Abstract
Calcium is a strong inducer of keratinocyte differentiation. We have previously demonstrated that extracellular calcium promotes keratinocyte differentiation via E-cadherin-catenin complex-mediated phospholipase C-γ1 (PLC-γ1) activation in the plasma membrane. However, it is unclear whether dietary calcium regulates keratinocyte proliferation, differentiation or carcinogenesis. To address this issue, the rates of oral tumor and levels of proliferation and differentiation in the oral epithelium were assessed in mice on different calcium diets and the carcinogen 4-nitroquinoline-1-oxide. The results showed that mice on the high calcium diet had lower rates of oral tumors, lower levels of proliferation and higher levels of differentiation in the normal oral epithelium than those on the normal calcium diet. Higher levels of E-cadherin, β-catenin, p120-catenin (p120), epidermal growth factor receptor (EGFR), and calcium and lower levels of PLC-γ1 were also noted in the normal oral epithelium in mice on high calcium diet than the control mice. In contrast, mice on low calcium diet had opposite effects. However, dietary calcium had no effect on the proliferation, differentiation or the levels of E-cadherin, β-catenin, p120, PLC-γ1 and EGFR in oral tumors. These data indicate that dietary calcium increases calcium levels in oral epithelium, suppresses oral carcinogenesis, inhibits proliferation and promotes differentiation of normal oral epithelium. Increased E-cadherin, β-catenin, p120 and EGFR and decreased PLC-γ1 may participate in the inhibitory effect of dietary calcium in oral carcinogenesis.
Keywords: Calcium, proliferation, differentiation, oral carcinogenesis
Introduction
Oral cancer is a malignant tumor of oral mucosa and has high mortality rates. It is the eighth leading cause of cancer and 263,900 new cases and 128,000 deaths occurred worldwide in 2008 [1]. Oral squamous cell carcinoma (OSCC) accounts for approximately 90% of oral cancers and it is frequently associated with smoking, excessive alcohol intake, chewing tobacco or betel nut and HPV infections [2,3]. These patients are often diagnosed at an advanced stage [4]. These necessitate the search for the identification and implementation of novel preventive and therapeutic strategies, and this, most certainly, calls for the thorough understanding of the mechanism of oral carcinogenesis.
Calcium is the most abundant mineral in the body. It combines with phosphorus to form calcium phosphate in bones and teeth. Calcium homeostasis is tightly regulated by the actions of 1,25-dihydroxycholecalciferol [1, 25-(OH)2D3], parathyroid hormone (PTH) and calcitonin and direct exchange with the bone matrix. Calcium plays a critical role in muscle contraction and relaxation, blood coagulation, nerve transmission and keratinocyte differentiation and proliferation [5,6]. It has been demonstrated that change in calcium concentration in the culture medium markedly alters the pattern of proliferation and differentiation of epidermal keratinocytes [7]. Our previous studies [8-12] indicate that calcium-induced formation of the E-cadherin-β-catenin-p120-catenin (p120) complex in the plasma membrane triggers an intracellular signaling pathway essential for keratinocyte differentiation.
Under high extracellular calcium (Cao) conditions, phosphatidylinositol-4-phosphate 5-kinase1α (PIP5K1α) and phosphatidylinositol 3-Kinase (PI3K) are recruited to the E-cadherin- β-catenin-p120 complex in the plasma membrane in which the synthesis of phosphatidylinositol (4,5)-bisphosphate (PIP2) and phosphatidylinositol (3,4,5)-triphosphate (PIP3) is induced respectively. Phospholipase C-γ1 (PLC-γ1) is then recruited to the plasma membrane and activated by PIP3, produces more inositol trisphosphate (IP3) that increases intracellular calcium (Cai) concentration by means of the calcium release from endoplasmic reticulum (ER) and Golgi and calcium influx through calcium channels. Increased Cai triggers keratinocyte differentiation. The relevance of calcium-induced differentiation in vitro to the in vivo situation is indicated by the steep gradient of calcium within the epidermis, with the highest levels in the uppermost (most differentiated) layers [13]. However, it is unclear whether dietary calcium regulates the level of calcium, differentiation and proliferation of keratinocytes in the squamous epithelium such as oral epithelium, and oral carcinogenesis. To address these issues, we fed mice with different levels of dietary calcium and assessed the levels of calcium, proliferation, differentiation and chemically induced carcinogenesis in the oral epithelium of these mice.
Materials and methods
4NQO-induced oral carcinogenesis model
Sixty-six weaned mice (C57BL/6) were randomly divided into the high (n=25), normal (n=19) and low calcium diet groups (n=22). Each group was fed with the diet containing 2.0% calcium, 1.3% calcium or 0.1% calcium (Harlan Lab) at 4 weeks of age. All mice were also fed with drinking water containing 4-nitroquinoline 1-oxide (4NQO, 100 μg/ml) for 16 weeks. 4NQO is a chemical carcinogen which is known to selectively induce oral carcinogenesis [14]. Our experiment showed that calcium does not precipitate 4NQO out of solution. The mice were then fed with normal drinking water for 12 weeks. At the end of 12 weeks, these mice were sacrificed and their tongues were removed for subsequent experiments. The study was approved by the Animal Care and Use Committee of the Second Xiangya Hospital of Central South University, China and San Francisco VA Medical Center, USA.
Determination of calcium levels in the oral epithelium
To determine the levels of calcium in the oral epithelium, samples of tongues in some mice were processed for ion-capture cytochemistry, as previously described by Bikle et al [15]. Samples were minced and immediately immersed in an ice-cold fixative containing 2% glutaraldehyde, 2% formaldehyde, 90 mM potassium oxalate, and 1.4% sucrose. After overnight incubation at 4°C in the fixative, samples were postfixed in 1% osmium tetroxide containing 2% potassium pyroantimonate at 4°C for 2 h, rinsed in cold distilled water (adjusted to pH 10 with KOH), and dehydrated, paraffin embedded and sectioned. Ultrathin sections were examined under a Zeiss electrons microscope.
Histological and immunohistochemical assessment
Tumors larger than 1 mm in diameter in the tongue were noted and the incidence of tumors was determined. The tongue tissues were fixed in formalin solution and embedded in paraffin blocks for routine histological and immunohistochemical analysis. Paraffin-embedded 4-micrometer-thick specimens were dewaxed in turpentine and rehydrated through decreased concentrations of ethanol. Endogenous peroxidase activity was blocked by using 3% H2O2 in methanol for 15 min. The sections were incubated with trisodium citrate dihydrate liquid (0.125%, pH 6.0) for 15 min, and then soaked with phosphate buffered saline (PBS) liquid (pH 7.2-7.4) three times for 5 min. The sections were then pre-incubated with sheep serum for 10 min to block non-specific antigen. The pretreated slides were incubated overnight at 4°C in a humidified chamber with rabbit polyclonal or monoclonal primary antibodies against mouse keratin 1, involucrin, loricrin, filaggrin, E-cadherin, β-catenin, p120, PLC-γ1, PIKE, or EGFR. Antibodies purchased from Santa Cruz Biotechnology (Santa Cruz, CA) include rabbit polyclonal antibodies against proliferating cell nuclear antigen (PCNA, cat# sc-7907), PLC-γ1 (cat# sc-81), E-cadherin (cat# sc-7870), p120 (cat# sc-13957), or EGFR (cat# sc-03) and rabbit monoclonal antibody against b-catenin (cat# sc-7199). Antibodies purchased from Covance Research Products (Denver, PA) include rabbit polyclonal antibodies against involucrin (cat# PRB-140c, dilution 1:1500), filaggrin (cat# PRB-417p-100, dilution 1:3000), keratin 1 (cat# PRB-1499-100, dilution 1:500), or loricrin (cat# PRB-145p-100, dilution 1:1000). Antibodies purchased from upstate biotechnology (Lakeplacid, NY) include rabbit polyclonal the antibody against PIKE (cat# 52-5817, dilution 1:100). After incubation with these antibodies, the slides were incubated at room temperature for 1 hour. After rinsing with PBS three times, the slides were incubated with appropriate biotinylated secondary antibodies for 20 min followed by avidin (Maixin Biological Technology Development Company) and diaminobenzidine (Maixin Biological Technology Development Company). Hematoxylin was used as counter-staining. In the negative controls, PBS (pH 7.4) was used instead of the primary antibody.
Determination of calcium and PTH levels in serum
At the end of 28 weeks, blood samples were obtained from sacrificed mice for serum calcium determination. Serum total calcium was measured by an ion-specific electrode (ABL 700, Radiometer, Copenhagen, Denmark). Serum PTH was measured by chemiluminescence.
Statistical analysis
Immunohistochemical results were assessed by counting number of cells stained and total number of cells in five representative regions of the sections. The percentages of positively stained cells were calculated [16]. For routine histological analysis, the results were examined under a light microscope (Olympus, Japan) and reviewed by two Board-certified pathologists at the Second Xiangya Hospital. Data are presented as mean ± standard deviation. Analysis of variance (ANOVA) and chi-square test were used to calculate differences among three groups. Results were considered statistically significant when the P value was less than 0.05.
Results
Inhibition of oral tumor formation by dietary calcium
High calcium intake is associated with low risk of some cancers [17]. Calcium is a strong inducer of differentiation of keratinocytes in the squamous epithelium [18]. However, the relationship between dietary calcium and squamous cell carcinoma is unknown. To determine the effects of dietary calcium on the development of squamous cell carcinoma, we fed mice with different calcium diets and 4NQO and examined the rates of tumors in the oral cavity. Tumors larger than 1 mm were counted. In the low calcium diet group, 64% mice (14 out of 22) developed oral tumors (Figure 1). In the normal calcium diet group, 36% mice (7 out of 19) developed oral tumors (Figure 1). In the high calcium diet group, only 12% mice (3 out of 25) developed oral tumors (Figure 1). The differences among three groups are significant (P < 0.05). These data indicate that dietary calcium inhibits oral tumor formation.
Figure 1.
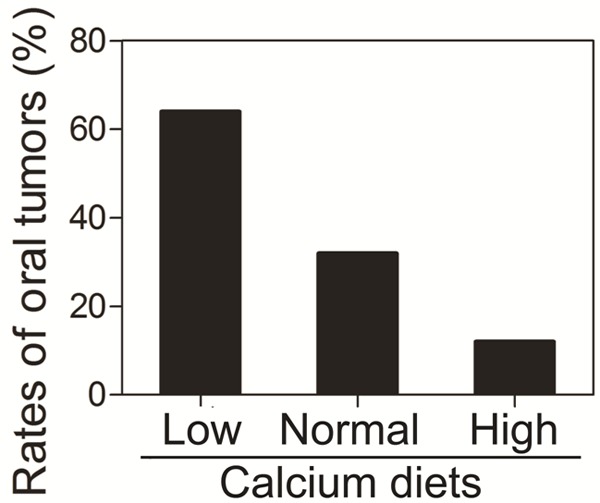
Effects of dietary calcium on the incidence of oral tumors. Sixty-six mice were divided into the low calcium diet group (n = 22), the normal calcium diet group (n = 19) and the high calcium diet group (n = 25). All the mice were fed with 4NQO and the diets containing high (2%), normal (1.3%) or low (0.01%) calcium. At the end of study, the mice were sacrificed and examined for oral tumors. The bar graph indicates the rates of tumors in the oral cavity of these mice. The data are expressed as mean ± SD, *P < 0.05 (compared with the normal calcium diet group).
Dietary calcium affected the calcium level in the oral epithelium but not the serum calcium and PTH
To determine whether dietary calcium affects calcium levels in the oral epithelium, we examined the level of calcium in the oral epithelium in mice on different calcium diets. The results showed that the low calcium diet reduced the level of calcium in the oral epithelium and the high calcium diet enhanced the level of calcium in the oral epithelium (Figure 2A). These data suggest that dietary calcium increases the level of calcium in the oral epithelium.
Figure 2.
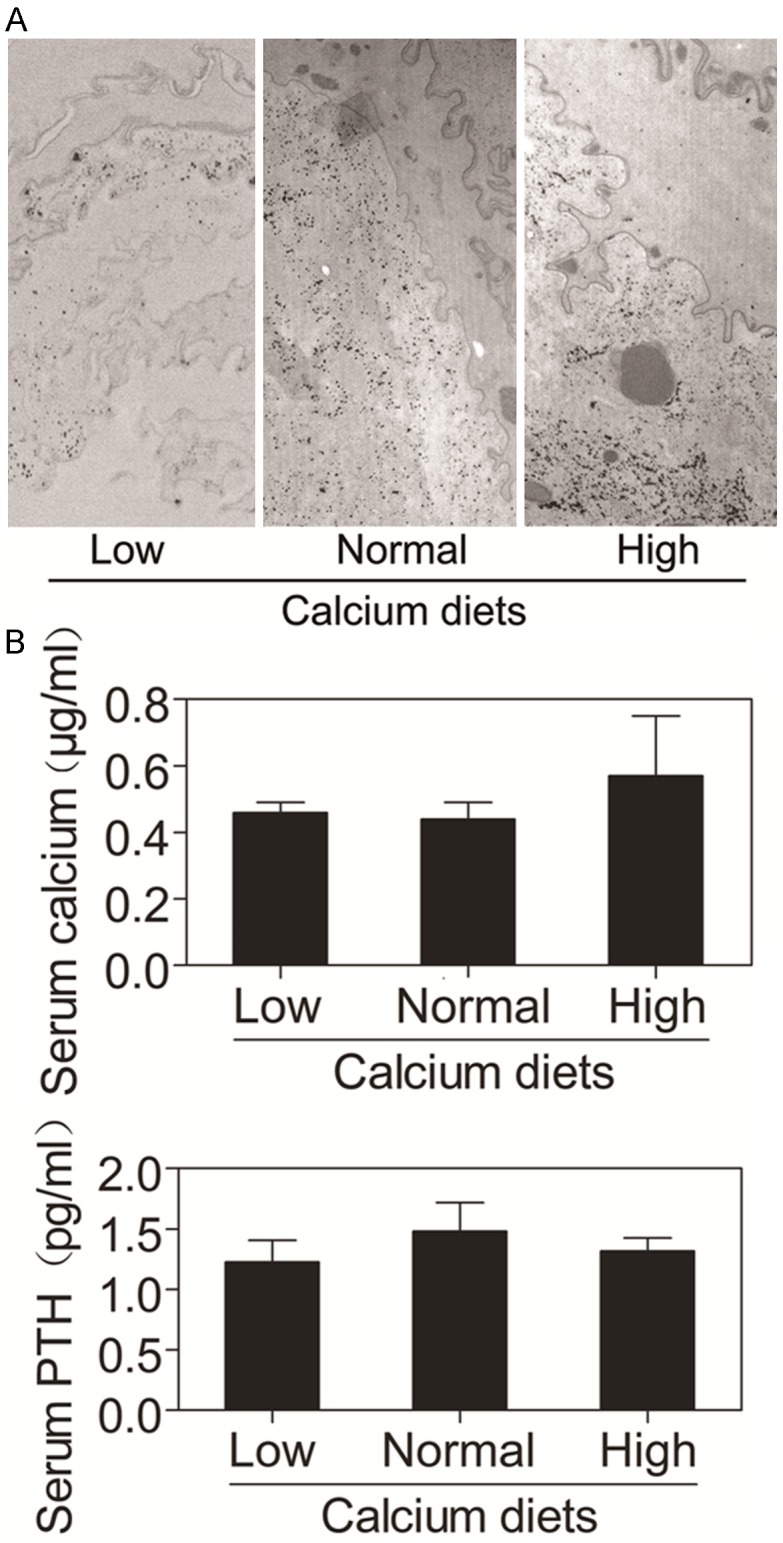
Effects of dietary calcium on calcium gradient in the normal oral epithelium, serum calcium and PTH. A. The graphs show electron micrographs of representative sections of the oral epithelial samples taken from the mice on different calcium diets. The calcium gradient (black granules) in the oral epithelium was determined by the ion-capture electron microscope cytochemistry. The left panel shows calcium precipitates in oral epithelium of mice on the low calcium diet. The middle panel shows calcium precipitates in oral epithelium of mice on the normal calcium diet. The right panel shows calcium precipitates in oral epithelium of mice on the high calcium diet. B. Serum was obtained from the mice described in Figure 1. The bar graph indicates the serum calcium and PTH concentration in these mice. The data are expressed as mean ± SD.
To determine whether serum calcium and PTH is affected by dietary calcium, we collected blood samples from these mice. The results showed that there was no significant difference in the levels of serum calcium or PTH among three groups on different calcium diets (Figure 2B). These results suggest that dietary calcium does not affect levels of serum calcium or PTH.
Dietary calcium inhibited proliferation of normal oral epithelium
To determine whether dietary calcium affects oral epithelial proliferation, the expression levels of PCNA in oral epithelium of mice on three different calcium diets were examined by immunohistochemistry. The results showed that the level of PCNA in normal oral epithelium was lower in mice on high calcium diet and higher in mice on low calcium diet compared to mice on normal calcium diet (P < 0.05, Figure 3A, 3B). However, there was no difference in the expression level of PCNA in papilloma or squmaous cell carcinoma (SCC) among three groups. These data indicate that dietary calcium inhibits proliferation of normal oral epithelium, but has no effect on proliferation of oral tumors.
Figure 3.
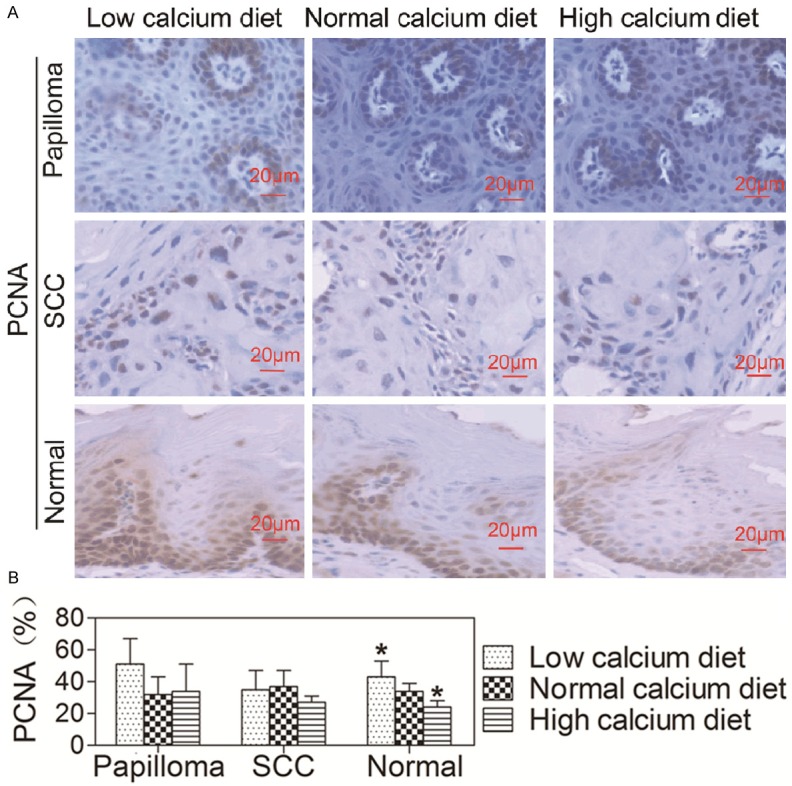
Effects of dietary calcium on the proliferation of oral epithelia. The tongue was removed from the mice described in Figure 1 and the tissue was fixed in formalin solution and embedded in paraffin blocks for pathological analysis and immunohistochemistry using the antibody against PCNA. A. Tissue sections were stained with the antibody against PCNA (brown) and counterstained with hematoxylin (blue). The representative section shows the average expression level of PCNA. B. Quantitation of the PCNA levels in the cells was shown as bar graphs. The quantitation for each section was obtained by counting the number of positive cells and total number of cells in the corresponding region in five representative regions in each section. The data are expressed as mean ± SD, *P < 0.05 (compared with the normal calcium diet group).
Dietary calcium promoted differentiation of normal oral epithelium
To determine whether dietary calcium affects oral epithelial differentiation, we examined expression levels of epidermal differentiation markers in oral papilloma, SCC and normal epithelium using immunohistochemistry (Figure 4A-D). The results showed that levels of differentiation markers (keratin 1, involucrin, filaggrin and loricrin) in the normal oral epithelium were higher in the mice on the high calcium diet and lower in the mice on the low calcium diet than mice fed with normal calcium diet (P < 0.05, Figure 4E). However, there were no differences in the expression levels of differentiation markers in papilloma or SCC among three groups. These results indicate that dietary calcium promotes differentiation of normal oral epithelium, but has no effect on 4NQO-induced differentiation of oral tumors.
Figure 4.
Effects of dietary calcium on the differentiation of oral epithelium. The tongue was removed from the mice described in Figure 1 and the tissue was fixed in formalin solution and embedded in paraffin blocks for routine histological and immunohistochemical analysis using antibodies against differentiation markers including keratin 1, involucrin, filaggrin and loricrin. Positive expression is shown in blown and the counterstaining is shown in blue. The figure shows the representative sections of oral papilloma, SCC and normal epithelium. The bar graph shows quantitation of the levels of differentiation markers in the cells. The quantitation was obtained as described in Figure 3. The data are expressed as mean ± SD, *P < 0.05 (compared with the normal calcium diet group).
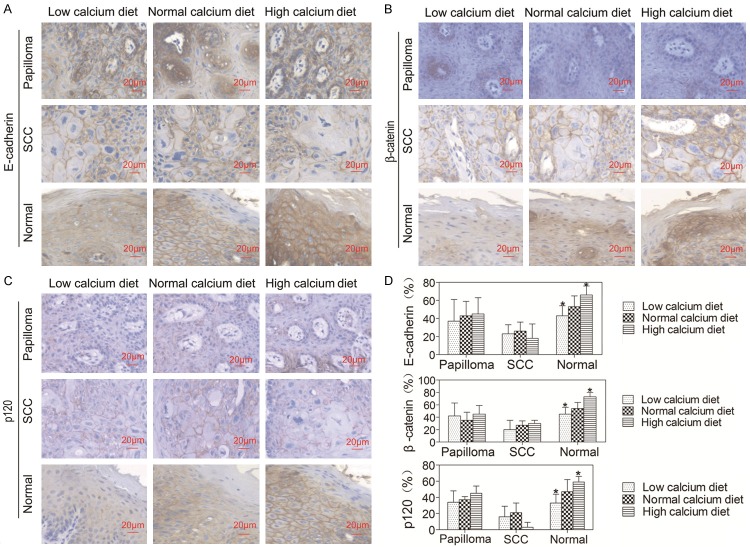
Dietary calcium stimulated expression of E-cadherin, β-catenin and p120 in normal oral epithelium
Upon Cao stimulation, the extracellular domain of E-cadherin interacts with the extracellular domain of E-cadherin molecules on the surface of neighboring cells, and its cytoplasmic tail interacts with β- (or γ-), and p120-catenins to form the core adhesive structure of adherent junctions [19]. Our previous studies [8-12] indicated that Cao induces E-cadherin-β-catenin-p120 complex formation in the plasma membrane. To investigate the effects of dietary calcium on the E-cadherin-β-catenin-p120 complex in the oral epithelium, the expression levels of E-cadherin, β-catenin and p120 in the oral papilloma, SCC and normal epithelium were examined by immunohistochemistry. The results showed that E-cadherin, β-catenin and p120 were mainly expressed in the plasma membrane, and barely expressed in the cytoplasm (Figure 5A-C). The levels of E-cadherin, β-catenin and p120 in the normal oral epithelium were higher in mice on the high calcium diet and lower in mice on the low calcium diet than mice on the normal calcium diet (P < 0.05, Figure 5D). However, there were no differences in the expression levels of E-cadherin, β-catenin or p120 in papilloma or SCC among three groups. The results indicate that dietary calcium increases expression of E-cadherin, β-catenin and p120 in the normal oral epithelium, but has no effect on expression of them in oral tumors.
Figure 5.
Effects of dietary calcium on the expression of E-cadherin, β-catenin and p120 in the oral epithelium. The tongue was removed from the mice described in Figure 1 and the tissue was fixed in formalin solution and embedded in paraffin blocks for routine histological and immunohistochemical analysis using antibodies against E-cadherin, β-catenin and p120. Positive expression is shown in blown and the counterstaining is shown in blue. The representative section shows the average level of E-cadherin, β-catenin and p120 in oral papilloma, SCC and normal epithelium. Quantitation of E-cadherin, β-catenin and p120 in the cells is shown in the bar graph. The quantitation was obtained as described in Figure 3. The data are expressed as mean ± SD, *P < 0.05 (compared with the normal calcium diet group).
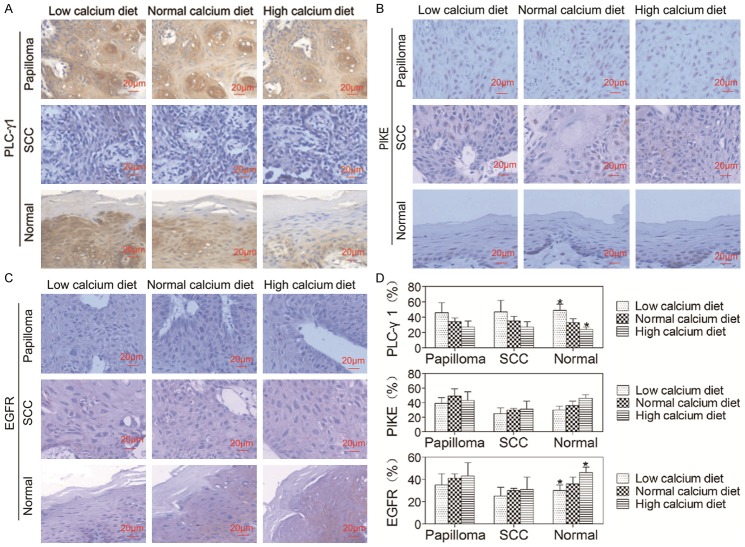
Dietary calcium inhibited expression of PLC-γ1 but did not affect the expression of PIKE
PLC-γ1 is the most abundant member of the phospholipase C family in keratinocytes and required for calcium-induced keratinocyte differentiation [8] as well as epidermal growth factor receptor (EGFR)-induced keratinocyte and SCC cell proliferation [20,21]. The SH3 domain of PLC-γ1 acts as a guanine nucleotide exchange factor for PIKE, the short form of which is a nuclear GTPase and enhances the activity of nuclear class Ia PI3K required for proliferation [22,23]. To determine the effects of dietary calcium on the expression of PLC-γ1 and the downstream signaling molecule PIKE in the oral epithelium, we examined expression levels of PLC-γ1 and PIKE in the oral papilloma, SCC and normal epithelium using immunohistochemistry. The results showed that PLC-γ1 was localized to the cytoplasm and the cell membrane of keratinocytes in the oral papilloma, SCC and normal epithelium (Figure 6A). The expression level of PLC-γ1 in the normal oral epithelium was lower in mice on the high calcium diet and higher in mice on the low calcium diet than that in mice on the normal calcium diet (P < 0.05, Figure 6D) PIKE was detected only in the nucleus (Figure 6B). However, there were no differences in the expression levels of PLC-γ1 in the oral papilloma or SCC epithelium and PIKE in the oral tumor or normal epithelium among three groups (P > 0.05) (Figure 6D). These data suggest that dietary calcium inhibits expression of PLC-γ1 in the normal oral epithelium, but has no effect on expression of PLC-γ1 in oral tumor epithelium and also no effect on expression of nuclear PIKE in the oral tumor and normal epithelium.
Figure 6.
Effects of dietary calcium on the expression of PLC-γ1, PIKE and EGFR in the oral epithelium. The tongue was removed from the mice described in Figure 1 and the tissue was fixed in formalin solution, embedded in paraffin blocks and sectioned for routine histological and immunohistochemical analysis using antibodies against PLC-γ1, PIKE and EGFR. Positive staining was shown in brown and counterstaining was shown in blue. The representative section shows the average expression levels of PLC-γ1, PIKE and EGFR in oral papilloma, SCC and normal epithelium. Quantitation of PLC-γ1, PIKE and EGFR in the cells is shown in the bar graph. The quantitation was obtained as described in Figure 3. The data are expressed as mean ± SD, *P < 0.05 (compared with the normal calcium diet group).
Dietary calcium stimulated expression of EGFR in normal oral epithelium
It is known that EGFR, a 170-kDa transmembrane tyrosine kinase receptor, is expressed in tissues of epithelial, mesenchymal and neuronal origin [24]. Upon activation by one of its ligands such as EGF or transforming growing factor-α, EGFR forms homo- or heterodimer leading to its autophosphorylation and triggers downstream signaling cascades including protein kinase C and Ras-activated ERK1/2 MAP kinase pathways [25,26]. EGFR-mediated signal transduction plays a major role in cell proliferation, migration and differentiation [27,28]. Therefore, EGFR is considered to be an important regulator of keratinocyte terminal differentiation [29] and proliferation [30]. To determine the effects of dietary calcium on the expression level of EGFR in the oral epithelium, we examined expression levels of EGFR in the oral papilloma, SCC and normal epithelium using immunohistochemistry. The results showed that EGFR was expressed in the plasma membrane (Figure 6C). The expression level of EGFR in the normal oral epithelium was lower in mice on the low calcium diet and higher in mice on the high calcium diet than that in mice on the normal calcium diet (P < 0.05, Figure 6D). However, there were no differences in the expression levels of EGFR in the oral papilloma or SCC (P > 0.05, Figure 6D). These data suggest that dietary calcium stimulates expression of EGFR in the normal oral epithelium, but has no effect on expression of EGFR in oral tumors.
Discussion
In the present study, we used the 4NQO-induced oral cancer mouse model to investigate the role of dietary calcium in regulating oral carcinogenesis. The results showed that there was a significant decrease in the incidence of oral tumors in mice on high calcium diet but an increment in mice fed on low calcium diet. Furthermore, the dietary calcium decreased proliferation and increased differentiation of keratinocytes in the normal oral epithelium. These results indicate that dietary calcium inhibits oral carcinogenesis probably by regulating proliferation and differentiation of oral epithelium.
Our results are consistent with a recent study which showed that dietary calcium suppresses DMBA-induced oral carcinogenesis in hamster [31]. The role of calcium in suppressing carcinogenesis is not only found in the oral epithelium, but also seen in other tissues [17]. More recently, it has been shown that calcium supplement reduced risk of head and neck cancers [32]. However, not all of studies suggest that calcium suppresses carcinogenesis. For example, a prospective cohort study showed no statistically significant association between dietary calcium and site-specific or overall cancer incidence or mortality [33]. The multi-factorial etiology of various cancers may account for the discrepancy between studies.
The rationale for conducting the present study was the association between dietary calcium and the incidence of oral cancers as well as the regulation of keratinocyte differentiation by extracellular calcium [17,34-39]. Our present results showed that dietary calcium increases the level of calcium in the normal oral epithelium. However, the serum calcium was not affected by the dietary calcium in our study. Serum calcium is maintained within narrow range under tightly homeostatic control of PTH, 1,25-(OH)2D3 and calcitonin (CT) and does not change as alteration of dietary calcium [40]. The dorsum of the tongue is covered by specialized epithelium as a mosaic of keratinized and non-keratinized epithelium. The non-keratinized epithelium allows penetration of small molecules into the extracellular space [41]. We cannot rule out the possibility that dietary calcium increases levels of calcium in the oral epithelium through direct penetration.
Our present studies show that dietary calcium suppresses keratinocyte proliferation and induces keratinocyte differentiation of normal oral epithelium but not papilloma or SCC epithelium. It is seems that dietary calcium has stronger impact on normal keratinocytes than on tumor keratinocytes. The anti-proliferative and pro-differentiative effects of calcium on keratinocytes may account for calcium inhibition of oral carcinogenesis.
Calcium-dependent adherence proteins or cadherins are a family of proteins essential for connecting the plasma membrane of adjacent cells. E-cadherin forms a complex with β-catenin. Loss of E-cadherin-β-catenin adhesion represents an important step in the progression of many epithelial malignancies [42]. Our previous studies [8-12] have indicated that the signaling pathway involving calcium-induced formation of the E-cadherin-β-catenin-p120 complex in the plasma membrane and subsequent activation of PLC-γ1 mediates calcium-induced human keratinocyte differentiation. Our present results showed that dietary calcium stimulated expression of E-cadherin, β-catenin and p120 in normal oral epithelium. However, the dietary calcium had no effect on the expression of these proteins in oral papilloma or SCC. It seems that dietary calcium promotes E-cadherin-β-catenin-p120-meditated signaling in normal keratinocytes but not in tumor keratinocytes.
PLC-γ1 is a critical component of the signaling pathway mediating calcium-induced keratinocyte differentiation via its mobilization of intracellular calcium [8-12], and also is required for EGFR-induced keratinocyte and SCC proliferation [20,21]. PLC-γ1 contains two Src homology (SH2), one SH3, one pleckstrin homology (PH) domain and two catalytic domains. Activation of the SH3 and catalytic domains of PLC-γ1 leads to proliferation and migration, respectively [21]. The activation of PLC-γ1 in the plasma membrane via increased PIP3 formation produced by c-src- and fyn-activated PI3K is required for calcium-induced human keratinocyte differentiation [10]. The activation of PIKE by PLC-γ1 is required for EGF-induced SCC cell proliferation [43]. Our results indicate that dietary calcium inhibits PLC-γ1 expression and stimulates EGFR expression, but does not affect PIKE expression. The functional link between the regulation of these molecules and inhibition of oral carcinogenesis by dietary calcium requires further investigation.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant numbers 81072219, 81272973 and 81471055). The author’s responsibilities were as follows-YJ, LL and CS contributed by performing the experiment, data analysis and manuscript preparation; DL, ML and YM contributed by performing immunohistochemistry and analyzing pathological results; DC contributed to the measurements of calcium in oral epithelium; LW contributed to the measurements of calcium and PTH in serum; ZX contributed to the experiment design, data analysis and manuscript preparation and all authors contributed to the data interpretation and manuscript preparation and approved the final version of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Hashibe M, Brennan P, Chuang SC, Boccia S, Castellsague X, Chen C, Curado MP, Dal Maso L, Daudt AW, Fabianova E, Fernandez L, Wunsch-Filho V, Franceschi S, Hayes RB, Herrero R, Kelsey K, Koifman S, La Vecchia C, Lazarus P, Levi F, Lence JJ, Mates D, Matos E, Menezes A, McClean MD, Muscat J, Eluf-Neto J, Olshan AF, Purdue M, Rudnai P, Schwartz SM, Smith E, Sturgis EM, Szeszenia-Dabrowska N, Talamini R, Wei Q, Winn DM, Shangina O, Pilarska A, Zhang ZF, Ferro G, Berthiller J, Boffetta P. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18:541–550. doi: 10.1158/1055-9965.EPI-08-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang SF, Li HF, Liao CT, Wang HM, Chen IH, Chang JC, Chen YJ, Cheng AJ. Association of HPV infections with second primary tumors in early-staged oral cavity cancer. Oral Dis. 2012;18:809–815. doi: 10.1111/j.1601-0825.2012.01950.x. [DOI] [PubMed] [Google Scholar]
- 4.Langevin SM, Michaud DS, Eliot M, Peters ES, McClean MD, Kelsey KT. Regular dental visits are associated with earlier stage at diagnosis for oral and pharyngeal cancer. Cancer Causes Control. 2012;23:1821–1829. doi: 10.1007/s10552-012-0061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hennings H, Kruszewski FH, Yuspa SH, Tucker RW. Intracellular calcium alterations in response to increased external calcium in normal and neoplastic keratinocytes. Carcinogenesis. 1989;10:777–780. doi: 10.1093/carcin/10.4.777. [DOI] [PubMed] [Google Scholar]
- 6.Menon GK, Elias PM, Lee SH, Feingold KR. Localization of calcium in murine epidermis following disruption and repair of the permeability barrier. Cell Tissue Res. 1992;270:503–512. doi: 10.1007/BF00645052. [DOI] [PubMed] [Google Scholar]
- 7.Hennings H, Michael D, Cheng C, Steinert P, Holbrook K, Yuspa SH. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980;19:245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- 8.Xie Z, Bikle DD. Phospholipase C-gamma1 is required for calcium-induced keratinocyte differentiation. J Biol Chem. 1999;274:20421–20424. doi: 10.1074/jbc.274.29.20421. [DOI] [PubMed] [Google Scholar]
- 9.Xie Z, Chang SM, Pennypacker SD, Liao EY, Bikle DD. Phosphatidylinositol-4-phosphate 5-kinase 1alpha mediates extracellular calcium-induced keratinocyte differentiation. Mol Biol Cell. 2009;20:1695–1704. doi: 10.1091/mbc.E08-07-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie Z, Singleton PA, Bourguignon LY, Bikle DD. Calcium-induced human keratinocyte differentiation requires src- and fyn-mediated phosphatidylinositol 3-kinase-dependent activation of phospholipase C-gamma1. Mol Biol Cell. 2005;16:3236–3246. doi: 10.1091/mbc.E05-02-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bikle DD, Ng D, Tu CL, Oda Y, Xie Z. Calcium- and vitamin D-regulated keratinocyte differentiation. Mol Cell Endocrinol. 2001;177:161–171. doi: 10.1016/s0303-7207(01)00452-x. [DOI] [PubMed] [Google Scholar]
- 12.Xie Z, Bikle DD. The recruitment of phosphatidylinositol 3-kinase to the E-cadherin-catenin complex at the plasma membrane is required for calcium-induced phospholipase C-gamma1 activation and human keratinocyte differentiation. J Biol Chem. 2007;282:8695–8703. doi: 10.1074/jbc.M609135200. [DOI] [PubMed] [Google Scholar]
- 13.Menon GK, Grayson S, Elias PM. Ionic calcium reservoirs in mammalian epidermis: ultrastructural localization by ion-capture cytochemistry. J Invest Dermatol. 1985;84:508–512. doi: 10.1111/1523-1747.ep12273485. [DOI] [PubMed] [Google Scholar]
- 14.Schoop RA, Noteborn MH, Baatenburg DJ. A mouse model for oral squamous cell carcinoma. J Mol Histol. 2009;40:177–181. doi: 10.1007/s10735-009-9228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bikle DD, Chang S, Crumrine D, Elalieh H, Man MQ, Choi EH, Dardenne O, Xie Z, Arnaud RS, Feingold K, Elias PM. 25 Hydroxyvitamin D 1 alpha-hydroxylase is required for optimal epidermal differentiation and permeability barrier homeostasis. J Invest Dermatol. 2004;122:984–992. doi: 10.1111/j.0022-202X.2004.22424.x. [DOI] [PubMed] [Google Scholar]
- 16.Li W, Cai JH, Zhang J, Tang YX, Wan L. Effects of cyclooxygenase inhibitors in combination with taxol on expression of cyclin d1 and ki-67 in a xenograft model of ovarian carcinoma. Int J Mol Sci. 2012;13:9741–9753. doi: 10.3390/ijms13089741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park Y, Leitzmann MF, Subar AF, Hollenbeck A, Schatzkin A. Dairy food, calcium, and risk of cancer in the NIH-AARP Diet and Health Study. Arch Intern Med. 2009;169:391–401. doi: 10.1001/archinternmed.2008.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pillai S, Bikle DD. Role of intracellular-free calcium in the cornified envelope formation of keratinocytes: differences in the mode of action of extracellular calcium and 1,25 dihydroxyvitamin D3. J Cell Physiol. 1991;146:94–100. doi: 10.1002/jcp.1041460113. [DOI] [PubMed] [Google Scholar]
- 19.Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005;6:328–340. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- 20.Xie Z, Chen Y, Liao EY, Jiang Y, Liu FY, Pennypacker SD. Phospholipase C-gamma1 is required for the epidermal growth factor receptor-induced squamous cell carcinoma cell mitogenesis. Biochem Biophys Res Commun. 2010;397:296–300. doi: 10.1016/j.bbrc.2010.05.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng J, Liao L, Chen Y, Pennypacker SD, Gao X, Zhou SH, Xie Z. Two distinct mechanisms by which phospholipase C-gamma1 mediates epidermal growth factor-induced keratinocyte migration and proliferation. J Dermatol Sci. 2012;67:199–202. doi: 10.1016/j.jdermsci.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Ye K, Aghdasi B, Luo HR, Moriarity JL, Wu FY, Hong JJ, Hurt KJ, Bae SS, Suh PG, Snyder SH. Phospholipase C gamma 1 is a physiological guanine nucleotide exchange factor for the nuclear GTPase PIKE. Nature. 2002;415:541–544. doi: 10.1038/415541a. [DOI] [PubMed] [Google Scholar]
- 23.Xie Z, Chen Y, Pennypacker SD, Zhou Z, Peng D. The SH3 domain, but not the catalytic domain, is required for phospholipase C-gamma1 to mediate epidermal growth factor-induced mitogenesis. Biochem Biophys Res Commun. 2010;398:719–722. doi: 10.1016/j.bbrc.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yano S, Kondo K, Yamaguchi M, Richmond G, Hutchison M, Wakeling A, Averbuch S, Wadsworth P. Distribution and function of EGFR in human tissue and the effect of EGFR tyrosine kinase inhibition. Anticancer Res. 2003;23:3639–3650. [PubMed] [Google Scholar]
- 25.Getsios S, Simpson CL, Kojima V, Harmon R, Sheu LJ, Dusek RL, Cornwell M, Green KJ. Desmoglein 1-dependent suppression of EGFR signaling promotes epidermal differentiation and morphogenesis. J Cell Biol. 2009;185:1243–1258. doi: 10.1083/jcb.200809044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 27.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 28.Pastore S, Mascia F, Mariani V, Girolomoni G. The epidermal growth factor receptor system in skin repair and inflammation. J Invest Dermatol. 2008;128:1365–1374. doi: 10.1038/sj.jid.5701184. [DOI] [PubMed] [Google Scholar]
- 29.Johnston A, Gudjonsson JE, Aphale A, Guzman AM, Stoll SW, Elder JT. EGFR and IL-1 signaling synergistically promote keratinocyte antimicrobial defenses in a differentiation-dependent manner. J Invest Dermatol. 2011;131:329–337. doi: 10.1038/jid.2010.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu W, Hsu DK, Chen HY, Yang RY, Carraway KR, Isseroff RR, Liu FT. Galectin-3 regulates intracellular trafficking of EGFR through Alix and promotes keratinocyte migration. J Invest Dermatol. 2012;132:2828–2837. doi: 10.1038/jid.2012.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lajolo C, Sgambato A, Maiorano E, Lucchese A, Capodiferro S, Favia G, Giuliani M. Calcium glucarate inhibits DMBA-induced oral carcinogenesis in the hamster: histomorphometric evaluation. Anticancer Res. 2010;30:843–849. [PubMed] [Google Scholar]
- 32.Li Q, Chuang SC, Eluf-Neto J, Menezes A, Matos E, Koifman S, Wunsch-Filho V, Fernandez L, Daudt AW, Curado MP, Winn DM, Franceschi S, Herrero R, Castellsague X, Morgenstern H, Zhang ZF, Lazarus P, Muscat J, McClean M, Kelsey KT, Hayes RB, Purdue MP, Schwartz SM, Chen C, Benhamou S, Olshan AF, Yu G, Schantz S, Ferro G, Brennan P, Boffetta P, Hashibe M. Vitamin or mineral supplement intake and the risk of head and neck cancer: pooled analysis in the INHANCE consortium. Int J Cancer. 2012;131:1686–1699. doi: 10.1002/ijc.27405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li K, Kaaks R, Linseisen J, Rohrmann S. Dietary calcium and magnesium intake in relation to cancer incidence and mortality in a German prospective cohort (EPIC-Heidelberg) Cancer Causes Control. 2011;22:1375–1382. doi: 10.1007/s10552-011-9810-z. [DOI] [PubMed] [Google Scholar]
- 34.Wang JL, Lin YW, Chen HM, Kong X, Xiong H, Shen N, Hong J, Fang JY. Calcium prevents tumorigenesis in a mouse model of colorectal cancer. PLoS One. 2011;6:e22566. doi: 10.1371/journal.pone.0022566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahearn TU, Shaukat A, Flanders WD, Rutherford RE, Bostick RM. A Randomized Clinical Trial of the Effects of Supplemental Calcium and Vitamin D3 on the APC/beta-Catenin Pathway in the Normal Mucosa of Colorectal Adenoma Patients. Cancer Prev Res (Phila) 2012;5:1247–1256. doi: 10.1158/1940-6207.CAPR-12-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacobson EA, James KA, Newmark HL, Carroll KK. Effects of dietary fat, calcium, and vitamin D on growth and mammary tumorigenesis induced by 7,12-dimethylbenz(a)anthracene in female Sprague-Dawley rats. Cancer Res. 1989;49:6300–6303. [PubMed] [Google Scholar]
- 37.McCullough ML, Rodriguez C, Diver WR, Feigelson HS, Stevens VL, Thun MJ, Calle EE. Dairy, calcium, vitamin D intake and postmenopausal breast cancer risk in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:2898–2904. doi: 10.1158/1055-9965.EPI-05-0611. [DOI] [PubMed] [Google Scholar]
- 38.Prineas RJ, Folsom AR, Zhang ZM, Sellers TA, Potter J. Nutrition and other risk factors for renal cell carcinoma in postmenopausal women. Epidemiology. 1997;8:31–36. doi: 10.1097/00001648-199701000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Yang CY, Cheng MF, Tsai SS, Hsieh YL. Calcium, magnesium, and nitrate in drinking water and gastric cancer mortality. Jpn J Cancer Res. 1998;89:124–130. doi: 10.1111/j.1349-7006.1998.tb00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 41.Squier CA, Lesch CA. Penetration pathways of different compounds through epidermis and oral epithelia. J Oral Pathol. 1988;17:512–516. doi: 10.1111/j.1600-0714.1988.tb01326.x. [DOI] [PubMed] [Google Scholar]
- 42.Natsugoe S, Uchikado Y, Okumura H Matsumoto M, Setoyama T, Tamotsu K, Kita Y, Sakamoto A, Owaki T, Ishigami S, Aikou T. Snail plays a key role in E-cadherin-preserved esophageal squamous cell carcinoma. Oncol Rep. 2007;17:517–523. [PubMed] [Google Scholar]
- 43.Xie Z, Jiang Y, Liao EY, Chen Y, Pennypacker SD, Peng J, Chang SM. PIKE mediates EGFR proliferative signaling in squamous cell carcinoma cells. Oncogene. 2012;31:5090–5098. doi: 10.1038/onc.2012.10. [DOI] [PubMed] [Google Scholar]