Abstract
Collagenous micronodules (CMs) are microscopic stromal nodular eosinophilic fibrillar collagen deposition of uncertain histogenesis seen in prostatic adenocarcinoma. Per the 2005 International Society of Urologic Pathology (ISUP) consensus conference, they are categorized as Gleason pattern 3. This study analyzes morphological and clinical features of CMs from a large series of radical prostatectomies. Hematoxylin and eosin stained slides for 129 radical prostatectomies for adenocarcinoma of prostate with CMs and for 93 prostatic adenocarcinoma cases without CMs as comparison were examined out of a total of 667 cases performed from January 2010 to December 2011 at Houston Methodist Hospital. CMs were identified in 19% of all radical prostatectomies (129/667 cases). Almost all tumors with CMs were located in the peripheral zone (98%) as single or multiple foci of prostatic cancer glands. The vast majority of cases (96%) were identified in association with mucinous secretion. A cribriform Gleason pattern 4 was associated in 86 cases (67%). The CMs were associated with glomerulation (42%) and amphophilic luminal secretion (59%). 88 cases (68%) showed tumor foci with Gleason pattern ≥ 4 in close association with CMs. Multivariate analysis revealed CMs of the prostatic adenocarcinoma are closely related to mucinous secretion, cribriform growth pattern, and Gleason pattern 4. This study suggests that CMs are more frequently associated with Gleason pattern 4 cancer warranting morphologic reappraisal of CMs, rather than the consensus assignment of Gleason pattern 3.
Keywords: Prostate adenocarcinoma, collagenous micronodules, mucinous fibroplasia, Gleason pattern 3, Gleason pattern 4
Introduction
Collagenous micronodules (CMs), also referred to as “mucinous fibroplasia”, are microscopic stromal eosinophilic fibrillary collagenous nodules of uncertain histogenesis and reported as a pathognomonic feature of prostate adenocarcinoma. CMs were first described by McNeal et al in 1991 [1] and were observed in 13 out of 33 mucin-producing prostatic adenocarcinomas. Based on the 2005 International Society of Urologic Pathology (ISUP) consensus conference [2]. CMs have been previously defined as being seen in association with Gleason pattern 3 [3]. The aim of this study is to analyze the incidence, morphological (including Gleason pattern) and clinical features related to CMs in a large series of radical prostatectomies.
Materials and methods
This study was approved by the Houston Methodist Hospital Institutional Review Board. Hematoxylin and eosin stained slides were reviewed in 667 consecutively performed radical prostatectomies for adenocarcinomas from January 2010 to December 2011 at Houston Methodist Hospital, Texas, USA for the presence of CMs. We also studied 93 cases of prostatic adenocarcinoma without CMs for comparison. We reviewed all sections (at least more than 30 sections per case) in whole mount prostatectomy specimens. Benign prostatic lesions seen in the cancer specimens were evaluated as internal control for the presence of CMs. The findings evaluated in conjunction with CMs in this study include Gleason pattern, mucin secretion, glomerulation, cribriform growth pattern, circumferential perineural invasion, capsular invasion, extraprostatic extension, seminal vesicle invasion and lymph node metastasis. Each case was assigned a Gleason grade, based on modified Gleason grading system proposed at the 2005 ISUP consensus meeting [4]. For analysis of the prostatic capsular invasion, we employed routinely assessed criteria [5-7]. The evaluation of mucinous adenocarcinoma was based on previously established criteria [8,9], and their Gleason patterns were assessed on underlying glandular architecture. Clinical factors studied include patients’ age, tumor size, location, lymph node and seminal vesicle status. Statistically, all descriptive data are presented as mean ± standard deviation (SD) for continuous variables and frequency and percentage for categorical variables. Data was compared by Student’s t-test or chi-square test as appropriate. Multiple logistic regression models were used to assess the association between Gleason grade and CMs after adjusting for covariates, including age, glomerulation, cribriform pattern, amphophilic secretion, circumferential perineural invasion, extraprostatic extension and seminal vesicle invasion. All analyses were performed using IBM SPSS Statistics 19.0 (IBM Corp., Armonk, NY, USA), and the significance test was carried out at P<0.05, and P values between 0.05 and 0.1 were considered as the borderline level of significance.
Results
The results of prostatic carcinoma cases with CMs are summarized in Table 1. CMs were identified in 129 cases of 667 radical prostatectomies (19%). CMs were exclusively found in association with prostatic adenocarcinoma with 100% specificity. These nodules were not identified in benign glandular lesions including benign prostatic glands, benign nodular glandular hyperplasia and high grade prostatic intraepithelial neoplasia. The nodules had fine textured, eosinophilic fibrillary loose connective tissue with elongated or serpiginous appearance as well as round to oval contours (Figure 1). These nodules were present in focal or aggregated forms and found immediately adjacent to the tumor glands (Figure 2). CMs were frequently associated with cribriform Gleason pattern 4 with ingrowths into the luminal spaces in 86 cases (67%) (Figure 3). Occasionally, these stromal nodules in pattern 4 areas were mimicking pattern 3, so-called “pseudo-grade 3” pattern (Figure 4). CMs were less commonly observed in areas of Gleason pattern 3 cancers (43 cases, 33%). Some prostatic adenocarcinoma glands, in which CMs were abutting on glandular lumens as an extraluminal subepithelial location, produced a crescentic configuration (Figure 5). 124 cases (96%) were identified in association with mucinous secretion (Figure 6). However, in our study there were no cases with mucinous differentiation which fulfilled the diagnostic criteria of the primary mucinous carcinoma of the prostate [8,9]. The CMs were associated with glomerulations in 53 cases (41%) (Figure 7), amphophilic luminal secretions in 89 cases (69%) and circumferential perineural invasion in 93 cases (72%) of prostatic adenocarcinoma (Figure 8). The age range was 43-72 years (mean 61). Almost all tumors with CMs (125 cases) were located in the peripheral zone (98%) as pure or mixed with other zonal cancers. Two tumors with CMs were exclusively located in the transition zone. The tumor size range was 0.5-4.0 cm (mean 1.7 cm). Extraprostatic extension was seen in 12 cases (9%), seminal vesicle involvement in 7 of 123 cases (6%, the seminal vesicle evaluation was possible in 123 cases) and nodal metastasis in 3 of 102 cases (3%, lymph node dissection performed in only 102 cases). In relation to Gleason pattern, the analysis of morphological and clinical factors between prostatic carcinoma with and without CMs showed a statistically significant correlation. In relationship to Gleason pattern, this study revealed significant association between Gleason pattern and CMs, mucin secretion, glomerulation, cribriform pattern of cancer, circumferential perineural invasion and extraprostatic extension (P value <0.001, Table 2) than cases without CMs. However, on adjusted odds ratio analysis for higher Gleason pattern (≥ 4), it was demonstrated that cribriform pattern had the greatest adjusted odds among covariates, with an odds ratio of 4.57 (95% CI: 2.25-9.27), followed by CMs. CMs were also associated with a borderline significantly increased risk of higher Gleason pattern (≥ 4) after adjusting for age, glomerulation, cribriform, amphophilic secretion, circumferential perineural invasion, extraprostatic extension and seminal vesicle invasion (adjusted OR=1.90, 95% CI: 0.90-4.02, P value=0.094, Table 3).
Table 1.
Clinicopathologic features in 129 patients’ prostate cancer with collagenous micronodules
| Clinicopathologic features | Adenocarcinoma with collagenous micronodules: number of cases (%) |
|---|---|
| Mucin secretion | 124 (96%) |
| Cribriform | 77 (60%) |
| Glomerulation | 53 (41%) |
| Circumferential perineural invasion | 93 (72%) |
| Dominant Gleason pattern | |
| Grade 3 | 43 (33%) |
| Grade 4 | 86 (67%) |
| Grade 5 | 0 (0%) |
| Eosinophilic secretion | 89 (69%) |
| Capsular invasion | |
| L0 | 15 (12%) |
| L1 | 9 (7%) |
| L2 | 93 (72%) |
| L3F/E | 12(9%) |
| Tumor location | |
| Pure peripheral | 93 (72%) |
| Pure transitional | 4 (3%) |
| Mixed peripheral and transitional | 32 (25%) |
| Central | 0 (0%) |
| Age | 43-72 years (Mean 61) |
| Tumor size | 0.9-4.8 cm (Mean 1.7 cm) |
| Lymph node invasion | 3/102 (3%) |
| Seminal vesicle invasion | 7/123 (6%) |
Figure 1.
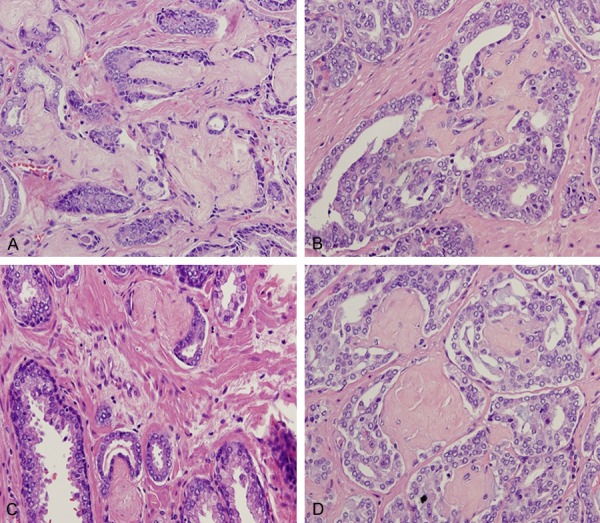
Collagenous micronodules with finely textured, eosinophilic fibrillary loose connective tissue with elongated or serpiginous appearance (A and B) as well as round to oval contours (C and D). Hematoxylin and eosin stain ×200.
Figure 2.
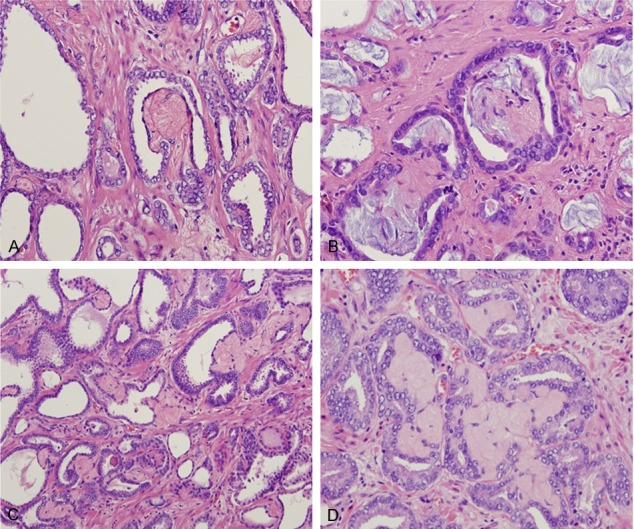
Collagenous micronodules present as single/focal (A and B) or multiple/aggregated forms (C and D) and found in the immediate vicinity of malignant prostate cancer glands. Hematoxylin and eosin stain ×200.
Figure 3.
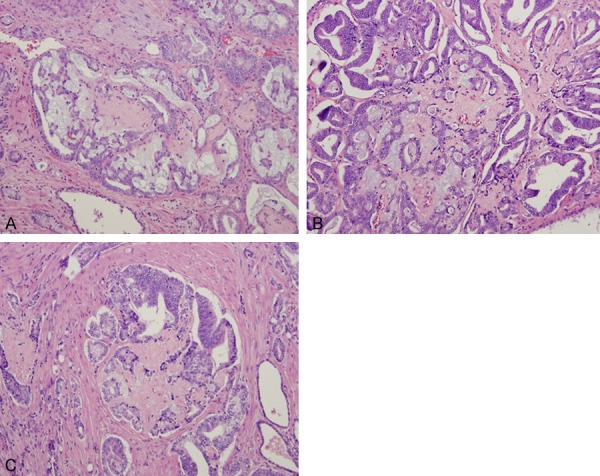
Collagenous micronodules seen in association with mucin-secreting cribriform Gleason grade 4 pattern with ingrowths into the luminal spaces (A-C). Hematoxylin and eosin stain ×100.
Figure 4.
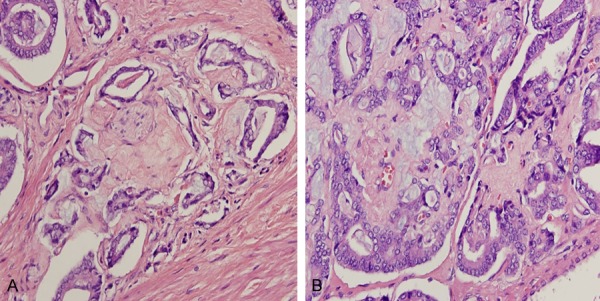
Occasionally, these stromal nodules in grade 4 areas showed “pseudo-grade 3” pattern resulting from collagen impinge on malignant glands (A and B). Hematoxylin and eosin stain ×200.
Figure 5.
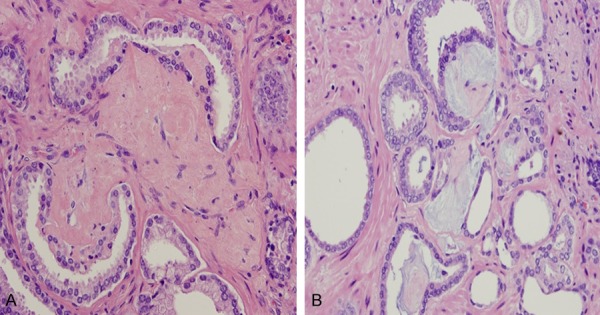
Collagenous or mucinous micronodules abutting on Gleason grade 3 cancer glandular lumina, resulting in a crescentic configuration (A and B). Hematoxylin and eosin stain ×200.
Figure 6.
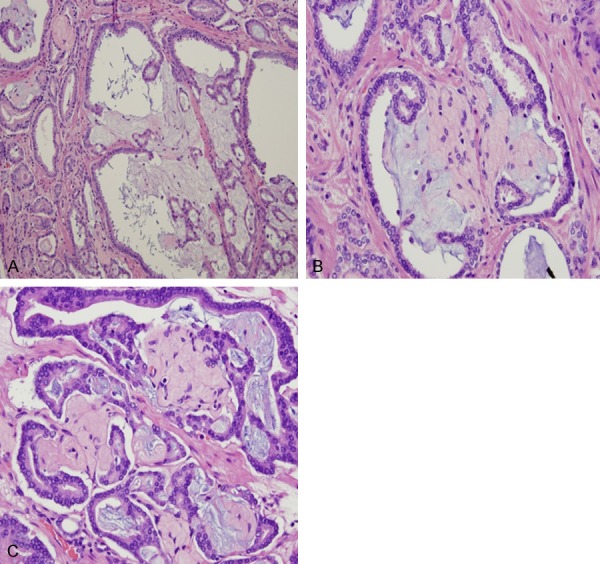
In our study, 96% of prostatic adenocarcinoma with collagenous micronodules were identified in association with mucinous secretion or mucinous lakes. The spectrum of intraluminal and extraglandular mucin deposit (A) and stromal collagenous replacement (B) and development of complete luminal collagenous tissue (C) is highlighted. Hematoxylin and eosin stain ×200.
Figure 7.
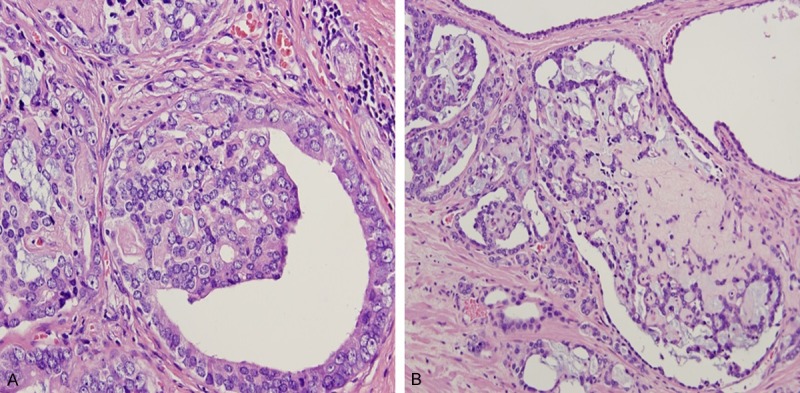
Collagenous micronodules seen in association with glomerulations (A and B). Hematoxylin and eosin stain × 200 for (A) and ×100 for (B).
Figure 8.
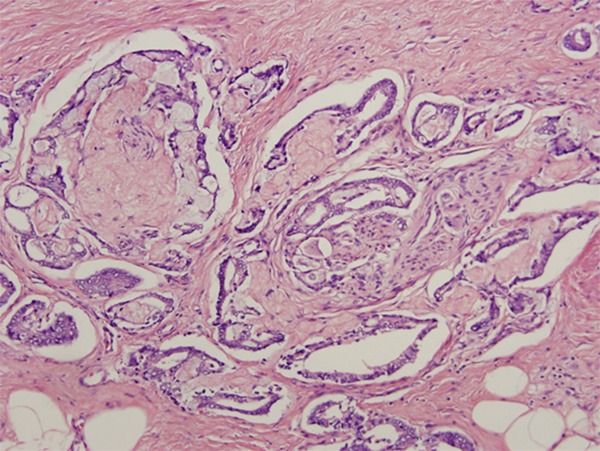
Circumferential perineural invasion identified in foci of prostatic adenocarcinoma with collagenous micronodules. Hematoxylin and eosin stain ×200.
Table 2.
General characteristics of the 222 patients by Gleason pattern
| Gleason pattern | P value | ||
|---|---|---|---|
|
|
|||
| Low (pattern 3) (N=90) | High (pattern 4) (N=132) | ||
| Age (years) | 60.9±7.8 | 60.9±7.4 | 0.991 |
| Collagenous micronodule | <0.001 | ||
| No | 49 (52.1%) | 45 (47.9%) | |
| Yes | 42 (32.6%) | 87 (67.4%) | |
| Glomerulation | <0.001 | ||
| No | 72 (47.7%) | 79 (52.3%) | |
| Yes | 19 (26.4%) | 53 (73.6%) | |
| Cribriform pattern | <0.001 | ||
| No | 48 (71.6%) | 19 (28.4%) | |
| Yes | 43 (27.6%) | 113 (72.4%) | |
| Amphophilic secretion | 0.231 | ||
| No | 45 (37.2%) | 76 (62.8%) | |
| Yes | 46 (45.1%) | 56 (54.9%) | |
| Circumferential perineural invasion | 0.001 | ||
| No | 51 (53.1%) | 45 (46.9%) | |
| Yes | 40 (31.7%) | 86 (68.3%) | |
| Extraprostatic extension | 0.039 | ||
| No | 78 (44.3%) | 98 (55.7%) | |
| Yes | 13 (27.7%) | 34 (72.3%) | |
| Seminal vesicle invasion | 0.103 | ||
| No | 85 (41.9%) | 118 (58.1%) | |
| Yes | 4 (22.2%) | 14 (77.8%) | |
Table 3.
Adjusted odds ratios (95% confidence intervals) for higher Gleason pattern (>4)
| Adjusted OR* (95% CI) | P value | |
|---|---|---|
| Age (years) | 1.02 (0.98-1.06) | 0.387 |
| Collagenous micronodule | ||
| No | Reference | |
| Yes | 1.90 (0.90-4.02) | 0.094 |
| Glomerulation | ||
| No | Reference | |
| Yes | 1.69 (0.85-3.38) | 0.134 |
| Cribriform pattern | ||
| No | Reference | |
| Yes | 4.57 (2.25-9.27) | <0.001 |
| Amphophilic secretion | ||
| No | Reference | |
| Yes | 0.53 (0.27-1.04) | 0.064 |
| Circumferential perineural invasion | ||
| No | Reference | |
| Yes | 1.38 (0.71-2.68) | 0.335 |
| Extraprostatic extension | ||
| No | Reference | |
| Yes | 1.42 (0.63-3.19) | 0.393 |
| Seminal vesicle invasion | ||
| No | Reference | |
| Yes | 1.59 (0.44-5.73) | 0.482 |
OR, odds ratio; CI, confidence interval.
Adjusted for age, glomerulation, Cribriform pattern, amphophilic secretion, circumferential perineural invasion, extraprostatic extension, and seminal vesicle invasion.
Discussion
Features considered pathognomonic for prostate cancer diagnosis include the identification of glomerulations, CMs, circumferential perineural invasion and presence of glands in adipose tissue [3,10]. CMs are a rare but significant diagnostic feature seen in association with prostatic adenocarcinoma [11,12]. These microscopic nodules have distinctive features with small, poorly cellular eosinophilic fibrillary stromal material found immediately adjacent to malignant neoplastic glands. CMs in prostatic adenocarcinoma were first described by McNeal et al. [1] in 1991 in association with mucin secreting prostatic adenocarcinoma. CMs were observed in 13 out of 33 mucin producing adenocarcinomas, especially embedded in mucinous lakes characteristic of colloid carcinoma, resulting in Gleason grade 4 cribriform glands mimicking a “pseudo grade 3” pattern. This phenomenon was also observed in our study. A close association between mucin producing tumor glands and CMs has also been reported by several authors [9,13-15]. However, only rare studies examining the possibility of an association between CMs and mucinous differentiation in prostate carcinoma have been undertaken and have reported no significant association between the two features [12]. The results of our study showed that 96% of the cases with CMs were associated with intraluminal acid mucin secretion of prostate carcinoma glands. In the study by Bostwick et al. [15] these nodules were reported to be present in 0.6% of needle biopsies and 12.7% of prostatectomy specimens. Varma et al. [16] noted collagenous micronodules in 2% of cases of prostatic adenocarcinoma in a study of prostatic needle biopsies. In our study, the frequency for identification of CMs was 19% in radical prostatectomy specimens which is slightly higher than that of other reports, possibly related to whole mount sections examination [15,16]. Most of the previously published studies do not elucidate the link between CMs and Gleason pattern [11]. In our study, CMs were found to be commonly associated with Gleason pattern 4. However, this is in contrast to prior studies performed by Bostwick et al. [15] and Jacob et al. [11] in which Gleason pattern 3 has been reported to be more commonly associated with CMs (57%). The histogenesis of CMs is unknown. Previous studies suggest that CMs are formed by collagen fragmentation secondary to stimulation of stroma by intraluminal acid mucin by production of collagenase from tumor cells and stromal response by TGF-β [14,15,17]. TMMPRSS2-ERG fusion prostatic cancer is significantly associated with morphologic features with a cribriform growth pattern (P=0.03), presence of blue-tinged mucin (P<0.01), macronucleoli (P=0.02) and CMs (P=0.04) [18,19]. In our experience, the presence of basophilic mucinous cytoplasm of tumor cells and dense stromal reaction intervening between foci of malignant glandular epithelium are clues for the presence of CMs. The main consideration in differential diagnoses of CMs is the prostate counterpart of collagenous spherulosis of the breast and adenoid cystic carcinoma of prostate with cribriform glands containing basement membrane-like material [20,21]. Both these entities may morphologically resemble CMs. CMs of the prostate are always associated with usual forms of prostate adenocarcinoma which are different from the pure form of adenoid cystic carcinoma. The distinct pathological and clinical features of CMs in prostate cancer in this study are similar with the findings in studies by other researchers [11,12,22].
In conclusion, on comprehensive review of prostatic adenocarcinomas with CMs in this study, these nodules were distinctive and exclusively seen in association with peripheral zone prostate cancers. There were a significant number of prostatic cancer cases with CMs in association with Gleason pattern 4 (score 4+3=7 or 4+4=8). These micronodules tend to be associated with the presence of mucin secretion, cribriform growth pattern, and circumferential perineural invasion. A strong relationship of CMs with these pathologic features may provide a diagnostic clue for the presence of CMs adjacent to the malignant glands. This study suggests that CMs are more closely associated with Gleason pattern 4, warranting reappraisal of CMs as opposed to their assignment of Gleason pattern 3 per the consensus. Further studies, including molecular analysis and assessment of ERG gene status are required to determine the prognostic significance of prostatic adenocarcinoma with CMs.
Disclosure of conflict of interest
None.
References
- 1.McNeal JE, Alloy J, Villars A, Redwine EA, Freiha FS, Stamey TA. Mucinous differentiation in prostatic adenocarcinoma. Hum Pathol. 1991;22:979–988. doi: 10.1016/0046-8177(91)90006-b. [DOI] [PubMed] [Google Scholar]
- 2.Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL ISUP Grading Committee. The 2005 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol. 2005;29:1228–1242. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 3.Ro JY, Shen S, Zhai QJ, Ayala AG. Advances in Surgical Pathology: Prostate Cancer. Wolters Kluwer/Lippincott Williams & Wilkins; 2012. pp. 127–230. [Google Scholar]
- 4.Lars E, Roberta M Rodolfo M. Implications of the International Society of Urological Pathology Modified Gleason Grading System. Arch Pathol Lab Med. 2012;136:426–434. doi: 10.5858/arpa.2011-0495-RA. [DOI] [PubMed] [Google Scholar]
- 5.Stamey TA, McNeal JE, Freiha FS, Redwine E. Morphometric and clinical studies on 68 consecutive radical prostatectomies. J Urol. 1988;199:1235–41. doi: 10.1016/s0022-5347(17)42876-x. [DOI] [PubMed] [Google Scholar]
- 6.Wheeler TM. Anatomic consideration in carcinoma in the prostate. Urol Clin North Am. 1989;16:623–634. [PubMed] [Google Scholar]
- 7.Ayala AG, Ro JY, Babaian R, Troncoso P, Grignon DJ. The prostatic capsule; Does it exist? Its importance in the staging and treatment of prostatic cancer. Am J Surg Pathol. 1989;13:21–27. [PubMed] [Google Scholar]
- 8.Osunkoya AO, Nielsen ME, Epstein JL. Prognosis of mucinous adenocarcinoma of the prostate treated by radical prostatectomy: a study of 47 cases. Am J Surg Pathol. 2008;32:468–472. doi: 10.1097/PAS.0b013e3181589f72. [DOI] [PubMed] [Google Scholar]
- 9.Bohman KD, Osunkoya AO. Mucin-producing tumors and tumor-like lesions involving the prostate: a comprehensive review. Adv Anat Pathol. 2012;19:374–387. doi: 10.1097/PAP.0b013e318271a361. [DOI] [PubMed] [Google Scholar]
- 10.Egevad L, Allsbrook WC Jr, Epstein JI. Current practice of diagnosis and reporting on needle biopsy among genitourinary pathologists. Hum Pathol. 2006;37:292–297. doi: 10.1016/j.humpath.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Jacob S, Mammen K. Collagenous micronodules in prostatic adenocarcinoma-a case report. Indian J Pathol Microbiol. 2004;47:406–407. [PubMed] [Google Scholar]
- 12.Arangelovich V, Tretiakova M, SenGupta E, Krausz T, Yang XJ. Pathogenesis and significance of collagenous micronodules of the prostate. Appl Immunohistochem Mol Morphol. 2003;11:15–19. doi: 10.1097/00129039-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Ro JY, Grignon DJ, Ayala AG, Fernandez PL, Ordonez NG, Wishnow KI. Mucinous adenocarcinoma of the prostate: Histochemical and immunohistochemical studies. Hum Pathol. 1990;21:593–600. doi: 10.1016/s0046-8177(96)90004-0. [DOI] [PubMed] [Google Scholar]
- 14.Epstein JI. Diagnosis and reporting of limited adenocarcinoma of the prostate on needle biopsy. Mod Pathol. 2004;17:307–315. doi: 10.1038/modpathol.3800050. [DOI] [PubMed] [Google Scholar]
- 15.Bostwick DG, Wollan P, Adlakha K. Collagenous micronodules in prostate cancer. A specific but infrequent diagnostic finding. Arch Pathol Lab Med. 1995;119:444–447. [PubMed] [Google Scholar]
- 16.Varma M, Lee MW, Tamboli P, Zarbo RJ, Jimenez RE, Salles PG, Amin MB. Morphologic criteria for the diagnosis of prostatic adenocarcinoma in needle biopsy specimens. A study of 250 consecutive cases in a routine surgical pathology practice. Arch Pathol Lab Med. 2002;126:554–561. doi: 10.5858/2002-126-0554-MCFTDO. [DOI] [PubMed] [Google Scholar]
- 17.Barron DA, Strand DW, Ressler SJ, Dang TD, Hayward SW, Yang F, Ayala GE, Ittmann M, Rowley DR. TGF β1 induces an age dependentinflammation of nerve ganglia and fibroplasia in the prostate gland stroma of a novel transgenic mouse. PLoS One. 2010;5:e13751. doi: 10.1371/journal.pone.0013751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosquera JM, Mehra R, Regan MM, Perner S, Genega EM, Bueti G, Shah RB, Gaston S, Tomlins SA, Wei JT, Kearney MC, Johnson LA, Tang JM, Chinnaiyan AM, Rubin MA, Sanda MG. Prevalence of TMPRSS2-ERG fusion prostate cancer among men undergoing prostate biopsy in the United States. Clin Cancer Res. 2009;15:4706–4711. doi: 10.1158/1078-0432.CCR-08-2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosquera JM, Perner S, Demichelis F Kim R, Hofer MD, Mertz KD, Paris PL, Simko J, Collins C, Bismar TA, Chinnaiyan AM, Rubin MA. Morphologic features of TMPRSS2-ERG gene fusion prostate cancer. J Pathol. 2007;212:91–101. doi: 10.1002/path.2154. [DOI] [PubMed] [Google Scholar]
- 20.Fine SW. Variants and unusual patterns of prostatic cancer: Clinicopathologic and differential diagnostic consideration. Adv Anat Pathol. 2012;19:204–216. doi: 10.1097/PAP.0b013e31825c6b92. [DOI] [PubMed] [Google Scholar]
- 21.Clemant PB, Young RH, Azzopardi JG. Collagenous spherulosis of the breast. Am J Surg Pathol. 1987;11:411–417. doi: 10.1097/00000478-198706000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Baisden BL, Kahane H, Epstein JI. Perineural invasion, mucinous fibroplasia, and glomerulation: diagnostic features of limited cancer on prostate needle biopsy. Am J Surg Pathol. 1999;23:918–924. doi: 10.1097/00000478-199908000-00009. [DOI] [PubMed] [Google Scholar]


