Abstract
Pituitary adenomas (PAs) are noncancerous tumors, and about 35% of those reported to be invasive have been classified as “invasive pituitary adenomas (IPAs)”. In clinical, operative complications, total resection failures, and high relapse rates result from invasive features during the therapeutic process. Invasive mechanism is a complex process, including metalloproteases, inhibitors and tumor microenvironment factors etc. Thus, studying invasive mechanism of PAs might contribute to understanding its biological behavior. In our research, three type tissue samples of human, pituitaries, PAs, IPAs, their mRNA expression of MMP1, MMP2, MMP9, MMP14 and MMP15 were measured using real-time PCR. MMP2 and MMP14 protein levels also were measured with immunohistochemistry in same samples. We confirmed that elevated matrix metalloproteinase-14 expression correlates with invasive characteristics of IPAs. To investigate molecular mechanism of how MMP14 contributes to invasiveness, an ATT20 cell was used in this study. After transient-transfection of the MMP14-shRNA expression vector into ATT20 cells, we observed that mRNA expression of PTTG, VEGF, and TGFβ was significantly suppressed in interference groups. Meanwhile, ATT20 cells in high concentration TIMP-1 environment exhibit reduced PTTG, VEGF, and TGFβ expression accompanied with the down-regulation of MMP14. Thus, we propose that MMP14 plays an important role in tumor invasion and angiogenesis and that a novel regulatory pathway for MMP14 may exist through VEGF and PTTG. In brief, MMP14 may be a target for therapeutic treatment.
Keywords: MMP14, PTTG, VEGF, ATT20, invasive pituitary adenomas
Introduction
Pituitary adenoma (PA) is a noncancerous tumor arising from the pituitary gland which can be categorized as a benign or invasive pituitary adenoma (IPA) or as histologically indistinct carcinomas. PAs are capable of proliferative expansion and the diagnosis of such is usually accompanied with intracranial invasion. Although invasive PAs are benign, they can invade surrounding structures as evidenced by magnetic resonance imaging (MRI). IPAs have such “malignant behavior” in 33.3% of patients, invading into the cavernous and sphenoid sinus, the orbit, and the clivus [1,2]. Except for prolactinomas which are sensitive to bromocriptine, therapeutic strategies of IPAs chiefly depend on surgical resection and postoperative radiotherapy. IPAs grow rapidly, recur postoperatively, and are poor prognosis [3]. Also, the molecular mechanism by which PAs invade surrounding tissue is unclear.
Matrix metalloproteinases (MMPs) are a broad family of zinc-binding endopeptidases that help degrade the extracellular matrix (ECM), which is associated with cancer cell invasion, metastasis, and angiogenesis [4]. Studies focused on PAs’ invasiveness chiefly center on MMP1, MMP2, MMP3, MMP9 and its inhibitors TIMP1 and TIMP2. Among these invasive factors, high expression of MMP2 and MMP9 is correlated with invasiveness of the dural or cavernous sinus [5,6]. Meanwhile, MMP14 has been identified to be a major physiological activator of pro-MMP-2 [7]. MMP14 is a membrane-type metalloproteinase with collagenase activity with potential roles in many biological processes in normal and cancerous tissues [8]. Most studies of MMP14 primarily focus on angiogenesis and invasion [9,10]. In addition to invasiveness, oncogenes and tumor suppressor genes associated with malignant transformation have received attention. Pituitary tumor transforming gene (PTTG) is highly expressed in PAs compared to normal pituitaries [11] and PTTG contributes to cell migration, invasion and angiogenesis by inducing MMP-2 secretion and expression [12]. Also, PTTG promotes angiogenesis of pituitary tumors by regulating relevant markers, particularly VEGF and bFGF [13,14]. Thus, we sought to determine whether expression of MMP14 may help with invasion and angiogenesis in IPAs and we explored a potential role for MMP14, VEGF and PTTG in ATT20 cells (mouse pituitary corticotrope tumor cell line, ATT20), to better understand the molecular mechanism of IPA invasiveness.
Materials and methods
Ethical statement
This study was approved by the Institutional Review Board of the First Affiliated Hospital of Soochow University. Written informed consents were signed and biological samples were donated by participants.
Patients
We studied PAs from 82 patients who visited the Department of Neurosurgery, First Affiliated Hospital, Soochow University, between January 2010 and March 2012. Patients included 35 males aged 21-76 years (average = 41.3 ± 9.2 years) and 47 females aged 18-68 years (average = 39.5 ± 10.2 years). The disease duration of the patients ranged between 5 days and 18 years. The maximum tumor diameter measured with MRI before surgery was less than 10 mm in 13 cases, 10-20 mm in 24 cases, 20-40 mm in 26 cases, and greater than 40 mm in 19 cases. With tumors classified in accordance with the results of immunohistochemical staining for endocrine hormones, there were 21 cases of nonfunctioning adenoma, 16 cases of multiple-hormone-type, 18 cases of PRL-type, 13 cases of GH-type, 6 cases of ACTH-type, 4 cases of TSH-type, and 4 cases of gonadotropin adenoma. Tumors were classified according to Knosp classification [15]. 12 cases were grade 0, 25 cases were grade I, 8 cases were grade II, 26 cases were grade III, 11 cases were grade IV. In this study, 37 cases were defined as invasive pituitary adenoma with knosp grade III or IV. In addition, 5 cases of normal pituitary tissue were derived from fresh corpses as control.
Cell culture
ATT20 cell is a mouse pituitary corticotrope tumor cell line, purchased from ATCC. All ATT20 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplement with 10% calf serum, 1.5 mM glutamine and penicillin-streptomycin (1:1000, 100 IU/mL; 100 g/mL). All cells cultivated at 37°C, 5% CO2 atmosphere and change culture medium once every two days.
Cell transfection
Four pieces of short hairpin RNA segments (shRNA fragments; See Table 1) for MMP14 gene mRNA were designed according to the MMP14 sequence in GenBank (NM_008608.3). Then, the recombined plasmid pGPU6/GFP/Neo-Mmp14 of four groups containing the interference fragment was constructed by GenePharma Co., Ltd. (Shanghai, China). ATT20 cells (1.5 × 104) in 6-well plates were transfected with recombinant plasmids using Lipofectamine™ 2000 (Invitrogen, USA). Transfected cells expressing GFP were measured with fluorescent microscopy (Zeiss; Gottingen, Germany) after 24-48 h. Then, transient transfected cells were counted to confirm transfection efficiency. The effective interference plasmid and concentration were selected using real-time PCR compared to untreated groups.
Table 1.
shRNA expression vector used in experiment
| shRNA expression vector | Template sequence of shDNA |
|---|---|
| pGPU6/GFP/Neo-MMP14-mus-1050 | 5’-CACCGGATGGACACAGAGAACTTCGTTCAAGAGACGAAGTTCTCTGTGTCCATCCTTTTTTG-3’ |
| 5’-GATCCAAAAAAGGATGGACACAGAGAACTTCGTCTCTTGAACGAAGTTCTCTGTGTCCATCC-3’ | |
| pGPU6/GFP/Neo-MMP14-mus-1246 | 5’-CACCGCTCCGAGGAGAGATGTTTGTTTCAAGAGAACAAACATCTCTCCTCGAGCTTTTTTG-3’ |
| 5’-GATCCAAAAAAGCTCCGAGGAGAGATGTTTGTTCTCTTGAAACAAACATCTCTCCTCGGAGC-3’ | |
| pGPU6/GFP/Neo-MMP14-mus-1358 | 5’-CACCGCATCCATCAATACTGCCTACTTCAAGAGAGTAGGCAGTATTGATGGATGCTTTTTTG-3’ |
| 5’-GATCCAAAAAAGCATCCATCAATACTGCCTACTCTCTTGAAGTAGGCAGTATTGATGGATGC-3’ | |
| pGPU6/GFP/Neo-MMP14-mus-537 | 5’-CACCGCTGTGGTGTTCCGGATAACTTTCAAGAGAACTTATCCGGAACACCACAGCTTTTTTG-3’ |
| 5’-GATCCAAAAAAGCTGTGGTGTTCCGGATAAGTTCTCTTGAAACTTATCCGGAACACCACAGC-3’ |
ATT20 cells treated with different concentration of TIMP-1
ATT20 cells were grown to confluence in three days. Then, cells were digested and plated into 6-well plate (1.5 × 104 cells/well). Three groups were divided as followed: controls (cells cultured with conditioned medium), a second group cultured with conditioned medium with TIMP1 (R&D, Minneapolis, MN) protein (10 μmol/mL), and a third group cultured with conditioned medium with TIMP1 protein (50 μmol/mL). All groups were cultured for 72-96 h.
Isolation of total RNA
Homogenize tissue samples were full ground in liquid nitrogen environment and the powder was dissolved in 0.75 ml of TRIzol (Invitrogen). Then, add 0.2 ml of chloroform into sample tubes and shake tubes for 25 times. After incubating them for 15 min at room temperature, centrifuge the sample for 15 min (12000 g, 4°C). The upper phenol-chloroform phases were collected into new tubes and add isometric alcohol volume and shake tubes for 25 times. After incubating them for 15 min at room temperature, centrifuge the sample for 10min (12000 g, 4°C). Then, remove the supernatant and wash the RNA deposit with 75% ethyl alcohol. Centrifuge and collect the RNA deposit for 5 min (7500 g, 4°C). Then, briefly air-dry the RNA deposit about 5 min and dissolved in RNasefree-water. The total RNA in cells is consistent with above steps except grinding process. Total RNA was detected by micro-spectrophotometer at 260/280 nm.
Gene expression analysis
cDNA was synthesized from 1 μg of total isolated RNA using an RNA reverse transcription diagnostic kit (TaKaRa). The 25 μL model of real-time PCR system: 12.5 μL SYBR Green, 9.5 μL ddH2O, 1 μL primer R, 1 μL primer F, 1 μL cDNA. Then, the sample in 96 well plates was mixed with centrifuge and was performed with the ABI PRISM 7500 sequence detection system (Applied Biosystems). PCR procedure was amplified in 40 cycles of denaturation (95°C, 60 s), annealing (95°C, 15 s), and extension (60°C, 60 s). Gene expression was measured using the 2-ΔCt method. Primer sequences used for real-time PCR are included in Tables 2, 3.
Table 2.
Sequences of primers used for tissue sample of human
| Gene for human | Oligonucleotide sequence (5’-3’) | |
|---|---|---|
| GAPDH | F: GGAGCGAGATCCCTCCAAAAT | R: GGCTGTTGTCATACTTCTCATGG |
| MMP1 | F: TCTATGATGGCACTGCTGAC | R: TGTCCAGGTTTCATCATCATC |
| MMP2 | F: AGATACCCTCAAGAAGATGC | R: AGCATCATCCACGGTTTCAG |
| MMP9 | F: AGTCTGGATAAGTTGGGTCT | R: AGATGTCGTGTGAGTTCCAG |
| MMP14 | F: AAACATCAAAGTCTGGGAAGG | R: ACTTGGGATACCCTGGCTCT |
| MMP15 | F: ATCCAGAACTACACTGAGAAG | R: TGGAAGCCAGAGGCAAAGAG |
Table 3.
Sequences of primers used for mouse
| Gene for mouse | Oligonucleotide sequence (5’-3’) | |
|---|---|---|
| GAPDH | F: TGCTGAGTATGTCGTGGAGT | R: AGTTGTCATATTTCTCGTGG |
| MMP1 | F: TCTATGATGGCACTGCTGAC | R: TGTCCAGGTTTCATCATCATC |
| MMP2 | F: AGATACCCTCAAGAAGATGC | R: AGCATCATCCACGGTTTCAG |
| MMP9 | F: AGTCTGGATAAGTTGGGTCT | R: AGATGTCGTGTGAGTTCCAG |
| MMP14 | F: AAACATCAAAGTCTGGGAAGG | R: ACTTGGGATACCCTGGCTCT |
| MMP15 | F: ATCCAGAACTACACTGAGAAG | R: TGGAAGCCAGAGGCAAAGAG |
| TGFβ-1 | F: ACCAAAGACATCTCACACAG | R: ACCAAGGTAACGCCAGGAAT |
| VEGF | F: ATGGATGTCTACCAGCGAAG | R: ATGGTGATGTTGCTCTCTGAC |
| P53 | F: CTACAAGAAGTCACAGCACAT | R: TGCCTGTCTTCCAGATACTC |
| PTTG | F: TGCCTAAAGCCAGCAGAAAG | R: ATCATCAGGAGCAGGAACAG |
Western blot assay
Total-proteins were exacted from ATT20 cells using lysis buffer containing 125 mM NaCl, 50 mM Tris-HCl, 1.5% Triton X-100. pH 7.2, 1 mM EDTA, 1 mM PMSF and placed in ice for 30 min. Then, 20 μg of proteins were mixed with loading buffer (1:5) and boiled for 5-10 min. Proteins were separated via 10% SDS-PAGE [16] in 40 min and transferred to nitrocellulose filter membrane (Millipore) (NC membrane) using a semi-dry process in 120 min. Then, the NC membrane was blocked with 5% fat-free milk for 1.5 h at 37°C. After washing with TBST three times, membrane was incubated with primary antibody at 4°C overnight. Then washing three times, extracted proteins were incubated with horseradish peroxidase (HRP)-conjugated mouse anti-rabbit IgG-AP at room temperature for 90 min. Detection and autoradiography were performed using an eECL Western Blotting kit (Cwbio, China) according to the manufacturer’s protocol. Blots were quantified using Quantity-one software. Mouse monoclonal MMP2 and rabbit monoclonal MMP14 were purchased from Abcam (Cambridge, UK). GAPDH antibody was purchased from Beyotime (Shanghai, China).
Transwell invasion assay
Three groups of ATT20 cells treated with TIMP1 proteins were grown to confluence and then digested in 0.25% trypsin. Then, ATT20 cells (1 × 105) were plated in the top chambers of 8-μm pore Transwells with serum-free DMEM medium, the lower stratum adding DMEM medium with 10% BSA. Cells and Matrigel in the upper chamber were removed after 24 h and cells at the filter bottom were fixed with 1% paraformaldehyde. After washing with PBS, the chamber was stained with Eosin dye liquor. Cell penetration through the membrane was detected by pathological microscopy in 10 fields (200 × magnification) per filter. This experiment was repeated 3 times each.
Histopathological examination
Specimens obtained from patients were fixed in 10% formalin and embedded into petrolin. Then, paraffin-embedded samples were cut in 3-4 μm slices and incubated at 65°C overnight. Sections were de-paraffinized and re-hydrated through xylene and alcohol gradient. After PBS washing 3 times, antigen retrieval was soaked in 3% H2O2 for 10 min. Then, washing three times, retorted in microwave oven with 0.05% phosphate buffer to exposure antigen sites. Then, sections were incubated with FBS at 37°C for 30 min. After PBS washing 3 times, Incubations with mouse monoclonal MMP2 (Abcam) or rabbit monoclonal MMP14 (Abcam) antibody (1:1,000) were carried out at 4°C overnight. 0.01 M PBS washed the tissue sections for three times. The sections were incubated with HRP-conjugated mouse anti-rabbit IgG-AP (1:500) at 37°C for 30 min. After washing, chromogenic agents were added as followed: 0.05% DAB, 5% H2O2, 0.05% phosphate buffer. Then, sections were washed, counterstained with hematoxylin, dehydrated and protected with coverslips. The results were observed and photographed under 200 × pathological microscopy. The gradational distinction was based on positivity cell numbers and staining intensity.
Statistical analysis
Data analyses were performed using the SPSS19.0 program. Three independent results are measured as mean ± SD. Statistical significance was achieved when the P value is < 0.05.
Results
Radiological results
Compared to benign PAs (Figure 1A), the radiological results of IPAs (Figure 1B) clearly invaded into the cavernous sinus on both sides with encasement of the internal carotid artery. In our research samples, it occurs in 37 of 82 patients and defined as invasive pituitary adenoma with knosp grade III and IV. Accordingly, in 18 of 37 patients, IPAs were incompletely surgically removed. Although IPAs were benign, they did migrate into surrounding structures.
Figure 1.
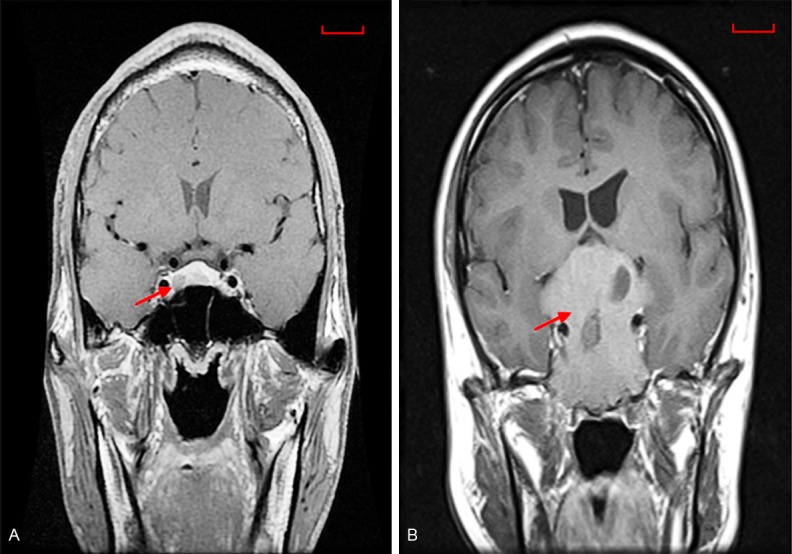
MRI depicts differences in benign PAs and IPAs. (A) benign PA (PA) in a 43-year-old male, tumor size: 0.69 × 1.46 × 1.2 cm; Knosp I; noninvasive (B) invasive PA in a 27-year-old male, tumor sizes: 7 × 6.5 × 6.2 cm; Knosp IV; invasion to both sides of the cavernous sinus with encasement of the internal carotid artery.
MMPs mRNA expression in pituitaries, PAs and IPAs
The expression of MMP1, MMP2, MMP9, MMP14 and MMP15 was measured in pituitaries, PAs and IPAs. As shown in Figure 2A, MMPs expressed in IPAs were higher than that of PAs and pituitaries using real-time PCR. And expression of MMP14 compared to other MMPs was elevated expressed (P < 0.01) in IPAs. Results revealed that MMP14 may play an important role in invasive PAs.
Figure 2.
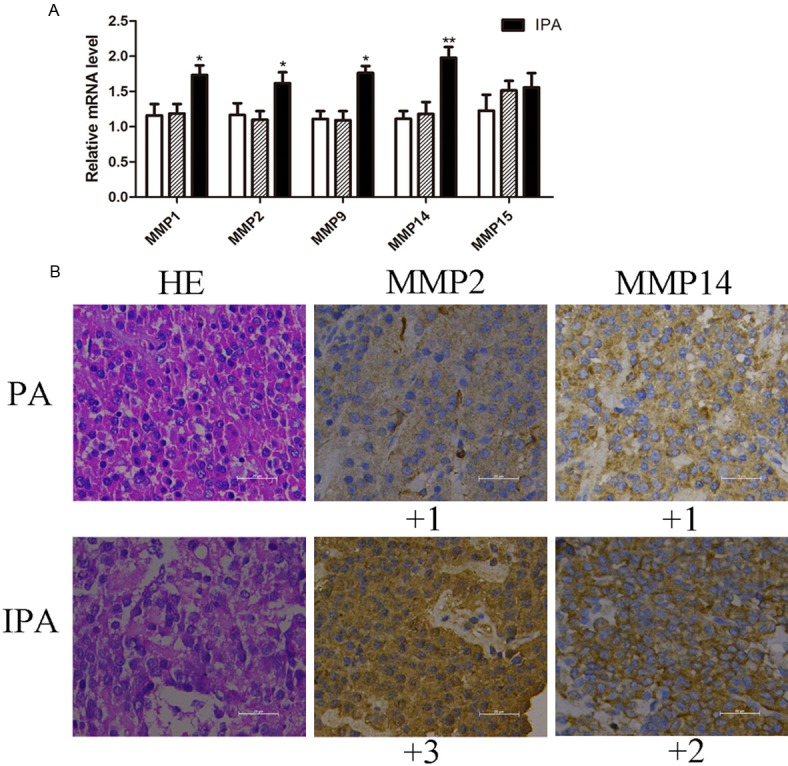
MMPs expression in pituitaries, PAs and IPAs. A. mRNA expression of MMP1, MMP2, MMP9, MMP14 and MMP15 was measured with real-time PCR. Each datum was mean values, including pituitarium, PAs and IPAs. B. PAs and IPAs were evaluated with hematoxylin and immunohistochemical staining of MMP2, MMP14. Score based on number of staining cells and intensity of staining color consisted of four levels: +0, achromaticity, < 5% cells; +1, faint yellow, 10-25% cells; +2, pale brown, 25%-40% cells; +3, tan, > 40% cells.
Immunohistochemistry
Because of high expressed MMP14 detected in mRNA level, we also measured the protein expression of MMP2 and MMP14 in IPAs (Figure 2B). We stained 82 PA samples with MMP2 and MMP14 antibodies (1:1,000) in duplicate. Data were plotted for representative images and MMP2/14-positive case distributions among clinical samples. As shown in Figure 2B, IPAs had more MMP14 staining (> 25%) than benign PAs, which has strong staining intensity located in cytomembrane. And the MMP2 staining was also obvious in cytoplasm. IPAs had high MMP14 immunoreactivity compared to benign PAs suggesting that high MMP14 correlated with pituitary tumor migration. Thus, elevated MMP14 in IPAs may co-relate with tumor invasiveness and migration.
MMPs, VEGF, TGFβ, P53 and PTTG mRNA expression in ATT20 cells
To investigate the potential regulatory mechanism behind IPAs, we measured MMP2, MMP9, MMP14, MMP15, TGFβ, VEGF, P53 and PTTG mRNA expression in ATT20 cells using real-time PCR (Figure 3). Using mouse pituitaries as controls, MMP14, TGFβ, VEGF and PTTG mRNA were significantly elevated (P < 0.05) compared to other genes. And MMP14 expression in ATT20 cells was higher than other MMPs suggesting that this gene may be important in tumorigenesis and invasiveness in vitro.
Figure 3.
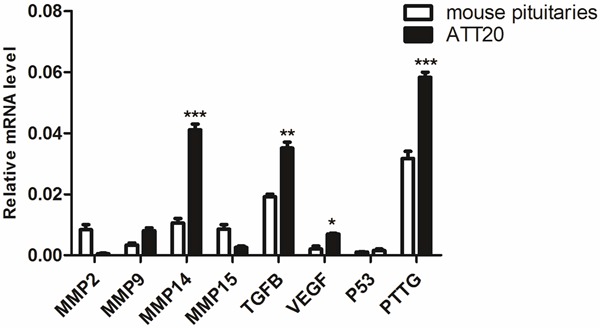
Differential expression of MMP2, MMP9, MMP14, MMP15, TGFβ, VEGF, P53 and PTTG in ATT20 cell lines and mouse pituitaries. mRNA of relative factors was measured with real-time PCR. mRNA intensity was normalized against GAPDH. Relative changes in gene expression were measured using the 2-Δ C T method and relative quantitative comparison mRNA expression was taken mouse pituitaries as control. Three dependent results were presented as mean ± SD, *P < 0.05 was considered significant.
Effect of downregulated MMP14 in ATT20 cells
To explore the role of MMP14 in ATT20 cells, pGPU6/GFP/Neo-MMP14 (shRNA carrier) were transfected into an ATT20 cell line. Transfection efficiency was measured using fluorescent microscopy to measure expression of the plasmid- encoded eGFP gene (Figure 4A). Then, the efficiency of different interference plasmids was measured with real-time RCR (Figure 4B). MMP14-mus-1246 groups had more significant interference effect than that of untreated groups (control) and other interference groups. Thus, pGPU6/GFP/Neo-MMP14-mus-1246 plasmid was used to investigate whether modulation of MMP14 expression affects tumorigenic properties of PAs. We then measured MMP2, MMP9, MMP15, TGFβ, VEGF, P53 and PTTG mRNA expression (Figure 4C). Compared with the untransfected group, MMP9, TGFβ, VEGF and PTTG (P < 0.05) were significantly suppressed in MMP14-downregulated cells. The expression of MMP14 has a potential relationship with MMP9, TGFβ, VEGF and PTTG.
Figure 4.
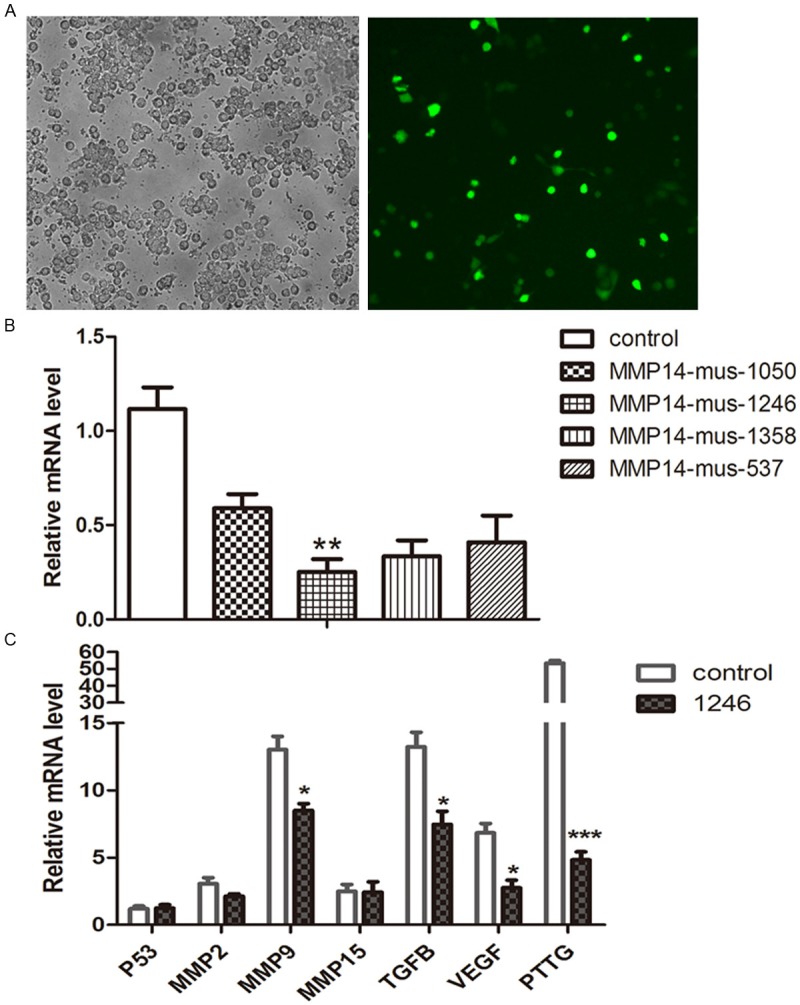
Selection transfectants and expression of MMP2, MMP9, MMP15, TGFβ, VEGF, P53 and PTTG in transiently transfected cells and controls. A. Transfection efficiency was detected with fluorescent microscopy (40 ×magnifications). B. Downregulated MMP14 mRNA efficiency was measured by real-time PCR in five interference plasmids. C. mRNA of relative factors was measured with real-time PCR. Results are presented as means ± SD from three independent data. *P < 0.05 was significant compared to control.
Inhibitory effect of TIMP-1 on cell invasive signaling in ATT20 cells
To investigate the effect of high concentration of TIMP-1 in ATT20 cells, several cell tumorigenic and invasive signals were measured with real-time PCR and Western blot. With increasing TIMP-1 concentration, MMP2, MMP9, MMP14, TGFβ, VEGF, P53 and PTTG mRNA expression were gradually suppressed (Figure 5A). MMP-2 and MMP-14 protein from different treatment groups were measured with Western blot (Figure 5B, 5C, respectively). MMP2 and MMP14 protein expression were decreased when treated with 10 and 50 μmol TIMP1 respectively. High concentration of TIMP-1 can inhibit MMP14 expression in ATT20 cells. And down-regulated expressed MMP14 may result to less expressing of TGFβ, VEGF and PTTG in mRNA level. And this was consistent with the transfected results above.
Figure 5.
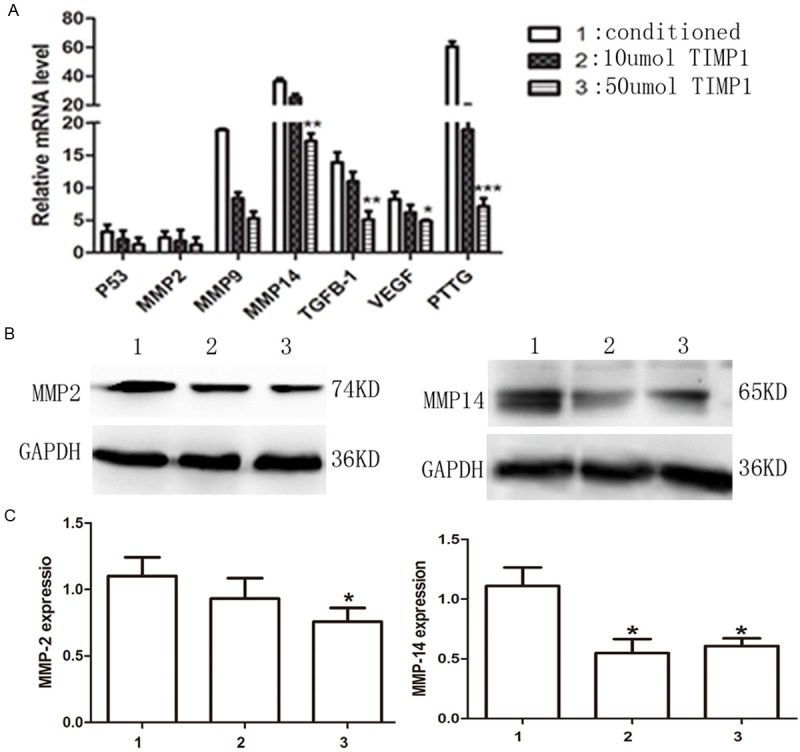
Analysis of MMP2, MMP9, MMP14, TGFβ, VEGF, P53 and PTTG expression in TIMP1 treated cells and controls. A. mRNA expression of relative factors was measured with real-time PCR. B, C. MMP2 and MMP14 protein were measured with Western blot. *P < 0.05 was significant.
Down-regulated MMP14 inhibits ATT20 cell migration and invasion
To confirm the invasion and migration of cell lines affected by disproportionated Matrix Metalloproteinases (MMPs) and Tissue Inhibitor of Metalloproteinases (TIMPs), transwell migration and invasion chambers coated with Matrigel were used. Cells were treated with 10 and 50 μmol TIMP1. As shown in Figure 6, the inhibited degree of invasive and metastasis ability highly increased with concentration of TIMP1 and treated times. Consistent with previous experimental results, down-regulated MMP14 affected by high concentration of TIMP-1 contributes to its invasive and metastasis behavior.
Figure 6.
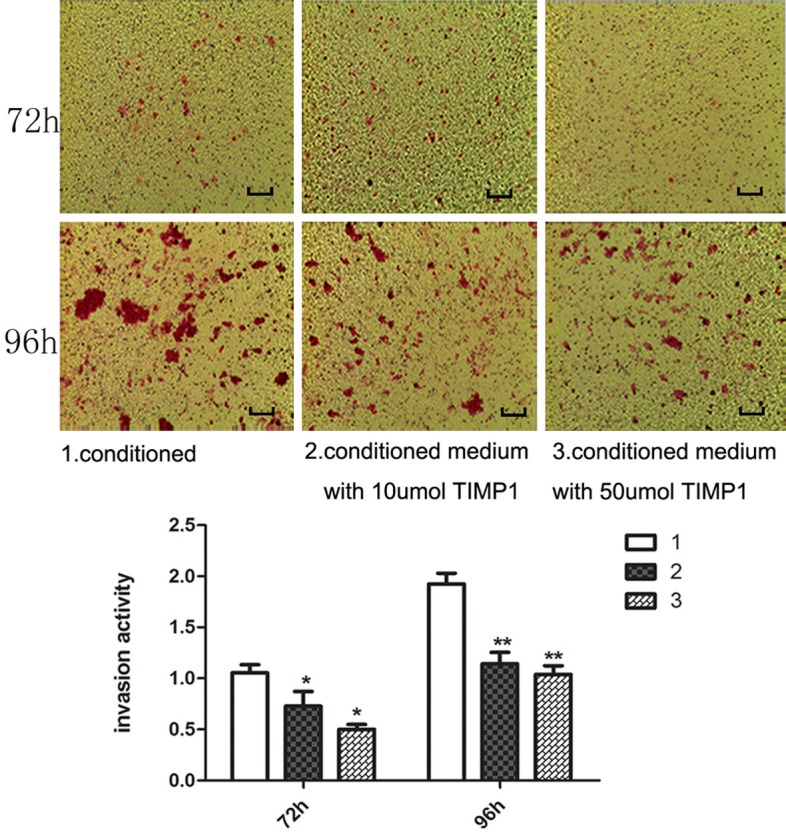
Comparison of in vitro cell invasiveness. In vitro invasion of ATT20 cells were measured by Matrigel-coated Transwell chambers (12-AM pore size). Data represent 3 independent experiments in 72 and 96h respectively. 1: control group: cultured with condition medium; 2: sample group: cultured with conditioned medium with 10 μmol of TIMP1; 3: sample group: cultured with conditioned medium with 50 μmol of TIMP1 (*P < 0.05, **P < 0.01 compared to the untreated group).
Discussion
PAs are benign brain tumors accounting for 10-15% of all primary brain tumors [17,18]. Jefferson first proposed the concept of an IPA, which was considered to possess histologic characteristics of non-infiltrative PAs but to act as a pituitary carcinoma with malignant biological behaviors [19]. As shown in Figure 1, IPAs invade into both sides cavernous sinus with encasement of the internal carotid artery, creating potentially fatal complications. Whether the PAs invades adjacent regions and relapses post-surgery depends on the extent of invasiveness [20,21]. Until now the molecular mechanisms of PAs invasiveness are unclear. Some factors including MMPs, growth factors, and TIMPs correlate with PAs invasive phenotypes. Specifically, high expression of MMP2 and MMP9 correlate with invasiveness of the dural or cavernous sinus in diverse PAs. It also predicted the importance of other MMPs such as MMP13 and MMP14 in PAs invasion [5,6]. Thus, we measured MMPs expression in pituitaries, PAs and IPAs. Both mRNA expression and immunoreactivity of MMP14 were higher in IPAs groups compared with other groups (Figure 2). This indicates the important role of MMP14 in IPAs.
MMP14 is a family of zinc-dependent endopeptidases, identified as an important membrane-type MMPs. It has been studied for its roles in many biological processes of normal or tumor tissues but has not been investigated in IPAs. MMP14 participate in mediating pericellular proteolysis of extracellular matrix (ECM) macromolecules and can assemble MMPs with different substrate specificities to degrade the ECM environment [22]. MMP14 can cleave type I, II and III collagen [23], MMP2 and MMP9 have type IV collagen activity, which effectively degrade basement membrane (BM) containing type IV collagen. These MMPs are usually secreted as pro-enzymes and are activated by MMP14 (particularly MMP2). Tumor invasion and metastasis is a complex process that may be accompanied by lymphatic induction and angiogenesis, which is co-regulated by various genes and proteins. MMP14 promotes angiogenesis, and vasculogenesis inhibits apoptosis by mediating VEGF in the cornea [24], glioma [25] and in breast carcinoma [26] etc.
To investigate whether MMP14 is a key enzyme driving tumor invasion and migration in invasive PAs, we selected ATT20 cell lines to study invasion mechanisms in vitro. As MMP2, MMP9, MMP14, TGFβ, VEGF, P53 and PTTG mRNA expression measured in ATT20 cell line, MMP14, PTTG, VEGF and TGFβ were highly expressed compared to other factors. Next, we measured MMP14 in the presence of an MMP14 shRNA vector and observed that PTTG, VEGF and TGFβ mRNA were significantly suppressed in MMP14 downregulated groups as measured by real-time PCR. The PTTG expression was associated with VEGF secretion from human pituitary adenomas, but their internal connection seems unclear [27]. VEGF, an important angiogenic cytokine, regulates physiological angiogenesis and mediating pathological angiogenesis such as tumor-associated neovascularization. In addition, MMP14 regulate tumor aggressiveness by modulating target molecules such as VEGF. And it together with TGFβ also maintains vessel stability and vascular response to tissue injury [28]. Thus, we proposed that high expression of PTTG in IPAs may promote MMP14 to induce VEGF expression, which eventually causes angiogenesis.
Increased MMPs activity and low tissue inhibitors of metalloproteinases cause robust ECM degrading activity. To confirm the effect of MMP14 and the function of TIMP-1 in ATT20 cells, TIMP-1, a inhibitor of MMP14 [29], was added to conditioned medium. Suppressed expression of MMP14 and MMP2 protein were observed as measured by Western blot. The C-terminal domain of TIMP-1 binds to hemopexin-like domain of pro-MMP2 and it affected the activation of MMP2 by MMP14 [30]. Down-regulated MMPs ultimately result to restrained migration and invasion ability of ATT20 cells. And in TIMP-1 treated groups and shRNA groups, a consistent gene expression of down-regulated VEGF, TGFβ and PTTG level was accompanied with inhibited MMP14 expression. This emphasizes the important relation of MMP14 to VEGF and PTTG. In addition, disproportionate MMPs and its inhibitors were also a cause for malignant phenotype. TIMP1 is not only an inhibitor of MMP14, but also has been recognized as a multifunctional protein which can inhibit new blood vessel formation and exert an anti-angiogenic activity both in vitro and in vivo [31,32]. It was reported that TIMP-1 endogenously regulates growth factors. Low expression of MMP14 may reduce tumor aggressiveness and invasion through feedback down-regulation of VEGF that is inhibited by high concentrations of TIMP1 protein, which may better illustrate the connection of MMP14 and VEGF.
Thus, we concluded that MMP14 is critical for tumor invasion and angiogenesis and we supposed a new regulatory pathway for MMP14, VEGF and PTTG. Our data lay the foundation for future studies to identify a novel therapeutic target for invasive PAs.
Acknowledgements
This work was mainly supported by the National Natural Science Foundation of China (No. 31170772) and Suzhou Special Project of Diagnosis and Treatment for key Clinical Disease (No. LCZX201403). And it was also partly supported by Six Talent Peaks Project in Jiangsu Province (No. WSW-045) and Science and Technology Development Plan of Suzhou City (No. SYS201326).
Disclosure of conflict of interest
None.
References
- 1.Sasagawa Y, Tachibana O, Doai M, Akai T, Tonami H, Iizuka H. Internal carotid arterial shift after transsphenoidal surgery in pituitary adenomas with cavernous sinus invasion. Pituitary. 2013;16:465–470. doi: 10.1007/s11102-013-0492-2. [DOI] [PubMed] [Google Scholar]
- 2.Goel A, Phalke U, Cacciola F, Muzumdar DP. Giant pituitary adenoma invading the clivus. Neurol India. 2005;53:105–107. doi: 10.4103/0028-3886.15073. [DOI] [PubMed] [Google Scholar]
- 3.Bronstein MD, Paraiba DB, Jallad RS. Management of pituitary tumors in pregnancy. Nat Rev Endocrinol. 2011;7:301–310. doi: 10.1038/nrendo.2011.38. [DOI] [PubMed] [Google Scholar]
- 4.Yadav L, Puri N, Rastogi V, Satpute P, Ahmad R, Kaur G. Matrix metalloproteinases and cancer - roles in threat and therapy. Asian Pac J Cancer Prev. 2014;15:1085–1091. doi: 10.7314/apjcp.2014.15.3.1085. [DOI] [PubMed] [Google Scholar]
- 5.Liu W, Matsumoto Y, Okada M, Miyake K, Kunishio K, Kawai N, Tamiya T, Nagao S. Matrix metalloproteinase 2 and 9 expression correlated with cavernous sinus invasion of pituitary adenomas. J Med Invest. 2005;52:151–158. doi: 10.2152/jmi.52.151. [DOI] [PubMed] [Google Scholar]
- 6.Hussaini IM, Trotter C, Zhao Y, Abdel-Fattah R, Amos S, Xiao A, Agi CU, Redpath GT, Fang Z, Leung GK, Lopes MB, Laws ER Jr. Matrix metalloproteinase-9 is differentially expressed in nonfunctioning invasive and noninvasive pituitary adenomas and increases invasion in human pituitary adenoma cell line. Am J Pathol. 2007;170:356–365. doi: 10.2353/ajpath.2007.060736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 8.Lehti K, Allen E, Birkedal-Hansen H, Holmbeck K, Miyake Y, Chun TH, Weiss SJ. An MT1-MMP-PDGF receptor-beta axis regulates mural cell investment of the microvasculature. Genes Dev. 2005;19:979–991. doi: 10.1101/gad.1294605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang WG, Davies G, Martin TA, Parr C, Watkins G, Mason MD, Mansel RE. Expression of membrane type-1 matrix metalloproteinase, MT1-MMP in human breast cancer and its impact on invasiveness of breast cancer cells. Int J Mol Med. 2006;17:583–590. [PubMed] [Google Scholar]
- 10.Sabeh F, Ota I, Holmbeck K, Birkedal-Hansen H, Soloway P, Balbin M, Lopez-Otin C, Shapiro S, Inada M, Krane S, Allen E, Chung D, Weiss SJ. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167:769–781. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanchez-Ortiga R, Sanchez Tejada L, Peiro Cabrera G, Moreno-Perez O, Arias Mendoza N, Aranda Lopez FI, Pico Alfonso A. Rol of pituitary tumour-transforming gene (PTTG) in the pituitary adenomas. Endocrinol Nutr. 2010;57:28–34. doi: 10.1016/S1575-0922(10)70006-1. [DOI] [PubMed] [Google Scholar]
- 12.Malik MT, Kakar SS. Regulation of angiogenesis and invasion by human Pituitary tumor transforming gene (PTTG) through increased expression and secretion of matrix metalloproteinase-2 (MMP-2) Mol Cancer. 2006;5:61. doi: 10.1186/1476-4598-5-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCabe CJ, Boelaert K, Tannahill LA, Heaney AP, Stratford AL, Khaira JS, Hussain S, Sheppard MC, Franklyn JA, Gittoes NJ. Vascular endothelial growth factor, its receptor KDR/Flk-1, and pituitary tumor transforming gene in pituitary tumors. J Clin Endocrinol Metab. 2002;87:4238–4244. doi: 10.1210/jc.2002-020309. [DOI] [PubMed] [Google Scholar]
- 14.McCabe CJ, Khaira JS, Boelaert K, Heaney AP, Tannahill LA, Hussain S, Mitchell R, Olliff J, Sheppard MC, Franklyn JA, Gittoes NJ. Expression of pituitary tumour transforming gene (PTTG) and fibroblast growth factor-2 (FGF-2) in human pituitary adenomas: relationships to clinical tumour behaviour. Clin Endocrinol (Oxf) 2003;58:141–150. doi: 10.1046/j.1365-2265.2003.01598.x. [DOI] [PubMed] [Google Scholar]
- 15.Knosp E, Steiner E, Kitz K, Matula C. Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery. 1993;33:610–617. doi: 10.1227/00006123-199310000-00008. discussion 617-618. [DOI] [PubMed] [Google Scholar]
- 16.Bode HJ. SDS - polyethyleneglycol electrophoresis: a possible alternative to SDS - polyacrylamide gel electrophoresis. FEBS Lett. 1976;65:56–58. doi: 10.1016/0014-5793(76)80620-5. [DOI] [PubMed] [Google Scholar]
- 17.Miller BA, Rutledge WC, Ioachimescu AG, Oyesiku NM. Management of large aggressive nonfunctional pituitary tumors: experimental medical options when surgery and radiation fail. Neurosurg Clin N Am. 2012;23:587–594. doi: 10.1016/j.nec.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 18.Sheehan JP, Jagannathan J, Pouratian N, Steiner L. Stereotactic radiosurgery for pituitary adenomas: a review of the literature and our experience. Front Horm Res. 2006;34:185–205. doi: 10.1159/000091581. [DOI] [PubMed] [Google Scholar]
- 19.Jefferson G. Extrasellar Extensions of Pituitary Adenomas: (Section of Neurology) Proc R Soc Med. 1940;33:433–458. [PMC free article] [PubMed] [Google Scholar]
- 20.Zada G, Kelly DF, Cohan P, Wang C, Swerdloff R. Endonasal transsphenoidal approach for pituitary adenomas and other sellar lesions: an assessment of efficacy, safety, and patient impressions. J Neurosurg. 2003;98:350–358. doi: 10.3171/jns.2003.98.2.0350. [DOI] [PubMed] [Google Scholar]
- 21.Wilson CB. A decade of pituitary microsurgery. The Herbert Olivecrona lecture. J Neurosurg. 1984;61:814–833. doi: 10.3171/jns.1984.61.5.0814. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe A, Hoshino D, Koshikawa N, Seiki M, Suzuki T, Ichikawa K. Critical role of transient activity of MT1-MMP for ECM degradation in invadopodia. PLoS Comput Biol. 2013;9:e1003086. doi: 10.1371/journal.pcbi.1003086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.d’Ortho MP, Will H, Atkinson S, Butler G, Messent A, Gavrilovic J, Smith B, Timpl R, Zardi L, Murphy G. Membrane-type matrix metalloproteinases 1 and 2 exhibit broad-spectrum proteolytic capacities comparable to many matrix metalloproteinases. Eur J Biochem. 1997;250:751–757. doi: 10.1111/j.1432-1033.1997.00751.x. [DOI] [PubMed] [Google Scholar]
- 24.Han KY, Fahd DC, Tshionyi M, Allemann N, Jain S, Chang JH, Azar DT. MT1-MMP modulates bFGF-induced VEGF-A expression in corneal fibroblasts. Protein Pept Lett. 2012;19:1334–1339. doi: 10.2174/092986612803521639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deryugina EI, Soroceanu L, Strongin AY. Up-regulation of vascular endothelial growth factor by membrane-type 1 matrix metalloproteinase stimulates human glioma xenograft growth and angiogenesis. Cancer Res. 2002;62:580–588. [PubMed] [Google Scholar]
- 26.Yao G, He P, Chen L, Hu X, Gu F, Ye C. MT1-MMP in breast cancer: induction of VEGF-C correlates with metastasis and poor prognosis. Cancer Cell Int. 2013;13:98. doi: 10.1186/1475-2867-13-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunter JA, Skelly RH, Aylwin SJ, Geddes JF, Evanson J, Besser GM, Monson JP, Burrin JM. The relationship between pituitary tumour transforming gene (PTTG) expression and in vitro hormone and vascular endothelial growth factor (VEGF) secretion from human pituitary adenomas. Eur J Endocrinol. 2003;148:203–211. doi: 10.1530/eje.0.1480203. [DOI] [PubMed] [Google Scholar]
- 28.Sounni NE, Dehne K, van Kempen L, Egeblad M, Affara NI, Cuevas I, Wiesen J, Junankar S, Korets L, Lee J, Shen J, Morrison CJ, Overall CM, Krane SM, Werb Z, Boudreau N, Coussens LM. Stromal regulation of vessel stability by MMP14 and TGFbeta. Dis Model Mech. 2010;3:317–332. doi: 10.1242/dmm.003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rostad S. Pituitary adenoma pathogenesis: an update. Curr Opin Endocrinol Diabetes Obes. 2012;19:322–327. doi: 10.1097/MED.0b013e328354b2e2. [DOI] [PubMed] [Google Scholar]
- 30.Ogier C, Bernard A, Chollet AM, LE Diguardher T, Hanessian S, Charton G, Khrestchatisky M, Rivera S. Matrix metalloproteinase-2 (MMP-2) regulates astrocyte motility in connection with the actin cytoskeleton and integrins. Glia. 2006;54:272–284. doi: 10.1002/glia.20349. [DOI] [PubMed] [Google Scholar]
- 31.Ikenaka Y, Yoshiji H, Kuriyama S, Yoshii J, Noguchi R, Tsujinoue H, Yanase K, Namisaki T, Imazu H, Masaki T, Fukui H. Tissue inhibitor of metalloproteinases-1 (TIMP-1) inhibits tumor growth and angiogenesis in the TIMP-1 transgenic mouse model. Int J Cancer. 2003;105:340–346. doi: 10.1002/ijc.11094. [DOI] [PubMed] [Google Scholar]
- 32.Martin DC, Sanchez-Sweatman OH, Ho AT, Inderdeo DS, Tsao MS, Khokha R. Transgenic TIMP-1 inhibits simian virus 40 T antigen-induced hepatocarcinogenesis by impairment of hepatocellular proliferation and tumor angiogenesis. Lab Invest. 1999;79:225–234. [PubMed] [Google Scholar]


