Abstract
The present study demonstrates the effect of DHPEA on suppression of irradiation-induced pulmonary fibrosis. A 60Co irradiator was used to induce pulmonary fibrosis in a rat model at a dose of 22 Gy. The rats of the treatment and positive control group were intraperitoneally injected DHPEA (10 mg/kg) or dexamethasone (DEX; 5 mg/kg) daily for 30 days. Hydroxyproline assay was used to evaluate the fibrosis of pulmonary and lung tissue sections after irradiation. Hematoxylin and eosin (H&E) and Masson’s trichrome stained lung section were used for alveolitis and fibrosis score analyses, respectively. Immunohistochemistry was used for surfactant protein-B (SPB) and α-SMA expression analysis. Western blot analysis was employed for analysis of nuclear transcription factor NF-E2-related factor 2 (Nrf-2) and its associated antioxidant enzymes like heme oxygenase-1 (HO-1) and NAD (P) H: quinone oxidoreductase-1 (NQO-1). Results revealed a significant decrease in mortality rates and lung index scores, decreased collagen deposition, reduced MDA content and enhanced superoxide dismutase (SOD) activity in DHPEA treated rats compared to DEX-treated rats. DHPEA treatment also inhibited (myo) fibroblast proliferation, and regulated serum levels of TGF-β1, IL-6, IL-10, and TNF-α. In addition, DHPEA-treatment activated Nrf-2 and its downstream antioxidant enzymes HO-1 and NQO-1. Thus DHPEA can be a promising agent for the suppression of irradiation-induced pulmonary fibrosis.
Keywords: Pulmonary fibrosis, antioxidant, transcription, virgin olive
Introduction
A number of secoiridoid compounds possesing p-hydroxyphenylethanol and 3,4-dihydroxyphenylethanol (DHPEA) motif are reported from the phenolics of olive fruits. DHPEA is the most active antioxidant of all the constituents present in the virgin olive oil [1-4]. The presence of two hydroxyl groups on ortho position of aromatic ring imparts stronger antioxidant activities to DHPEA compared to α-tocopherol [5]. DHPAE and α-tocopherol are shown to exhibit synergistic effect when used in combination [6].
Nuclear accidents, total body irradiation for hematopoietic stem cell transplantation, thoracic radiotherapy for lung, breast, thymoma, and lymphoma cancers are accompanied by radiation-induced pulmonary fibrosis [7,8]. Radiation-induced pulmonary fibrosis begins to develop after 6 months and 100-120 days in humans and C57BL/6J mouse model respectively [8]. It is reported that 20.3-36.9%of the radiation treated cancer patients develop radiation-induced pulmonary injury [9-12]. The currently available drugs only decrease acute pneumonitis for 2-3 months after irradiation but fail to suppress fibrosis [13]. Exposure to radiation induces oxidative stress through activation of oxidant-generating enzymes, mitochondrial leakage, and the activation of the respiratory burst in the phagocytic cells that infiltrate damaged tissue [14]. Thus irradiation-induced pulmonary fibrosis treatment strategies involve use of antioxidants like epigallocatechin-3-gallate [15]. Therefore, the present study is designed to examine the efficacy of DHPEA with regard to preventing or treating irradiation-induced pulmonary fibrosis. In response to oxidative stress by reactive oxygen species (ROS), endogenous antioxidant enzymes get activated in the lung cells. Nuclear transcription factor NF-E2-related factor 2 (Nrf2) plays a vital role in the regulation of transcription of the cytoprotective enzymes. A negative regulator of Nrf2 transcription, Kelchlike ECH-associated protein 1 (Keap1) suppresses the transcription of Nrf2 under normal conditions. It is reported that Nrf2 dissociates from cytosolic Keap1 and translocates to nucleus, binds to antioxidant response element (ARE) in the promoter regions of the genes encoding antioxidant enzymes leading to their transcription [16]. The enzymes comprising antioxidant enzyme system regulated by the Nrf2-ARE signaling pathway are heme oxygenase-1 (HO-1), γ-glutamine cysteine synthetase (γ-GCS), NAD (P) H: quinone oxidoreductase-1 (NQO-1) and superoxide dismutase (SOD).
In the present study, we hypothesized that the administration of DHPEA (Figure 1) would significantly inhibit irradiation-induced pulmonary fibrosis. We first evaluated the efficacy of DHPEA to suppress irradiation-induced pulmonary fibrosis and then examined whether DHPEA treatment influenced Nrf-2, HO-1 and NQO-1 levels in irradiated rats. To the best of our knowledge, this study is the first to assess and highlight the efficacy of DHPEA in the treatment of irradiation-induced pulmonary fibrosis.
Figure 1.
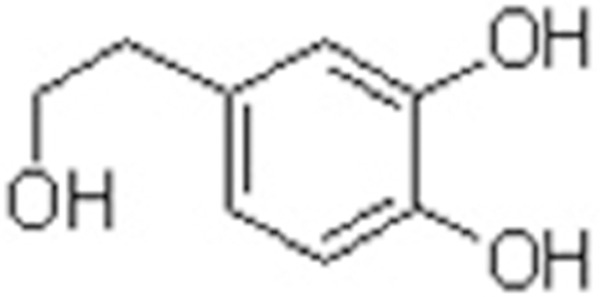
Structure of 3,4-dihydroxyphenylethanol (DHPEA).
Materials and methods
Animals
Ten week old male, adult SpragueDawley rats (Chengdu Dashuo Biological Technology Co., Ltd., Chengdu, China) were maintained according to the guidelines of the National Institutes of Health and Academy of Military Medical Sciences for the Care and Use of Laboratory Animals. Rats were provided with pathogen-free water and food with a 12 h light/dark cycle and were observed daily up to 4 months after irradiation. The study was approved by the Committee on the Ethics of Animal Experiments of the Affiliated Hospital of Academy of Military Medical Sciences.
Irradiation and treatment
Among 4 groups of rats with 20 each, three were exposed to 22 Gy doses of radiations at a rate of 290 cGy/min using 60Co irradiator (Reviss Services (UK), Ltd., Buckinghamshire, UK) on entire thorax after anesthetization. The treatment and positive control group of irradiated rats were intraperitoneally injected 10 mg/kg DHPEA (Sigma, St. Louis, MO, USA) and 5 mg/kg dexamethasone (Tianjing Pharmaceuticals Group Corp., Tianjing, China) respectively, daily for 20 days. Untreated rats received only radiations without any treatment. The control group of rats were not irradiated.
Specimen processing and histopathology
On day 10, 20, 40 or 80 after irradiation six rats from each group were sacrificed after recording the body weight. The right lungs were fixed with 4%paraformaldehyde, dehydrated in ethanol and embedded in paraffin. Lung sections (2 μm) were stained with hematoxylin and eosin (H&E) and Masson’s trichrome (Masson). Blood samples from heart were centrifuged after clotting to obtain serum samples. The right lungs were dehydrated in ethanol and embedded in paraffin.
Lung index measurement
The lung wet weight (mg) was divided by body weight (g) to get lung index.
Measurement of collagen content in the lungs
For determination of lung collagen content using the hydroxyproline (Hyp) assay (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), 120 mg of left lung tissue was hydrolyzed in 1 ml of lysis buffer solution at 100°C for 30 min and the absorbance was measured.
Malondialdehyde (MDA) content and SOD activity measurements in serum
Serum samples were assayed for MDA content and SOD activity using kits and protocol from Nanjing Jiancheng Bioengineering Institute. MDA, an end product of ROS-induced peroxidation of cell membrane lipids, is a marker of oxidative damage. MDA content was determined by reacting MDA with 2-thiobarbituric acid and measuring chromogen generation. For SOD activity the capacity of sample to suppress ferricytochrome c was measured.
Immunohistochemical analyses
Lung sections (2 μm) were deparaffinized, rehydrated, and exposed to antigen retrieval with citrate buffer. Endogenous peroxidases were quenched using 3% H2O2 for 10 min. The sections were incubated with surfactant protein-B (SPB) or α smooth muscle actin (α-SMA) (Boster Biological Technology, Wuhan, China) antibodies at 37°C for 2 h. Washing with PBS was followed by incubation with poly-peroxidase-conjugated anti-mouse/rabbit IgG for 20 min. Diaminobenzidine (Dako, Glostrup, Denmark) and Mayer’s hematoxylin were used for visualization and counterstaining of the slides.
Serum cytokine levels
TGF-β1 ELISA kit (Boster Biological Technology) was used for determination of serum levels of TGF-β1. Serum levels of IL-6, IL-10, and TNF-α were measured using flow cytometric bead assays (BD™ CBA Flex Set; BD, Sparks, MD, USA).
Western blot analyses
The frozen lungs were washed with ice-cold phosphate-buffered saline and then lysed in lysis buffer. The lysates were sonicated for 10 s, centrifuged for 20 min at 15000 rpm and then stored at -70°C. A BCA protein assay kit (Beyotime Institute of Biotechnology, Jiangsu, China) was used to determine protein concentrations. Equal amounts of protein were separated by SDS-PAGE, transferred to a PVDF membrane (Millipore Corp., Billerica, MA, USA), and incubated with 5% BSA at room temperature for 3 h to block non-specific binding. The membranes were then incubated with Nrf-2, HO-1, NQO-1 or β-actin (Cell Signaling Technology, Inc., Danvers, MA, USA) for 3 h. After washing with TBS-T, the membranes were incubated with HRP-conjugated secondary antibodies. The protein bands were visualized using enhanced chemiluminescence with a Super Signal detection kit (Boster Biological Technology).
Morphometric analyses
To determine alveolitis and fibrosis score, lung sections were stained with H&E or Masson’s trichrome respectively. Ten fields were selected per section/rat and examined under Olympus microscope (Olympus, Tokyo, Japan) and the mean score of each group was determined. For immunohistochemical analyses of SPB and α-SMA, staining density was determined using Image Proplus software in one field with a prominent DAB reaction for each section. The scale of 0-3 score where grade 0 for normal lung; grade 1 for minimal lesion; grade 2 for moderate lesion; or grade 3 for severe lesion was used.
Statistical analysis
All the results are expressed as the mean ± S. D. Two-way ANOVA and Student’s t-tests were used for data analysis. The differences were considered statistically significant for P < 0.05.
Results
Effect of DHPEA on mortality and fibrous nodules formation in pulmonary tissues
The mortality rate in DHPEA (15%) treated rats was significantly lower compared with DEX-treated (30%) and untreated rats (30%). In DHPEA-treated rats the bleeding sites in pulmonary tissues were absent and signs of congested edema were also not observed after 80 days of irradiation compared with DEX-treated and untreated rats (Figure 2).
Figure 2.
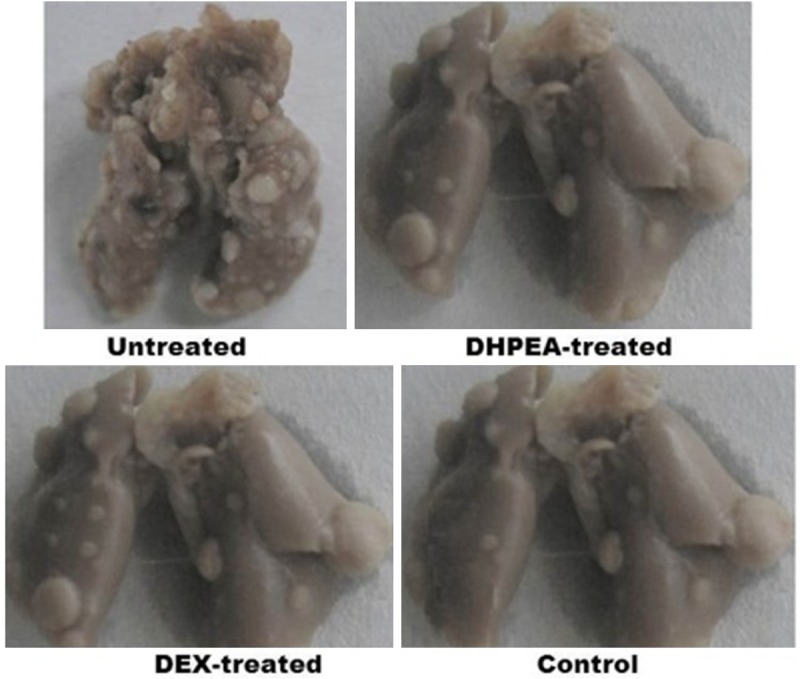
Effect of DHPEA on the lung appearance after 80 days of irradiation. DHPEA-treated animals showed no lung collapse and gray fibrous nodules.
Effect of DHPEA on lung index score
Lung index was significantly lower in DHPEA-treated animals relative to DEX-treated animals at 10, 20, 40 and 80 days after irradiation (Figure 3). This result was consistent with morphometric observations of lung appearance in DHPEA-treated animals.
Figure 3.
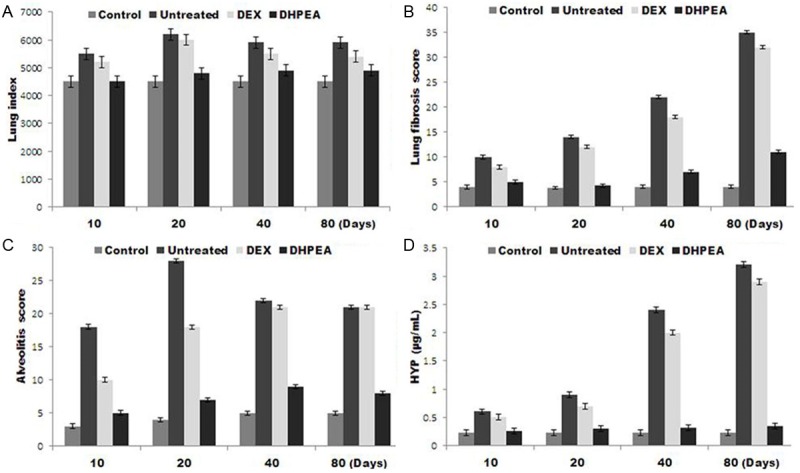
Effect of DHPEA on the lung index score, combined alveolitis score, combined fibrosis score, and hydroxyproline (Hyp) content at 10, 20, 40 and 80 days after-irradiation.
Effect of DHPEA on histological changes and collagen deposition in pulmonary tissues
Examination of H&E-stained irradiated pulmonary tissues revealed significantly thickened alveolar walls, extensive deposition of collagen, and regional fibrotic foci after 80 days of irradiation. Treatment with DHPEA significantly improved irradiation-induced regional fibrotic foci and collagen depositions of the lung sections (Figure 4). The alveolitis and fibrosis scores of DHPEA-treated animals were significantly lower than those of DEX-treated animals at 10, 40 and 80 days after irradiation. The alveolitis score of the latter group was significantly lower than that of the untreated animals at 10 and 20 days but were similar at 80 days after irradiation (Figure 3B, 3C).
Figure 4.
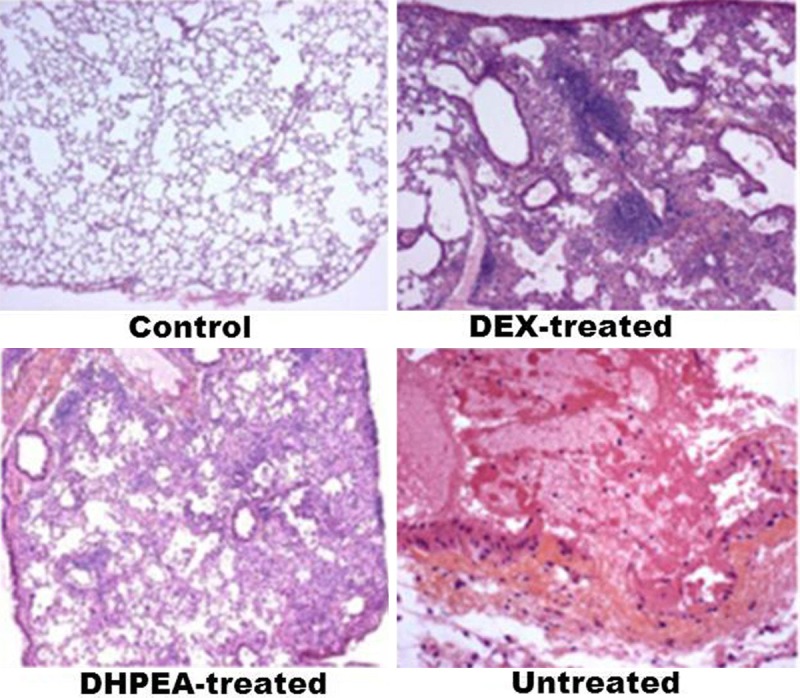
Effect of DHPEA on the histological changes in lung tissue after 80 days of irradiation.
The amount of Hyp, a major constituent of collagen was significantly lower in DHPEA-treated animals than DEX-treated animals at 40 and 80 days after irradiation. The Hyp content of the latter group was significantly lower than that of the untreated animals at 10 and 20 days after irradiation but was similar at 40 and 80 days (Figure 3D).
Effect of DHPEA on serum redox state
Investigation of the serum MDA concentration revealed a significantly lower level in DHPEA-treated animals than DEX-treated animals at 40 and 80 days after irradiation. The MDA concentration in DEX-treated animals was similar to that of the untreated animals at 10, 20, 40 and 80 days (Figure 5). In DHPEA-treated animals serum SOD activity was significantly higher compared with DEX-treated animals at 10, 20, 40 and 80 days after irradiation. Levels of SOD activity in the latter group were significantly higher than those of the untreated animals at 20 days after irradiation but similar at 10, 40 and 80 days (Figure 5).
Figure 5.
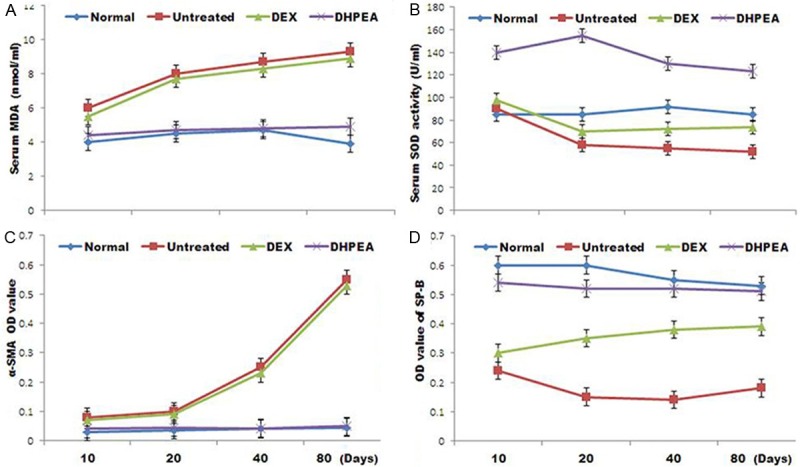
Effect of DHPEA on serum malondialdehyde (MDA) concentrations, superoxide dismutase (SOD) activity, α smooth muscle actin (α-SMA) levels and surfactant protein-B (SPB) levels at 10, 20, 40 and 80 days after irradiation.
Effect of DHPEA on (myo) fibroblast proliferation and alveolar epithelial type II (AE2) cells from injury
We observed a significant inhibition of α-SMA expression and promotion of SPB expression in DHPEA-treated rats compared to that in DEX-treated animals at 80 days post-irradiation. In DHPEA-treated animals OD value for α-SMA immunohistochemistry was significantly lower compared with the DEX-treated animals at 40 and 80 days after irradiation. The OD value for α-SMA in DEX-treated and untreated animals were similar at 10, 30, 40 and 80 days after irradiation (Figure 5). On the other hand treatment with DHPEA led to OD higher value for SPB immunohistochemistry compared with the DEX-treated animals at 10, 20, 40 and 80 days after irradiation. The OD value for SPB immunohistochemistry in the latter group was significantly higher than that of the untreated animals at 10, 20 and 80 days after irradiation (Figure 5).
Effect of DHPEA on serum cytokine levels
Examination of the serum levels of TGF-β1 using ELISA as well as IL-6, IL-10, and TNF-α using the BD™ CBA Flex Set revealed a significant decrease in serum level of TGF-β1 in the DHPEA-treated animals compared with the DEX-treated animals at 10, 40 and 80 days after irradiation. However the level observed in the DEX-treated animals was similar to that of the untreated animals at the same time points (Figure 6A).
Figure 6.
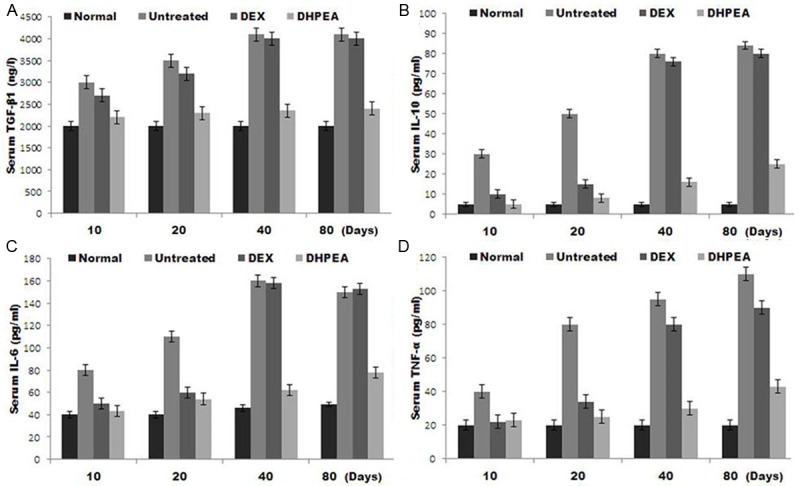
Effect of DHPEA on the serum levels of transforming growth factor β1 (TGF-β1), interleukin (IL)-6, IL-10, and tumor necrosis factor α (TNF-α) at 10, 20, 40 and 80 days after irradiation.
DHPEA-treatment also caused a significant decrease in serum levels of IL-6, IL-10 and TNF-α compared to the DEX-treated animals at 40 and 80 days after irradiation. The levels of IL-6 and TNF-α in untreated group were significantly higher compared to DEX-treated group at 10 and 20 days after irradiation. IL-10 was significantly reduced in the DEX-treated animals compared with the untreated animals at 20 days after irradiation. At days 40 and 80 after irradiation the levels of IL-6, IL-10 and TNF-α in the DEX-treated and untreated animals were similar (Figure 6B-D).
Effect of DHPEA on Nrf-2 and its downstream antioxidant enzymes
Investigation of the effect of DHPEA on Nrf-2 signaling and its associated antioxidant enzymes using Western blotting revealed a significant enhancement of Nrf-2, HO-1, and NQO-1 protein expression in DHPEA-treated animals compared to DEX at 10 days after irradiation (Figure 7).
Figure 7.
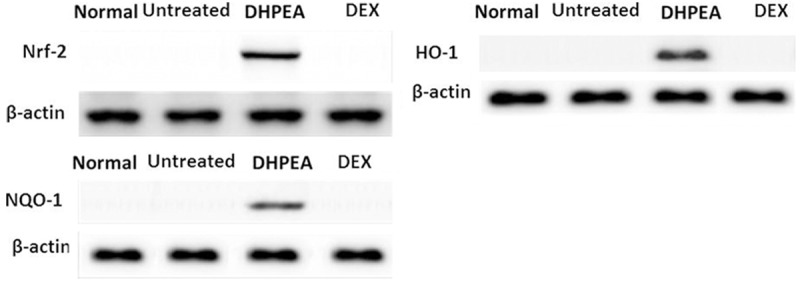
DHPEA activates nuclear transcription factor NF-E2-related factor 2 (Nrf-2), heme oxygenase-1 (HO-1) and NAD (P) H: quinone oxidoreductase-1 (NQO-1) protein expression as detected by western blot analysis of lung tissue extracts.
The Nrf-2, HO-1, and NQO-1 protein expression was significantly higher in DHPEA-treated animals compared to those of the DEX-treated animals at 10, 20, 40 and 80 days after irradiation. However the Nrf-2, HO-1 and NQO-1 expression in the DEX-treated group was significantly higher than those of the untreated group at 10 and 20 days but similar at 40 and 80 days after irradiation (Figure 8).
Figure 8.
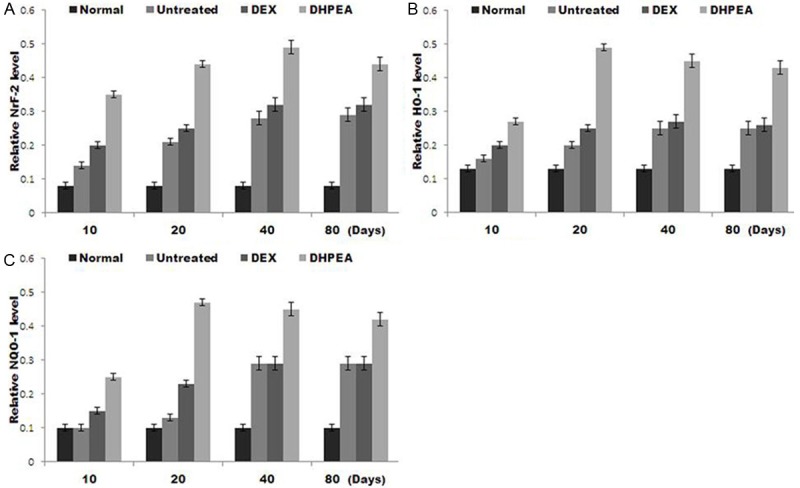
The protein levels of nuclear transcription factor NF-E2-related factor 2 (Nrf-2), heme oxygenase-1 (HO-1) and NAD (P) H: quinone oxidoreductase-1 enzyme (NQO-1) were compared using western blot analysis of lung tissue extracts at 10, 20, 40 and 80 days after irradiation.
Discussion
In radiation-induced pulmonary fibrosis oxidative stress and ROS directly and indirectly play a vital role [17]. The inflammatory cells on activation by ROS induce expression of a variety of intracellular oxidative enzymes and synthesis of reactive nitrogen species (RNS) to remove necrotic tissue [18,19]. Change in oxidant/antioxidant balance in the lung increases tissue damage. Thus the present study demonstrates the effect of DHPEA on inhibition of irradiation-induced pulmonary fibrosis. The present study demonstrates that effect of irradiation-induced pulmonary fibrosis in rats is suppressed by DHPEA treatment. DHPEA treatment led to reduction of mortality rate, lung index score, lung histological damage, collagen deposition, inhibited (myo) fibroblast proliferation, and regulated the serum levels of TGF-β1, IL-6, IL-10, and TNF-α. DHPEA treatment also activated Nrf-2 and its downstream antioxidant enzymes HO-1 and NQO-1. The DEX treatment of irradiation-induced pulmonary fibrosis did not produce similarly effects. Thus DHPEA treatment significantly suppresses irradiation-induced pulmonary fibrosis.
The level of serum MDA indicates degree of organic lipid peroxidation causing damage to cell membranes [20]. SOD is reported to maintain organic oxidant/antioxidant balance and neutralize free radicals, thereby protecting cells from oxidative damage. The levels of MDA and activity of SOD in serum are therefore indicators of oxidative stress and the body’s capacity to respond to induced oxidative stress. The results from our study demonstrate that a decrease of MDA and inflammatory cytokines and increase of serum SOD activity in DEX-treated animals compared with untreated animals at 15 and 30 days after irradiation. DHPEA-treated rats showed even greater SOD activity non-irradiated normal controls, at all the time points. Our results prove confirm that DHPEA is superior to DEX with regard to increasing the body’s capacity to handle oxidative stress due to ROS/RNS.
TGF-β1, a powerful cytokine promotes proliferation and maturation of fibroblast, thus accelerating the development of pulmonary fibrosis [21]. There is correlation between TGF-β1 levels and incidence of radiation-treatment-induced lung injury among lung cancer patients [22]. TNF-α stimulates the proliferation of fibroblasts and the secretion of proinflammatory cytokines, including IL-1 and IL-6, from neutrophils and macrophages [23]. It is suggested that IL-6 leads to inflammation and fibrosis associated with hypersensitivity pneumonitis in mice. These results suggest a close relationship in IL-6 and pneumonitis and fibrotic development [24]. IL-10 may inhibit monocytes, macrophages, and Th1 cells as well as enhance Bcell immune regulation function [25]. In addition, IL-10 is a T-cell-derived cytokine of the Th-2 family that suppresses inflammation by inhibiting numerous pro-inflammatory cytokines [26].
To investigate the effects of DHPEA treatment on systemic inflammation, we measured the serum levels of key inflammatory cytokines including TGF-β1, IL-6, IL-10, and TNF-α. These cytokines were significantly reduced in the DHPEA-treated animals compared with the untreated and steroid-treated rats, and this effect lasted for months after treatment ceased. The lower alveolitis score of the DHPEA-treated rats also suggested that DHPEA reduces the infiltration of inflammatory immune cells. Results of this study are in agreement with those of previous studies that have shown significant anti-inflammatory effects from the administration of DHPEA [27-29].
Results of the SPB staining analysis in the lung revealed that DHPEA-treated rats showed a more normalized distribution of AE2 cells in the parenchyma compared with untreated and DEX-treated rats. These results suggest that DHPEA protected parechymal and AE2 cells from free radical damage. In addition, the myofibroblast proliferation in the lung (as demonstrated by α-SMA staining) observed in the untreated and DEX-treated groups was significantly reduced in rats treated with DHPEA at 40 and 80 days after irradiation, suggesting that DHPEAinhibited pulmonary fibrosis partially inhibits myofibroblast transformation and proliferation.
Nrf2 plays a critical role in the regulation of the major antioxidant enzymes HO-1 and NQO-1. Our western blot analysis results revealed that DHPEA significantly enhanced the expression levels of Nrf-2, HO-1, and NQO-1 in rat lung tissues compared with untreated and DEX-treated rats thereby supporting the hypothesis that DHPEA relieves oxidative stress by activating Nrf2 and its associated antioxidant enzymes.
Since glucocorticosteroids are commonly used to treat irradiation-induced pulmonary fibrosis and other forms of lung fibrosis in humans, DEX was selected as a baseline to compare the efficacy of DHPEA using various measures of lung inflammation, the oxidative stress response, and fibrosis. A marginal effectiveness was achieved with DEX therapy at 10 and 20 days after irradiation; however, these improvements ceased following discontinuing steroid therapy (i.e., at 40 and 80 days postirradiation). The measures of lung inflammation, oxidative stress, and fibrosis among the DEX-treated rats were similar to those of the untreated group at 40 and 80 days, suggesting a lack of persistent therapeutic effects. Conversely, our results demonstrate that DHPEA was superior to glucocorticoids with regard to reducing inflammation, fibrosis, and oxidative stress during the treatment period (20 days after irradiation). In addition, the therapeutic effects of DHPEA were sustained even after treatment ceased.
Conclusion
The results from our study demonstrate that DHPEA treatment induces antioxidant, antiinflammatory, and anti-proliferative effects that protect against irradiation-induced pulmonary fibrosis in rats.
Acknowledgements
This project was supported by the funding for construction of national key clinical specialty.
Disclosure of conflict of interest
None.
References
- 1.Baldioli M, Servili M, Perretti G, Montedoro GF. Antioxidant Activity of tocopherols and phenolic compounds of virgin olive oil. J Am Oil Chem Soc. 1996;73:1589. [Google Scholar]
- 2.Morelló JR, Vuorela S, Romero MP, Motilva MJ, Heinonen MJ. Antioxidant activity of olive pulp and olive oil phenolic compounds of the arbequina cultivar. J Agric Food Chem. 2005;53:2002–8. doi: 10.1021/jf048386a. [DOI] [PubMed] [Google Scholar]
- 3.Papadopoulos G, Boskou D. Antioxidant effect of natural phenols on olive oil. J Am Oil Chem Soc. 1991;68:669–71. [Google Scholar]
- 4.Montedoro G, Servili M, Baldioli M, Selvaggini R, Miniati E, Maccioni AJ. Simple and hydrolyzable compounds in virgin olive oil. Agric Food Chem. 1993;41:2228–34. [Google Scholar]
- 5.Gordon MH, Paiva-Martins F, Almeida MJ. Antioxidant activity of hydroxytyrosol acetate compared with that of other olive oil polyphenols. J Agric Food Chem. 2001;49:2480–85. doi: 10.1021/jf000537w. [DOI] [PubMed] [Google Scholar]
- 6.Servili M, Baldioli M, Miniati E, Montedoro GF. Antioxidant activity of new phenolic compounds extracted from virgin oil and their interaction with α-tocopherol and β-carotene. Rev Ital Sost Grasse. 1996;73:55. [Google Scholar]
- 7.Almeida C, Nagarajan D, Tian J, Leal SW, Wheeler K, Munley M, Blackstock W, Zhao W. The role of alveolar epithelium in radiation-induced lung injury. PLoS One. 2013;8:e53628. doi: 10.1371/journal.pone.0053628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epperly MW, Guo H, Gretton JE, Greenberger JS. Bone marrow origin of myofibroblasts in irradiation pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003;29:213–24. doi: 10.1165/rcmb.2002-0069OC. [DOI] [PubMed] [Google Scholar]
- 9.Matsuo Y, Shibuya K, Nakamura M, Narabayashi M, Sakanaka K, Ueki N, Miyagi K, Norihisa Y, Mizowaki T, Nagata Y, Hiraoka M. Dose-volume metrics associated with radiation pneumonitis after stereotactic body radiation therapy for lung cancer. Int J Radiat Oncol Biol Phys. 2012;83:545–549. doi: 10.1016/j.ijrobp.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 10.Minor GI, Yashar CM, Spanos WJ Jr, Jose BO, Silverman CL, Carrascosa LA, Farmer M, Paris KJ. The relationship of radiation pneumonitis to treated lung volume in breast conservation therapy. Breast J. 2006;12:48–52. doi: 10.1111/j.1075-122X.2006.00180.x. [DOI] [PubMed] [Google Scholar]
- 11.Rosenzweig KE, Zauderer MG, Laser B, Krug LM, Yorke E, Sima CS, Rimner A, Flores R, Rusch V. Pleural intensitymodulated radiotherapy for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys. 2012;83:1278–1283. doi: 10.1016/j.ijrobp.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu HW, Seftel MD, Rubinger M, Szwajcer D, Demers A, Nugent Z, Schroeder G, Butler JB, Cooke A. Total body irradiation compared with BEAM: long-term outcomes of peripheral blood autologous stem cell transplantation for non-Hodgkin’s lymphoma. Int J Radiat Oncol Biol Phys. 2010;78:513–20. doi: 10.1016/j.ijrobp.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 13.Mak JC. Potential role of green tea catechins in various disease therapies: progress and promise. Clin Exp Pharmacol Physiol. 2012;39:265–73. doi: 10.1111/j.1440-1681.2012.05673.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Zhang X, Rabbani ZN, Jackson IL, Vujaskovic Z. Oxidative stress mediates radiation lung injury by inducing apoptosis. Int J Radiat Oncol Biol Phys. 2012;83:740–48. doi: 10.1016/j.ijrobp.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.You H, Wei L, Sun WL, Wang L, Yang ZL, Liu Y, Zheng K, Wang Y, Zhang WJ. The green tea extract epigallocatechin-3-gallate inhibits irradiation- induced pulmonary fibrosis in adult rats. Int J Mol Med. 2014;34:92–102. doi: 10.3892/ijmm.2014.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Vries HE, Witte M, Hondius D, Rozemuller AJ, Drukarch B, Hoozemans J, van Horssen J. Nrf2-induced antioxidant protection: a promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Radic Biol Med. 2008;45:1375–83. doi: 10.1016/j.freeradbiomed.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Williams JP, Johnston CJ, Finkelstein JN. Treatment for radiation-induced pulmonary late effects: spoiled for choice or looking in the wrong direction? Curr Drug Targets. 2010;11:1386–94. doi: 10.2174/1389450111009011386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rizvi M, Pathak D, Freedman JE, Chakrabarti S. CD40-CD40 ligand interactions in oxidative stress, inflammation and vascular disease. Trends Mol Med. 2008;14:530–38. doi: 10.1016/j.molmed.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Mikkelsen RB, Wardman P. Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene. 2003;22:573454. doi: 10.1038/sj.onc.1206663. [DOI] [PubMed] [Google Scholar]
- 20.Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316–28. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Martin M, Lefaix J, Delanian S. TGF-beta1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys. 2000;47:277–90. doi: 10.1016/s0360-3016(00)00435-1. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Qiao XY, Lu FH, Zhou ZG, Song YZ, Huo JJ, Liu X. TGF-beta1 in serum and induced sputum for predicting radiation pneumonitis in patients with non-small cell lung cancer after radiotherapy. Chin J Cancer. 2010;29:325–29. doi: 10.5732/cjc.009.10454. [DOI] [PubMed] [Google Scholar]
- 23.Wynn TA. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011;208:1339–50. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denis M. Interleukin-6 in mouse hypersensitivity pneumonitis: changes in lung free cells following depletion of endogenous IL-6 or direct administration of IL-6. J Leukoc Biol. 1992;52:197–201. doi: 10.1002/jlb.52.2.197. [DOI] [PubMed] [Google Scholar]
- 25.Zdanov A. Structural features of the interleukin-10 family of cytokines. Curr Pharm Des. 2004;10:3873–3884. doi: 10.2174/1381612043382602. [DOI] [PubMed] [Google Scholar]
- 26.Alhamad EH, Cal JG, Shakoor Z, Almogren A, Alboukai AA. Cytokine gene polymorphisms and serum cytokine levels in patients with idiopathic pulmonary fibrosis. BMC Med Genet. 2013;14:66. doi: 10.1186/1471-2350-14-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donà M, Dell’Aica I, Calabrese F, Benelli R, Morini M, Albini A, Garbisa S. Neutrophil restraint by green tea: inhibition of inflammation, associated angiogenesis, and pulmonary fibrosis. J Immunol. 2003;170:4335–4341. doi: 10.4049/jimmunol.170.8.4335. [DOI] [PubMed] [Google Scholar]
- 28.Bae HB, Li M, Kim JP, Kim SJ, Jeong CW, Lee HG, Kim WM, Kim HS, Kwak SH. The effect of epigallocatechin gallate on lipopolysaccharide-induced acute lung injury in a murine model. Inflammation. 2010;33:82–91. doi: 10.1007/s10753-009-9161-z. [DOI] [PubMed] [Google Scholar]
- 29.Sun Q, Zheng Y, Zhang X, Hu X, Wang Y, Zhang S, Zhang D, Nie H. Novel immunoregulatory properties of EGCG on reducing inflammation in EAE. Front Biosci (Landmark Ed) 2013;18:332–342. doi: 10.2741/4104. [DOI] [PubMed] [Google Scholar]


