Abstract
DNA polymerase iota (Polι) can repair several types of DNA damage but has extremely low fidelity. Previous studies have shown an aberrantly elevated Polι expression in human esophageal squamous cell cancer tissues. However, there were few reports describing the role of Polι in esophageal cancer progression. Based on Real-time PCR assay, we found Polι expression was up-regulated in esophageal cancer tissues compared to adjacent normal tissues and overexpression of Polι was correlated to lymph node metastasis. Clonogenic assay and transwell chamber assay showed that overexpression of Polι had higher clongenic capability and invasive tendency in human esophageal squamous cell cancer cells. Expression of cyclin D1, an important cell cycle regulator, was found to be associated with that of Polι in tissue samples and cancer cells as analyzed by real-time PCR, immunohistochemistry, Western blotting and imunofluorescence assay. Flow cytometry analysis further showed that cell cycle distribution was altered in Polι overexpressing cells. These results indicated that expression of Polι correlates significantly with tumor proliferation and invasion. We conclude that Polι is involved in the degree of aggressiveness of human esophageal squamous cell cancer.
Keywords: Esophageal cancer, DNA polymerase iota, cyclin D1, proliferation, metastasis
Introduction
DNA polymerase iota (Polι, hRAD30B), encoding an 80KD protein, is a conserved Y family DNA polymerase that participates in translesion DNA synthesis (TLS). Polι was found to be a remarkably error-prone human DNA polymerase when replicating undamaged DNA [1]. The error-prone character of Polι may lead to accumulation of DNA mutations thereby affecting genomic stability. Several studies reported up-regulation of Polι expression in human uveal melanoma, breast cancer cells and bladder cancer [2-4] (4). However, down-regulation of Polι expression was also found in human stomach, lung and colorectal cancers [5]. Several lines of evidence supported the Polι gene as a candidate in the mouse pulmonary adenoma resistance 2 locus (PAR2) responsible for higher tumor susceptibility [6]. While the controversial results may imply that Polι expression pattern is tissue-specific, the relationship between Polι and cancer progression has not been reported in any tissue types. Furthermore, the role of Polι in esophageal cancer progression has not been elucidated. Because of the error-prone DNA replication features of Polι, dysregulation of Polι may contribute to the acquisition of mutated phenotype that, along with the defective cell cycle control or disruption of other genome stability pathways, could facilitate or accelerate tumor progression. Hence, Polι may be involved in the acquisition of aggressive phenotypes of human esophageal squamous cell cancer.
Malignant nature of cancer is due to uncontrolled cell proliferation and invasive potential. Cyclin D1 is known as a key cell cycle regulator that contributes to cancer cell proliferation. Recent studies also revealed that cyclin D1 plays an essential role in cellular adhesion and migration. Cyclin D1 deficiency conferred a dramatic morphological phenotype that overrides the significant CSF-1-regulated morphological changes observed in WT macrophages [7-9]. Cyclin D1 stabilized p27Kip1, thereby inhibiting the RhoA-inducing Rho-associated protein kinase and myosin light chain kinase, and promoting cell migration [8-10]. Cyclin D1/p21 signaling axis was also found to be related to tumor growth initiation and local tumor cell invasion [11].
We have previously reported that the mRNA expression of Polι was 7.2-fold elevated in human esophageal cancer tissues compared with normal controls [12]. However, the role of Polι in esophageal cancer progression remains unknown. In this study, we analyzed the expression of Polι in esophageal cancer tissues and adjacent tissues, as well as its association with clinicopathological parameters. We further elucidated the role of Polι in esophageal cancer progression and its underlying mechanisms.
Materials and methods
Tissue samples
68 human esophageal squamous cell cancer tissues and 48 adjacent tissues used in this study were obtained from patients who had not received chemotherapy and radiation therapy before surgery in 2008 at the Gastrointestinal Center, Jiangbin Hospital (Zhenjiang, China), and were immediately frozen and stored in -80°C refrigerator. All the tissues used for scientific research were collected only after signing informed consent from the patients. The study was approved by the Institutional Ethics Committee of Jiangbin Hospital. Histological features and immunohistochemical conclusions were microscopically evaluated by two pathologists according to the classification of the World Health Organization [13].
RNA extraction and real-time PCR assay
Total RNA from frozen tissues was extracted using Trizol (Life Technologies, Grand Island, NY, USA) and cDNA was synthesized from total RNA using an oligo (dT)12 primer and Superscript II (Life Technologies, Grand Island, NY, USA). The SYBR green dye (Life Technologies, Grand Island, NY, USA) was used in real-time PCR reactions with a Real-Time PCR System (ABI PLUS ONE, Life Technologies, Grand Island, NY, USA). The sequences of the primers were shown in Table 1. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression was analyzed for normalization of real-time PCR data.
Table 1.
Primer sequences for real-time PCR analysis
| Forward primer | Reverse primer | |
|---|---|---|
| GAPDH | 5’-GAAGGTGAAGGTCGGAGTC-3’ | 5’-GAAGATGGTGATGGGATTTC-3’ |
| Polι | 5’-ACAAACCGGGATTTCCTACC-3’ | 5’-TCACACTTCCTTTCCCTTGAA-3’ |
| Cyclin D1 | 5’-CCCTCGGTGTCCTACTTCAAATGT-3’ | 5’-GGAAGCGGTCCAGGTAGTTCAT-3’ |
| MMP7 | 5’-GGAACAGGCTCAGGACTATCTCAA-3’ | 5’-GCAACATCTGGCACTCCACATCT-3’ |
| MMP9 | 5’-TATGGTCCTCGCCCTGAACCT-3’ | 5’-GCACAGTAGTGGCCGTAGAAGG-3’ |
Immunohistochemistry
The formalin-fixed, paraffin-embedded tissue sections were deparaffinized, rehydrated in graded alcohols series. Tissue sections were washed in distilled water and PBS, and treated with 0.03% hydrogen peroxide for 5 min to block endogenous peroxidase activity. Then the sections were incubated with anti-Polι antibody (Proteintech, Chicago, IL, USA) and anti-Cyclin D1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted 1:400 for 60 min, washed in PBS, and incubated with labeled HRP-conjugated anti-rabbit antibody (Beyotime, Haimen, China) for 30 min. The sections were washed and incubated with diaminobenzene for 10 min, followed by counterstaining with hemtoxylin, dehydration and mounting. Scoring was performed blind without clinical data. Positive cell rates of 0-10, 11-25, 26-50, 51-75, and > 75% were scored 0, 1, 2, 3 and 4, respectively. The staining intensity was graded no staining (score 0); pale yellow staining (score 1); buffy staining (score 2); strongly brown staining (score 3). For Polι, the final score was defined low expression level (≤ 4 score) and high expression level (> 4 score). For Cyclin D1, the final score was defined low expression level (< 2 score) and high expression level (≥ 2 score).
Cell culture
Human esophageal cancer cell lines ECA-109 and TE-1 were obtained from Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China) and cultured in DMEM with 10% FBS at 37°C with 5% CO2.
Construction of Polι expression vector
The cDNA encoding human full-length Polι gene was amplified by PCR using the following primers: Forward, 5’-TTTGGATCCATGGAGAAGCTGGGGGT-3’; Reverse, 5’-GCCCTCGAGTTATTTATGTCCAATGTGG-3’. The PCR product was cloned into the pcDNA-3.1 vector (Life Technologies, Grand Island, NY, USA). The cloned fragment was verified by DNA sequencing. The pcDNA-3.1 vector served as control.
Western blotting
The cells were lysed with RIPA (Beyotime, Haimen, China) and the protein concentration was measured by Pierce BCA protein assay kit (Thermo Fisher Scientific, Rockford, IL, USA). Protein (60 μg) was resolved by electrophoresis in a 10% SDS-PAGE gel, transferred onto PVDF membrane (Millipore, Billerica, MA, USA) and blocked with 5% skimmed milk. Membranes were incubated with antibodies against Polι (Abcam, Cambridge, MA, USA), Cyclin D1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and GAPDH (Proteintech, Chicago, IL, USA) respectively, and then incubated with HRP-conjugated anti-rabbit or anti-mouse antibodies (Beyotime, Haimen, China). Protein bands were visualized using ECL solution (Beyotime, Haimen, China).
Clonogenic assay
Approximately1,000 cells were cultured in a 6-well plate for 14 days. The colony was defined to consist of at least 50 cells. Colonies were fixed with glutaraldehyde, stained with crystal violet and counted under a microscope.
Immunofluorescence
Cells were fixed in 4% paraformaldehyde for 10 minutes at room temperature and permeabilized with 1% Triton X-100 in PBS for 5 min. Samples were then blocked with blocking solution (PBS containing 10% BSA and 1% triton-X-100) at 37°C for 60 min and incubated overnight at 4°C with the Polι antibody (Proteintech, Chicago, IL, USA). This was followed by 3 washes with PBS and then incubated with secondary Rhodamine-labeled goat anti-rabbit antibody (KPL, Gaithersburg, MD, USA) for 1 hour at room temperature. Cells were visualized using a fluorescence microscope (Eclipse 80i, Nikon, Tokyo, Japan).
Cell-cycle analysis
Cell cycle was analyzed as previously described [14]. In brief, cells were permeabilized by ethanol and treated with RNase A. Propidium iodide (Beyotime, Haimen, China) was applied to stain cellular DNA. Samples (1 × 105 cells/well) were analyzed by flow cytometry (Beckman Coulter, Brea, CA, USA).
Transwell chamber assay
Invasive ability of cells was evaluated in 24-well transwell chambers (Corning, Corning, NY, USA). The polycarbonate filters containing 8 mm pores were covered with 30 μl of Matrigel (BD Biosciences, Bedford, MA, USA) at 5 mg/ml. 1% BSA was used for blocking at 37°C for 1 h. Cells (106/ml) were placed in upper chamber in serum-free culture medium. Medium with 10% FBS (600 μl) was added in lower chamber. After 48 h incubation, lower surface of the filter were fixed in ethanol and stained with hematoxylin-eosin. Cells invaded were counted in 5 random microscopic fields at a magnification of × 200 (DMIL LED, Leica, Wetzlar, German).
Statistical analysis
Statistical significance for two experimental groups was analyzed using Mann-Whitney U test or Student’s t-test. For more than two groups, a Kruskal-Wallis H test was adopted. Correlation analysis of mRNA expression was analyzed using Pearson test. Correlation analysis between IHC staining was analyzed using Chi-square test. All tests were two-sided. The data were considered significant if P < 0.05.
Results
Elevated DNA polymerase iota (Polι) in human esophageal cancer
To compare expression differences between cancer and adjacent tissues, reverse transcriptase PCR and real-time PCR were used to evaluate the mRNA levels of Polι expression in 68 human esophageal squamous cell cancer tissues and 48 adjacent tissues. As shown in Figure 1A and 1B, the expression of Polι in esophageal squamous cell cancer tissues was significantly higher than in adjacent tissues (P < 0.05). This observation is consistent with our recent report showing that Polι is over-expressed in esophageal cancer as analyzed by immunohistochemistry and PCR [12].
Figure 1.

Overexpression of Polι in human esophageal cancer tissues. A. RT-PCR analysis of the expression of Polι in 4 pairs of human esophageal cancer tissues and adjacent tissues. “A”, adjacent tissues. “T”, tumor tissues. B. Real-time PCR assay of the expression of Polι in cancer and adjacent tissues (*P < 0.05, Mann-Whitney U test, n=3). C. Real-time PCR assay of the expression of Polι in pN(-) and pN(+) groups (*P < 0.05, Mann-Whitney U test, n=3). D. Real-time PCR assay of the expression of Polι in early stages and advanced stages of esophageal cancer tissues (*P < 0.05, Mann-Whitney U test, n=3). GAPDH was used for normalization.
Polι expression was correlated with lymph node metastasis and clinical stages
To evaluate whether Polι expression was related to clinicopathological features, the characteristics of 68 esophageal squamous cell cancer patients included in this study were described in Table 2. As Mann-Whitney U test showed, Polι expression was positively correlated with lymph node metastasis (Figure 1C, P < 0.05). Furthermore, the tumors at higher clinical stages (IIb and III) showed higher Polι expression than that of the lower stages (I and IIa) (Figure 1D, P < 0.05). In contrast, pT, as well as ages, gender, and histological grade were found no statistical differences among the groups of patients. Collectively, these findings indicated that overexpression of Polι in esophageal cancer is associated with lymph node metastasis and tumor progression.
Table 2.
Relationship between clinicopathological parameters and TNM stages in oesophageal squamous cell carcinoma (n=68)
| Clinicopathological parameters | Relative Polι expression (relative to GAPDH) | P value | |
|---|---|---|---|
|
|
|||
| Case | Median (Q3-Q1) | ||
| Age (years) | |||
| < 60 | 28 | 1.587 (19.557) | 0.636 |
| ≥ 60 | 40 | 1.206 (4.264) | |
| Gender | |||
| Male | 50 | 1.293 (5.613) | 0.675 |
| Female | 18 | 1.495 (7.805) | |
| Histological grade | |||
| Poorly | 9 | 0.941 (60.491) | 0.938 |
| Moderately | 33 | 1.315 (9.435) | |
| Well | 26 | 1.587 (8.747) | |
| pT | |||
| pT1-2 | 25 | 1.810 (9.648) | 0.252 |
| pT3-4 | 43 | 1.237 (5.103) | |
| pN | |||
| (--) | 28 | 1.133 (1.016) | 0.006 |
| (+) | 40 | 2.361 (19.337) | |
| Stage | |||
| I--IIa | 26 | 1.128 (0.940) | 0.002 |
| IIb--III | 42 | 2.541 (17.338) | |
Elevated Polι expression promoted cancer cell clonal formation and invasion
To understand whether overexpression of Polι in esophageal squamous cancer cells promote tumor progression, the human Polι expression vector pcDNA3.1-Polι was transfected into TE-1 and ECA-109 cells to up-regulate Polι expression. Clonogenic assay demonstrated a higher clonal formation efficiency in pcDNA3.1-Polι-transfected cells compared with control cells (Figure 2A, P < 0.05). The transwell chamber assay was used to explore the relationship between high Polι expression and cell invasion. As shown in Figure 2B, cells transfected with pcDNA3.1-Polι were more invasive than the control vector-transfected cells in both cell lines (Figure 2B, P < 0.05). Together these results indicated that cells with higher Polι expression exhibit more aggressive phenotypes, consistent with our observations obtained from cancer tissue samples.
Figure 2.

Overexpression of Polι promoted esophageal squamous cancer cell proliferation and invasion. A. Clonogenic assay of TE-1 and ECA-109 48 h after transfecting Polι expression vector (*P < 0.05, Student’s t-test). B. Transwell chamber assay showed that cells in pcDNA3.1-Polι transfected cells were more invasive than control group in both cell lines (*P < 0.05, Student’s t-test). Data are presented as the mean ± standard deviation for at least three independent experiments.
Overexpression of Polι enhanced Cyclin D1 expression in esophageal squamous cancer cells
To characterize the molecular mechanism underlying the induction of cell proliferation and invasion by overexpression of Polι, we examined expression of several genes related to tumor metastasis using real-time PCR assay in TE-1 and ECA-109 cells. We found that there was no difference of MMP1, MMP2, MMP7, MMP9, E-cadherin, VEGFA, VEGFB and VEGFC expression between pcDNA3.1-Polι-transfected cells and control vector-transfected cells (data not shown). However, overexpression of Polι increased the expression of Cyclin D1 at both mRNA and protein levels in human esophageal squamous cancer cells (Figure 3A and 3B). Immunofluorescence staining showed that Polι was mainly located in nucleus while Cyclin D1 was expressed in both nucleus and cytoplasm (Figure 3C). Therefore, Polι may accelerate cell cycle progression by up-regulating Cyclin D1 expression.
Figure 3.

Transfection of the Polι expression vector enhanced cyclin D1 expression in TE-1 and ECA-109 cells. A. Real-time PCR assay of the expression of Polι and cyclin D1 after transfecting the Polι expression vector (*P < 0.05, **P < 0.01, Student’s t-test, n=3). B. Western blotting analysis of the expression of Polι and cyclin D1 after transfecting the Polι expression vector. C. Cell immunofluorescence of Polι and cyclin D1 after transfection of the Polι expression vector.
Overexpression of Polι increased the S phase of esophageal cancer cells
To test whether overexpression of Polι alters cell cycle progression, a cell cycle analysis was performed using flow cytometry. Detailed analysis showed that overexpression of Polι increased the proportion of S phase from 20% to 35% in TE-1 (P= 0.006) cells and 22% to 41% in ECA-109 (P =0.002) cells. This was accomplished by a significant depletion of G2/M phase from 26% to 15% in TE-1 (P=0.003) cells and 27% to 7% in ECA-109 (P=0.003) cells. G1 phase was found no statistical difference between Polι overexpression and control cells in both cell lines (Figure 4). This observation support the conclusion that overexpression of Polι accelerates cell cycle progression.
Figure 4.
Cell-cycle analysis after transfection of the Polι expression vector in TE-1 and ECA-109 cells. Polι overexpression cells had higher percentage of S phase in both TE-1 and ECA-109 cell lines and lower percentage of G2/M phase (**, P < 0.01, Student’s t-test, n=3).
Correlation between expression of Polι and Cyclin D1 in esophageal squamous cell cancer tissues
To verify the findings from our cell culture experiments, expression of MMP7 and MMP9 was determined in 48 esophageal squamous cell cancer tissues by real-time PCR. We found no correlation between expression levels of Polι and MMP7 or MMP9 (Figure 5). However, a significant association was found between expression levels of Polι and Cyclin D1 (Figure 6A, r=0.833, P < 0.001). Furthermore, we analyzed protein expression of Cyclin D1 and Polι using IHC staining. As shown in Figure 6B, the expression of Cyclin D1 was mostly in agreement with that of Polι. The expression of Cyclin D1 was positively associated with elevated Polι expression as analyzed by Chi-square test (r=0.55, P < 0.05). Taken together, these data, along with our observations from cell culture experiments, suggested that Polι regulates Cyclin D1 expression in esophageal squamous cell cancer.
Figure 5.
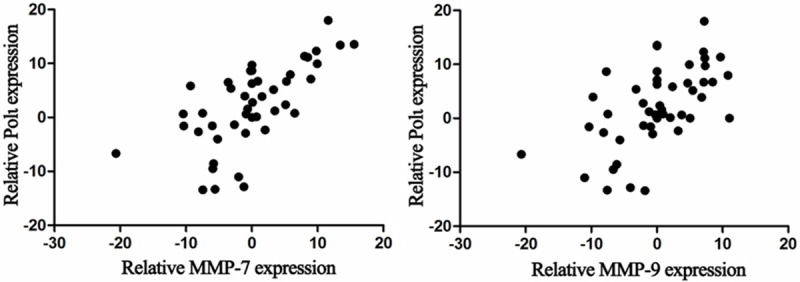
No correlation between Polι and MMP7 or MMP9 expression in 48 esophageal squamous cancer tissues analyzed by real-time PCR (P > 0.05, Pearson test, n=48).
Figure 6.
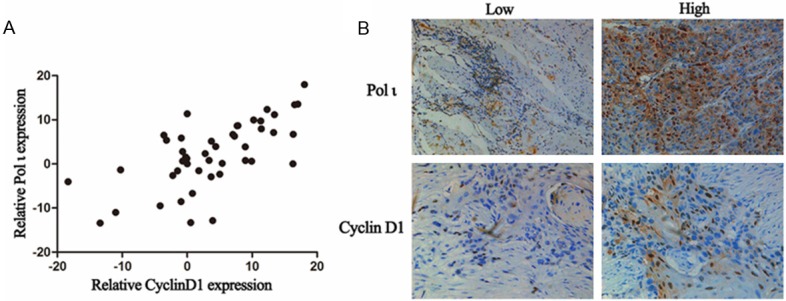
Expression of Polι was correlated with expression of cyclin D1 in human esophageal cancer tissues. A. Real-time PCR assay of the expression of Polι and cyclin D1 (r=0.833, P < 0.001, Pearson test, n=48). B. Immunohistochemical staining of Polι and cyclin D1 (r=0.55, P < 0.05, Chi-square test, n=48).
Discussion
Esophageal squamous cell carcinoma stands the eighth place in incidence and sixth place in cancer-related deaths worldwide. According to recent reports, three hundred thousand people died of esophageal cancer each year with 70% cases occurred in China [15,16]. Multiple factors contribute to tumorigenesis such as genetic alterations, epigenetic modifications and dysregulation of key molecules [17]. Genetic alterations can be repaired by the DNA repair system involving base excision repair (BER), nucleotide excision repair (NER), mismatch repair (MMR) and translesion DNA synthesis (TLS). Polι is known to participate in TLS and has the lowest fidelity with T-G misincorporation at a frequency of approximately 6.7 × 10-1. Recent studies showed that TLS across a particular DNA lesion may involve different TLS polymerases including Polι [18]. Despite its error-prone characters, the involvement of Polι in cancer development had not been clarified. Previous controversial reports [2-6] implied that Polι may act in a tissue-specific manner.
Previously, we have reported that mRNA expression of Polι was significantly up-regulated in human esophageal cancer tissues compared with normal controls [12]. In this study, overexpression of Polι in esophageal squamous cell cancer tissues was confirmed at both mRNA and protein levels, indicating the possible involvement of Polι in esophageal cancer progression. In addition, Polι expression is significantly correlated with pN and clinical stages. Thus, Polι expression may have prognostic potential for esophageal cancer outcome. Our findings were consistent with a previous report in which overexpression of Polι was positively correlated with the clinical tumor grade in bladder cancer [2].
The epithelial-mesenchymal transition (EMT) is a process by which epithelial cells lose their cell polarity and cell-cell adhesion, and gain migratory and invasive properties to become mesenchymal stem cells and is essential in the initiation of metastasis for cancer progression[19]. These processes involve various factors such as cell adhesion molecules, enzymes and cytokines such as matrix metalloproteinases (MMPs), E-cadherin, and vascular endothelial growth factor (VEGF) [20]. However, we found no evidence from both tissue culture and tissue sample studies to support the association of MMP1, MMP2, MMP7, MMP9, E-cadherin, VEGFA, VEGFB, or VEGFC with Polι during esophageal cancer progression. Hence it is highly likely that other cellular mechanisms relating to tumor metastasis are involved in Polι’s action in esophageal cancer. In this context, the correlation between Polι and cyclin D1 expression was found in esophageal cancer tissues and further confirmed by in vitro experiments, suggesting that Polι and cyclin D may act in synergy to promote esophageal cancer progression.
Cyclin D1 functions as a regulatory subunit of CDK4 or CDK6 and controls cell cycle progression. It is apparent that cyclin D1 promotes cell growth and acts as an oncogene [21]. However, results from several studies supported the concept that the oncogenic effects of cyclin D1 may not be simply attributed to an enhanced tumor proliferation. Studies with large samples (over 100 patients) indicated that cyclin D1 is likely an independent esophageal squamous cancer prognostic factor [22,23]. In bladder cancer and oral squamous cell carcinoma, cyclin D1 was found to be correlated with lymphoid node metastasis [24,25]. The results from our clonogenic assay and transwell assay indicated that overexpression of Polι promotes esophageal cancer cell proliferation and invasion. Flow cytometry studies further showed an S-phase accumulation in Polι overexpressing cells. Given that overexpression of Polι also enhanced cyclin D1 expression as determined by western blot analysis, it is logical to conclude that cyclin D1 mediates Polι’s action in promoting esophageal cancer growth and metastasis. This is consistent with previous findings showing that cyclin D1 could interact with p27Kip1, in mammary epithelial cells or cooperate with p21 in breast cancer cells, which plays an essential role in regulating cellular adhesion and migration [9,11].
Translesion synthesis by DNA polymerases was an important mechanism by which cancer cells could tolerate DNA damages and help bypass cell cycle arrest [26]. As a post-replication repair DNA polymerase, Polι has been shown to be accumulated in cells entering S-phase with DNA damage [27,28]. Hence overexpression of Polι may repair certain DNA damages and help the fulfillment of DNA replication, which promotes cell cycle progression. This is in agreement with our observations that overexpression of Polι promotes cell proliferation and metastasis. However, the molecular mechanism of how Polι might interact with cyclin D1 to regulate this process requires further investigation.
In summary, Polι expression was significantly up-regulated in esophageal squamous cell cancer tissues and was positively correlated with lymph node metastasis and clinical stages. Polι overexpression contributed to cancer proliferation and metastasis at least in part through induction of cyclin D1. These findings illustrated a role of Polι in human esophageal cancer progression, which may serve as a therapeutic target for esophageal cancer.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81372433 and 81472920), the Natural Science Foundation of Jiangsu Province of China (BK20131149) and the Suzhou Administration of Science & Technology (SYS201360 and SYS201254).
Disclosure of conflict of interest
None.
References
- 1.Tissier A, McDonald JP, Frank EG, Woodgate R. Poliota, a remarkably error-prone human DNA polymerase. Gene Dev. 2000;14:1642–1650. [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan F, Xu Z, Yang M, Wei Q, Zhang Y, Yu J, Zhi Y, Liu Y, Chen Z, Yang J. Overexpressed DNA polymerase iota regulated by jnk/c-jun contributes to hypermutagenesis in bladder cancer. PLos One. 2013;8:e69317. doi: 10.1371/journal.pone.0069317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gening LV, Grishina EE, Petrochenkov AN, Tarantul VZ. [Association between high activity of DNA polymerase iota and the development of human uveal melanoma] . Genetika. 2006;42:98–103. [PubMed] [Google Scholar]
- 4.Yang J, Chen Z, Liu Y, Hickey RJ, Malkas LH. Altered DNA polymerase iota expression in breast cancer cells leads to a reduction in DNA replication fidelity and a higher rate of mutagenesis. Cancer Res. 2004;64:5597–5607. doi: 10.1158/0008-5472.CAN-04-0603. [DOI] [PubMed] [Google Scholar]
- 5.Pan Q, Fang Y, Xu Y, Zhang K, Hu X. Down-regulation of DNA polymerases kappa, eta, iota, and zeta in human lung, stomach, and colorectal cancers. Cancer Lett. 2005;217:139–147. doi: 10.1016/j.canlet.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 6.Lee GH, Nishimori H, Sasaki Y, Matsushita H, Kitagawa T, Tokino T. Analysis of lung tumorigenesis in chimeric mice indicates the pulmonary adenoma resistance 2 (par2) locus to operate in the tumor-initiation stage in a cell-autonomous manner: Detection of polymorphisms in the poli gene as a candidate for par2. Oncogene. 2003;22:2374–2382. doi: 10.1038/sj.onc.1206387. [DOI] [PubMed] [Google Scholar]
- 7.Neumeister P, Pixley FJ, Xiong Y, Xie H, Wu K, Ashton A, Cammer M, Chan A, Symons M, Stanley ER, Pestell RG. Cyclin d1 governs adhesion and motility of macrophages. Mol Biol Cell. 2003;14:2005–2015. doi: 10.1091/mbc.02-07-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Z, Wang C, Jiao X, Lu Y, Fu M, Quong AA, Dye C, Yang J, Dai M, Ju X, Zhang X, Li A, Burbelo P, Stanley ER, Pestell RG. Cyclin d1 regulates cellular migration through the inhibition of thrombospondin 1 and rock signaling. Mol Cell Biol. 2006;26:4240–4256. doi: 10.1128/MCB.02124-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z, Jiao X, Wang C, Ju X, Lu Y, Yuan L, Lisanti MP, Katiyar S, Pestell RG. Cyclin d1 induction of cellular migration requires p27 (kip1) Cancer Res. 2006;66:9986–9994. doi: 10.1158/0008-5472.CAN-06-1596. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Wang C, Jiao X, Katiyar S, Casimiro MC, Prendergast GC, Powell MJ, Pestell RG. Alternate cyclin d1 mrna splicing modulates p27kip1 binding and cell migration. J Biol Chem. 2008;283:7007–7015. doi: 10.1074/jbc.M706992200. [DOI] [PubMed] [Google Scholar]
- 11.Dai M, Al-Odaini AA, Fils-Aime N, Villatoro MA, Guo J, Arakelian A, Rabbani SA, Ali S, Lebrun J. Cyclin d1 cooperates with p21 to regulate tgfbeta-mediated breast cancer cell migration and tumor local invasion. Breast Cancer Res. 2013;15:R49. doi: 10.1186/bcr3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou J, Zhang S, Xie L, Liu P, Xie F, Wu J, Cao J, Ding WQ. Overexpression of DNA polymerase iota (poliota) in esophageal squamous cell carcinoma. Cancer Sci. 2012;103:1574–1579. doi: 10.1111/j.1349-7006.2012.02309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sobin LH, Wittekind C. Tnm Classification of Malignant Tumor. 6th edition 2002. [Google Scholar]
- 14.Nunez R. DNA measurement and cell cycle analysis by flow cytometry. Curr Issues Mol Biol. 2001;3:67–70. [PubMed] [Google Scholar]
- 15.Chen W, Zheng R, Zhang S, Zhao P, Li G, Wu L, He J. The incidences and mortalities of major cancers in china, 2009. Chin J Cancer. 2013;32:106–112. doi: 10.5732/cjc.013.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 17.Kuwano H, Kato H, Miyazaki T, Fukuchi M, Masuda N, Nakajima M, Fukai Y, Sohda M, Kimura H, Faried A. Genetic alterations in esophageal cancer. Surg Today. 2005;35:7–18. doi: 10.1007/s00595-004-2885-3. [DOI] [PubMed] [Google Scholar]
- 18.Livneh Z, Ziv O, Shachar S. Multiple two-polymerase mechanisms in mammalian translesion DNA synthesis. Cell Cycle. 2010;9:729–735. doi: 10.4161/cc.9.4.10727. [DOI] [PubMed] [Google Scholar]
- 19.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 20.Groblewska M, Siewko M, Mroczko B, Szmitkowski M. The role of matrix metalloproteinases (mmps) and their inhibitors (timps) in the development of esophageal cancer. Folia Histochem Cytobiol. 2012;50:12–19. doi: 10.2478/18691. [DOI] [PubMed] [Google Scholar]
- 21.Eeckhoute J, Carroll JS, Geistlinger TR, Torres-Arzayus MI, Brown M. A cell-type-specific transcriptional network required for estrogen regulation of cyclin d1 and cell cycle progression in breast cancer. Gene Dev. 2006;20:2513–2526. doi: 10.1101/gad.1446006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikeguchi M, Sakatani T, Ueta T, Kaibara N. Cyclin d1 expression and retinoblastoma gene protein (prb) expression in esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2001;127:531–536. doi: 10.1007/s004320100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prognostic significance of cyclind1 and e-cadherin in patients with esophageal squamous cell carcinoma: Multiinstitutional retrospective analysis. Research committee on malignancy of esophageal cancer, japanese society for esophageal diseases. J Am Coll Surgeons. 2001;192:708–718. [PubMed] [Google Scholar]
- 24.Zhao Y, Yu D, Li H, Nie P, Zhu Y, Liu S, Zhu M, Fang B. Cyclin d1 overexpression is associated with poor clinicopathological outcome and survival in oral squamous cell carcinoma in asian populations: Insights from a meta-analysis. PLos One. 2014;9:e93210. doi: 10.1371/journal.pone.0093210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kopparapu PK, Boorjian SA, Robinson BD, Downes M, Gudas LJ, Mongan NP, Persson JL. Expression of cyclin d1 and its association with disease characteristics in bladder cancer. Anticancer Res. 2013;33:5235–5242. [PMC free article] [PubMed] [Google Scholar]
- 26.Cruet-Hennequart S, Villalan S, Kaczmarczyk A, O’Meara E, Sokol AM, Carty MP. Characterization of the effects of cisplatin and carboplatin on cell cycle progression and DNA damage response activation in DNA polymerase eta-deficient human cells. Cell Cycle. 2009;8:3039–3050. [PubMed] [Google Scholar]
- 27.Petta TB, Nakajima S, Zlatanou A, Despras E, Couve-Privat S, Ishchenko A, Sarasin A, Yasui A, Kannouche P. Human DNA polymerase iota protects cells against oxidative stress. EMBO J. 2008;27:2883–2895. doi: 10.1038/emboj.2008.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kakar S, Watson NB, McGregor WG. Rad18 signals DNA polymerase iota to stalled replication forks in cells entering s-phase with DNA damage. Adv Exp Med Biol. 2008;614:137–143. doi: 10.1007/978-0-387-74911-2_16. [DOI] [PubMed] [Google Scholar]



