, 15, 16]. However, increased MICs of doxycycline have been reported in a study from Mexico [17].
The World Health Organization (WHO) recommends doxycycline (6 weeks) in combination with aminoglycosides (streptomycin or gentamicin for 2–3 weeks) or rifampicin (6 weeks) [4]. The most preferred and effective aminoglycoside in treatment of brucellosis is streptomycin [4, 18, 19]. Combination with streptomycin was found to be superior as compared to rifampicin, in terms of relapse or treatment failure [18]. Even though gentamicin is more active in vitro and is associated with fewer side effects than streptomycin, there are not enough studies, which justify the replacement of streptomycin [4, 19]. In the present study, all the strains were susceptible to streptomycin (0.125–4 mg/L) and gentamicin (0.064–2 mg/L) and found to be in the range described previously [9, 15]. Nevertheless, a high MIC90 of streptomycin (8 mg/L) was reported from Turkey [16].
Another commonly used drug in treatment of brucellosis in combination with doxycycline is rifampicin. In spite of the proven efficacy with aminoglycoside combination, due to the need for parenteral administration, convenience and poor patient compliance, healthcare professionals prefer the all-oral regimen of doxycycline/rifampicin [18, 20]. A meta-analysis on various combinations used for the treatment of brucellosis, has shown higher relapse rate with doxycycline/rifampicin when compared to doxycycline/streptomycin regimen [21]. It has been suggested that rifampicin may enhance the plasma clearance of doxycycline resulting in lower doxycyline levels [22] and the possibility that its use could contribute to emergence of rifampicin- resistant Mycobacterium tuberculosis in endemic countries [23]. For in-vitro susceptibility testing, MIC breakpoints of rifampicin against Brucella has not been established and therefore these organisms cannot be confidently characterised as susceptible, intermediate or resistant. However, when compared with CLSI reference values for H.influenzae [8], in the present study, 48 % of Brucella strains showed elevated MIC’s of rifampicin (>1 mg/L). Higher MIC’s to rifampicin has been reported previously from Egypt (64 %) [9] and Malaysia (70 %) [15]. However, the impact of high MIC’s on clinical outcome in these countries is not known. In Qatar, in a retrospective cohort study, combination of doxycyline with streptomycin was found to be the preferred regimen followed by doxycyline with rifampicin and no relapse or therapeutic failures were detected [24].
Mutations conferring rifampin resistance are confined almost exclusively to the rpoB gene in most organisms and result in a decreased affinity of the DNA-dependent RNA polymerase to the antibiotic. Alternative mechanisms of rifampin resistance also have been observed [25]. Along with mutations in the rpoB gene, excitation of several metabolic processes may also be a contributing factor in conferring rifampicin resistant in Brucella [26]. We did not find any mutations in the rpoB gene in isolates with elevated MIC’s in this study, findings consistent with previous studies [14, 27, 11]. However, further research is required to find possible other mechanisms of rifampicin resistance in Brucella.
Trimethoprim/sulfamethoxazole (TMP/SMX) is an alternative agent recommended for treatment of brucellosis in pregnancy and in children under 8 years old. It has also been recommended for treatment of osteo-articular complications, neurobrucellosis and endocarditis, in combination with doxycycline, aminoglycoside and rifampicin [4]. TMP/SMX, when used in combination with rifampicin, was found to be effective in pediatric patients (8 weeks regimen). High relapse rates were noted when TMP/SMX was used as a monotherapy in pediatric brucellosis [28]. Development of resistance to TMP/SMX to B. melitensis is an important issue. High rate of resistance to TMP/SMX has been reported from previous studies [17, 29]. In the present study, TMP/SMX was found to be susceptible with low MIC50 (0.064 mg/L) and MIC90 (0.25 mg/L) when compared to other antimicrobials which is consistent with previous reports [9, 13, 15].
Ciprofloxacin is a potential alternative therapeutic option in the treatment of brucellosis with excellent oral bioavailability and reaching high concentrations in phagocytic cells [30]. However, ciprofloxacin is not recommended for monotherapy due to the lack of bactericidal activity at the intracellular acidic pH [31], high rate of relapse and the risk of development of overall fluoroquinolone resistance in the community [32]. Therefore, its use in combination with doxycycline is recommended as an acceptable alternative but not as first line regimen [29, 33]. We found ciprofloxacin MICs (0.064–0.5 mg/L) in the susceptible range when compared to MIC breakpoint criteria of slow growing bacteria and these findings are consistent with previous studies [9, 16]. Ciprofloxacin based therapy might play a role as an alternative regimen especially in patients with intolerance to commonly used drugs and relapsed disease [30].
Conclusion
In summary, this study identified B. melitensis as the etiological agent of brucellosis in Qatar. No resistant isolates were detected among conventional antimicrobial agents. Periodic monitoring of antimicrobial resistance in the Middle East, especially in the Gulf Cooperating Council (GCC) in light of the emerging resistance and development of CLSI or EUCAST interpretive criteria for rifampicin are needed.
Acknowledgements
Funding- this research project was supported by NPRP grant 5-746-3-176 from Qatar National Research Foundation (QNRF). The content and statement made herein are solely the responsibility of the authors.
The authors would like to thank the Bacterial Special Pathogen Branch at Center for Disease Control and Prevention, USA, for providing the Brucella Maldi-TOF database.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AD participated in the design of the study, coordinated all work related to the study, performed data analysis and drafted the manuscript; FH did the rpoB PCR and participated in data analysis; OAS and MA executed the work related to identification of Brucella and antimicrobial susceptibility test; GW, SD and MAM participated in the design of the study; JFM participated in the design of the study, performed data analysis and critically reviewed the manuscript. All authors read and approved the final manuscript.
Contributor Information
Anand Deshmukh, Email: adeshmukh@hamad.qa.
Ferry Hagen, Email: F.Hagen@cwz.nl.
Ola Al Sharabasi, Email: oalsharabasi@hmc.org.qa.
Mariamma Abraham, Email: MABRAHAM2@hmc.org.qa.
Godwin Wilson, Email: gwilson@hmc.org.qa.
Sanjay Doiphode, Email: sdoiphode@hmc.org.qa.
Muna Al Maslamani, Email: malmaslamani@hmc.org.qa.
Jacques F Meis, Email: jacques.meis@gmail.com.
References
- 1.World Health Organization (WHO) Fact sheet N173. Geneva, Switzerland: WHO; 1997. p. ᅟ. [Google Scholar]
- 2.Seleem MN, Boyle SM, Sriranganathan N. Brucellosis: a re-emerging zoonosis. Vet Microbiol. 2010;140:392–398. doi: 10.1016/j.vetmic.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. 2006;6:91–99. doi: 10.1016/S1473-3099(06)70382-6. [DOI] [PubMed] [Google Scholar]
- 4.Corbel MJ. Brucellosis in humans and animals. Geneva (Switzerland): World Health Organization; 2006.
- 5.Robichaud S, Libman M, Behr M, Rubin E. Prevention of laboratory-acquired brucellosis. Clin Infect Dis. 2004;38:e119–e122. doi: 10.1086/421024. [DOI] [PubMed] [Google Scholar]
- 6.Lista F, Reubsaet FA, De Santis R, Parchen RR, de Jong AL, Kieboom J, et al. Reliable identification at the species level of Brucella isolates with MALDI-TOF-MS. BMC Microbiol. 2011;11:267. doi: 10.1186/1471-2180-11-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CLSI . Methods of antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria. Approved guidelines- second edition. CLSI document M45-A2. Wayne, PA: Clinical and Laboratory Standard Institute; 2010. [Google Scholar]
- 8.CLSI . Performance standard for antimicrobial susceptibility testing: twenty-fourth informational supplement. CLSI document M100-S24. Wayne, PA: Clinical and Laboratory Standard Institute; 2014. [Google Scholar]
- 9.Abdel-Maksoud M, House B, Wasfy M, Abdel-Rahman B, Pimentel G, Roushdy G, et al. In vitro antibiotic susceptibility testing of Brucella isolates from Egypt between 1999 and 2007 and evidence of probable rifampin resistance. Ann Clin Microbiol Antimicrob. 2012;11:24. doi: 10.1186/1476-0711-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marianelli C, Ciuchini F, Tarantino M, Pasquali P, Adone R. Genetic bases of the rifampicin resistance phenotype in Brucella spp. J Clin Microbiol. 2004;42:5439–5443. doi: 10.1128/JCM.42.12.5439-5443.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valdezate S, Navarro A, Medina-Pascual MJ, Carrasco G, Saéz-Nieto JA. Molecular screening for rifampicin and fluoroquinolone resistance in a clinical population of Brucella melitensis. J Antimicrob Chemother. 2010;65:51–53. doi: 10.1093/jac/dkp389. [DOI] [PubMed] [Google Scholar]
- 12.Gür D, Kocagöz S, Akova M, Unal S. Comparison of E test to microdilution for determining in vitro activities of antibiotics against Brucella melitensis. Antimicrob Agents Chemother. 1999;43:2337. doi: 10.1128/aac.43.9.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayram Y, Korkoca H, Aypak C, Parlak M, Cikman A, Kilic S, et al. Antimicrobial susceptibilities of Brucella isolates from various clinical specimens. Int J Med Sci. 2011;8:198–202. doi: 10.7150/ijms.8.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marianelli C, Graziani C, Santangelo C, Xibilia MT, Imbriani A, Amato R, et al. Molecular epidemiological and antibiotic susceptibility characterization of Brucella isolates from humans in Sicily, Italy. J Clin Microbiol. 2007;45:2923–2928. doi: 10.1128/JCM.00822-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashim R, Ahmad N, Mohamed Zahidi J, Tay BY, Mohd Noor A, Zainal S, et al. Identification and in vitro antimicrobial susceptibility of Brucella species isolated from human brucellosis. Int J Microbiol. 2014;2014:596245. doi: 10.1155/2014/596245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaya O, Akçam FZ, Yaylı G. Investigation of the in vitro activities of various antibiotics against Brucella melitensis strains. Turk J Med Sci. 2012;42:145–148. [Google Scholar]
- 17.López-merino A, Contreras-rodríguez A, Migranas-ortiz R, Orrantia-gradín R, Hernández-oliva GM, Torres Y, et al. Susceptibility of Mexican Brucella isolates to moxifloxacin, ciprofloxacin and other antimicrobials used in the treatment of human brucellosis. Scand J Infect Dis. 2004;36:636–638. doi: 10.1080/00365540410020767. [DOI] [PubMed] [Google Scholar]
- 18.Solís García del Pozo J, Solera J. Systematic review and meta-analysis of randomized clinical trials in the treatment of human brucellosis. PLoS One. 2012;7:e32090. doi: 10.1371/journal.pone.0032090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ariza J, Bosilkovski M, Cascio A, Colmenero JD, Corbel MJ, Falagas ME, et al. Perspectives for the treatment of brucellosis in the 21st century: the Ioannina recommendations. PLoS Med. 2007;4:e317. doi: 10.1371/journal.pmed.0040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pappas G, Siozopoulou V, Akritidis N, Falagas ME. Doxycycline-rifampicin: physicians’ inferior choice in brucellosis or how convenience reigns over science. J Infect. 2007;54:459–462. doi: 10.1016/j.jinf.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 21.Skalsky K, Yahav D, Bishara J, Pitlik S, Leibovici L, Paul M. Treatment of human brucellosis: systematic review and meta-analysis of randomised controlled trials. BMJ. 2008;336:701–704. doi: 10.1136/bmj.39497.500903.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colmenero JD, Fernandez-Gallardo LC, Agundez JA, Sedeno J, Benitez J, Valverde E. Possible implications of doxycycline-rifampin interaction for treatment of brucellosis. Antimicrob Agents Chemother. 1994;38:2798–2802. doi: 10.1128/AAC.38.12.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grayson ML, Kucers A. Kucers’ the use of antibiotics a clinical review of antibacterial, antifungal, antiparasitic, and antiviral drugs. 6. London: Hodder Arnold; 2010. [Google Scholar]
- 24.Rahil AI, Othman M, Ibrahim W, Mohamed MY. Brucellosis in Qatar: a retrospective cohort study. Qatar Med J. 2014;1:25. doi: 10.5339/qmj.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldstein BP. Resistance to rifampicin: a review. J Antibiot (Tokyo) 2014;67:625–630. doi: 10.1038/ja.2014.107. [DOI] [PubMed] [Google Scholar]
- 26.Sandalakis V, Psaroulaki A, De Bock PJ, Christidou A, Gevaert K, Tsiotis G, et al. Investigation of rifampicin resistance mechanisms in Brucella abortus using MS-driven comparative proteomics. J Proteome Res. 2012;11:2374–2385. doi: 10.1021/pr201122w. [DOI] [PubMed] [Google Scholar]
- 27.Sayan M, Kilic S, Uyanik MH. Epidemiological survey of rifampicin resistance in clinic isolates of Brucella melitensis obtained from all regions of Turkey. J Infect Chemother. 2012;18:41–46. doi: 10.1007/s10156-011-0281-7. [DOI] [PubMed] [Google Scholar]
- 28.Lubani MM, Dudin KI, Sharda DC, Ndhar DS, Araj GF, Hafez HA, et al. A multicenter therapeutic study of 1100 children with brucellosis. Pediatr Infect Dis J. 1989;8:75–78. doi: 10.1097/00006454-198901001-00025. [DOI] [PubMed] [Google Scholar]
- 29.Kinsara A, Al-Mowallad A, Osoba AO. Increasing resistance of Brucellae to co-trimoxazole. Antimicrob Agents Chemother. 1999;43:1531. doi: 10.1128/aac.43.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Easmon CS, Crane JP, Blowers A. Effect of ciprofloxacin on intracellular organisms: in-vitro and in-vivo studies. J Antimicrob Chemother. 1986;18(Suppl D):43–48. doi: 10.1093/jac/18.supplement_d.43. [DOI] [PubMed] [Google Scholar]
- 31.Akova M, Gur D, Livermore DM, Kocagoz T, Akalin HE. In vitro activities of antibiotics alone and in combination against Brucella melitensis at neutral and acidic pHs. Antimicrob Agents Chemother. 1999;43:1298–1300. doi: 10.1128/aac.43.5.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pappas G, Christou L, Akritidis N, Tsianos EV. Quinolones for brucellosis: treating old diseases with new drugs. Clin Microbiol Infect. 2006;12:823–825. doi: 10.1111/j.1469-0691.2006.01442.x. [DOI] [PubMed] [Google Scholar]
- 33.Falagas ME, Bliziotis IA. Quinolones for treatment of human brucellosis: critical review of the evidence from microbiological and clinical studies. Antimicrob Agents Chemother. 2006;50:22–33. doi: 10.1128/AAC.50.1.22-33.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


