Abstract
Morpho-quantitative studies of the spleen indicate that the proportions of the compartments and sub-compartments are stable in normal conditions. However, disorders due to stress can influence the number and function of the immune cells in this organ. The aim of this study was to determine, through the model of altering the early mother-infant bond and altering the late social bond through isolation, the effect on the morpho-quantitative characteristics of the spleen in adult Sprague-Dawley rats subjected to intermittent chronic stress in adulthood. Twenty-five newborn female rats were used, kept under the standardized lactation and feeding conditions. The rats were assigned randomly to 2 control groups (C1 and C2) and 3 experimental groups, exposed to early (E1), late (E2) or early-late (E3) adverse experiences and then subjected to intermittent chronic stress in adulthood (C2, E1, E2 and E3). The spleen of each animal was isolated and its morphometric characteristics were determined: volume density (Vv) of the red pulp, white pulp, marginal zone, splenic lymph nodule, periarterial lymphatic sheath and germinal center; areal number density (Na), surface density (Sv), number density (Nv), diameter (D) and total number of splenic lymph nodules. The mass of each compartment was also determined. A one-way analysis of variance (ANOVA) and Scheffé’s post hoc test were used for the statistical analysis. The p values were considered significant when they were less than 0.05 (*) and very significant at less than 0.025 (**). There were significant differences in the Vv of the red pulp, white pulp and their sub-compartments between the control and experimental groups. The white pulp increased significantly (P = 0.000) in E1, E2 and E3 compared to C1 and C2. The average Na and D values of the splenic lymph nodules were also higher in the experimental groups. The ANOVA for the mass of the spleen and the red pulp revealed no differences between the groups. The mass of the white pulp and its subcompartments was greater in the experimental groups. A higher proportion of white pulp in the experimental groups could be associated with an increase in spleen immune activity, with alterations depending on certain cell subsets. The chronic stress produced morpho-quantitative changes in the rat spleen, and these depended on the animal’s history of stress, whether it had been previously stressed or not, with further exposure to stress in adulthood.
Keywords: Adverse experience, chronic stress, rat, spleen
Introduction
Stress is defined as the body’s non-specific response to any real or perceived threat or external force that triggers the general adaptation syndrome in the body [1]. This stressful event may increase vulnerability to certain diseases, producing for example changes of balance between the secretion of proinflammatory and immunoregulatory cytokines and/or producing effects on leukocyte transport and distribution [2-4]. Stress-induced immunological alterations manifest essentially in those pathologies linked directly to the immune mechanisms, such as autoimmune infections, diseases and neoplasias [5,6].
To better understand the mechanisms that underlie these alterations, it is important to consider that depending on the type of stressor or its duration, it is possible to produce immunosuppression or enhance the immune response [7]. Moreover, the changes observed in the immune function will depend on the type of response evaluated (innate or acquired) and on the different components of the immune system (IS) studied, such as primary or secondary lymphoid organs [8].
Stress, can induce a modulation of the IS through the hypothalamic pituitary adrenal (HPA) axis and the adrenal gland. In this context, the main mediators of stress are the corticotropin-releasing hormone (CRH) in the paraventricular nucleus of the hypothalamus, adrenocorticotropic hormone (ACTH) in the anterior pituitary, and catecholamines and glucocorticoids in the adrenal glands [9].
Both physical and psychosocial stress alter the functioning of the IS [10]. In humans, it has been found that the total number of leukocytes in the blood as well as the levels of adrenalin and noradrenaline increased after a period of psychological or physical stress, with the number of monocytes, K lymphocytes and B lymphocytes increasing significantly with physical stress [11]. By contrast, psychological stress can modulate cell-mediated immunity by suppressing lymphocyte proliferation and activating K lymphocytes, reducing the number of CD4+ lymphocytes in the peripheral blood and altering the proportion of T CD4+/CD8+ lymphocytes [12,13]. A variant of psychological stress is social stress, which has an impact on the susceptibility to enteropathogens, decreasing the proportion of T CD4+/CD8+ lymphocytes in the spleen [14].
In addition, the stress response and its effects on the immune function can be viewed in the context of a stress-immune spectrum [7]. One region of this spectrum is characterized by acute stress, short in duration, in which a rapid response to physiological stress is observed in the presence of the stressor, followed by a rapid cessation of the response when the tension stops. In this context, studies have shown that the response to acute stress is an evolutionary, physiological adaptation of survival that can promote health, improving the immune response. It has been suggested that an important function of the neuroendocrine mediators released under conditions of acute stress may be to guarantee that the appropriate leukocytes are in the right place at the right time to respond to an immune challenge that could be initiated by the induction of a stressor [15,16].
During acute stress, biphasic changes are produced in the number of cells in the blood. In the initial phase, shortly after the appearance of the tension, there is an increase in the total number of leukocytes due to the action of the catecholamines, with the increase in K lymphocytes and granulocytes being more prominent [12,15,17,18]. As the stress response continues, activation of the HHA axis releases glucocorticoids, which induces the leukocytes, mainly lymphocytes and monocytes, to leave the blood and take up position in organs and/or tissues that serve as defense barriers for the body, such as the skin and pulmonary, gastrointestinal and urogenital epithelia [3,16,19]. During acute stress, both the spleen and the blood are temporarily depleted of leukocytes. During this period, these compartments are prone to immunosuppression, whereas the skin, which is the route of entry for some pathogens, shows an immunological improvement [4,17]. These results are correlated positively with studies by Zalcman and Anisman [20], who reported that acute stress suppresses splenic immunoglobulin (Ig) M plaque-forming cell response.
In contrast to acute stress, it has been shown that chronic stress produces deregulation of the immune response, both innate and adaptive [21,22], suppresses immunity by decreasing the number, transport and function of immune cells in peripheral blood [7], promotes proinflammatory activity driven by type 2 cytokines [23] and suppresses protective T cells, increasing the function of suppressor T cells in the skin [24]. It has also been observed that chronic stress suppresses immunoprotective parameters such as cell immunity driven by type 1 cytokines [25] and the number of monocytes and K lymphocytes in peripheral blood [26]. Nevertheless, it has been reported that chronic stress improves mitogenic activity by inducing proliferation of the splenocytes and splenic IgM production [20], being correlated with the positive regulatory role that endogenous glucocorticoids play in the production of in vivo antibodies in peripheral blood against certain antigens [27] and a higher percentage of T-helper cells in the spleen [26].
Although the spleen is the largest secondary lymphoid organ in the body, there are very few studies that have investigated the effects of chronic stress on its morphology. The spleen has two main compartments: the red pulp, related to the destruction of aged and damaged erythrocytes, and the white pulp, which initiates the immune responses to foreign antigens. The proportions of the compartments and subcompartments are stable under normal conditions; however, they can change, reflecting functional and structural deteriorations in the IS as the result of a disease [28]. In this context, histoquantitative studies into the normal and altered morphology of the spleen could make possible a more precise evaluation of the pathological states that affect this organ under different stress conditions. According to Furrianca et al. [29], in clinically healthy adult rats the proportions of the main compartments of the spleen were 53.9% red pulp, 25.75% white pulp and 15.87% marginal zone. In 6-month-old Wistar rats, the red and white pulp presented in a proportion of 41.10% and 28.56%, respectively. In the white pulp, 15.77% corresponded to the periarterial lymphatic sheath (PALS) and 12.79% to the splenic lymph nodules. The marginal zone represented 22.77% of the splenic tissue [30]. On the other hand, under conditions of chronic stress, Wistar-Kyoto rats presented alterations in the structure and size of the follicles as well as in the morphology of the splenic parenchyma associated with changes in the plasma corticosterone levels [31]. Moreover, Wistar rats presented a higher percentage of follicles and marginal zone in the white pulp compared to the control groups when treated chronically with immunosuppressants [32].
This is particularly important for understanding the mechanisms involved in the regulation of the IS under stress conditions; however, the role of chronic stress in the regulation of the immune response of the spleen as it pertains to the morphological changes of this organ remains to be ascertained in greater detail. Additionally, studies have shown that the etiology of some diseases not only develops due to a genetic predisposition or adult lifestyle, but the moment at which the stressor occurs to alter the immune response must also be considered [33]. Consequently, it would be interesting to know the morphological changes the spleen might undergo when exposure to stress occurs at different points in an individual’s development. The aim of our study was to determine, through the model of altering the early mother-infant bond and altering the late social bond through isolation, the effect on the morpho-quantitative characteristics of the spleen in adult Sprague-Dawley rats subjected to intermittent chronic stress in adulthood.
Materials and methods
Animals
Twenty-fiveSprague-Dawley female albino newborn rats were used, taken from the Experimental Surgery Unit of the Doctorate in Morphological Sciences in the Faculty of Medicine at the Universidad de La Frontera, Chile. They were kept in controlled environmental conditions in terms of temperature, environmental noise and a 12/12 h light-dark cycle. The experiments were conducted according to the Guide for the Care and Use of Laboratory Animals [34]. The project was approved by the Scientific Ethics Committee of the Universidad de La Frontera (ED11-0054).
The rats were divided into five groups of five animals each, randomly assigned.
Experimental group 1 (E1): subjected to early adverse experience.
Experimental group 2 (E2): subjected to late adverse experience.
Experimental group 3 (E3): subjected to early-late adverse experience.
Control group 1 (C1): not subjected to early or late adverse experiences; No exposure to chronic intermittent stress.
Control group 2 (C2): not subjected to early or late adverse experiences.
Early adverse experience (E1): (alteration of the mother-infant bond through reduction of the lactation period).
Newborn rats with 18 days of lactation, separated from their mother and kept in a cage under conditions of social interaction, water and food (pellets) ad libitum for a period of 110 days.
Late adverse experience (E2): (alteration of the social bond through isolation in adulthood).
Newborn rats with 23 days of lactation, separated from their mother and kept in a cage under conditions of social interaction, water and food (pellets) ad libitum for a period of 80 days. Then, they were placed in individual cages, restraining all types of social interaction for 110 days. During this period, they had access to water and food (pellets) ad libitum.
Early-late adverse experience (E3): (alteration of the mother-infant bond through reduction of the lactation period and rupture of the social bond through isolation in adulthood).
Newborn rats with 18 days of lactation, separated from their mother and kept in a cage under conditions of social interaction, water and food (pellets) ad libitum for a period of 80 days. Then, they were placed in individual cages, restraining all types of social interaction for 110 days. During this period they had access to water and food (pellets) ad libitum.
Control group 1 (C1): Group without early or late adverse experiences, without exposure to intermittent chronic stress (normal morphological control of the spleen).
Newborn rats with 23 days of lactation were separated from their mother and kept in a cage under conditions of social interaction, water and food (pellets) ad libitum for the entire experiment (116 days).
Control group (C2): Newborn rats with 23 days of lactation, separated from their mother and kept in a cage under conditions of social interaction, water and food (pellets) ad libitum for a period of 110 days.
Measuring intake behavior
At the end of the 110-day period, for the following six days, the members of each group were deprived of food for 20 h a day. Afterwards, for two hours (anticipatory period), in the presence of a visual stimulus, they were provided with a diet of 50 g of pellets and 50 g of Quaker Quadritos® (oatmeal squares) with 200 ml of water. At the end of this period, the food and water were removed to measure intake. Then, for another two hours (stress stage) the rats were given the same diet, but this time in the presence of the stress stimulus. At the end of this period, the stress stimulus was removed, and the food and water intake was quantified. Intake behavior was evaluated by determining the number of calories consumed per each gram contributed by the diet.
Diet
Pellet: 5% fiber and 20% protein (3.375 cal/g).
Quaker Quadritos®: 4.5% fat, 11% protein and 70% carbohydrate (3.640 cal/g).
Anticipatory stage
For a period of two hours a day for six consecutive days, a visual stimulus (red light) was applied to each rat as previously described prior to intermittent chronic stress.
Intermittent chronic stress stage
For a period of 2 hours a day after the anticipatory stage and for 6 consecutive days, a stress stimulus (tail pinch) was applied to each rat that consisted of placing a metallic clamp approximately 2 cm distal to the base of the tail [35].
Morphometric and stereological analysis
On post-natal day 116, the rats were sacrificed by cervical dislocation to extract the spleen. The maximum value of length, width and thickness was determined, as well as the mass of each organ and the volume quantified using the Scherle’s method [36]. For the stereological study, five pieces were obtained from each spleen following the rules of the Orientator [37]. Then, the samples were fixed in 10% buffered formalin for 24 h, dehydrated and fixed in paraffin (Histosec, Merck). Once the blocks were made, 4-µm-thick sections were taken using a Microm HM 325 microtome. From each block, five histological sections were made separated from one another by 200 µm to be then stained with hematoxylin and eosin (H-E). Five fields were observed for each section; 125 in total. The slides were analyzed in a Motic® SMZ-171 stereo zoom microscope with a magnification of 30X and photographed with Moticam® 2300 camera. The images were projected onto a View Sonic® flat screen monitor. The multipurpose M42 test was used to make the stereological determinations. The stereological parameters evaluated were: 1) volume density of the red pulp (VvRP), white pulp (VvWP), marginal zone (VvMZ), splenic lymph nodules (VvSL), PALS (VvPALS) and germinal center (VvGC); 2) areal number density of the splenic lymph nodule (Na); 3) surface density of the splenic lymph nodule (Sv); 4) number density of the splenic lymph nodule (Nv) and 5) average diameter of the splenic lymph nodule (D). The number density of splenic lymph nodules per 1 mm3 of tissue was obtained using the following equation [38]:
Nv = 1/β × K × √(Na3/VvSL)
where Na = number of splenic lymph nodules per mm2 of spleen sections; VvSL = volume density of splenic lymph nodules; ß = 0.87 (splenic lymph nodule form factor); K = 1.06 (size factor of the reduction of splenic lymph nodules). The average diameter of splenic lymph nodules (D) was calculated using the equation based on the assumption that the splenic lymph nodules are spherical [38]:
D = 2 × 2√(3/4π × VvSL/Nv)
The total number of splenic lymph nodules (Nt) was calculated by applying the following equation [38]:
Nt = W/s × f3 × Nv
where W = mass of the spleen; s = specific gravity of the spleen tissue (= 1.27); f3 = factor for tissue contraction in cubic dimensions (= 0.59).
The mass (mg) of each compartment of spleen tissue was also determined; these tissues were calculated by multiplying the corresponding Vv by the spleen mass.
Statistical analysis
The statistical analysis took place with IBM SPSS Statistic 21© software, and the assumptions were verified with the one-sample Kolmogorov-Smirnov test (data normality test) and Levene’s test (homoscedasticity analysis). For the analysis of the differences between groups, a one-way analysis of variance (ANOVA) and Scheffe’s post hoc test were used. The P values were considered significant when they were less than 0.05 (*) and very significant at less than 0.025 (**).
Results
The compartments of the spleen, red pulp and white pulp with their subcompartments appeared well-defined and easily observed in the control groups (C1 and C2) and the experimental groups (E1, E2 and E3) (Figure 1). However, both C2 and the experimental groups presented reactive congestion in the red pulp and the splenic lymph nodules were hyperplastic, with larger germinal centers; these changes were more evident in the experimental groups (Figure 2).
Figure 1.
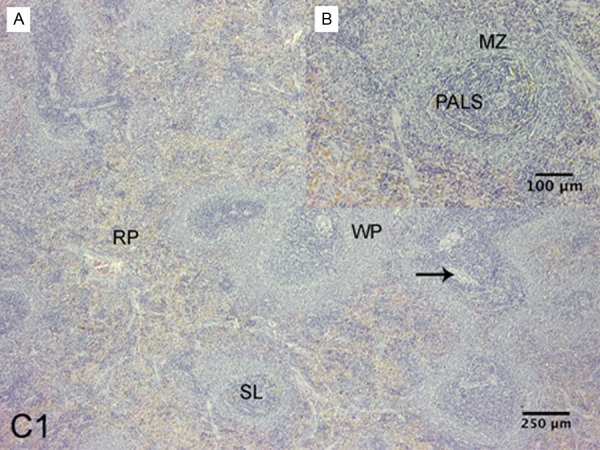
Spleen of Sprague-Dawley rat. C1 group. A: Shown the red pulp (RP) and white pulp (WP) with subcompartments: splenic lymph nodules (SL) and lymphonodular artery (arrow), B: Marginal zone (MZ), periarterial lymphatic sheath (PALS), (H-E).
Figure 2.
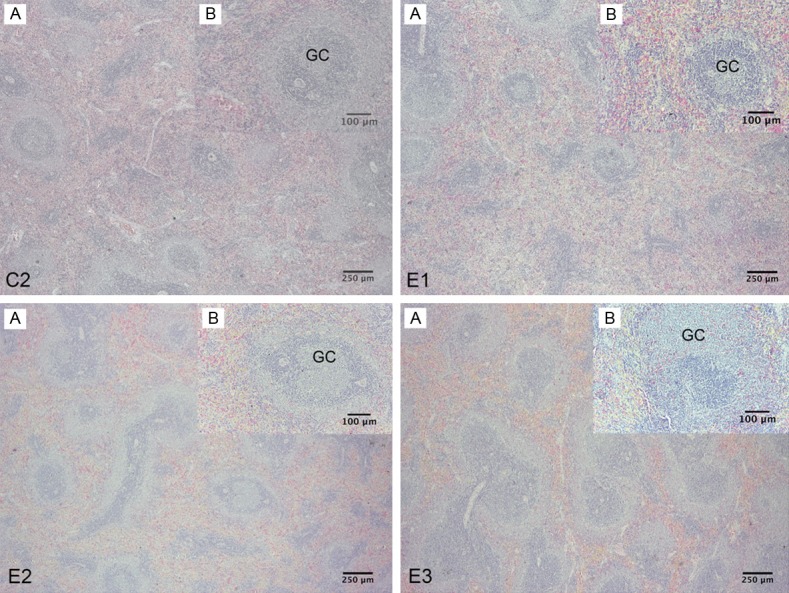
Spleen of Sprague-Dawley rat. C2 Group. A: Shown the proportion of the compartments of the spleen, with congestive red pulp, B: Splenic lymph nodules with germinal center (GC). E1 Group. A: Shown the proportion of the compartments of the spleen, with congestive red pulp and greater number of splenic lymph nodules with germinal centers, B: Splenic lymph nodules with germinal center. E2 group. A: Shown congestive red pulp and increased the proportion of white pulp and subcompartments, B: Splenic lymph nodules with larger germinal center. E3 Group. A: Shown congestive red pulp and increased the proportion of white pulp and subcompartments with closer together lymph nodules, B: Splenic lymph nodules hyperplastic with larger germinal center (H-E).
Morphometry of the spleen
Of the morphometric parameters assessed, only the thickness presented statistically significant intergroup differences (P = 0.037). Scheffé’s post hoc analysis revealed that this difference was produced between E1 and E2 (P = 0.039). The morphometric measurements of average length, width, thickness and volume of the spleen in the different groups are presented in Table 1.
Table 1.
Morphometric analysis of the spleen in female Sprague-Dawley rats subjected to early, late and early-late adverse experience and later intermittent chronic stress
| Variable | Mean SD | P | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| C1 | C2 | E1 | E2 | E3 | ||||
| Length (mm) | 33.45±2.16 | 33.60±1.41 | 34.92±0.71 | 32.50±0.90 | 33.46±0.52 | 0.098 | ||
| Width (mm) | 8.87±0.35 | 8.87±0.57 | 8.96±0.37 | 8.80±0.33 | 9.10±0.57 | 0.855 | ||
| Thickness (mm) | 5.08±0.13 | 5.03±0.41 | 4.74±0.45 | 5.41±0.12 | 5.01±0.24 | 0.037 | ||
| Volume (mm3) | 0.53±0.05 | 0.50±0.06 | 0.50±0.024 | 0.51±0.06 | 0.52±0.05 | 0.832 | ||
Stereology of the spleen
The ANOVA for the average volume density of the spleen tissue compartments showed that there was at least one group that differed from another (P = 0.000). Scheffé’s post hoc test for the analysis of multiple comparisons of the means of the groups revealed significant differences between the control and experimental groups (Figure 3).
Figure 3.
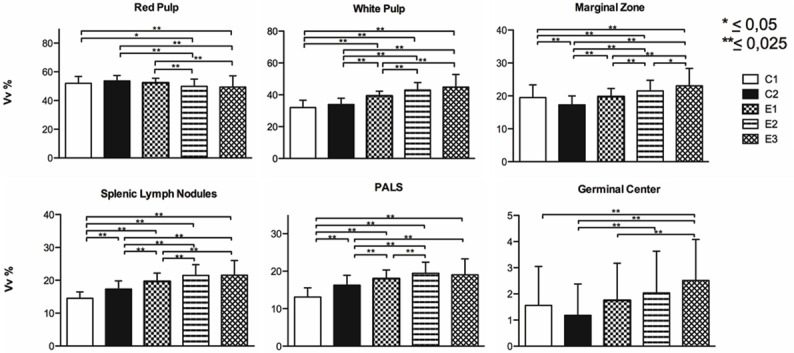
Volume density of rat spleen. Average volume density of the spleen tissue compartments of female Sprague-Dawley rats previously subjected to adverse experiences: early (E1), late (E2) and early-late (E3) and subsequent intermittent chronic stress in adulthood.
With respect to the stereological parameters of the splenic lymph nodules, the ANOVA showed at least one group that differed from another (P<0.025). Scheffé’s post hoc test again revealed significant differences between the control and experimental groups (Figure 4). The average total number of splenic lymph nodules for different groups was C1 = 144.9 (SD = 35.28); C2 = 131.52 (SD = 35.48; E1 = 139.09 (SD = 35.47); E2 = 149.82 (SD = 32.65); E3 = 144.69 (SD =40.90). The only significant differences were presented between C2 and E2 (P = 0.003).
Figure 4.
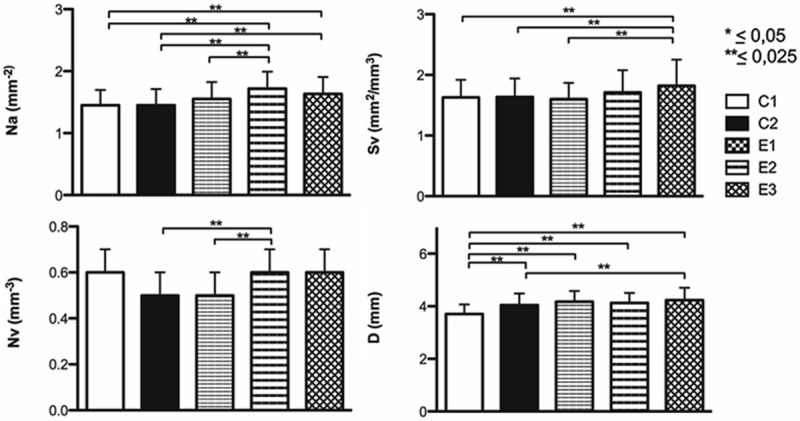
Stereological characteristics of the splenic lymph nodules in rats. Stereological parameters of splenic lymph nodules of female Sprague-Dawley rats previously subjected to adverse experiences: early (E1), late (E2) and early-late (E3) and subsequent intermittent chronic stress in adulthood.
The ANOVA of the spleen mass, showed that although there were differences, these were not significant (P>0.05). The average mass of the spleen for the different groups was C1 = 553.40 (SD = 43.09); C2 = 543.20 (SD = 25.43); E1 = 556.00 (SD = 12.41); E2 = 539.40 (SD = 41.37); E3 = 555.20 (SD = 53.57). With respect to the mass of the white pulp and its subcompartments, the ANOVA reflected that there was at least one group that differed from another (P<0.025), no significant differences were found in the mass of the red pulp (P>0.05). Scheffé’s post hoc test showed significant differences for the average mass between the control and experimental groups in the white pulp and subcompartments (Figure 5).
Figure 5.
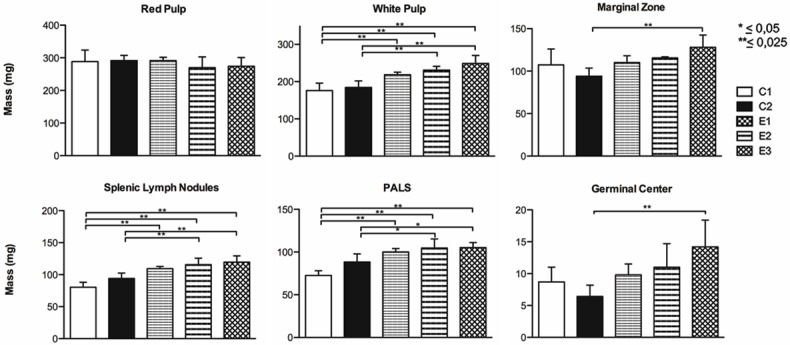
Mass of the spleen compartments. Average mass of the spleen tissue compartments of female Sprague-Dawley rats previously subjected to adverse experiences: early (E1), late (E2) and early-late (E3) and subsequent intermittent chronic stress in adulthood.
Discussion
The criteria for evaluating the defense capabilities of the IS are variable and complementary. The morphological evaluation of the IS organs is one way to understand the effects of stress on these structures. In this context, the morphometric indicators of length, width, weight and volume did not present any significant intergroup differences, which may indicate that the chronic stress applied in this experimental model, unlike neoplasias and infections [39], does not produce morphological changes associated with splenomegaly, one of the most important pathological signs of this organ. However, these observations do not discount possible changes or alterations in the morphology and function of the spleen under stress conditions. Stress determines an endocrine response, releasing catecholamines and glucocorticoids that can bring about immunosuppression or enhance the innate or acquired immune response in different compartments of the body, including the spleen, which will depend on the nature of the stressor and its duration, among other factors [2-4,7,22-25].
The microscopic analysis of the spleen showed an alteration in the proportion of the compartments of the red pulp and white pulp, with their subcompartments and hyperplasia of the splenic lymph nodules and germinal centers, compared to C1, which was not chronically stressed. Thus, these observations are consistent with the stereological analysis of the spleen, where the white pulp in experimental groups E1 (39.33%, SD = 2.98), E2 (42.95%, SD = 4.76) and E3 (44.84%, SD = 7.95) was significantly greater (P = 0.000) than C1 (31.96%, SD = 4.63); conversely, although C2 (33.92%, SD = 3.89) presented a higher percentage than C1, this was not significant (P = 0.057). By contrast, the percentage of red pulp decreased in the experimental groups E2 (49.89%, SD = 5.07) and E3 (49.41%, SD = 7.76) compared to C1 (52.04%, SD = 4.81) and C2 (53.66%, SD = 3.73).
These results suggest an increase in the immune activity of the spleen associated with morphological changes that depended on the animals being chronically stressed with only a physical stressor, a tail pinch [35], in adulthood, or being stressed previously with different early, late or early-late adverse experiences.
The increased parameters in the experimental groups compared to C2 demonstrate the importance of being exposed to stress prior to a later event. In this model, the rats were stressed at an early age by separating them early of their mother (E1), stressed later in adulthood by keeping them isolated for 30 consecutive days (E2), or a combination of the two. Thus, being re-exposed to a chronic stressor in adulthood, the experimental groups showed a more pronounced response in the percentage of white pulp in the spleen compared to C2. These results were correlated with the observations reported by Miller et al. [26], who indicated that relevant physiological chronic concentrations of natural adrenal steroids, such as corticosterone, had powerful effects on the distribution of immune cells in rat spleen. While it is true that these authors observed a decrease in the total number of leukocytes, the results also indicated that there were absolute and relative increases in some cell subsets, such as an increase in the number of neutrophils and a higher percentage of K lymphocytes, compared to the control group, along with a higher percentage of T-helper cells, after the chronic administration of type II adrenal steroid receptor agonists.
Studies suggest that genetics and environmental factors, i.e. nature and nurture, fulfill an important role in establishing individual differences in the psychophysiological response to stress and in the effects that the response can have on a body or an individual [40]. In this context, our results differ from those reported by Hernandez et al. [31] regarding the changes produced in the proportions of the red pulp vs. white pulp. They observed that Wistar-Kyoto rats experienced an increase in the percentage of red pulp and a decrease in the white pulp compared to the control group after subjecting the animals to chronic stress by immobilization, administration of hydrocortisone or both (immobilization + hydrocortisone). These discrepancies may be due, on the one hand, to the Wistar-Kyoto rat being a strain in which the HPA axis is overactive and, on the other, to applied chronic stress lasting days to weeks, which resulted in a reduction in plasma corticosterone levels.
With respect to the volume density of the marginal zone, splenic lymph nodules, PALS and germinal center of the white pulp, the experimental groups presented a higher value than C1, which revealed a common pattern in the morphological changes of these compartments after applying the experimental model, characterized by greater volume density in those more vulnerable groups. By contrast, C2 followed a different pattern; the marginal zone (17.30% SD = 2.69) and germinal center (1.18%, SD = 1.20) presented a lower volume density than C1 (19.49%, SD = 3.85 and 1.56%, SD = 1.49, respectively), with significant differences for the marginal zone (P = 0.000). The splenic lymph nodules and PALS in C2 showed a higher volume density than C1; however, percentage-wise it did not exceed the experimental groups. These results illustrate how physical and psychosocial stress produce different effects on the immune function [10]. However, in the literature few studies give account of the effects that chronic physical and psychosocial stress has on the spleen; rather they refer to the transport, number and function of cell-mediated immunity in peripheral blood [11-13], which makes it difficult to better understand the spleen’s role in the immune response when the individual is under different situations of chronic stress. In this respect, studies performed on the spleen of DBA/2 mice showed that stress due to social conflict (intruder/resident) increased plasma corticosterone levels, decreased the proportion of T CD4+/CD8+ lymphocytes, significantly increased the percentage of CD3+ lymphocytes and reduced the ability of splenocytes to proliferate in vitro in the presence of lipopolysaccharides [14]. Nevertheless, other models of chronic stress due to social conflict (fights among peers) showed that the spleen had a greater number of mononuclear cells, a significant increase in monocytes and neutrophils, and a decrease in the percentage of CD4 lymphocytes. In addition, this type of stress increased the proliferation and reduced the sensitivity of the splenocytes stimulated with lipopolysaccharides to the anti-proliferative effects of corticosterone, which suggests fights among peers induced a state of resistance to the glucocorticoid in splenocytes [41]. Additionally, the repeated social defeat in mice during a period of 2, 4 or 6 consecutive days was associated with cell mobilization and the increase in myelopoiesis in the bone marrow, an increase accompanied by an accumulation of neutrophils and monocytes in circulation and in the spleen. The substantial depletion of B cells in the bone marrow and blood was associated with an increase in splenic B cells, which indicates a reorientation of this type of cells in the spleen. By contrast, T cells were remarkably reduced in these immune compartments [42].
Our results are consistent with those reported by Avitsur et al. [41] and Engler et al. [42]. Along with an increase in white pulp in the experimental groups, the splenic lymph nodules showed greater areal number density, surface density and diameter compared to C1; E3 always showed significant differences (P = 0.000) in these parameters. C2 showed significantly higher values than C1 only in the diameter of the splenic lymph nodules (P = 0.000). In addition, the mass of the white pulp, splenic lymph nodules and PALS was also greater in the experimental groups compared to C1; however, C2 presented a non-significant increase in mass compared to C1 in these compartments. Furthermore, the mass of the marginal zone and germinal center in the experimental groups and C2 did not present significant differences compared to C1, which suggests to us that the chronic stress applied in this model could specifically alter certain cell populations in each subcompartment of the spleen.
Apparently, these results are contradictory with respect to the immunosuppressive effects that chronic stress has on the immune response, innate as well as adaptive [7,21-25]. However, other factors that can influence the direction (improvement vs. suppression) of the effects of stress or stress hormones and the nature of the immune response affected (immunoprotective, immunoregulatory/inhibitory or immunopathological) must be considered [4]. In the immune response of the spleen, it is important to recognize the effects of stress on the leukocyte distribution in the body [2,3,16,17,19], the differential effects of the physiological vs. pharmacological concentrations of glucocorticoids [26], the differential effects of endogenous glucocorticoids (cortisol and corticosterone) as opposed to synthetic (dexamethasone, RU28362 and FK506) [27,32,43], whether the studies are conducted in vivo or in vitro [44], types of adrenal receptors involved (type I or II) [16,26], among others.
Animal studies have shown greater immune activity after prolonged situations of stress. In this vein, an increase in the proliferative response of mononuclear cells of the spleen after a slight chronic stress [45], improvement in mitogenic activity of splenic immunoglobulin IgM plaque-forming cells when chronic stress was applied before and after immunization with sheep red blood cells [20] and the positive correlation between the plasma corticosterone levels after immunization and keyhole limpet hemocyanin, and the stimulation of splenic IgM and IgG2a in vivo [27] have been found.
In an agreement with what was proposed before and with the analysis of the results of this study, it is possible to conclude that chronic stress can alter the normal morphology of the spleen and subsequently its immune function. It is important to consider that the morphological changes observed depended on the animal’s history of stress, i.e., whether or not they had been previously stressed, after a second exposure to stress in adulthood. The morphological changes observed are characterized by alteration in the proportions of the red pulp land white pulp and their subcompartments, with a positive balance of the white pulp that could be associated with an increase in the immune activity of the spleen. Finally, the adaptation related to a regimen of repeated stress should be considered. Therefore, the adaptation of chronic stress applied in this model seems dependent upon the spleen compartments, since it specifically alters certain cell populations.
Disclosure of conflict of interest
None.
References
- 1.Goldstein D, Kopin I. Evolution of concepts of stress. Stress. 2007;10:109–120. doi: 10.1080/10253890701288935. [DOI] [PubMed] [Google Scholar]
- 2.Dhabhar FS. Stress-induced augmentation of immune function-the role of stress hormones, leukocyte trafficking, and cytokines. Brain Behav Immun. 2002;16:785–798. doi: 10.1016/s0889-1591(02)00036-3. [DOI] [PubMed] [Google Scholar]
- 3.Dhabhar FS. Stress leukocyte trafficking and the augmentation of skin immune function. Ann N Y Acad Sci. 2003;992:205–217. doi: 10.1111/j.1749-6632.2003.tb03151.x. [DOI] [PubMed] [Google Scholar]
- 4.Dhabhar FS. Ahassle adaymaykeep thepathogens away: the fight-or-flight stress response and the augmentation of immune function. Integr Comp Biol. 2009;49:215–236. doi: 10.1093/icb/icp045. [DOI] [PubMed] [Google Scholar]
- 5.Godbout JP, Glaser R. Stress-induced immune dysregulation: implications for wound healing, infectious disease and cancer. J Neuroimmune Pharmacol. 2006;1:421–427. doi: 10.1007/s11481-006-9036-0. [DOI] [PubMed] [Google Scholar]
- 6.Sánchez M, González RM, Marsán V, Macías C. Asociación entre el estrés y las enfermedades infecciosas, autoinmunes, neoplásicas y cardiovasculares. Rev Cubana Hematol Inmunol Med Transf. 2006:22. [Google Scholar]
- 7.Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- 8.Dhabhar FS. Effects of stress on immune function: the good, the bad, and the beautiful. Immunol Res. 2014;58:193–210. doi: 10.1007/s12026-014-8517-0. [DOI] [PubMed] [Google Scholar]
- 9.Dhabhar FS, McEwen BS. Changes in blood leukocyte distribution: interactions between catecholamine and glucocorticoid hormones. Neuroimmunomodulation. 1999;6:213. [Google Scholar]
- 10.Nadal R, Armario A. Mecanismos de susceptibilidad al estrés. Hipertens Riesgo Vasc. 2010;27:117–124. [Google Scholar]
- 11.Landmann LM, Müller FB, Perini CH, Wesp M, Erne P, Bühler FR. Changes of immunoregulatory cells induced by psychological and physical stress: relationship to plasma catecholamines. Clin Exp Immunol. 1984;58:127–135. [PMC free article] [PubMed] [Google Scholar]
- 12.Schedlowski M, Falk A, Rohne A, Wagner TO, Jacobs R, Tewes U, Schmidt RE. Catecholamines induce alterations of distribution and activity of human natural killer (NK) cells. J Clin Immunol. 1993;13:344–351. doi: 10.1007/BF00920243. [DOI] [PubMed] [Google Scholar]
- 13.Engler H, Dawils L, Hoves S, Kurth S, Stevenson JR, Schauenstein K, Stefanski V. Effect of social stress on blood leukocyte distribution: the role of alpha- and beta-adrenergic mechanisms. J Neuroimmunol. 2004;156:153–162. doi: 10.1016/j.jneuroim.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Dréau D, Sonnenfeld G, Fowler N, Morton DS, Lyte M. Effects of social conflict on immune responses and E. coli growth within closed chambers in mice. Physiol Behav. 1999;67:133–140. doi: 10.1016/s0031-9384(99)00072-4. [DOI] [PubMed] [Google Scholar]
- 15.Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Effects of stress on immune cell distribution: dynamics and hormonal mechanisms. J Immunol. 1995;154:5511–5527. [PubMed] [Google Scholar]
- 16.Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Stress-induced changes in blood leukocyte distribution: role of adrenal steroid hormones. J Immunol. 1996;157:1638–1644. [PubMed] [Google Scholar]
- 17.Dhabhar FS, McEwen BS. Stress-induced enhancement of antigen-specific cell-mediated immunity. J Immunol. 1996;156:2608–2615. [PubMed] [Google Scholar]
- 18.Benschop RJ, Rodriguez-Feuerhahn M, Schedlowski M. Catecholamine-induced leukocytosis: early observations, current research, and future directions. Brain Behav Immun. 1996;10:77–91. doi: 10.1006/brbi.1996.0009. [DOI] [PubMed] [Google Scholar]
- 19.Bowers SL, Bilbo SD, Dhabhar FS, Nelson RJ. Stressor-specific alterations in corticosterone and immune responses in mice. Brain Behav Immun. 2008;22:105–113. doi: 10.1016/j.bbi.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zalcman S, Anisman H. Acute and chronic stressor effects on the antibody response to sheep red blood cells. Pharmacol Biochem Behav. 1993;46:445–452. doi: 10.1016/0091-3057(93)90377-6. [DOI] [PubMed] [Google Scholar]
- 21.Chrousos GP, Kino T. Glucocorticoid action networks and complex psychiatric and/or somatic disorders. Stress. 2007;10:213–219. doi: 10.1080/10253890701292119. [DOI] [PubMed] [Google Scholar]
- 22.Dhabhar FS. Psychological stress and immunoprotection versus immunopathology in the skin. Clin Dermatol. 2013;31:18–30. doi: 10.1016/j.clindermatol.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Marshall GD, Agarwal SK. Stress, immune regulation, and immunity: applications for asthma. Allergy Asthma Proc. 2000;21:241–246. doi: 10.2500/108854100778248917. [DOI] [PubMed] [Google Scholar]
- 24.Saul AN, Oberyszyn TM, Daugherty C, Kusewitt D, Jones S, Jewell S, Malarkey WB, Lehman A, Lemeshow S, Dhabhar FS. Chronic stress and susceptibility to skin cancer. J Natl Cancer Inst. 2005;97:1760–1767. doi: 10.1093/jnci/dji401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glaser R, MacCallum RC, Laskowski BF, Malarkey WB, Sheridan JF, Kiecolt-Glaser JK. Evidence for a shift in the Th-1 to Th-2 cytokine response associated with chronic stress and aging. J Gerontol A Biol Sci Med Sci. 2001;56:M477–82. doi: 10.1093/gerona/56.8.m477. [DOI] [PubMed] [Google Scholar]
- 26.Miller AH, Spencer RL, Hasset J, Kim C, Rhee R, Cira D, Dhabhar FS, McEwen BS, Stein M. Effects of selective type I and type II adrenal steroid receptor agonists on immune cell distribution. Endocrinology. 1994;135:1934–1944. doi: 10.1210/endo.135.5.7956914. [DOI] [PubMed] [Google Scholar]
- 27.Fleshner M, Deak T, Nguyen KT, Watkins LR, Maier SF. Endogenous glucocorticoids play a positive regulatory role in the anti-keyhole limpet hemocyanin in vivo antibody response. J Immunol. 2001;166:3813–3819. doi: 10.4049/jimmunol.166.6.3813. [DOI] [PubMed] [Google Scholar]
- 28.Milićević NM, Trbojević-Stanković JB, Drachenberg CB, Milićević Z. Stereologic analysis of tissue compartments of gunshot-injured and blunt-injured spleen. Pathol Oncol Res. 2010;16:69–73. doi: 10.1007/s12253-009-9189-2. [DOI] [PubMed] [Google Scholar]
- 29.Furrianca MC, Vásquez B, del Sol M. Comparative stereology between the spleen of the guinea pig (Cavia porcellus) and the rat (Rattus novergicus, Sprague Dawley) Int J Morphol. 2008;26:529–532. [Google Scholar]
- 30.Milićević Z, Slepcević V, Nikolić D, Zivanović V, Milićević NM. Effects of cis-diamminedichloroplatinum II (cisplatin) on the splenic tissue of rats: a histoquantitative study. Exp Mol Pathol. 1994;61:77–81. doi: 10.1006/exmp.1994.1027. [DOI] [PubMed] [Google Scholar]
- 31.Hernandez ME, Martinez-Mota L, Salinas C, Marquez-Velasco R, Hernandez-Chan NG, Morales-Montor J, Pérez-Tapia M, Streber ML, Granados-Camacho I, Becerril E, Javier BH, Pavón L. Chronic stress induces structural alterations in splenic lymphoid tissue that are associated with changes in corticosterone levels in wistar-kyoto rats. Biomed Res Int. 2013;2013:868742. doi: 10.1155/2013/868742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milićević NM, Milićević Z. Stereological study of splenic tissue compartments in FK506-treated rats. Histol Histopathol. 1997;12:995–1001. [PubMed] [Google Scholar]
- 33.Yan W. Impact of prenatal stress and adulthood stress on immune system: a review. Biomedical Research. 2012;23:315–320. [Google Scholar]
- 34.Guide for the Care and Use of Laboratory animals. 8th edition. Washington (DC): The National Academies Press; 2011. Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research (ILAR), Division on Earth and Life Studies (DELS), National Research Council. [Google Scholar]
- 35.Katz RJ, Roth K. Tail pinch induced stress-arousal facilitates brain stimulation reward. Physiol Behav. 1979;22:193–194. doi: 10.1016/0031-9384(79)90422-0. [DOI] [PubMed] [Google Scholar]
- 36.Scherle W. A simple method for volumetry of organs in quantitative stereology. Mikroskopie. 1970;26:57–63. [PubMed] [Google Scholar]
- 37.Mattfeldt T, Mall G, Gharehbaghi H, Möller P. Estimation of surface area and length with the orientator. J Microsc. 1990;159:301–317. doi: 10.1111/j.1365-2818.1990.tb03036.x. [DOI] [PubMed] [Google Scholar]
- 38.Weibel ER. Practical methods for biological morphometry. Vol 1. Academic. New York: 1979. [Google Scholar]
- 39.González G, Escobar A. Estrés y sistema inmune. Rev Mex Neuroci. 2006;7:30–38. [Google Scholar]
- 40.Dhabhar FS, McEwen BS, Spencer RL. Adaptation to prolonged or repeated stress-comparison between rat strains showing intrinsic differences in reactivity to acute stress. Neuroendocrinology. 1997;65:360–368. doi: 10.1159/000127196. [DOI] [PubMed] [Google Scholar]
- 41.Avitsur R, Stark JL, Dhabhar FS, Sheridan JF. Social stress alters splenocyte phenotype and function. J Neuroimmunol. 2002;132:66–71. doi: 10.1016/s0165-5728(02)00310-7. [DOI] [PubMed] [Google Scholar]
- 42.Engler H, Bailey MT, Engler A, Sheridan JF. Effects of repeated social stress on leukocyte distribution in bone marrow, peripheral blood and spleen. J Neuroimmunol. 2004;148:106–115. doi: 10.1016/j.jneuroim.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 43.Dhabhar FS, McEwen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc Natl Acad Sci U S A. 1999;96:1059–1064. doi: 10.1073/pnas.96.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sterzer P, Wiegers GJ, Reul JM. Long-term in vivo administration of glucocorticoid hormones attenuates their capacity to accelerate in vitro proliferation of rat splenic T cells. Endocrinology. 2004;145:3630–3638. doi: 10.1210/en.2003-1578. [DOI] [PubMed] [Google Scholar]
- 45.Azpiroz A, Fano E, Garmendia L, Arregi A, Cacho R, Beitia G, Brain PF. Effects of chronic mild stress (CMS) and imipramine administration, on spleen mononuclear cell proliferative response, serum corticosterone level and brain norepinephrine content in male mice. Psychoneuroendocrinology. 1999;24:345–361. doi: 10.1016/s0306-4530(98)00084-5. [DOI] [PubMed] [Google Scholar]


