Abstract
Introduction
Current guidelines and consensus recommend arterial and venous samples as equally acceptable for blood glucose assessment in point-of-care devices, but there is limited evidence to support this recommendation. We evaluated the accuracy of two devices for bedside point-of-care blood glucose measurements using arterial, fingerstick and catheter venous blood samples in ICU patients, and assessed which factors could impair their accuracy.
Methods
145 patients from a 41-bed adult mixed-ICU, in a tertiary care hospital were prospectively enrolled. Fingerstick, central venous (catheter) and arterial blood (indwelling catheter) samples were simultaneously collected, once per patient. Arterial measurements obtained with Precision PCx, and arterial, fingerstick and venous measurements obtained with Accu-chek Advantage II were compared to arterial central lab measurements. Agreement between point-of-care and laboratory measurements were evaluated with Bland-Altman, and multiple linear regression models were used to investigate interference of associated factors.
Results
Mean difference between Accu-chek arterial samples versus central lab was 10.7 mg/dL (95% LA -21.3 to 42.7 mg/dL), and between Precision PCx versus central lab was 18.6 mg/dL (95% LA -12.6 to 49.5 mg/dL). Accu-chek fingerstick versus central lab arterial samples presented a similar bias (10.0 mg/dL) but a wider 95% LA (-31.8 to 51.8 mg/dL). Agreement between venous samples with arterial central lab was the poorest (mean bias 15.1 mg/dL; 95% LA -51.7 to 81.9). Hyperglycemia, low hematocrit, and acidosis were associated with larger differences between arterial and venous blood measurements with the two glucometers and central lab. Vasopressor administration was associated with increased error for fingerstick measurements.
Conclusions
Sampling from central venous catheters should not be used for glycemic control in ICU patients. In addition, reliability of the two evaluated glucometers was insufficient. Error with Accu-chek Advantage II increases mostly with central venous samples. Hyperglycemia, lower hematocrit, acidosis, and vasopressor administration increase measurement error.
Introduction
Although tight glucose control has failed to improve survival in critically ill patients [1], glucose control protocols are needed, since very high blood glucose levels [2], hypoglycemia [3] and glucose variability [4] may increase mortality. Point-of-care glucometers are the most commonly used devices for blood glucose measurement and guide glucose management in intensive care units (ICU) [5].
Point-of-care glucometers were developed to improve the self-management of blood glucose by diabetic patients [6], and their reliability may be insufficient for managing blood glucose in critically ill patients [5–7]. Portable glucometers are affected by environmental and therapeutic factors, such as humidity and temperature [6], ascorbic acid [8], acetaminophen and mannitol [9], and by innumerous patient-related conditions, such as hypoxemia [10,11], acidosis [10,11], hypotension [12], hematocrit [11,13] and hypoglycemia [14]. Additionally, according to the manufacturers, high triglycerides and bilirubin levels may increase the glucometers inaccuracy.
Several studies have addressed the performance of different point-of-care glucometers in critically ill patients [15]. However, those studies usually enrolled a small number of patients [16,17], were retrospective with non-concurrent blood draw from different sites [18], and/or used repeated samplings in the same subjects without accounting for the lack of independence of blood glucose values within subject [19,20].
Additionally, central venous sampling for point of care assessment of blood glucose is a common practice when arterial sampling is not promptly available, and current guidelines for the management of hyperglycemia in critically ill patients recommend either arterial or venous samples for blood glucose assessment [21]. Nevertheless, the available evidence supporting this recommendation is scarce. Only few studies have directly compared the accuracy of arterial versus venous samples [22], and many have reported the results including both sources indistinctly [23–25].
Our aim was to evaluate the reliability of two point-of-care devices for blood glucose measurement of samples collected simultaneously from arterial, fingerstick and venous blood of critically ill patients. Additionally, we sought to determine the influence of clinical and environmental factors on the performance of point-of-care glucometers in the intensive care unit setting.
Methods
Participants
We performed a cross-sectional study between March 2006 and July 2007, with prospective data collection, in critically ill patients admitted to a 41-bed intensive care unit (ICU). Inclusion criteria were admission to the ICU and periodic blood glucose measurements requested by the attending physician. There were no exclusion criteria. The local hospital ethics committee approved the protocol, and informed consent was provided by each patient or next of kin before enrollment.
Measurements
The reference method used was the central lab measurement in serum of arterial blood glucose (dry chemistry, Vitros, Jonhson&Jonhson, New Jersey, USA) [26]. We compared whole blood glucose assessments, involving arterial measurements obtained with Precision PCx (glucose oxidase, Abbott, Illinois, USA) [27] to the arterial central lab measurement. We also compared arterial, fingerstick and venous measurements of blood glucose obtained with Accu-chek Advantage II glucometer (glucose dehydrogenase, and bioamperometry, pyrroloquinolinequinone strips; Roche, Basel, Switzerland) [28] to the arterial central lab measurement. Samples from arterial (indwelling catheter), venous (central venous catheter [CVC], double or triple lumen, 20 cm, 7Fr., 14–18 Ga.) and fingerstick blood were obtained simultaneously, and once per patient.
To avoid hemodilution and contamination of the arterial samples, 3 mL of arterial blood was collected from the indwelling arterial catheter and discarded [29]. Then, an additional 5 mL of arterial blood was drawn and immediately analyzed. One drop of the arterial blood sample was analyzed at the bedside with Precision PCx glucometer and another drop with Accu-chek Advantage II glucometer. The remaining arterial blood was sent to the central lab for blood glucose assessment. Additionally, triglycerides and total bilirubin levels were measured in the arterial blood sample. All samples were obtained using vacuum tubes containing fluoride oxalate. Right after sampling, the blood tubes were systematically sent to the central lab through a pneumatic tube transport system, to be immediately analyzed.
To avoid hemodilution by, and contamination with intravenous fluids, 5 mL of venous blood were collected from the distal lumen of the CVC and discarded prior sampling [29]. Afterwards, an additional 5 mL of venous blood was drawn and analyzed immediately with the Accu-chek Advantage II glucometer. Fingerstick blood samples were obtained from the patient’s fingertip with a lancet device, and were analyzed with the Accu-chek Advantage II glucometer. One of the investigators (F.P.A), a senior critical care nurse, supervised all procedures.
Data collected included: demographic data, the Sequential Organ Failure Assessment score (SOFA score) [30], Acute Physiology and Chronic Health Evaluation II (APACHE II score) [31], mean arterial blood pressure, peripheral body temperature, hematocrit level, arterial pH, arterial oxygen saturation, room temperature and humidity (Humidity Sensor System, Model NR 5026, Gibeck Respiration AB, Stockholm, Sweden), total bilirubin levels, triglycerides, use of vasopressors (norepinephrine and dopamine), acetaminophen, ascorbic acid, mannitol and mechanical ventilation.
Statistical Analysis
We planned to enroll 140 patients to have adequate power to run multiple linear models with up to seven variables [32].
Categorical variables were presented as absolute and relative frequencies. Continuous variables were presented as mean and standard deviation, or as median and interquartile range (IQR).
Agreements between the point-of-care assessments (arterial measurements with Precision PCx and Accu-chek Advantage II, and fingerstick and venous measurements with Accu-chek Advantage II) and the arterial central lab measurement (reference method) were evaluated by calculating the mean differences (bias) and 95% limits of agreement according to the Bland-Altman method [33]. The 95% limits of agreement indicate the interval in which one expects that 95% of the differences between pairs of blood glucose measurements (e.g., point-of-care arterial blood versus central lab arterial blood) will lie within.
We compared the magnitude of bias between the different methods of blood glucose measurement against arterial central lab measurement [e.g., arterial blood point-of-care against arterial central lab measurement (reference) compared to fingerstick blood point-of-care against arterial central lab measurement (reference)] using paired Student’s t test. The variability in the differences between point-of-care measurements minus arterial central lab measurements were compared among different point-of-care methods (e.g., arterial blood point-of-care versus fingerstick point-of-care) using the F test for the homogeneity of variance.
Univariate and multivariate linear regression models were used to assess which clinical and environmental factors led to biased point-of-care measurements and to estimate the magnitude of bias. All suspect variables (mean arterial blood pressure, peripheral body temperature, hematocrit, arterial pH, arterial oxygen saturation, room temperature, room air humidity, total bilirubin levels, triglycerides, use of vasopressors, acetaminophen, ascorbic acid or mannitol) were tested in univariate models. Those with p-value ≤0.10 were included in a multiple linear regression model. A p value of <0.05 was considered significant. All analyses were performed with STATA SE 9.0 (StataCorp, Texas, USA).
Results
One hundred forty five consecutive critically ill patients were enrolled in this study. The mean age was 60.9±20.5 years and the mean APACHE II score was 23.7±9.0. Baseline characteristics of study patients are presented in Table 1.
Table 1. Baseline characteristics.
| Age (years) | 60.9 ± 20.5 |
| Male gender, n (%) | 94 (64.8) |
| APACHE II score | 23.7 (9.0) |
| SOFA score | 6 (4–11) |
| Diagnostic category, n (%) | |
| Respiratory | 35 (24.2) |
| Severe sepsis / septic shock | 24 (16.6) |
| Solid organ transplantation | 24 (16.6) |
| Neurologic | 16 (11.7) |
| Postoperative | 16 (11.7) |
| Cardiovascular | 10 (6.9) |
| Renal and metabolic | 9 (6.3) |
| Others | 11(6.0) |
| Mean arterial blood pressure (mm Hg) | 85 (74–97) |
| Body temperature (°C) | 36.3 (36.0–36.9) |
| Hematocrit (%) | 27.7 (24.6–31.5) |
| Arterial pH | 7.39 (7.33–7.43) |
| Arterial oxygen saturation (%) | 98 (96–100) |
| Room temperature (°C) | 23.5 (22.8–24.4) |
| Room air humidity (%) | 61.4 (57.3–61.7) |
| Total bilirubin (mg/dl) | 0.9 (0.6–2.8) |
| Triglycerides (mg/dl) | 97 (69–137) |
| Therapy | |
| Mechanical ventilation, n° (%) | 90 (62.1) |
| Vasopressors, n (%) | 57 (39.3) |
| Norepinephrine, n (%) | 56 (38.6) |
| Median dose (mcg/kg/min) | 0.15 (0.07–0.42) |
| Acetaminophen, n (%) | 8 (5.5) |
| Ascorbic acid, n (%) | 1 (0.7) |
| Mannitol, n (%) | 2 (1.4) |
Values are mean±SD, median (IQR) or n (%). APACHE II = Acute Physiology and Chronic Health Evaluation II (The score ranges from 0 to 71, with higher scores indicating more severe illness), SOFA score = Sepsis-related Organ Failure Assessment (The score ranges from 0 to 20, with higher scores indicating more severe organ failure).
Mean arterial central lab blood glucose was 146±91 mg/dl. The mean Precision PCx arterial blood glucose was 160±71 mg/dl. The mean Accu-chek Advantage II arterial, fingerstick and venous blood glucose were 151±69 mg/dl, 151±74 mg/dl and 162±90 mg/dl, respectively.
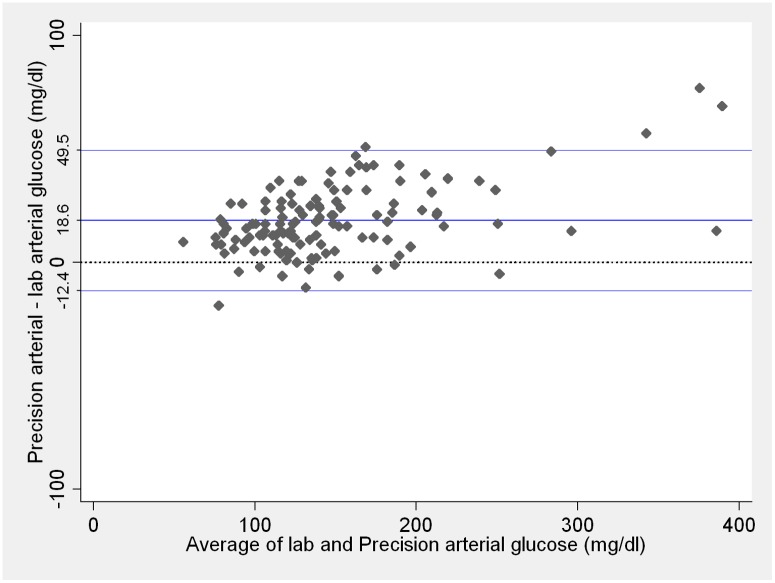
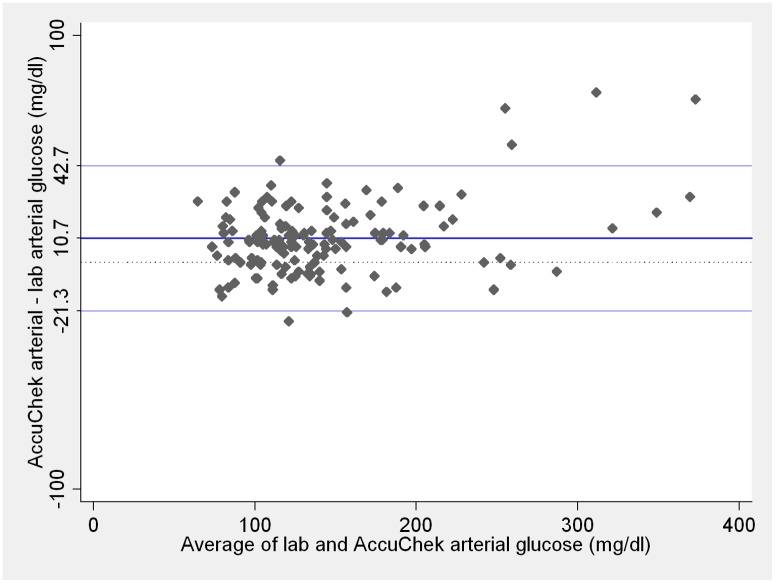
Mean difference (bias) between arterial blood glucose measurements performed with Precision PCx compared to arterial central lab and between arterial samples analyzed with Accu-chek Advantage II compared to arterial central lab are presented on Figs 1 and 2, respectively. Bias was greater for arterial blood glucose measurements performed with Precision PCx than measurements performed with Accu-chek Advantage II (p < 0.001), although the variability of differences between the two point-of-care methods against arterial central lab was similar (p = 0.74).
Fig 1. Comparison between central lab arterial and Precision PCx arterial blood glucose measurements.
Blue lines represent the mean difference and 95% limits of agreement. Dotted line represents zero. Lab and Precision PCx measurements ≤ 20 mg/dL were considered as 20 mg/dL and measurements ≥ 600mg/dL considered as 600 mg/dL. These are the Precision PCx limits of detection according to the manufacturer.
Fig 2. Comparison between central lab arterial and Accu-chek Advantage II arterial blood glucose measurements.
Blue lines on the plot represent the mean difference and 95% limits of agreement. Dotted line represents zero. Lab and Accu-chek Advantage II measurements ≤ 10mg/dL were considered as 10 mg/dL and measurements ≥ 600 mg/dL considered as 600 mg/dL. These are the Accu-chek Advantage II limits of detection according to the manufacturer.
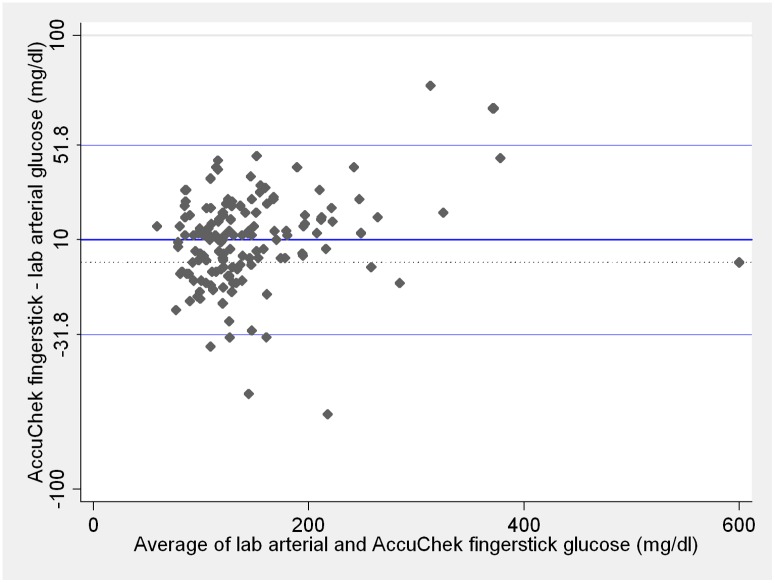
Mean bias between fingerstick samples analyzed with Accu-chek Advantage II versus arterial central lab measurements is presented on Fig 3. Although the mean bias of fingerstick blood glucose measurements with Accu-chekAdvantage II was similar to the bias for arterial blood glucose measurements with the same point-of-care device (p = 0.65), variability of differences was wider for fingerstick than for arterial measurements (p = 0.002).
Fig 3. Comparison between central lab arterial and Accu-chek Advantage II fingerstick glucose measurements.
Blue lines on the plot represent the mean difference and 95% limits of agreement. Dotted line represents zero. Lab and Accu-chek Advantage II measurements ≤ 10mg/dL were considered as 10 mg/dL and measurements ≥ 600 mg/dL considered as 600 mg/dL. These are the Accu-chek Advantage II limits of detection according to the manufacturer.
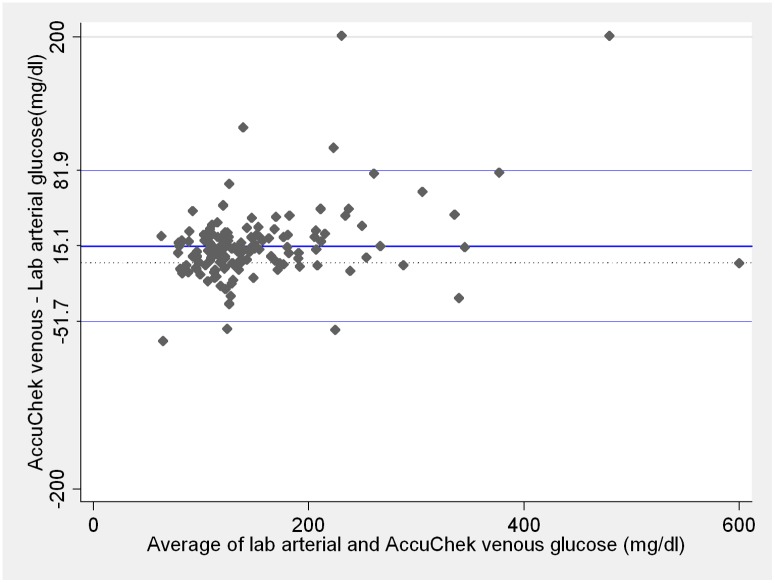
The worst agreement was detected between venous samples analyzed with Accu-chek Advantage II versus arterial central lab measurements (Fig 4). We observed venous blood glucose values varying between 69 mg/dL lower than the central lab arterial measurement up to 474 mg/dL higher. Mean bias of venous blood glucose analyzed with Accu-chek Advantage II did not differ from mean bias of arterial blood analyzed with the same device (p = 0.17). Nevertheless, variability was wider for venous samples in comparison to arterial blood analyzed with Accu-chek Advantage II (p < 0.001).
Fig 4. Comparison between central lab arterial and Accu-chek Advantage II venous blood glucose measurements.
Blue lines on the plot represent the mean difference and 95% limits of agreement. Dotted line represents zero. Lab and Accu-chek Advantage II measurements ≤ 10mg/dL were considered as 10 mg/dL and measurements ≥ 600 mg/dL considered as 600 mg/dL. These are the Accu-chek Advantage II limits of detection according to the manufacturer.
Factors associated with measurement errors for arterial blood glucose analyzed with Precision PCx, and arterial, fingerstick and venous blood glucose analyzed with Accu-chek Advantage II are presented in Table 2. Blood glucose levels were associated with error for both point-of-care glucometers and at all sampling sites (Table 2). For instance, for each 1.0 mg/dL increase in arterial blood glucose, the mean error of arterial blood glucose measurement with Precision PCx will increase by 0.12 mg/dL (95% confidence interval: 0.08 to 0.16; p < 0.001) and by 0.10 mg/dL (95% confidence interval: 0.06 to 0.15; p < 0.001) with Accu-chek Advantage II (Table 2). Therefore, when blood glucose of a patient was 300 g/dL the mean arterial blood glucose value with Precision PCx and Accu-chek Advantage II would be 326 mg/dL and 317 mg/dL, respectively.
Table 2. Multiple linear regression models of clinical and environmental factors, which are independently associated with bias in blood glucose measurement with point-of-care glucometers.
| Glucometer | Sampling site | Clinical and environmental factors | Measurement error (95%CI) § | P-value |
|---|---|---|---|---|
| Precision PCx | Arterial | Mean blood glucose (mg/dl) † | 0.12 (0.08 to 0.16) | <0.001 |
| Hematocrit (%) | -0.89 (-1.38 to -0.40) | <0.001 | ||
| Accu-chekAdvantage II | Arterial | Mean blood glucose (mg/dl) † | 0.10 (0.06 to 0.15) | <0.001 |
| Hematocrit (%) | -0.74 (-1.29 to -0.19) | 0.009 | ||
| Arterial pH | -34.8 (-62.8 to -6.7) | 0.020 | ||
| Accu-chekAdvantage II | Fingerstick | Mean blood glucose (mg/dl) † | 0.12 (0.06 to 0.17) | <0.001 |
| Use of norepinephrine | -25.8 (-40.1 to -11.5) | 0.001 | ||
| Hematocrit (%) | -1.04 (-1.75 to -0.32) | 0.005 | ||
| Accu-chekAdvantage II | Venous | Mean blood glucose (mg/dl) † | 0.22 (0.13 to 0.30) | <0.001 |
| Arterial pH | -77.4 (-135.7 to -19.1) | 0.010 |
§ = Measurement error represents the change in the difference of blood glucose between point-of-care glucometer and arterial central lab measurement for one unit increase in the clinical or environmental factor;
† = mean blood glucose is the mean between the point-of-care and arterial central lab blood glucose values; CI = confidence interval.
Other factors which interfered with point-of-care measurements were hematocrit for arterial blood measurements performed with Precision PCx (p < 0.001) and arterial (p = 0.009) and fingerstick (p = 0.005) blood measurements performed with Accu-chek Advantage II; arterial pH for arterial (p = 0.02) and venous (p = 0.01) blood samples analyzed with Accu-chek Advantage II and finally, norepinephrine administration for fingerstick measurements performed with Accu-chek Advantage II (p = 0.001; Table 2).
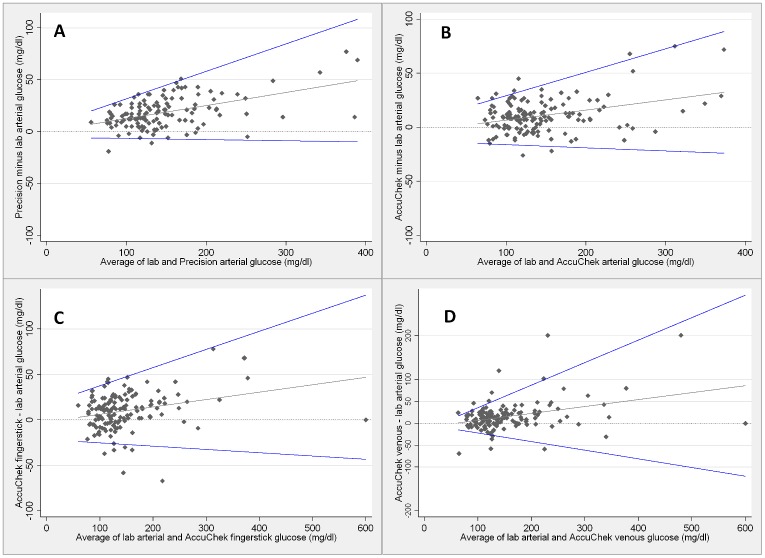
In addition to being associated with mean bias, increasing blood glucose values are also associated with larger 95% limits of agreement, implying that precision decreases during hyperglycemia (Fig 5).
Fig 5. Relationship between the blood glucose values and the point-of care performance.
Comparisons between arterial central lab and Precision PCx arterial (A), Accu-chek Advantage II arterial (B), fingerstick (C) and venous (D) blood glucose measurements. The gray and blue lines are the mean difference and 95% limits of agreement, respectively. Precision PCx measurements ≤ 20 mg/dL were considered as 20 mg/dL and measurements ≥ 600mg/dL considered as 600 mg/dL. These are the Precision PCx limits of detection according to the manufacturer. Accu-chek Advantage II measurements ≤ 10mg/dL were considered as 10 mg/dL and measurements ≥ 600 mg/dL considered as 600 mg/dL. These are the Accu-chek Advantage II limits of detection according to the manufacturer.
Discussion
The most intriguing result of this study was the lack of accuracy of point-of-care venous blood glucose measurements. We strictly followed a routine care to avoid contamination with I.V. fluids, discarding 5 mL of blood before the measurements. The International Federation of Clinical Chemistry recommends withdrawing a volume equal to three times the “dead space” of the catheter prior to blood sampling [29], or even less for arterial catheters [34]. Assuming that the priming volume of a double or triple lumen catheter corresponds to 0.4–0.6 cc [35], 5 mL would be more than enough to rule out sampling contamination. Analysis of catheter venous samples in portable glucometers in ICU, e.g. after laboratory sampling for other tests in patients without indwelling arterial catheters is a common issue, and raising awareness about this limitation should change clinical practice in future.
Current international guidelines consider arterial and venous samples as equivalent [21], or venous samples at least as the second option in case of arterial line unavailability [36]. However, the evidence to support that is rather limited. Several studies assumed arterial-venous glucose differences as insignificant and sampled arterial or venous sites, indistinctly [18,24,25,37], or included only venous samples as the reference to the central lab measurements [20,38,39]. One study directly compared venous to arterial blood glucose measurements in 20 patients [22]. In spite of the small sample size, authors reported a larger variability in the venous to central lab differences (95% limits of agreement: -30 to 60) compared to the arterial to central lab differences (95% limits of agreement: -5 to 35) [22]. About potential mechanisms involved, another study, in which catheter venous samples were compared to samples obtained through venipuncture, ruled out exogenous blood glucose contamination as the cause of error, and suggested that properties unique to CVC blood may interfere with Accu-Chek meter measurements [40]. Results of our study corroborate this finding.
Our study has limitations. We had limited power to estimate the effect of factors that might interfere with blood glucose measurements, in particular those with low prevalence in the population we sampled, for example use of acetaminophen or ascorbic acid. Previous data and recommendations from previous guidelines suggest that all factors we have considered may interfere in the accuracy of glucose measurements [9,41]. We have also not observed hypoglycemia in our sample. Therefore, it is possible that levels of bias and limits of agreement might be different from those we have found in lower levels of blood glucose. Finally, our findings of lower accuracy of fingerstick and venous point-of-care blood glucose measurements compared to arterial sampling are likely due to sampling site properties, but as we only assessed those samples with one type of glucometer (Accu-Check Advantage II) one can not assure those findings would be similar with Precision PCx or other devices.
There are some strengths in our work. It was a prospective study testing two devices in real conditions. We considered only one sample of each site (arterial, venous or fingerstick) per patient so the statistical assumption of independence of observations was truly held. All measurements were collect simultaneously, by a senior trained nurse, following a standardized protocol. Finally, the evaluation was not only restricted to the reliability of two glucometers and different sampling sites, but we also assessed which factors increased errors in point-of-care blood glucose measurements, being able to identify and dissect the potential sources of error during measurements, including the device itself (intrinsic error), the sample site, and the environmental and other associated factors.
Conclusions
Due to high variability, sampling from central venous catheters should not be used for glycemic control in ICU patients. In addition, we found that the reliability of two commonly used point-of-care glucometers (Accu-chek Advantage II and Precision PCx) was insufficient in critical care, especially when using catheter venous samples. Error with Accu-chek Advantage II increases further with sampling from fingerstick blood and, most profoundly, with central venous catheter sampling. Hyperglycemia, lower hematocrit and lower pH are additional sources of error. Arterial samples seem to be the only site sufficiently accurate to be used with point-of-care glucometers.
Acknowledgments
The authors would like to thank Lucas Petri Damiani, MSc, statistician of the Research Institute of the HCor Hospital (São Paulo, Brazil), Hospital do Coração, (São Paulo, Brazil), and Institute of Mathematics and Statistics of the University of São Paulo (São Paulo, Brazil), for the independent statistical revision of the manuscript.
Data Availability
All relevant data are available from Dryad (doi:10.5061/dryad.sr7c).
Funding Statement
This work was funded by "Instituto de Ensino e Pesquisa (IIEP) - Hospital Israelita Albert Einstein" (grant number: 80-05; responsible for the grant: AJP). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, et al. : Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009, 360:1283–1297. 10.1056/NEJMoa0810625 [DOI] [PubMed] [Google Scholar]
- 2. Krinsley JS, Egi M, Kiss A, Devendra AN, Schuetz P, Maurer PM, et al. : Diabetic status and the relation of the three domains of glycemic control to mortality in critically ill patients: an international multicenter cohort study. Crit Care 2013, 17:R37 10.1186/cc12547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Finfer S, Liu B, Chittock DR, Norton R, Myburgh JA, McArthur C, et al. : Hypoglycemia and risk of death in critically ill patients. N Engl J Med 2012, 367:1108–1118. [DOI] [PubMed] [Google Scholar]
- 4. Egi M, Bellomo R, Stachowski E, French CJ, Hart G: Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology 2006, 105:244–252. [DOI] [PubMed] [Google Scholar]
- 5. Finfer S, Wernerman J, Preiser JC, Cass T, Desaive T, Hovorka R, et al. : Clinical review: Consensus recommendations on measurement of blood glucose and reporting glycemic control in critically ill adults. Crit Care 2013, 17:229 10.1186/cc12537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Kirkman MS,et al. : Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem 2011, 57:e1–e47. 10.1373/clinchem.2010.161596 [DOI] [PubMed] [Google Scholar]
- 7. Scott MG, Bruns DE, Boyd JC, Sacks DB: Tight glucose control in the intensive care unit: are glucose meters up to the task? Clin Chem 2009, 55:18–20. 10.1373/clinchem.2008.117291 [DOI] [PubMed] [Google Scholar]
- 8. Strijdom JG, Marais BJ, Koeslag JH: Ascorbic acid causes spuriously low blood glucose measurements. S Afr Med J 1993, 83:64–65. [PubMed] [Google Scholar]
- 9. Tang Z, Du X, Louie RF, Kost GJ: Effects of drugs on glucose measurements with handheld glucose meters and a portable glucose analyzer. Am J Clin Pathol 2000, 113:75–86. [DOI] [PubMed] [Google Scholar]
- 10. Tang Z, Louie RF, Payes M, Chang KC, Kost GJ: Oxygen effects on glucose measurements with a reference analyzer and three handheld meters. Diabetes Technol Ther 2000, 2:349–362. [DOI] [PubMed] [Google Scholar]
- 11. Watkinson PJ, Barber VS, Amira E, James T, Taylor R, Young JD: The effects of precision, haematocrit, pH and oxygen tension on point-of-care glucose measurement in critically ill patients: a prospective study. Ann Clin Biochem 2012, 49:144–151. 10.1258/acb.2011.011162 [DOI] [PubMed] [Google Scholar]
- 12. Atkin SH, Dasmahapatra A, Jaker MA, Chorost MI, Reddy S: Fingerstick glucose determination in shock. Ann Intern Med 1991, 114:1020–1024. [DOI] [PubMed] [Google Scholar]
- 13. Tang Z, Lee JH, Louie RF, Kost GJ: Effects of different hematocrit levels on glucose measurements with handheld meters for point-of-care testing. Arch Pathol Lab Med 2000, 124:1135–1140. [DOI] [PubMed] [Google Scholar]
- 14. Trajanoski Z, Brunner GA, Gfrerer RJ, Wach P, Pieber TR: Accuracy of home blood glucose meters during hypoglycemia. Diabetes Care 1996, 19:1412–1415. [DOI] [PubMed] [Google Scholar]
- 15. Inoue S, Egi M, Kotani J, Morita K: Accuracy of blood-glucose measurements using glucose meters and arterial blood gas analyzers in critically ill adult patients: systematic review. Crit Care 2013, 17:R48 10.1186/cc12567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ray JG, Hamielec C, Mastracci T: Pilot study of the accuracy of bedside glucometry in the intensive care unit. Crit Care Med 2001, 29:2205–2207. [DOI] [PubMed] [Google Scholar]
- 17. Kanji S, Buffie J, Hutton B, Bunting PS, Singh A, McDonald K, et al. : Reliability of point-of-care testing for glucose measurement in critically ill adults. Crit Care Med 2005, 33:2778–2785. [DOI] [PubMed] [Google Scholar]
- 18. Finkielman JD, Oyen LJ, Afessa B: Agreement between bedside blood and plasma glucose measurement in the ICU setting. Chest 2005, 127:1749–1751. [DOI] [PubMed] [Google Scholar]
- 19. Meynaar IA, van Spreuwel M, Tangkau PL, Dawson L, Visser SS, Rijks L, et al. : Accuracy of AccuChek glucose measurement in intensive care patients. Crit Care Med 2009. [DOI] [PubMed] [Google Scholar]
- 20. Critchell CD, Savarese V, Callahan A, Aboud C, Jabbour S, Marik P: Accuracy of bedside capillary blood glucose measurements in critically ill patients. Intensive Care Med 2007, 33:2079–2084. [DOI] [PubMed] [Google Scholar]
- 21. Jacobi J, Bircher N, Krinsley J, Agus M, Braithwaite SS, Deutschman C, et al. : Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med 2012, 40:3251–3276. 10.1097/CCM.0b013e3182653269 [DOI] [PubMed] [Google Scholar]
- 22. Karon BS, Gandhi GY, Nuttall GA, Bryant SC, Schaff HV, McMahon MM, et al. : Accuracy of roche accu-chek inform whole blood capillary, arterial, and venous glucose values in patients receiving intensive intravenous insulin therapy after cardiac surgery. Am J Clin Pathol 2007, 127:919–926. [DOI] [PubMed] [Google Scholar]
- 23. Petersen JR, Graves DF, Tacker DH, Okorodudu AO, Mohammad AA, Cardenas VJ Jr.: Comparison of POCT and central laboratory blood glucose results using arterial, capillary, and venous samples from MICU patients on a tight glycemic protocol. Clin Chim Acta 2008, 396:10–13. 10.1016/j.cca.2008.06.010 [DOI] [PubMed] [Google Scholar]
- 24. Lacara T, Domagtoy C, Lickliter D, Quattrocchi K, Snipes L, Kuszaj J, et al. : Comparison of point-of-care and laboratory glucose analysis in critically ill patients. Am J Crit Care 2007, 16:336–346. [PubMed] [Google Scholar]
- 25. Desachy A, Vuagnat AC, Ghazali AD, Baudin OT, Longuet OH, Calvat SN, et al. : Accuracy of bedside glucometry in critically ill patients: influence of clinical characteristics and perfusion index. Mayo Clin Proc 2008, 83:400–405. 10.4065/83.4.400 [DOI] [PubMed] [Google Scholar]
- 26. Test Methodology, VITROS Chemistry Products. August 1997, Johnson & Johnson Clinical Diagnostics, Inc. [Google Scholar]
- 27. Balion C, Grey V, Ismaila A, Blatz S, Seidlitz W: Screening for hypoglycemia at the bedside in the neonatal intensive care unit (NICU) with the Abbott PCx glucose meter. BMC Pediatr 2006, 6:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill, B. [Available: http://www.currentseparations.com/issues/21-2/cs21-2c.pdf]. Last accessed in August 5th, 2014.
- 29. Burnett RW, Covington AK, Fogh-Andersen N, Kulpmann WR, Maas AH, Muller-Plathe O, et al. : International Federation of Clinical Chemistry (IFCC). Scientific Division. Committee on pH, Blood Gases and Electrolytes. Approved IFCC recommendations on whole blood sampling, transport and storage for simultaneous determination of pH, blood gases and electrolytes. Eur J Clin Chem Clin Biochem 1995, 33:247–253. [PubMed] [Google Scholar]
- 30. Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et at;: The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996, 22:707–710. [DOI] [PubMed] [Google Scholar]
- 31. Knaus WA, Draper EA, Wagner DP, Zimmerman JE: APACHE II: a severity of disease classification system. Crit Care Med 1985, 13:818–829. [PubMed] [Google Scholar]
- 32. Katz MH. Multivariate analysis: a primer for readers of medical research. Ann Intern Med. 2003; 138(8):644–650. [DOI] [PubMed] [Google Scholar]
- 33. Bland JM, Altman DG: Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1:307–310. [PubMed] [Google Scholar]
- 34. Rickard CM, Couchman BA, Schmidt SJ, Dank A, Purdie DM: A discard volume of twice the deadspace ensures clinically accurate arterial blood gases and electrolytes and prevents unnecessary blood loss. Crit Care Med 2003, 31:1654–1658. [DOI] [PubMed] [Google Scholar]
- 35.Arrow multi-lumen central venous catheter—Nursing Care Guidelines. 1996.
- 36. Finfer S, Wernerman J, Preiser JC, Cass T, Desaive T, Hovorka R, et al. : Clinical review: Consensus recommendations on measurement of blood glucose and reporting glycemic control in critically ill adults. Crit Care 2013, 17:229 10.1186/cc12537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hoedemaekers CW, Klein Gunnewiek JM, Prinsen MA, Willems JL, Van der Hoeven JG: Accuracy of bedside glucose measurement from three glucometers in critically ill patients. Crit Care Med 2008, 36:3062–3066. 10.1097/CCM.0b013e318186ffe6 [DOI] [PubMed] [Google Scholar]
- 38. Meex C, Poncin J, Chapelle JP, Cavalier E: Analytical validation of the new plasma calibrated Accu-Chek Test Strips (Roche Diagnostics). Clin Chem Lab Med 2006, 44:1376–1378. [DOI] [PubMed] [Google Scholar]
- 39. Boyd R, Leigh B, Stuart P: Capillary versus venous bedside blood glucose estimations. Emerg Med J 2005, 22:177–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Karon BS, Koch CD, Wockenfus AM, Brown JK: Accuracy of whole blood glucose measurement when venous catheter blood samples are used on glucose meters. Diabetes Technol Ther 2009, 11:819–825. 10.1089/dia.2009.0074 [DOI] [PubMed] [Google Scholar]
- 41. Sacks DB, Bruns DE, Goldstein DE, Maclaren NK, McDonald JM, Parrott M: Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem 2002, 48:436–472. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are available from Dryad (doi:10.5061/dryad.sr7c).