Abstract
Introduction
Vitamin D deficiency has been associated with the development of myocardial hypertrophy and inflammation. These findings suggest that vitamin D status and vitamin D receptor (VDR) genomics may play a role in myocardial fibrosis. The aim of this pilot study was to determine the association between vitamin D levels, VDR polymorphisms, and biomarkers of left ventricular remodeling and hemodynamics.
Methods
In a cross-sectional pilot study, patients with ejection fraction (EF) <40% (and New York Heart Association ≥ II) undergoing right heart catheterization were included in the study. Blood was collected for determination of 25-hydroxyvitamin D level (antibody competitive immunoassay), VDR genotypes (BsmI, ApaI, TaqI, and FokI), and biomarkers (N-terminal propeptide of collagen type III [PIIINP], matrix metalloproteinase 2, and galectin 3). The vitamin D genotypes were determined through the use of pyrosequencing.
Results
A total of 30 patients with a mean EF of 17% ± 8% were enrolled. There was a significant association between the BsmI C allele, ApaI G allele, and TaqI A allele, which formed a haplotype block (CGA) for analysis. There were no differences in baseline parameters between patients with the VDR haplotype block (n = 20) and those without (n = 10). Individual genotypes were not associated with any biomarker or hemodynamics. Patients with the CGA haplotype demonstrated significantly higher log PIIINP values (1.74 ± 0.32 mcg/mL vs 1.36 ± 0.31 mcg/mL, P = .0041). When evaluating vitamin D levels below and above the median level (19 ng/mL), there was no significant difference between these 2 groups in regard to biomarker levels for left ventricular remodeling.
Conclusion
This study has shown that a biomarker for collagen type III synthesis, PIIINP, was associated with the CGA haplotype of BsmI, ApaI, and TaqI single nucleotide polymorphisms on the VDR. These findings suggest that VDR genetics may play a role in myocardial fibrosis in patients with systolic heart failure.
Keywords: vitamin D, systolic heart failure, biomarkers, hemodynamics
Introduction
Heart failure (HF) is a major and growing public health problem in the United States. Approximately 5 million patients in the United States have HF and over 550 000 patients are newly diagnosed with HF on an annual basis. In addition, there are significant mortality (range from 5%–50% on an annual basis) and morbidity associated with systolic HF. Given the number of patients and the mortality and morbidity associated with systolic HF, it is important to understand the pathophysiology of HF. One area that has recently gained attention is the role of vitamin D in the pathophysiology of HF. There is increasing evidence that vitamin D may play an important role in HF. Early investigations have shown that in vitamin D receptor (VDR) knockout (KO) mice, there is an increase in renin production and blood pressure compared to normal mice. Over time, the VDR KO mice develop typical signs of HF with cardiac hypertrophy and fibrosis.1–6 Vitamin D is also involved in immune function and inflammatory response as shown in human in vitro7,8 and animal studies.9 In fact, these findings suggest that vitamin D may play a role in HF and especially in regard to myocardial fibrosis. Data from human studies have also linked vitamin D levels to HF. For example, a prospective cohort study in patients scheduled for coronary angiography demonstrated that low 25-hydroxyvitamin D (25[OH]D) levels were independently associated with deaths due to HF and sudden cardiac death.10 More recently, low 25(OH)D levels have been associated with poor prognosis in patients with systolic HF11; however, not all studies evaluating vitamin D levels and cardiovascular outcomes have shown an association.12–14 For example, the findings from a population-based study in 1125 patients with coronary heart disease did not show that baseline vitamin D levels were prognostic for secondary cardiovascular events or all-cause mortality.12 In addition, a recent systematic review and meta-analysis of 51 trials did not show an association between vitamin D and reduction in mortality or cardiovascular risk.13
Vitamin D effects are translated at the cellular level through the activation of the VDR by the active metabolite of vitamin D, 1,25-dihydroxyvitamin D (1,25[OH]2D). The VDR heterodimerizes with the retinoid X receptor and can affect the transcription of over 200 genes. The VDRs are located throughout the body including the myocardium. The VDR gene is located on chromosome 12q12-q14 in humans. Several polymorphisms in the gene encoding the VDR have been identified and include BsmI, ApaI, TaqI (located at the 3′ end of the VDR), and FokI (located at the 5′ end of the VDR).15 These polymorphisms and their combinations (haplotypes) have been associated with alterations in bone metabolism and increased risk of myocardial infarction, diabetes, and cancer.16–20 In addition, VDR polymorphisms have also been associated with plasma renin activity (PRA) and left ventricular hypertrophy in animal studies4 and PRA in human and animal studies.21,22 Overall, these findings in addition to vitamin D effects in regard to inflammation suggest that VDR polymorphisms may play an important role in the remodeling of the myocardium in patients with systolic HF. One approach to assess remodeling of the myocardium and especially the progression of fibrosis is to evaluate biomarkers that reflect turnover of the myocardium’s extracellular matrix (ECM). A basic structural protein of the ECM of the heart is collagen that supports myocytes and fibroblasts. There is a continual balance between synthesis and degradation of the ECM. When this balance is disrupted and there is a change in the ECM turnover, fibrosis can develop. Biomarkers of ECM turnover (formation and degradation), which can be measured in the blood and has been associated with clinical outcomes, include N-terminal propeptide of collagen type III (PIIINP) and matrix metalloproteinase 2 (MMP2). Currently, little data exist on the effect of VDR genetics on biomarkers reflecting the ECM and hemodynamic parameters in patients with systolic HF. The aim of this pilot study was to determine whether there is an association between vitamin D levels, VDR genetics, and biomarkers of left ventricular remodeling or hemodynamics in patients with systolic HF.
Methods
Patients
We performed a cross-sectional analysis of patients with systolic HF presenting for routine right heart catheterization (RHC). Patients were enrolled if they had a left ventricular ejection fraction (EF) <40% within the last 6 months, an attempt had been made to optimize their medical therapy for HF as noted in patient records, and they were scheduled for an RHC. Patients were excluded if they were <18 years of age, were unable to give consent, had primary valvular HF, had a heart transplant or left ventricular assist device, were pregnant, or had renal dysfunction (serum creatinine >2 mg/dL at the time of RHC). The University of Michigan Institutional Review Board approved this study and informed consent was obtained.
Patients were recruited at the time of scheduled RHC. After obtaining informed consent and meeting inclusion/exclusion criteria, approximately 30 mL of blood was collected at the time of catheterization for determination of 25(OH)D level, VDR genotypes, and biomarkers.
Hemodynamics
Hemodynamic parameters obtained during the catheterization were utilized to correlate with VDR genotypes. Specifically, pulmonary capillary wedge pressure and cardiac index were obtained at the time of RHC in the University of Michigan Cardiac Catheterization Laboratory. All measurements were taken with the patient in the fasting state. All pressure measurements were taken during end expiration. Cardiac output was measured using the Fick principle, with collection of a mixed venous sample from the pulmonary artery and using an assumption of oxygen consumption where oxygen consumption is equal to 125 mL O2/m2.
Genetics
The VDR polymorphisms we evaluated were based on previous studies suggesting that these polymorphisms may play a role in cardiovascular disease or has been shown to have a strong linkage disequilibrium, specifically BsmI, ApaI, TaqI, and FokI polymorphisms.4,16–22 Genomic DNA was isolated from whole blood with the salt precipitation method. Genotyping was done with Pyrosequencing Technology. Polymerase chain reaction primers were designed using Oligo 6 (MBI, Cascade, Color-ado). Pyrosequencing primers were designed using Pyrosequencing Primer Design Version 1.01 software.23 BsmI, ApaI, TaqI, and FokI single-nucleotide polymorphisms (rs1544410, rs7975232, rs10735810, and rs2228570) in VDR were analyzed. Specific details regarding these assays are available upon request.
Circulating Biomarkers
Biomarkers such as PIIINP, MMP2, and galectin 3 which represent the ECM (including formation and degradation) and remodeling were evaluated. Blood was collected during RHC and centrifuged within 4 hours. Plasma was extracted and stored at −70°C until batched after study completion. Laboratory analysis was performed at the University of Michigan CLASS laboratory.
N-Terminal propeptide of collagen type III
The Orion Diagnostica (Espoo, Finland) UniQ PIIINP RIA kit is based on the competitive radioimmunoassay technique. A known amount of labeled PIIINP and an unknown amount of unlabeled PIIINP in the sample compete for the limited number of high affinity binding sites of the antibody. After separating the free antigen, the amount of labeled PIIINP in the sample tube is inversely proportional to the amount of PIIINP in the sample. The assay range is 1 to 50 μg/L and the lower limit of detection (LLD) is 0.3 μg/L. The interassay coefficient of variation is 3.1% (4.6 μg/L).
Matrix metalloproteinase 2
The Human MMP2 immunoassay (DMP2F0) from R&D Systems (Minneapolis, Minnesota) uses the quantitative sandwich enzyme immunoassay technique. A polyclonal antibody specific for MMP2 has been precoated onto a microplate. Standards and samples are pipetted into the wells and any MMP2 present is bound by the immobilized antibody. After washing away any unbound substances, an enzyme-linked polyclonal antibody specific for MMP2 is added to the wells. Following a wash to remove any unbound antibody–enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of MMP2 bound in the initial step. The color development is stopped and the intensity of the color is measured. The assay range is 0.78 to 50 ng/mL and the LLD is 0.047 ng/mL. The interassay coefficients of variation are 3.0% (3.9 ng/mL), 4.2% (11.8 ng/mL), and 0.3% (17.9 ng/mL).
Galectin 3
The Human Galectin-3 Assay kit from Immuno-Biological Laboratories Co (Takasaki-Shi, Gunma) is a solid phase sandwich enzyme-linked immunosorbent assay using 2 kinds of high specific antibodies. Standards and samples are pipetted into the wells and any galectin 3 present is bound by the immobilized antibody. After washing away any unbound substances, an enzyme-linked polyclonal antibody specific for galectin 3 is added to the wells. Following a wash to remove any unbound antibody–enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of galectin 3 bound in the initial step. Tetramethylbenzidine is used as coloring agent. The color development is stopped and the intensity of the color is measured. The assay range is 117.19 to 7500 pg/mL and the LLD is 44 pg/mL. The interassay coefficients of variation are 1.2% (4395 pg/mL) and 0.8% (5267 pg/mL).
Vitamin D Analysis
For this study, we measured 25(OH)D since this is the major circulating form of vitamin D in the body and is representative of total vitamin D stores. The ADVIA Centaur Vitamin D Total assay is used for quantitative determination of total 25(OH)D. The assay is a 1-pass, 18-minute antibody competitive immunoassay that uses an antifluorescein monoclonal mouse antibody covalently bound to paramagnetic particles, an anti-25(OH)D monoclonal mouse antibody labeled with acridinium ester and a vitamin D analog labeled with fluorescein. An inverse relationship exists between the amount of vitamin D present in the patient sample and the amount of relative light units detected by the system.
Statistical Analysis
Results are presented as mean ± standard deviation, and statistical comparisons were made using the unpaired Student t test. A value of P < .05 was considered statistically significant. Biomarkers and hemodynamics were tested for distribution normality with the Shapiro-Wilk test. Due to lack of visual and statistical normality of PIIINP, MMP2, galectin 3, Fick CI, and PCWP, these values were converted to logarithm base 10 for statistical analysis. All statistical analyses were performed using SAS software version 9.2 (SAS Institute Inc; Cary, North Carolina). Linkage disequilibrium between the genotypes and the haplotype analyses was determined using haploview.24
Results
Clinical characteristics of the study patients are shown in Table 1. Of the 30 patients, 46.7% had ischemic HF and most had class III symptoms with a mean EF of 16.8%± 8%. The mean age was 52 years with most (73.3%) patients being male.
Table 1.
Baseline Demographics.
| Variable | All Patients (N = 30) | Haplotype (N = 20) | No Haplotype (N = 10) | P Value |
|---|---|---|---|---|
| Age, years | 52.1 ± 12.8 | 51.4 ± 14.8 | 53.6 ± 7.6 | .59 |
| Male | 22 (73.3) | 13 (59.1) | 9 (40.9) | .21 |
| Type of HF | ||||
| Ischemic | 14 (46.7) | 10 (50) | 4 (40) | .80 |
| Nonischemic | 16 (53.3) | 10 (50) | 6 (60) | |
| NYHA class | ||||
| II | 12 (40) | 7 (35) | 5 (50) | .73 |
| III | 16 (53.3) | 11 (55) | 5 (50) | |
| IV | 2 (6.7) | 2 (10) | 0 (0) | |
| EF | 16.8 ± 8 | 15 ± 6.2 | 20.1 ± 10.3 | .11 |
| ACEi/ARB | 27 (90) | 17 (85) | 10 (100) | .53 |
| β-Blocker | 27 (90) | 18 (90) | 9 (90) | 1 |
| Aldosterone antagonist | 22 (73.3) | 15 (75) | 7 (70) | 1 |
| Loop diuretic | 30 (100) | 20 (100) | 10 (100) | 1 |
| Thiazide diuretic | 7 (23.3) | 6 (20) | 1 (10) | .37 |
| Digoxin | 22 (73.3) | 16 (80) | 6 (60) | .38 |
| SBP | 107.6 ± 15.8 | 109.2 ± 17 | 104.5 ± 13.1 | .46 |
| SCr | 1.36 ± 0.43 | 1.39 ± 0.34 | 1.32 ± 0.58 | .69 |
| CrCl, mL/min | 66.4 ± 28.1 | 63 ± 28.3 | 73 ± 27.6 | .36 |
| 25-Hydroxyvitamin D | 20.1 ± 9.1 | 20.7 ± 9.2 | 18.8 ± 9.2 | .59 |
| log PIIINP, mcg/mL | 1.6 ± 0.36 | 1.74 ± 0.32 | 1.36 ± 0.31 | .0041 |
| log MMP2, ng/mL | 5.4 ± 0.23 | 5.43 ± 0.24 | 5.34 ± 0.19 | .36 |
| log galectin 3, pg/mL | 9.23 ± 0.27 | 9.22 ± 0.29 | 9.24 ± 0.26 | .82 |
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CrCl, creatinine clearance; EF, ejection fraction; HF, heart failure; MMP2, matrix metalloproteinase 2; NYHA, New York Heart Association; PIIINP, N-terminal propeptide of collagen type III; SBP, systolic blood pressure; SCr, serum creatinine.
Vitamin D receptor Genotypes
When evaluating the VDR genotypes, 3 of the variants examined were in linkage disequilibrium (BsmI ApaI, and TaqI, dD’0.83, r2 = .64). The most common haplotype block formed was CGA, which was present in 0.47% of the population. This was followed by the TTG (present in 25%) and CTA (present in 17%). All other haplotypes occurred at a frequency of <0.05%. The most common haplotype (CGA) was used for association with outcomes (see Figure 1). The FokI polymorphism was not shown to be in linkage disequilibrium with the other polymorphisms evaluated. The distribution of VDR genotypes is reported in Table 2.
Figure 1.
Haplotype block.
Table 2.
Frequency of Genotype.
| Fokl polymorphism | |
| CC/CT/TT | 15/11/4 |
| BsmI polymorphism | |
| TT/TC/CC | 5/10/15 |
| ApaI polymorphism | |
| TT/TG/GG | 8/16/6 |
| TaqI polymorphism | |
| AA/AG/GG | 17/9/4 |
Genomics, Baseline Parameters, Biomarkers, and Hemodynamics
In regard to baseline parameters, there were no significant differences in those patients with the VDR haplotype block as compared to those without the VDR haplotype (Table 1). Individual genotypes were not associated with log PIIINP, log MMP2, log galectin 3, log Fick CI, or log PCWP. The CGA haplotype of BsmI, ApaI, and TaqI was associated with significantly higher log PIIINP (1.74 ± 0.32 mcg/mL vs 1.36 ± 0.31 mcg/mL, P = .0041; Figure 2) and a trend toward a higher log PCWP (3.03 ± 0.43 mm Hg vs 2.72 ± 0.69 mm Hg, P = .14) than those without that haplotype. No association was seen when log Fick CI was evaluated, so overall no associations were seen with hemodynamic parameters. In addition, when evaluating vitamin D levels below and above the median level (19 ng/mL) there was no significant difference between these 2 groups in regard to log PIIINP, log MMP2, log galectin 3, log Fick CI, or log PCWP. Serum 25(OH)D levels were low for both groups and the baseline population was vitamin D insufficient. There was a nonsignificant decrease in serum 25(OH)D in the no-haplotype group compared to the haplotype group.
Figure 2.
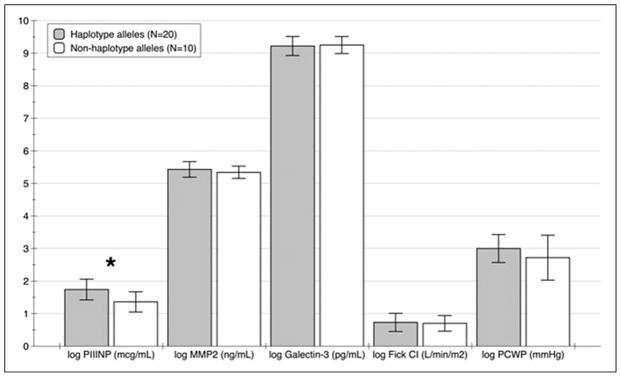
Biomarker comparison by CGA haplotype and non CGA haplotype, *P = .0041.
Discussion
In this preliminary study, we have demonstrated that a biomarker for collagen type III synthesis, PIIINP, was associated with the CGA haplotype of BsmI, ApaI, and TaqI single-nucleotide polymorphisms on the VDR. Specifically, patients with this haplotype had significantly higher levels of PIIINP, suggesting higher degree of fibrosis. These findings are consistent with previous findings, suggesting that vitamin D may play a role in fibrosis. More so, we have shown that VDR genetics may also play a role in fibrosis and potentially HF progression.
Collagen is the basic structural protein in the ECM of the heart that supports myocytes and fibroblasts. There is a continual balance between synthesis and degradation of the ECM. When this balance is disrupted and there is a change in the ECM turnover, fibrosis may develop. Fibrosis is thought to be an important driving force in the progression of HF. One of the markers for ECM turnover which may provide insight into the extent of myocardial fibrosis is PIIINP.25,26 This biomarker has been associated with poor outcomes in patients with HF.27,28 In addition, reduction in PIIINP levels with drug therapy has been associated with improved outcomes.27,29 The synthesis and accumulation of collagen type III is mediated by a number of factors including mechanical, cytokine, and neurohormonal stress. In regard to neurohormones, angiotensin II and aldosterone appear to be important in regard to collagen synthesis.30–32 Initial experiments in animals have demonstrated that vitamin D and the VDR inhibit renin expression.21,33 These findings are consistent with human studies showing that there is an inverse relationship between 1,25(OH)2D, 25(OH)D, and PRA.34 Based on these findings, it is plausible that patients with the CGA haplotype may have limited ability to suppress renin production resulting in greater ECM turnover and high PIINP levels. We did not evaluate PRA and therefore this assertion is only conjecture; however, another study evaluating only the FOK1 polymorphism found that receptor genomics did affect PRA.22 Specifically, PRA was attenuated in both patients with hypertension and normotensive patients with the minor allele (T) of the FOK1 polymorphism. Although our study did not find that FOK1 polymorphisms influence any of the ECM biomarkers, this may suggest that either CGA haplotype has greater influence on PRA or other more important pathways that promote fibrosis are influenced by receptor genomics.
As previously mentioned there are a number of pathways that may promote ECM turnover and fibrosis including inflammation. There are a number of studies demonstrating that vitamin D may attenuate proinflammatory response. For example, in a study on type 2 diabetic patients, 1,25(OH) 2D was able to downregulate the expression of tumor necrosis factor α, inter-leukin (IL) 6, IL-1, and IL-8 in monocytes.8 In addition to vitamin D levels, VDR polymorphism has also been linked to markers of systemic inflammation such as C-reactive protein levels and periodontal disease.35–37 It is plausible that patients with the CGA haplotype may have a higher state of inflammation, which may promote fibrosis and higher PIIINP levels.
Interestingly, the 1 marker we evaluated which is expressed by activated macrophages, galectin 3, was not influenced by receptor genomics. Galectin 3 is highly expressed and secreted by macrophages and has been shown to be involved in the regulation of myocardial fibrosis.38 In addition, galectin 3 levels have been shown to be elevated in patients with HF.39,40 In our study, VDR receptor genomics was not shown to be associated with galectin 3 levels. The reason for this is unknown. There are data suggesting that vitamin D levels may be able to down-regulate the inflammatory response associated with macrophages.41 Whether or not receptor polymorphisms play a role is unknown. There are no data to our knowledge which have directly evaluated VDR genetics and macrophage activation. It is also unknown whether galectin 3 is a mediator or a marker for HF. If galectin 3 is a marker then our findings are not surprising since from a clinical viewpoint patients with and without the defined haplotype block were similar. In addition, galectin 3 has been shown to be elevated in a number of other fibrotic conditions which may make using this biomarker to track changes in myocardial remodeling challenging. Additional larger studies evaluating patients with HF at different stages of HF are required to determine whether vitamin D and VDR polymorphisms are important in determining galectin 3 expression.
Our findings showing VDR genomics may influence myocardial structure are consistent with a previous study evaluating left ventricular mass index (LVMI) in patients with end-stage renal disease.42 In this study, the role of Bsml VDR polymorphism on LVMI progression was evaluated in 182 dialysis patients over an 18-month period. A total of 175 healthy individuals were used as a reference group. The results of this study demonstrated that the number of B alleles was associated with changes in LVMI. Unfortunately, no other receptor polymorphisms or ECM biomarkers were evaluated in the study. These findings, similar to ours, support the hypothesis that VDR genomics may play a role in myocardial remodeling.
There are a number of significant limitations with this study. Being a pilot study, the number of patients evaluated is too low for any conclusive findings. In addition, the type of biomarkers we evaluated demonstrates significant variation. However, despite these limitations we were still able to suggest that receptor genomics may play role in development of fibrosis. Although we demonstrated association with receptor genomics and log PIIINP concentrations, the clinical application of this association (and a log difference of 0.38 mcg/mL) still needs to be determined. Overall, understanding the clinical utility of using biomarkers for fibrosis such as PIIINP and MMP is not known at this time. Another limitation of this study is that the patients in this study tended to be sicker patients as seen by New York Heart Association classification, relatively low EF, and blood pressure. How VDR genomics influence HF progression in less sick patients still needs to be addressed.
Overall, the results of this study suggest that VDR receptor polymorphisms may affect fibrosis in patients with systolic HF as measured by ECM turnover (PIIINP). Given the wide range of effects that vitamin D has on the body, receptor genomics should influence disease progression. In fact, the interplay between VDR receptor genomics and disease progression has been observed not only in regard to myocardial remodeling but in a number of disease conditions. Disease states and conditions in which VDR receptor genomics may play role include cardiovascular disease, osteoporosis, diabetes, and cancer.16–20 In this context, the results of this investigation is very plausible and hypothesis generating. Our findings need to be confirmed in a larger cohort of patients. Perhaps most importantly, future studies will need to address whether or not vitamin D supplementation can be beneficial in attenuating HF progression in patients with at-risk genotype.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: Funding support for this study was provided in part by Michigan Institute for Clinical & Health Research and the National Center for Research Resources, Grant UL1RR024986 and National Center for Advancing Translational Sciences, Grant 2UL1TR000433. National Institutes of Health NIH grant HL074894 (R.U. Simpson). Dr. Simpson received funding from Abbott Laboratories and is a shareholder of Cardiavent, Inc.
Footnotes
The work was performed at University of Michigan Health System, 1500 East Medical Center Drive, Ann Arbor, Michigan, 48109, USA
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Weishaar RE, Kim SN, Saunders DE, Simpson RU. Involvement of vitamin D3 with cardiovascular function. III. Effects on physical and morphological properties. Am J Physiol. 1990;258(1 pt 1):E134–E142. doi: 10.1152/ajpendo.1990.258.1.E134. [DOI] [PubMed] [Google Scholar]
- 2.Weishaar RE, Simpson RU. Vitamin D3 and cardiovascular function in rats. J Clin Invest. 1987;79(6):1706–1712. doi: 10.1172/JCI113010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weishaar RE, Simpson RU. Involvement of vitamin D3 with cardiovascular function II. Direct and indirect effects. Am J Physiol Endocrinol Metab. 1987;253(6 pt 1):E675–E683. doi: 10.1152/ajpendo.1987.253.6.E675. [DOI] [PubMed] [Google Scholar]
- 4.Xiang W, Kong J, Chen S, et al. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab. 2005;288(1):E125–E132. doi: 10.1152/ajpendo.00224.2004. [DOI] [PubMed] [Google Scholar]
- 5.Rahman A, Hershey S, Ahmed S, Nibbelink K, Simpson RU. Heart extracellular matrix gene expression profile in the vitamin D receptor knockout mice. J Steroid Biochem Mol Biol. 2007;103(3–5):416–419. doi: 10.1016/j.jsbmb.2006.12.081. [DOI] [PubMed] [Google Scholar]
- 6.Nemerovski CW, Dorsch MP, Simpson RU, Bone HG, Aaronson KD, Bleske BE. Vitamin D and Cardiovascular Disease. Pharmacotherapy. 2009;29(6):691–708. doi: 10.1592/phco.29.6.691. [DOI] [PubMed] [Google Scholar]
- 7.Müller K, Haahr PM, Diamant M, Rieneck K, Kharazmi A, Bendtzen K. 1,25-Dihydroxyvitamin D3 inhibits cytokine production by human blood monocytes at the posttranscriptional level. Cytokine. 1992;4(6):506–512. doi: 10.1016/1043-4666(92)90012-g. [DOI] [PubMed] [Google Scholar]
- 8.Giulietti A, Etten Ev, Overbergh L, Stoffels K, Bouillon R, Mathieu C. Monocytes from type 2 diabetic patients have a pro-inflammatory profile. 1,25-Dihydroxyvitamin D3 works as anti-inflammatory. Diabetes Res Clin Pract. 2007;77(1):47–57. doi: 10.1016/j.diabres.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Zhu Y, Mahon BD, Froicu M, Cantorna T. Calcium and 1-alpha,25-dihydroxyvitamin D3 target the TNF-alpha pathway to suppress experimental inflammatory bowel disease. Eur J Immunol. 2005;35(1):217–224. doi: 10.1002/eji.200425491. [DOI] [PubMed] [Google Scholar]
- 10.Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168(12):1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 11.Gotsman I, Shauer A, Zwas DR, Hellman Y, Keren A, Lotan C, Admon D. Vitamin D deficiency is a predictor of reduced survival in patients with heart failure; vitamin D supplementation improves outcomes. Eur J Heart Fail. 2012;14(4):357–366. doi: 10.1093/eurjhf/hfr175. [DOI] [PubMed] [Google Scholar]
- 12.Grandi NC, Breitling LP, Vossen CY, et al. Serum vitamin D and risk of secondary cardiovascular disease events in patients with stable coronary heart disease. Am Heart J. 2010;159(6):1044–1051. doi: 10.1016/j.ahj.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 13.Elamin MB, Abu Elnour NO, Elamin KB, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96(7):1931–1942. doi: 10.1210/jc.2011-0398. [DOI] [PubMed] [Google Scholar]
- 14.van Ballegooijen AJ, Visser M, Cotch MF, et al. Serum vitamin D and parathyroid hormone in relation to cardiac structure and function: the ICELAND-MI substudy of AGES-Reykjavik. J Clin Endocrinol Metab. 2013;98(6):2544–2552. doi: 10.1210/jc.2012-4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uitterlinden AG, Fang Y, van Meurs JBJ, Pols HAP, van Leeuwen JPTM. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338(2):143–156. doi: 10.1016/j.gene.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Uitterlinden AG, Ralston SH, Brandi ML, et al. The association between common vitamin D receptor gene variations and osteoporosis: a participant-level meta-analysis. Ann Intern Med. 2006;145(4):255–264. doi: 10.7326/0003-4819-145-4-200608150-00005. [DOI] [PubMed] [Google Scholar]
- 17.Ortlepp JR, Krantz C, Kimmel M, et al. Additive effects of the chemokine receptor 2, vitamin D receptor, interleukin-6 polymorphisms and cardiovascular risk factors on the prevelance of myocardial infarction in patients below 65 years. Int J Cardiol. 2005;105(1):90–95. doi: 10.1016/j.ijcard.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Ortlepp JR, Lauscher J, Hoffmann R, Hanrath P, Joost HG. The vitamin D receptor gene variant is associated with the prevalence of type 2 diabetes mellitus and coronary artery disease. Diabetic Med. 2001;18(10):842–845. doi: 10.1046/j.1464-5491.2001.00585.x. [DOI] [PubMed] [Google Scholar]
- 19.Falleti E, Bitetto D, Fabris C, et al. Vitamin D receptor gene polymorphisms and hepatocellular carcinoma in alcoholic cirrhosis. World J Gastroenterol. 2010;16(24):3016–3024. doi: 10.3748/wjg.v16.i24.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levin GP, Robinson-Cohen C, Boer IH, et al. Genetic variants and associations of 25-hydroxyvitamin D concentrations with major clinical outcomes. JAMA. 2012;308(18):1898–1905. doi: 10.1001/jama.2012.17304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-dihydroxyvitamin D3 is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110(2):229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaidya A, Sun B, Forman JP, et al. The Fok1 vitamin D receptor gene polymorphism is associated with plasma renin activity in Caucasians. Clin Endocrinol (Oxf) 2011;74(6):783–790. doi: 10.1111/j.1365-2265.2011.03991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiagen - Sample and Assay Technologies. [Accessed January 20, 2014]; Available at: http://www.qiagen.com/products/catalog/automated-solutions/pyrosequencing/pyromark-q96-id.
- 24.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 25.Lopez B, Gonzalez A, Querejeta R, et al. The use of collagen-derived serum peptides for the clinical assessment of hypertensive heart disease. J Hypertens. 2005;23(8):1445–1451. doi: 10.1097/01.hjh.0000173780.67308.f1. [DOI] [PubMed] [Google Scholar]
- 26.Zannad F, Rossignol P, Iraqi W. Extracellular matrix fibrotic markers in heart failure. Heart Fail Rev. 2010;15(4):319–329. doi: 10.1007/s10741-009-9143-0. [DOI] [PubMed] [Google Scholar]
- 27.Zannad F, Alla F, Dousset B, Perez A, Pitt B. Limitations of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the randomized aldactone evaluation study (RALES). Rales Investigators. Circulation. 2000;102(22):2700–2706. doi: 10.1161/01.cir.102.22.2700. [DOI] [PubMed] [Google Scholar]
- 28.Radauceanu A, Ducki C, Virion JM, et al. Extracellular matrix turnover and inflammatory markers independently predict functional status and outcome in chronic heart failure. J Card Fail. 2008;14(6):467–474. doi: 10.1016/j.cardfail.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 29.Pitt B, Reichek N, Willlenbrock R, et al. Effects of eplerenone, enalapril, and eplerenone/enalapril in patients with essential hypertension and left ventricular hypertrophy: the 4E-left ventricular hypertrophy study. Circulation. 2003;108(15):1831–1838. doi: 10.1161/01.CIR.0000091405.00772.6E. [DOI] [PubMed] [Google Scholar]
- 30.Weber KT, Sun Y, Guarda E, et al. Myocardial fibrosis in hypertensive heart disease: an overview of potential regulatory mechanisms. Eur Heart J. 1995;16(suppl C):24–28. doi: 10.1093/eurheartj/16.suppl_c.24. [DOI] [PubMed] [Google Scholar]
- 31.Stein M, Boulaksil M, Jansen JA, et al. Reduction of fibrosis related arrhythmias by chronic renin-angiotensin-aldosterone-system inhibitors in an aged mouse model. Am J Physiol. 2010;299(2):H310–H321. doi: 10.1152/ajpheart.01137.2009. [DOI] [PubMed] [Google Scholar]
- 32.Wright JW, Mizutani S, Harding JW. Pathways involved in the transition from hypertension to hypertrophy to heart failure. Treatment strategies. Heart Fail Rev. 2008;13(3):367–375. doi: 10.1007/s10741-007-9060-z. [DOI] [PubMed] [Google Scholar]
- 33.Kong J, Qiao G, Zhang Z, Liu SQ, Li YC. Targeted vitamin D receptor expression in juxtaglomerular cells suppresses renin expression independent of parathyroid hormone and calcium. Kidney Int. 2008;74(12):1577–1581. doi: 10.1038/ki.2008.452. [DOI] [PubMed] [Google Scholar]
- 34.Burgess ED, Hawkins RG, Watanabe M. Interaction of 1,25-dihydroxyvitamin D and plasma renin activity in high renin essential hypertension. Am J Hypertens. 1990;3(12 pt 1):903–905. doi: 10.1093/ajh/3.12.903. [DOI] [PubMed] [Google Scholar]
- 35.Pacini S, Punzi T, Gulisano M, et al. Vitamin D receptor alleles and C-reactive protein in hemodialysis patients. Ital J Anat Embryol. 2008;113(1):55–62. [PubMed] [Google Scholar]
- 36.Punzi T, Fabris A, Morucci G, et al. C-reactive protein levels and vitamin d receptor polymorphisms as markers in predicting cachectic syndrome in cancer patients. Mol Diagn Ther. 2012;16(2):115–124. doi: 10.1007/BF03256436. [DOI] [PubMed] [Google Scholar]
- 37.Chen LL, Li H, Zhang PP, Wang SM. Association between vitamin D receptor polymorphisms and periodontitis: a meta-analysis. J Periodontol. 2012;83(9):1095–1103. doi: 10.1902/jop.2011.110518. [DOI] [PubMed] [Google Scholar]
- 38.Sharma UC, Pokharel S, van Brakel TJ, et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Ciruclation. 2004;110(19):3121–3218. doi: 10.1161/01.CIR.0000147181.65298.4D. [DOI] [PubMed] [Google Scholar]
- 39.de Boer RA, Lok DJA, Jaarsma T, et al. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann Med. 2011;43(1):60–68. doi: 10.3109/07853890.2010.538080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Felker GM, Fiuzat M, Shaw L, et al. Galectic-3 in ambulatory patients with heart failure. Results from the HF-ACTION study. Cir Heart Fail. 2012;5(1):72–78. doi: 10.1161/CIRCHEARTFAILURE.111.963637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Villaggio B, Soldano S, Cutolo M. 1,25-dihydroxyvitamin D3 downregulates aromatase expression and inflammatory cytokines in human macrophages. Clin Exp Rheumatol. 2012;30(6):934–938. [PubMed] [Google Scholar]
- 42.Testa A, Mallamaci F, Benedetto FA, et al. Vitamin D receptor (VDR) gene polymorphism is associated with left ventricular (LV) mass and predicts left ventricular hypertrophy (LVH) progression in end-stage renal disease (ESRD) patients. J Bone Miner Res. 2010;25(2):313–319. doi: 10.1359/jbmr.090717. [DOI] [PubMed] [Google Scholar]



