Abstract
The radiotherapy as a local and regional modality is widely applied in treatment of glioma, but most glioblastomas are commonly resistant to irradiation treatment. It remains challengeable to seek out efficient strategies to conquer the resistance of human glioblastoma cells to radiotherapy. Leucine-rich repeats and immunoglobulin-like domains protein 1 (LRIG1) is a newly discovered tumor suppressor which involved in regulation of chemosensitivity in various human cancer cells. In the present study, we established a radioresistant U251 cell line (U251R) to investigate the role of LRIG1 in regulation of radiosensitivity in human glioblastoma cells. Significantly decreased expression level of LRIG1 and enhanced expression of EGFR and phosphorylated Akt were detected in U251R cells compared with the parental U251 cells. U251R cells exhibited an advantage in colony formation ability, which accompanied by remarkably reduced X-ray-induced γ-H2AX foci formation and cell apoptosis. LRIG1 overexpression significantly inhibited the colony formation ability of U251R cells and obviously enhanced X-ray-inducedγ-H2AX foci formation and cell apoptosis. In addition, up-regulated expression of LRIG1 suppressed the expression level of EGFR and phosphorylated Akt protein. Our results demonstrated that LRIG1 expression was related to the radiosensitivity of human glioblastoma cells and may play an important role in the regulation of cellular radiosensitivity of human glioblastoma cells through the EGFR/Akt signaling pathway.
Keywords: LRIG1, glioblastoma, radiosensitivity, γ-H2AX, apoptosis, EGFR signaling
Introduction
Gliomas count for approximately 80% of all malignant brain tumors in adults [1]. The glioblastomas (WHO grade IV) are the most malignant gliomas with the highest morbidity and mortality. Less than 3% of glioblastoma patients can survive more than 5 years after diagnosis [2]. Consistent with the dismal prognosis and poor quality of life, glioblastoma remains a challenging disease to treat. The current standard of care for newly diagnosed glioblastoma is surgical resection followed by radiotherapy plus concomitant and adjuvant temozolomide [3-5]. At the same time, many other therapeutic approaches such as immune and gene therapy have been improving [6]. However, these therapy strategies benefits patients pettiness due to the fact that resistance of glioblastoma cells to treatment especially irradiation therapy [7]. Thus it is imperative to understand the mechanism of resistance to irradiation therapy and seek out efficient radiosensitizers to improve the delivery of radiation treatments of glioblastoma.
Leucine-rich repeats and immunoglobulin-like domains protein 1 (LRIG1) is a transmembrane protein that is widely expressed in human tissues and organs [8]. LRIG1 is considered to be a potential tumor suppressor, and it has been found to be under-expressed in some human tumor cell lines [9]. Down-regulation of LRIG1 in tumor tissues is significantly associated with the poor prognosis of patients [10,11]. In addition, LRIG1 negatively regulates aggressive properties and enhances the chemosensitivity of tumor cells [12,13]. Moreover, previous studies showed that LRIG1 plays an important role in regulation of cancer cell biological behaviors such as cell growth and invasion through binding to EGFR family members and down-regulating EGFR signaling pathway [14,15].
Although the role of LRIG1 in tumor inhibition and regulation of chemosensitivity of human cancers have been elucidated, there is a paucity of literature on the association between LRIG1 and the radiosensitivity of the tumor cells, especially glioblastoma cells. In this study, we established a radioresistant human glioblastoma cell line and evaluated the role of LRIG1 in regulation of radiosensitivity of human glioblastoma cell line U251 by up-regulation of LRIG1 expression.
Material and methods
Cell culture and transfection
The human glioma cell line U251 was purchased from State Key Laboratory of Molecular Biology, Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghai, China). The cells were maintained in DMEM medium containing 10% fetal bovine serum, at 37°C in humidified air containing 5% carbon dioxide. The medium was replaced every 3 days. Cells were checked routinely until they reached 80-90% confluence. For transfection of LRIG1 plasmids, the cells were trypsinized and seeded in 6-well plates, and transfection with Lipofectamine was performed under conditions described by the manufacturer (Invitrogen, Carlsbad, CA). After media was replaced with serum-free Opti-MEM (Gibco, Invitrogen), the transfection complexes were added. And the final concentration of LRIG1 plasmid was 1.5 μg/mL. At 6 hours after the incubation, the medium was replaced with the standard culture medium as described. After another incubation of 48-72 hours, the cells were used in the following tests.
Establishment of radioresistant cell line
Cells growing in T-75 flasks were irradiated at room temperature using a linear accelerator 6-MV X-rays at dose rate of 4 Gy/min. After irradiation, the culture medium was replaced immediately, and the cells were returned to the incubator. Cultures were progressively treated with daily rounds of radiation over a 5-month period, starting with 1 Gy/fraction and ending with 10 Gy/fraction. Irradiation was stopped, as necessary, to allow for cell monolayer recovery. The procedure was continued until a total of 62 Gy had been delivered.
Cell viability assay
The cell viability was examined via a cell counting kit-8 (CCK-8 kit) purchased from Dojindo Molecular Technologies (Kumamoto, Japan) according to the manufacturer’s instructions. Briefly, with or without radiation, approximately 5×103 cells were seeded in a volume of 100 μl DMEM on each well of a 96-well plate. The cells were continuously cultured for different times. Subsequently, 100 μl of fresh medium containing 10 μl of the CCK-8 solution was added to each well and incubated at 37°C for 1 h. The absorbance at 450 nm was measured on a spectrophotometric plate reader.
Colony formation assay
Cell survival after X-ray irradiation was measured by colony formation assay. The cells were counted and plated in 6-well plates (100 cells for 0 Gy, 200 cells for 1 Gy, 400 cells for 2 Gy, 600 cells for 4 Gy, and 800 cells for 6 Gy), and were irradiated at different doses ranging from 0 to 10 Gy. After irradiation in a single fraction, these cells were immediately were returned to the 37°C incubator for 14 days without movement. After fixation with 4% paraformaldehyde and staining with 0.1% crystal violet, colonies consisting of 50 cells or more were counted under a light microscope. The survival curves were then determined and fitted to a linear quadratic model using the GraphPad prism software (version 5.0).
Apoptosis analysis
Apoptosis was assessed by using the Annexin-V/PI apoptosis kit (MultiSciences Biotechnology, Zhejiang, China) and flow cytometry according to the manufacturer’s instruction. Briefly, approximately 5×105 cells were trypsinized and seeded in 6-well plates. Cells were treated with or without radiation (6 Gy) 24 hours before harvesting. Both adherent and detached cells were collected, centrifuged, and double stained with Annexin V and propidium iodide (PI). Then, treated cells were quantified with flow cytometry using a BD Accuri C6 Flow Cytometer (BD Biosciences, USA).
Immunofluorescence assay
Cells were seeded in 6-well plates on 22-cm2 coverslips and incubated for 24 hours. They were then treated with or without 6 Gy of irradiation and harvested at 24 h. The cell climbing coverslips were fixed in 4% formaldehyde in phosphate buffered saline (PBS) for 30 min at room temperature and then incubated with anti-γ-H2AX rabbit monoclonal antibody (Cell Signaling Technology, USA) overnight at 4°C. After washing twice with PBS, cells were incubated with fluorescein isothiocyanate-labeled mouse anti-rabbit antibody for 1 h and washed twice with PBS. Nuclei were counterstained with Hoechst 33258 (Beyotime Institute of Biotechnology, Jiangsu, China) in PBS for 30 min before cells were covered by anti-fade solution and observed with an Olympus fluorescent microscope. For quantification of foci, clear and easily distinguished dots of a pre-determined brightness level were counted as positive foci. The number of foci was counted in 100 cells per sample for each time point by visual inspection and average number of foci per cell was calculated.
Western blot analysis
Cell proteins were extracted with RIPA (radioimmunoprecipitation assay) lysis buffer and determined by the standard BCA method (Beyotime Institute of Biotechnology, Jiangsu, China). Equal amounts of protein were separated by SDS-PAGE and electro-blotted onto polyvinylidene difluoride membranes (Millipore Corp., Bedford, MA, USA). Membranes were blocked in TBS containing 0.1% Tween-20 and 5% powdered milk, and probed with primary antibody. Primary antibody directed against EGFR, LRIG1, p-Akt (Ser308), Akt (Cell Signaling Technology, USA), and GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used at a dilution of 1:1000. Blots were visualized by LI-COR Odyssey Infrared Imaging System with Alex Fluor 680/790-labeled goat anti-rabbit IgG (LI-COR Biosciences, USA) used as second antibody.
Statistical analysis
All experiments were performed at least three times. Results were expressed as means ± SD. Statistically significant differences in mean values were tested by Student t test or analysis of variance. Differences at P < 0.05 were considered statistically significant. The data were analyzed using SPSS 17.0.
Results
Measurement of the radiosensitivity of U251 and U251R cells
Human glioblastoma cell line U251 was used to develop cells resistant to X-ray irradiation. Exponentially growing cells were irradiated 10 times with crescent X-ray doses from 1 Gy/fraction to 10 Gy/fraction. The radioresistant subline (U251R) was generated from the surviving fraction of U251 cells treated with a total of 62 Gy of fractionated X-ray irradiation for approximate 5 months (Figure 1A). The U251R cells exhibited an advantage in cell survival compared with parental U251. As shown in Figure 1B, cell viability assay indicated that U251R cells displayed higher cell growth viability than normal U251 cells with or without irradiation exposure. In addition, colony formation assay showed that U251R cells exhibited higher colony formation capacity compared with parental U251 cells (Figure 1C, 1D). These results indicated that U251R cells had higher radioresistance compared with the parental U251 cells.
Figure 1.
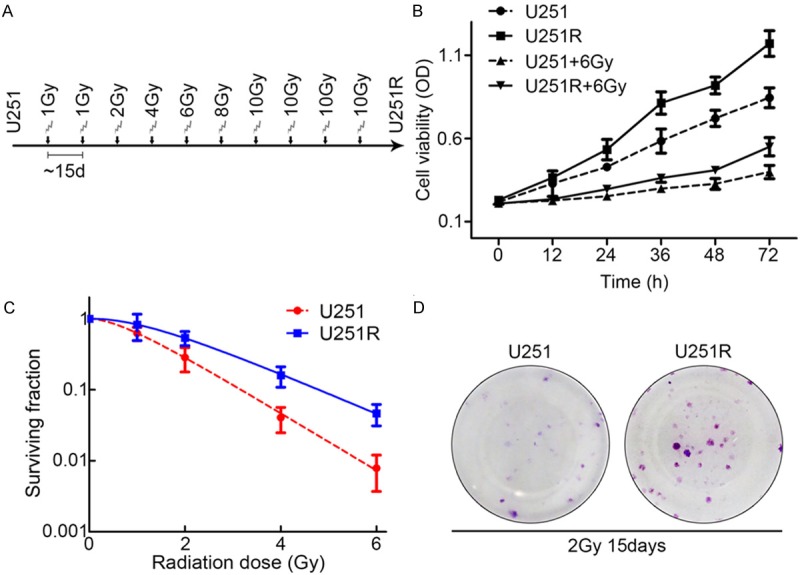
Long-term irradiation induction promotes glioblastoma cell proliferation and colony formation. A. Schematic diagram depicts the procedure of establishment of radioresistant subline. B. Long-term irradiation induction promoted cell proliferation of U251 cells with or without irradiation treatment. C. Colony formation assay of U251 and U251R cells. D. Representative photograph of colonies of U251 and U251R cells 15 days after 2 Gy irradiation exposures in colony formation assay. Three independent experiments were conducted.
X-ray-induced DNA damage and cell apoptosis were reduced in U251R cells
Radiation is a stress that induces apoptosis and death of cancer cells. To assess the effect of X-ray irradiation on U251 and U251R cells, the cells all exposed to X-ray at a dose of 6 Gy. Apoptosis analysis showed that X-ray-induced apoptosis in U251R cells was lower than in U251 cells at 24 h after irradiation treatment (Figure 2A, 2B). We then evaluated the X-ray-induced DNA damage of the two cell lines by immunofluorescent staining of γ-H2AX foci. The U251R cells showed a stronger capability to repair the dsDNA breaks (DSBs) with fewer γ-H2AX foci compared with the parental U251 cells at 24 h after 6Gy of radiation (Figure 2C, 2D).
Figure 2.
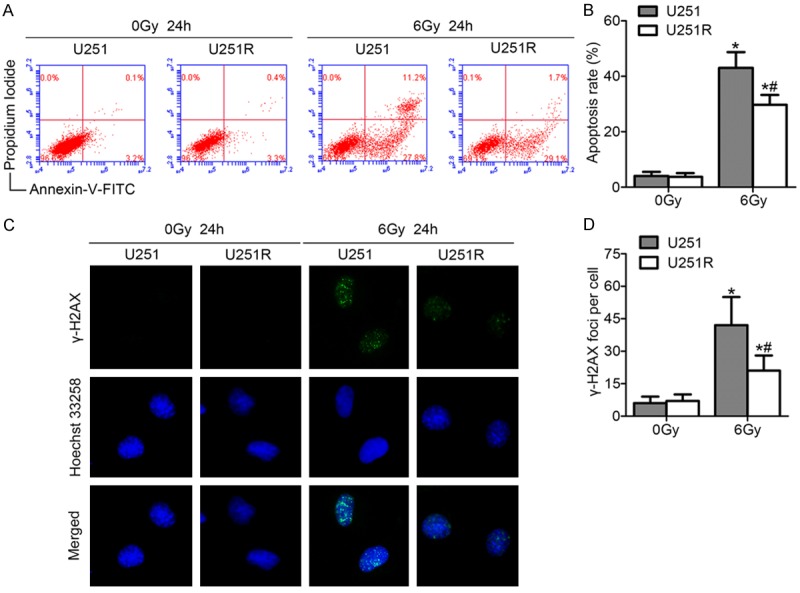
Long-term irradiation induction reduces irradiation-induced apoptosis and enhances DNA damage repair. (A) Representative plots of showed Annexin-V/PI staining in U251 and U251R cells 24 h after treatment with or without ionizing radiation of 6 Gy. (B) The histogram represented quantitative analysis of the apoptotic rates as shown in (A). (C) Examples showed residual immunofluorescence staining against γ-H2AX foci (green) and nucleus (blue); magnification, ×400. (D) Quantitative analysis of the number of γ-H2AX foci per cell. *P < 0.05 compared with U251 cells without irradiation treatment. *#P < 0.05 compared with U251R cells with irradiation treatment.
LRIG1 expression was down-regulated in U251R cells accompanied by upregulation of EGFR and phosphorylated Akt expression
The expression of LRIG1 protein was analyzed in U251R and U251 cells by Western blotting. The results revealed that long-term irradiation induction down-regulated the expression levels of LRIG1 protein in glioblastoma cells (Figure 3A, 3B). Studies indicated that LRIG1 is a natural antagonist of EGFR. Therefore, the expression level of EGFR protein was further detected. As expected, the expression level of EGFR protein increased with the increase of total dose of irradiation induction (Figure 3C, 3D). EGFR expression in U251R cells was approximately 2-fold higher than parental U251 cells. It is well known that the PI3K/Akt pathway is involved in resistance to radiation treatment. As shown in Figure 3E, 3F, the phosphorylation of Akt was increased in line with EGFR expression in the U251R cells. The differential expression pattern of LRIG1, EGFR and p-Akt protein between U251R and U251 cells suggested that the expression status of LRIG1 was closely related to the radiation sensitivity of U251R and U251 cells.
Figure 3.
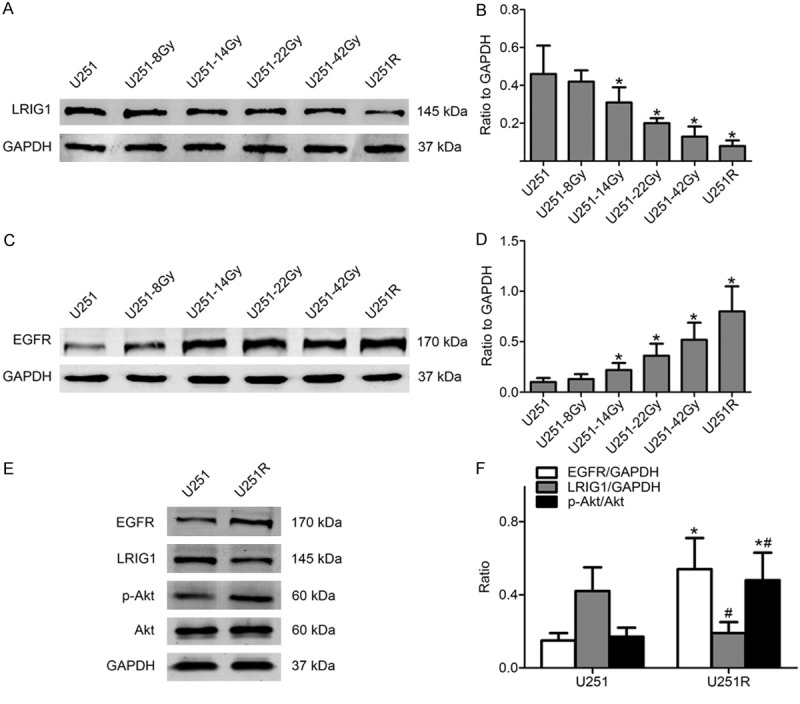
LRIG1 expression level is down-regulated in established radioresistant cells accompanied with EGFR and phosphorylated Akt expression level up-regulated. (A) LRIG1 expression level gradually decreased in irradiation-treated cells. (B) Quantitative analysis of the bands from Western blotting was shown in (A). (C) EGFR protein level gradually increased in irradiation-treated cells. (D) Quantitative analysis of the bands from Western blotting for EGFR protein. (E) EGFR and Akt phosphorylation level in U251R cells significantly increased while LRIG1 level decreased. (F) The histogram represented quantitative analysis of EGFR, LRIG1, total and phosphorylated Akt. GAPDH protein was used as loading control. *P < 0.05 compared with U251 cells; *#P < 0.05 compared with U251 cells.
Overexpression of LRIG1 restored the radiosensitivity of U251R cells
To investigate whether LRIG1 could restore the radioresistance of U251R cells, the plasmid transfection was performed to up-regulate the LRIG1 expression. The transfection efficiency of LRIG1 plasmid and subsequent protein expression was evaluated by Western blotting. The results showed that the expression of LRIG1 in the U251R cells transfected with LRIG1 plasmid was significantly increased compared with the empty vector-transfected U251R cells (Figure 4A). As shown in Figure 4B, LRIG1 overexpression reduced the cell viability of U251R cells compared with empty vector-transfected U251R cells under normal circumstances or exposure to irradiation of 6 Gy. The radiation survival curves of the colony formation assay showed that overexpression of LRIG1 sensitized U251R cells to the ionizing radiation (Figure 4C, 4D). The number of clones from LRIG1-transfected cells was significantly fewer than that of control cells.
Figure 4.
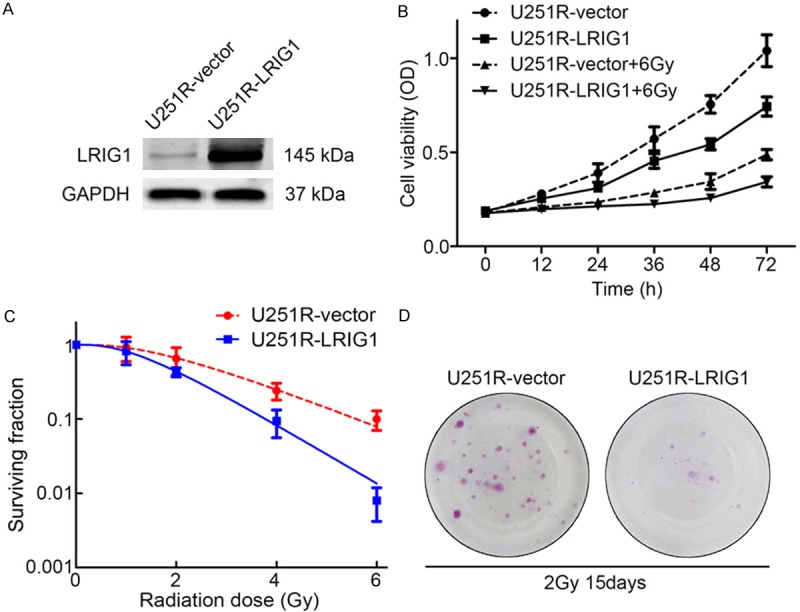
Overexpression of LRIG1 inhibits cell proliferation and colony formation of U251R cells. A. The protein expression level of LRIG1 in U251R cells after plasmid transfection. B. Overexpression of LRIG1 inhibited cell proliferation of U251R cells with or without irradiation treatment. C. Colony formation assay of U251R-vector and U251R-LRIG1 cells. D. Representative photograph of colonies of U251R-vector and U251R-LRIG1 cells 15 days after 2 Gy irradiation exposures in colony formation assay. Three independent experiments were conducted.
Overexpression of LRIG1 enhanced the DNA damage and cell apoptosis in U251R cells
As shown in Figure 5A and 5B, LRIG1 overexpression enhanced the apoptosis of U251R cells whether exposure to irradiation or not, the apoptotic rate in LRIG1-transfected U251R cells was markedly increased by approximately 12% in response to irradiation with 6 Gy at 24 h compared with control cells. As expected, upregulation of LRIG1 caused remarkable prolongation of radiation-induced γ-H2AX foci formation, indicating delayed DNA damage repair 24 h after irradiation exposure in LRIG1-transfected U251R cells (Figure 5C, 5D).
Figure 5.
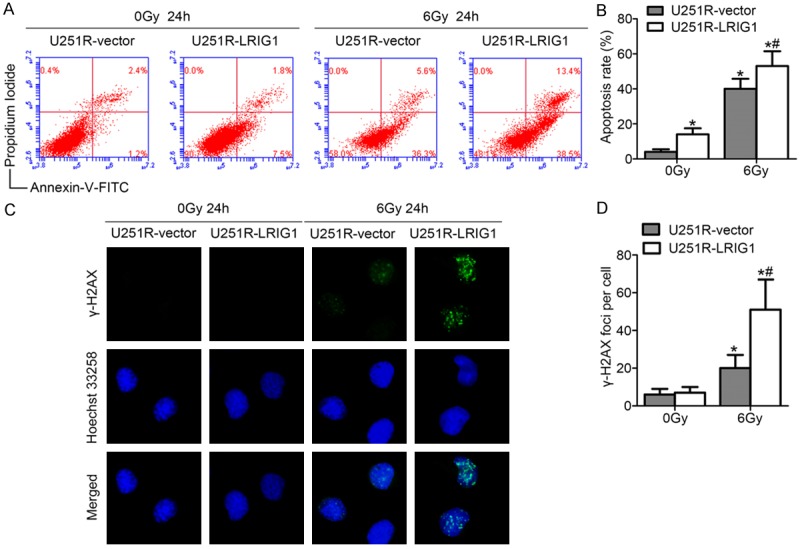
Overexpression of LRIG1 enhances irradiation-induced apoptosis and DNA damage of U251R cells. (A)Representative plots of show Annexin-V/PI staining in U251R-vector and U251R-LRIG1 cells 24 h after treatment with or without ionizing radiation of 6 Gy. (B) The histogram represented quantitative analysis of the apoptotic rates as shown in (A). (C) Examples show residual immunofluorescence staining against γ-H2AX foci (green) and nucleus (blue); magnification, ×400. (D) Quantitative analysis of the number of γ-H2AX foci per cell. *P < 0.05 compared with U251R-vector cells without irradiation treatment. *#P < 0.05 compared with U251R-vector cells with irradiation treatment.
Overexpression of LRIG1 suppressed EGFR expression and Akt phosphorylation in U251R cells
To further characterize how LRIG1 participates in regulation of radiosensitivity of glioblastoma cells, EGFR and p-Akt expression were assessed in LRIG1 overexpressed U251R cells by western blotting. Upregulation of LRIG1 dramatically attenuated the expression level of EGFR and p-Akt in U251R cells (Figure 6A, 6B). These data indicated that the LRIG1-mediated radiosensitization of U251 glioblastoma cells may be related to the inhibition of the EGFR/Akt signaling pathway.
Figure 6.
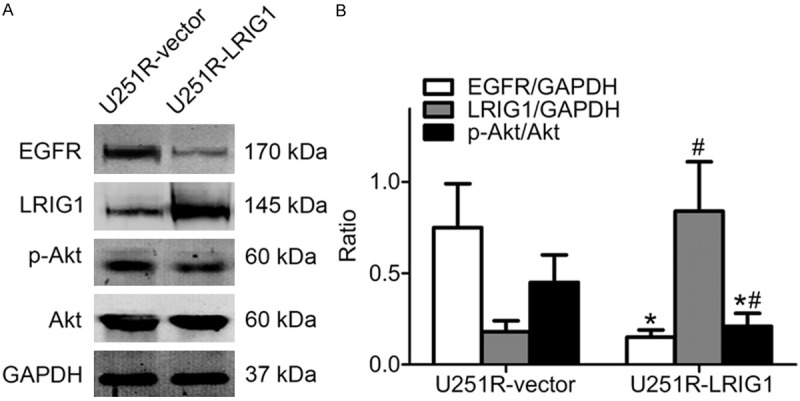
LRIG1 regulates EGFR expression and Akt phosphorylation level. Equal amounts of cell protein were separated by SDS-PAGE and probed by anti-LRIG1, anti-EGFR, anti-Akt and anti-phosphorylated Akt antibody. GAPDH protein was used as loading control. A. Western blotting detection of proteins in U251R-vector and U251R-LRIG1 cells without treatment with ionizing radiation. B. The histogram represents quantitative analysis of EGFR, LRIG1, total and phosphorylated Akt. *P < 0.05 compared with U251R-vector cells; #P < 0.05 compared with U251R-vector cells; *#P < 0.05 compared with U251R-vector cells.
Discussion
Resistance of glioblastoma cells to ionizing radiation affects the efficacy of the radiotherapy. Therefore, to understand the regulation mechanism of the radiosensitivity of glioblastoma would contribute to improve the therapeutic effect of radiotherapy for glioblastoma. Establishing radioresistant cancer cell lines is a useful method to explore the regulatory mechanism of radiosensitivity of cancer. Previous studies demonstrated that several human cancer cell lines especially the glioblastoma cell lines themselves have distinct degrees of radioresistance.The most common method to isolate a radioresistant cell line is to treat cancer cells with long-term fractionated irradiation at a same dose [16-19]. In the present study, we established a much more radioresistant cell line U251R utilizing repeated X-ray irradiation treatment in a new dose-escalation manner. In line with the previous study, we found that the newly established radioresistant cell strain U251R exhibited more advantages in colony formation capacity and DSBs repair.
Previous study showed that LRIG1 expression is down-regulated in chemoresistant cells derived from MDA-MB-231 and SKOV-3 cells treated with SM-164 in vitro [20], but no research has ever evaluated the expression level of LRIG1 in radioresistant cells. In the present study, we first revealed that LRIG1 was down-regulated in U251R cells, which were more resistant to irradiation. Further study with the LRIG1 plasmid transfection demonstrated that the radiosensitivity was restored when LRIG1 overexpressed in U251R cells. These results indicated that LRIG1 expression level is associated with the radiosensitivity of glioblastoma cells and plays an important role in regulation of radiosensitivity.
A previous study reported that LRIG1 could regulate chemosensitivity and proliferation of glioma cells through inhibiting EGFR signaling [21]. However, how LRIG1 regulated radiosensitivity remains mysterious. Recent studies provided evidences that EGFR also participates in regulation of radiosensitivity of human cancer cells. Fedrigo CA et al [22] reported that the EGFR levels were significantly increased in glioblastoma spheroids after treatment with irradiation at a dose of 5 Gy. Qu Y et al [23] observed that humanized monoclonal antibody specifically targeting EGFR could enhance radiosensitivity of human cancer cells. Consistent with the aforementioned studies, our Western blotting results revealed that LRIG1 expression was down-regulated in U251R cells accompanied by up-regulation of EGFR compared with parental glioblastoma cells. In addition, we observed that up-regulation LRIG1 expression by plasmid transfection in U251R cells can attenuate the long-term irradiation-induced up-regulated EGFR expression, which further confirmed that LRIG1 may regulate radiosensitivity of glioblastoma cells through EGFR signaling pathway.
The tumoricidal effect of radiation therapy is largely attributed to the induction of dsDNA breaks [24]. Sensing of DNA lesions initiates a highly complex DNA damage response (DDR). This response involves signaling cascades that activate appropriate damage repair pathways, arrest the cell cycle, and ultimately determine cell survival or death [25]. Histone protein H2AX which was used as a biomarker and powerful tool to monitor DNA DSBs from cancer therapy, a key component in DNA repair, becomes rapidly phosphorylated at serine 139 by PI3-kinases to form γ-H2AX foci at nascent DSB sites [26], followed by initiation of DNA damage repair pathways mainly composed of homologous recombination (HR) and nonhomologous end-joining (NHEJ) [27]. Several studies confirmed the role of Akt signal in regulation of DNA damage. Ionizing radiation stimulates the activation of Akt and its phosphorylation at threonine 308 and serine 473 in a PDK1- and DNA-PKcs-dependent manner [28,29]. In human cancer cells, dampening of PI3K/Akt signaling using small-molecule inhibitors or small interfering RNA (siRNA) impairs DSBs repair [30-32]. Previous studies demonstrated that Akt was a downstream molecule of EGFR and EGFR/Akt signaling pathway seems to positively regulate both HR and NHEJ in U87 glioma cells [33]. Further study has verified the role of EGFR/Akt signaling pathway in regulation of radiosensitivity of some cancer cells. Li B et al [34] indicated that the PI3K/Akt pathway is involved in radioresistance of cells of astrocytic origin. Consistent with these discoveries, we found that up-regulated LRIG1 expression lead to increased γ-H2AX foci numbers and apoptotic rates in U251R cells in response to irradiation treatment. Meanwhile, LRIG1 overexpression significantly restored the EGFR expression and Akt phosphorylation level. These results indicate that LRIG1 may enhance radiosensitivity of glioblastoma cells through impairing DSB repair and inducing apoptosis via inhibiting the EGFR/Akt signaling pathway.
In conclusion, this study illustrated that repeated exposure to irradiation could lead to resistance of the human glioblastoma U251 cells to ionizing radiation. In contrast, LRIG1 can reverse the radioresistance through attenuating the EGFR/Akt pathway. However, these data are only sufficient to elucidate the initial understanding of the mechanism involved in LRIG1-mediated sensitization the glioblastoma cells to radiation treatment. In the future studies, it would be meaningful to explore the effect of LRIG1 on cell cycle checkpoints and gain a deep understanding of the mechanisms involved in the response to ionizing radiation in tumor xenografts.
Acknowledgements
This work was supported by the National Natural Science Foundation of China, No. 81372683 and the CHINESE ANTI-CANCER ASSOCIATION, CSNO-2013-MSD 004.
Disclosure of conflict of interest
None.
References
- 1.Goodenberger ML, Jenkins RB. Genetics of adult glioma. Cancer Genetics. 2012;205:613–621. doi: 10.1016/j.cancergen.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Ohgaki H, Dessen P, Jourde B, Horstmann S, Nishikawa T, Di Patre PL, Burkhard C, Schüler D, Probst-Hensch NM, Maiorka PC, Baeza N, Pisani P, Yonekawa Y, Yasargil MG, Lütolf UM, Kleihues P. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64:6892–6899. doi: 10.1158/0008-5472.CAN-04-1337. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. New Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Jiang T. Understanding high grade glioma: Molecular mechanism, therapy and comprehensive management. Cancer Lett. 2013;331:139–146. doi: 10.1016/j.canlet.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 5.Carlsson SK, Brothers SP, Wahlestedt C. Emerging treatment strategies for glioblastoma multiforme. EMBO Mol Med. 2014;6:1359–1370. doi: 10.15252/emmm.201302627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Omuro A. Glioblastoma and other malignant gliomas. JAMA. 2013;310:1842. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- 7.Atkins RJ, Ng W, Stylli SS, Hovens CM, Kaye AH. Repair mechanisms help glioblastoma resist treatment. J Clin Neurosci. 2015;22:14–20. doi: 10.1016/j.jocn.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson J, Vallbo C, Guo D, Golovleva I, Hallberg B, Henriksson R, Hedman H. Cloning, characterization, and expression of human LIG1. Biochem Bioph Res Co. 2001;284:1155–1161. doi: 10.1006/bbrc.2001.5092. [DOI] [PubMed] [Google Scholar]
- 9.Hedman H, Nilsson J, Guo D, Henriksson R. Is LRIG1 a tumour suppressor gene at chromosome 3p14.3? Acta Oncol. 2002;41:352–354. doi: 10.1080/028418602760169398. [DOI] [PubMed] [Google Scholar]
- 10.Thomasson M, Hedman H, Guo D, Ljungberg B, Henriksson R. LRIG1 and epidermal growth factor receptor in renal cell carcinoma: a quantitative RT-PCR and immunohistochemical analysis. Brit J Cancer. 2003;89:1285–1289. doi: 10.1038/sj.bjc.6601208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagata M, Nakamura T, Sotozono C, Inatomi T, Yokoi N, Kinoshita S. LRIG1 as a Potential Novel Marker for Neoplastic Transformation in Ocular Surface Squamous Neoplasia. PLoS One. 2014;9:e93164. doi: 10.1371/journal.pone.0093164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu X, Hedman H, Bergqvist M, Bergström S, Henriksson R, Gullbo J, Lennartsson J, Hesselius P, Ekman S. Expression of EGFR and LRIG proteins in oesophageal carcinoma with emphasis on patient survival and cellular chemosensitivity. Acta Oncol. 2012;51:69–76. doi: 10.3109/0284186X.2011.562239. [DOI] [PubMed] [Google Scholar]
- 13.Sheu JJ, Lee CC, Hua CH, Li CI, Lai MT, Lee SC, Cheng J, Chen CM, Chan C, Chao SC, Chen JY, Chang JY, Lee CH. LRIG1 modulates aggressiveness of head and neck cancers by regulating EGFR-MAPK-SPHK1 signaling and extracellular matrix remodeling. Oncogene. 2013;33:1375–1384. doi: 10.1038/onc.2013.98. [DOI] [PubMed] [Google Scholar]
- 14.Johansson M, Oudin A, Tiemann K, Bernard A, Golebiewska A, Keunen O, Fack F, Stieber D, Wang B, Hedman H, Niclou SP. The soluble form of the tumor suppressor Lrig1 potently inhibits in vivo glioma growth irrespective of EGF receptor status. Neuro Oncol. 2013;15:1200–1211. doi: 10.1093/neuonc/not054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu L, Teixeira VH, Yuan Z, Graham TA, Endesfelder D, Kolluri K, Al-Juffali N, Hamilton N, Nicholson AG, Falzon M, Kschischo M, Swanton C, Wright NA, Carroll B, Watt FM, George JP, Jensen KB, Giangreco A, Janes SM. LRIG1 regulates cadherin-dependent contact inhibition directing epithelial homeostasis and pre-invasive squamous cell carcinoma development. J Pathol. 2013;229:608–620. doi: 10.1002/path.4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desoubzdanne D, Claparols C, Martins-Froment N, Zedde C, Balayssac S, Gilard V, Tercé F, Martino R, Malet-Martino M. Analysis of hydrophilic and lipophilic choline compounds in radioresistant and radiosensitive glioblastoma cell lines by HILIC-ESI-MS/MS. Anal Bioanal Chem. 2010;398:2723–2730. doi: 10.1007/s00216-010-4196-4. [DOI] [PubMed] [Google Scholar]
- 17.Wu F, Hu Y, Long J, Zhou YJ, Zhong YH, Liao ZK, Liu SQ, Zhou FX, Zhou YF, Xie CH. Cytotoxicity and radiosensitization effect of TRA-8 on radioresistant human larynx squamous carcinoma cells. Oncol Rep. 2009;21:461–465. [PubMed] [Google Scholar]
- 18.Lee YS, Oh J, Yoon S, Kwon MS, Song CW, Kim KH, Cho MJ, Mollah ML, Je YJ, Kim YD, Kim CD, Lee JH. Differential Gene Expression Profiles of Radioresistant Non-Small-Cell Lung Cancer Cell Lines Established by Fractionated Irradiation: Tumor Protein p53-Inducible Protein 3 Confers Sensitivity to Ionizing Radiation. Int J Radiat Oncol Biol Phys. 2010;77:858–866. doi: 10.1016/j.ijrobp.2009.12.076. [DOI] [PubMed] [Google Scholar]
- 19.Wouters BG, Sy AM, Skarsgard LD. Low-dose hypersensitivity and increased radioresistance in a panel of human tumor cell lines with different radiosensitivity. Radiat Res. 1996;146:399–413. [PubMed] [Google Scholar]
- 20.Bai L, McEachern D, Yang CY, Lu J, Sun H, Wang S. LRIG1 modulates cancer cell sensitivity to Smac mimetics by regulating TNF expression and receptor tyrosine kinase signaling. Cancer Res. 2012;72:1229–1238. doi: 10.1158/0008-5472.CAN-11-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi XC, Xie DJ, Yan QF, Wang YR, Zhu YX, Qian C, Yang SX. LRIG1 dictates the chemo-sensitivity of temozolomide (TMZ) in U251 glioblastoma cells via down-regulation of EGFR/topoisomerase- 2/Bcl-2. Biochem Biophys Res Commun. 2013;437:565–572. doi: 10.1016/j.bbrc.2013.06.116. [DOI] [PubMed] [Google Scholar]
- 22.Fedrigo CA, Grivicich I, Schunemann DP, Chemale IM, Santos D, Jacovas T, Boschetti PS, Jotz GP, Braga Filho A, da Rocha AB. Radioresistance of human glioma spheroids and expression of HSP70, p53 and EGFr. Radiation Oncology. 2011;6:156. doi: 10.1186/1748-717X-6-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qu YY, Hu SL, Xu XY, Wang RZ, Yu HY, Xu JY, Chen L, Dong GL. Nimotuzumab Enhances the Radiosensitivity of Cancer Cells In Vitro by Inhibiting Radiation-Induced DNA Damage Repair. PLoS One. 2013;8:e70727. doi: 10.1371/journal.pone.0070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kesari S, Advani SJ, Lawson JD, Kahle KT, Ng K, Carter B, Chen CC. DNA damage response and repair: insights into strategies for radiation sensitization of gliomas. Future Oncology. 2011;7:1335–1346. doi: 10.2217/fon.11.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Stechow L, van de Water B, Danen EH. Unraveling DNA damage response-signaling networks through systems approaches. Arch Toxicol. 2013;87:1635–1648. doi: 10.1007/s00204-013-1106-5. [DOI] [PubMed] [Google Scholar]
- 26.Kinner A, Wu W, Staudt C, Iliakis G. Gramma-H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res. 2008;36:5678–5694. doi: 10.1093/nar/gkn550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartlerode AJ, Scully R. Mechanisms of double-strand break repair in somatic mammalian cells. Biochem J. 2009;423:157–168. doi: 10.1042/BJ20090942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boehme KA, Kulikov R, Blattner C. p53 stabilization in response to DNA damage requires Akt/PKB and DNA-PK. Proc Natl Acad Sci U S A. 2008;105:7785–7790. doi: 10.1073/pnas.0703423105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bozulic L, Surucu B, Hynx D, Hemmings BA. PKBα/Akt1 Acts downstream of DNA-PK in the DNA double-strand break response and promotes survival. Mol Cell. 2008;30:203–213. doi: 10.1016/j.molcel.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 30.Gupta AK, Cerniglia GJ, Mick R, McKenna WG, Muschel RJ. HIV protease inhibitors block Akt signaling and radiosensitize tumor cells both in vitro and In vivo. Cancer Res. 2005;65:8256–8265. doi: 10.1158/0008-5472.CAN-05-1220. [DOI] [PubMed] [Google Scholar]
- 31.Toulany M, Kehlbach R, Florczak U, Sak A, Wang S, Chen J, Lobrich M, Rodemann HP. Targeting of AKT1 enhances radiation toxicity of human tumor cells by inhibiting DNA-PKcs-dependent DNA double-strand break repair. Mol Cancer Ther. 2008;7:1772–1781. doi: 10.1158/1535-7163.MCT-07-2200. [DOI] [PubMed] [Google Scholar]
- 32.Chang L, Graham PH, Hao J, Ni J, Bucci J, Cozzi PJ, Kearsley JH, Li Y. PI3K/Akt/mTOR pathway inhibitors enhance radiosensitivity in radioresistant prostate cancer cells through inducing apoptosis, reducing autophagy, suppressing NHEJ and HR repair pathways. Cell Death and Disease. 2014;5:e1437. doi: 10.1038/cddis.2014.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Golding SE, Morgan RN, Adams BR, Hawkins AJ, Povirk LF, Valerie K. Pro-survival AKT and ERK signaling from EGFR and mutant EGFRvIII enhances DNA double-strand break repair in human glioma cells. Cancer Biol Ther. 2009;8:730–738. doi: 10.4161/cbt.8.8.7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li B, Yuan M, Kim I, Chang C, Bernhard EJ, Shu HG. Mutant epidermal growth factor receptor displays increased signaling through the phosphatidylinositol-3 kinase/AKT pathway and promotes radioresistance in cells of astrocytic origin. Oncogene. 2004;23:4594–4602. doi: 10.1038/sj.onc.1207602. [DOI] [PubMed] [Google Scholar]


