Abstract
Introduction: The pathological diagnosis of papillary thyroid carcinoma (PTC) is generally easy on routine sections stained with hematoxylin and eosin (H&E). However, the differentiation of the follicular variant of PTC (FVPTC) from other suspected follicular-patterned lesions of the thyroid is highly difficult. Among these, the lesions for which FVPTC cannot be excluded are classified as well-differentiated tumors of uncertain malignant potential (WDT-UMP). The most common immunohistochemical (IHC) markers used in the differential diagnosis include HBME-1, galectin-3, and CK19. However, none of these markers provide a 100% differential diagnosis. Objective: The present study compared the diagnostic value of CD56 and E-cadherin for the differentiation of FVPTC from the other benign follicular-patterned lesions, with HBME-1, galectin-3, and CK19. Using these markers, the controversial cases within the WDT-UMP group were reclassified. Additionally, the relationship between the reductions in E-cadherin expression with poor prognostic factors was investigated. Materials and methods: The IHC expressions of CD56, E-cadherin, HBME-1, galectin-3, and CK19 were evaluated in 181 thyroid lesions, including 101 PTCs (45 classical variant PTCs and 56 FVPTCs), 20 WDT-UMPs, 20 follicular adenomas (FAs), 20 hyperplastic nodules (HN), and 20 hyperplastic foci of lymphocytic thyroiditis. The results were statistically compared via SPSS. Results: The expressions of all of the markers were statistically significantly different in PTC and follicular-patterned lesions (P<0.05). It was found that the only marker with both sensitivity and specificity above 90% was CD56 negativity (sensitivity 91.1%, specificity 91.7%). The most sensitive and also the most specific double panel was CD56 negativity and galectin-3 positivity (sensitivity 96%, specificity 85%), and the most sensitive and specific triple panel was CD56 negativity, HBME-1 positivity, and galectin-3 positivity (97% and 70%, respectively).
Keywords: CD56, E-cadherin, HBME-1, galectin-3, thyroid papillary cancer, follicular-patterned lesions
Introduction
Papillary thyroid carcinoma (PTC) is the most common malignant tumor of the thyroid gland and endocrine organs. Thyroid cancer accounts for 1%-2% of all malignant tumors [1,2] and is more commonly seen in females. It can occur at every age, but is more common in young and middle-aged adults; the median age at diagnosis is 40 years. It is caused by environmental, genetic, and hormonal factors. In children especially, the etiology is associated with radiation to the neck area [3-5].
Although its incidence is increasing all over the world, the mortality rate of PTC is decreasing [5]. This increase in the incidence is not an actual increase in the number of thyroid cancers; rather, it is due to the increased rate of detecting hidden thyroid cancers through detailed evaluation of non-palpable thyroid nodules with advanced assessments [2,6].
PTC may be easily diagnosed due to the highly characteristic nuclear features accompanying its structural features (transparency, overlapping, grooves, and pseudoinclusions) [3-5]. It has a large number of morphological variants. The most common of these is the follicular variant of PTC (FVPTC), and its differentiation from the other benign follicular-patterned lesions of the thyroid is usually very difficult. This causes diagnostic differences among pathologists [2,6-8].
The most common follicular-patterned lesions with similarities to FVPTC are follicular adenomas (FAs), hyperplastic nodules (HNs), and dysplastic foci of lymphocytic thyroiditis (LT) [3,4]. Among these, the lesions from which FVPTC cannot be excluded are classified as well-differentiated tumors of uncertain malignant potential (WDT-UMP) [3,4,7]. This group causes difficulty for clinicians in the treatment and follow-up of patients, and the uncertainty of the differential diagnosis may lead to anxiety in some patients.
The morphological diagnosis on routine section stained with hematoxylin and eosin (H&E) is the gold standard for the diagnosis of thyroid nodules [3-5,7,9]. Some facilitative immunohistochemical (IHC) markers are used in the differential diagnosis. The most commonly used among these include HBME-1, galectin-3, and CK19 [3-5]. However, none of these markers provide a 100% differential diagnosis [7].
The present study aimed at IHC staining of CD56 and E-cadherin for the differentiation of these lesions that are difficult to differentiate from other follicular-patterned lesions, in particular. These two markers were compared, based on the knowledge in the literature, with three previously used markers: HBME-1, galectin-3, and CK19. WDT-UMPs that were diagnosed within the gray zone and benign follicular lesions showing dysplastic modifications were reclassified and an attempt was made to identify the cases requiring treatment.
CD56 is a neural cell adhesion molecule found in normal thyroid follicular epithelial cells. It regulates cell motility and affects the migration capacity of tumor cells [2,8]. E-cadherin is a transmembrane glycoprotein found primarily in the epithelial cells. It has a role in cell polarity and tissue architecture formation. Its damage is associated with the development and progression of human cancers [1].
The Hector Battifora mesothelial cell (HBME-1) is a marker demonstrated in normal mesothelial cells by Battifora et al. [8].
Galectin-3 is a member of the galactoside-binding animal lectins. It regulates the cell-cell and cell-matrix relationships, cell growth, neoplastic transformation, and apoptosis [2]. CK19 is an intermediate filament protein and the least-known keratin [2].
Materials and methods
The study included a total of 181 cases, with 56 FVPTCs, 45 classical variant PTCs (CVPTCs), 20 WDT-UMPs, 20 FAs, 20 HNs, and 20 cases of LT (involving areas with suspected nuclear features), diagnosed between 2007 and 2012 at Istanbul Medeniyet University Goztepe Training and Research Hospital, Department of Pathology. Each patient’s age and gender, as well as the tumor diameter, capsule invasion status, presence or absence of lymph node and organ metastases, number of foci, and thyroiditis status on the pathology reports were included in the study as data.
The pathology reports of all cases and the HBME-1, galectin-3, and CK19 reactivities from the archival samples were noted. For the cases where these antibodies were not studied, new IHC studies were done on the paraffin blocks prepared via the tissue microarray method to remove the missing parts. CD56 and E-cadherin were applied to all samples included in the study. Sections with a thickness of 4-5 microns from the formalin-fixed paraffin-embedded blocks of the thyroidectomy materials were used.
Immunohistochemical method
IHC staining was performed in the Leica BOND-MAX automated staining device. In all cases, IHC procedures were performed for CD56, E-cadherin, HBME-1, galectin-3, and CK19. For the formalin-fixed paraffin-embedded tissue sections (thickness 4-5 um), as well as the suitable positive and negative controls, the standard technique (avidin-biotin-peroxidase) was applied. The primary antibodies CD56 (mouse, clone 123C3.D5, dilution 1:50-1:100, Thermo Scientific, Fremont, CA, USA), E-cadherin (mouse monoclonal, clone GM016, dilution 1:100, Genemed Biotechnologies, Inc., South San Francisco, CA, USA), HBME-1 (mouse, clone HBME-1, dilution 1:25-1:50, Thermo Scientific, Fremont, CA, USA), CK19 (mouse, clone RCK108, ready-to-use, BioGenex, San Ramon, CA, USA), and galectin-3 (mouse monoclonal, clone 9C4, dilution 1:100-1:200, Novocastra, Newcastle, UK) were used.
Evaluation
For the light microscope semi-quantitative evaluation, the scoring was made semi-quantitatively as positive and negative based on the cytoplasmic and membranous staining for CD56. Scoring was 0 (<5%, none), 1 (5%-25%, mild), 2 (25%-50%, moderate), or 3 (>50%, severe), based on the rate and intensity of cytoplasmic membranous staining for E-cadherin. The scores 0, 1, and 2 were considered negative, and 3 was positive. For CD56, focal cytoplasmic and membranous reactivity of up to 10% was considered negative. Normal thyroid tissue was used as a positive control for CD56, and breast invasive ductal carcinoma was used for E-cadherin.
HBME-1, galectin-3, and CK19 immunoreactivity scoring was based on cytoplasmic and/or membranous staining. As cutoff values, ≤10% was negatively semi-quantitative and >10% was positively semi-quantitative. The positive controls were selected from PTCs for HBME-1 and galectin-3, and from colon mucosa for CK19.
Statistical evaluation
Statistical analysis was performed using SPSS software (version 10.0, SPSS Inc., Chicago, IL, USA). Chi-square or Fisher’s exact tests were applied in order to compare frequencies between the groups. The sensitivity, specificity, and accuracy of the markers and their combination in the diagnosis of PTCs and benign follicular-patterned lesions were compared. Probability values of <0.05 were considered statistically significant.
Results
The evaluation of 101 PTC cases indicated that 45 were CVPTC and 56 were FVPTC. The mean age was 45 [11-82] years, 86% were females and 14% were males, 25% had capsule invasion, 13% had lymph node metastasis, 43% had multiple foci, and 34% had concomitant thyroiditis. Of 60 benign lesions, 20 were FAs, 20 were HNs, and 20 were LT (showing suspected nuclear features). Additionally, there were 20 lesions from the WDT-UMP group.
1. CD56 negativity (91.1%) and E-cadherin loss (rate: 86.1%, density: 92.1%) was significantly increased in PTC (CV and FV) (P<0.005), whereas CD56 positivity (91.7%) and E-cadherin positivity (rate: 63.3%, density: 61.7%) was significantly increased in follicular lesions and dysplastic nodules (P<0.005). Again in PTC, CK19, HBME-1, and galectin-3 positivity (84.2%, 71.3%, and 65.3%, respectively) was significantly increased, which was consistent with the literature (Table 1).
Table 1.
Immunohistochemical expression of markers in thyroid lesions
| Diagnosis | N | CD56 negativity % | E-cad loss range-density % | HBME-1 positivity % | Galectin 3 positivity % | CK19 positivity % |
|---|---|---|---|---|---|---|
| Papillary thyroid carcinoma (CVPTC+FVPTC) | 101 (45+56) | 91.1* | 86.1*-92.1* | 71.3* | 65.3* | 84.2* |
| BL (FA, HN, LT with suspicious nuclear features) | 60 | 8.3 | 36.7-38.3 | 21.7 | 46.7 | 63.3 |
| WDT-UMP | 20 | 65 | 60-65 | 50 | 20 | 70 |
P<0.05, PTC vs. BL. PTC, papillary thyroid carcinoma;
CVPTC, classical variant papillary thyroid carcinoma; FVPTC, follicular variant papillary thyroid carcinoma; BL, benign lesion; FA, follicular adenoma; HN, hyperplastic nodule; LT, lymphocytic thyroiditis; WDT-UMP, well-differentiated tumor of uncertain malign potential; values are presented as percentages.
2. The markers with the highest sensitivity were CD56 negativity (91.1%) and CK19 positivity (84.2%) in PTC. The markers with the highest specificity were galectin-3 positivity (93.3%) and CD56 negativity (91.7%). In other words, the most important marker with the highest sensitivity (91.1%) and also the highest specificity (91.7%) was found to be CD56 (Table 2).
Table 2.
Sensitivity, specificity, positive and negative predictable values, and diagnostic accuracy of markers in differentiating PTC from other suspected follicular-patterned lesions
| Marker | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|
| CD56 | 91.1 | 91.7 | 85.9 | 94.8 | 91.30 |
| E-cadherin (D) | 61.7 | 92.1 | 82.2 | 80.2 | 80.7 |
| E-cadherin (R) | 63.3 | 86.1 | 73.1 | 79.8 | 77.6 |
| HBME-1 | 71.3 | 78.8 | 84.7 | 61.8 | 73.9 |
| Galectin-3 | 65.3 | 93.3 | 94.2 | 61.5 | 75.8 |
| CK19 | 84.2 | 36.7 | 69.1 | 57.8 | 72.7 |
PPV, positive predictive value; NPV, negative predictive value; CK19, cytokeratin 19; values are presented as percentages.
3. When evaluated for double markers, the markers with the highest sensitivity and also the highest specificity in PTC were CD56 negativity and galectin-3 positivity (sensitivity: 96%, specificity: 85%), followed by CD56 negativity and HBME-1 positivity (sensitivity: 96%, specificity: 75%) (Table 3).
Table 3.
Sensitivity, specificity, positive and negative predictable values, and diagnostic accuracy of markers in differentiating PTC from other suspected follicular-patterned lesions using double, triple, quadruple, and five-fold markers together
| Marker | Sensitivity % | Specificity % | PPV % | NPV % | Accuracy |
|---|---|---|---|---|---|
| Double markers | |||||
| CD56 + Gal-3 | 96 | 85 | 91.5 | 92.7 | 91.9 |
| CD56 + HBME-1 | 96 | 75 | 86.6 | 91.8 | 87.6 |
| Gal-3 + HBME-1 | 85.3 | 73.3 | 84.3 | 74.6 | 80.7 |
| E-cad + HBME-1 | 94.1 | 55 | 77.9 | 84.6 | 79.5 |
| E-cad + Gal-3 | 88.1 | 58.3 | 78.1 | 74.5 | 77 |
| Triple Markers | |||||
| CD56 + E-cad + Gal-3 | 99 | 55 | 78.7 | 97.1 | 82.6 |
| CD56 + HBME-1 + Gal-3 | 97 | 70 | 84.5 | 93.3 | 87 |
| E-cad + HBME-1 + Gal-3 | 94.1 | 50 | 76 | 83.3 | 77.6 |
| Quad markers | |||||
| CD56 + E-cad + Gal-3 + HBME-1 | 99 | 50 | 76.9 | 96.8 | 80.7 |
| Five-Fold Markers | |||||
| CD56 + E-cad + Gal-3 + HBME-1 + CK19 | 100 | 16.7 | 66.9 | 100 | 68.9 |
PPV, positive predictive value; NPV, negative predictive value; Gal-3, galectin 3; E-cad, E-cadherin; CK19, cytokeratin 19; values are presented as percentage.
4. When evaluated for triple markers, the markers with the highest sensitivity and also the highest specificity in PTC were CD56 negativity, HBME-1, and galectin-3 positivity (sensitivity: 97%, specificity: 70%) (Table 3).
5. When CD56 and E-cadherin negativity and galectin-3 and HBME-1 positivity were evaluated in terms of the quadruple markers in PTC, sensitivity was 99% but specificity was only 50% (Table 3).
6. In patients with E-cadherin loss, the lymph node metastases, capsule invasions, multiple foci, or tumor diameters of >10 mm were significantly increased (P<0.05) (Table 4).
Table 4.
Immunohistochemical expression of E-cadherin in PTC with poor prognostic factors (capsular invasion, lymph node metastasis, multiple foci, tumor size >1 cm
| N | E-cad (-) | E-cad (+) | |
|---|---|---|---|
| Capsular invasion | 25 | 23 (92%) | 2 (8%) |
| LNM | 13 | 13 (100%) | 0 (0%) |
| Multiple foci | 43 | 42 (95.3%) | 1 (4.7%) |
| Tumor size >1 cm | 75 | 70 (93.3%) | 5 (6.7%) |
LNM, lymph node metastasis; E-cad, E-cadherin.
CD56 negativity was observed in 92 (91.1%) of 101 PTCs, 5 (8.3%) of 60 benign lesions (papillary carcinoma-like suspected nuclear features on HN, FA, and LT), and 13 (65%) of 20 WDT-UMPs.
E-cadherin loss (loss of staining rate and loss of density, respectively) was observed in 87 (86.1%) and 93 (92.1%) of 101 PTCs, 22 (36.7%) and 23 (38.3%) of 60 benign lesions, and 12 (60%) and 13 (65%) of 20 WDT-UMPs.
HBME-1 positivity was identified in 72 (71.3%) of 101 PTCs, 13 (21.7%) of 60 benign lesions, and 10 (50%) of 20 WDT-UMPs.
Galectin-3 positivity was identified in 66 (65.3%) of 101 PTCs, 4 (6.7%) of 60 benign lesions, and 4 (20%) of 20 WDT-UMPs.
CK19 positivity was identified in 85 (84.2%) of 101 PTCs, 38 (63.3%) of 60 benign lesions, and 15 (75%) of 20 WDT-UMPs (Table 1).
The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy rates for the markers CD56, E-cadherin, HBME-1, galectin-3, and CK19 are presented in Table 2.
The sensitivity, specificity, PPV, NPV, and accuracy rates for many double and triple combinations of the five markers, as well as the CD56, E-cadherin, HBME-1, galectin-3 quartet, and joint combinations of five markers, are presented in Table 3.
Among the PTC cases, the loss of IHC staining with E-cadherin (density or rate) was found in 23 (92%) of 25 cases with capsule invasion, 13 (100%) of 13 cases with lymph node metastasis, 42 (95.3%) of 43 cases with multiple foci, and 70 (93.3%) of 75 cases with a tumor diameter of >1 cm (Table 4).
Discussion
The diagnosis of PTC is generally easy on routine sections stained with H&E. However, the definitive differential diagnosis of some cases, in which the characteristic features of FVPTC are uncertain and there are no papillary structures from the other benign lesions, is very difficult. Such lesions are hyperplastic adenomatous nodules, especially the hyperplastic foci observed in LT, FAs with nuclear atypia, and WDT-UMPs that cannot be included in any group.
The present study aimed at facilitating this differentiation and establishing the diagnosis in order to guide the clinician more clearly, by reclassifying the controversial lesions within the WDT-UMP group in particular.
It is reported that CD56 is expressed at high levels in normal thyroid tissue and benign follicular lesions of the thyroid, such as FAs and HNs, whereas there are high rates of negativity in PTC [1,2,6-15]. Consistent with this, CD56 was found to be the most sensitive and also the most specific marker in the present study. In our study, high rates (91.7%) of diffuse, strong, positive staining were observed with CD56 in HNs, FAs, and LT, and in the normal thyroid tissues around the PTC areas, whereas there were high rates (91.1%) of CD56 negativity in PTC (CV, FV, and micropapillary) (Figure 1). The strong, diffuse CD56 staining, especially in the follicular-patterned benign lesions with suspected nuclear features, provides convenience in the differentiation of these lesions from FVPTC, which have similar morphological features.
Figure 1.
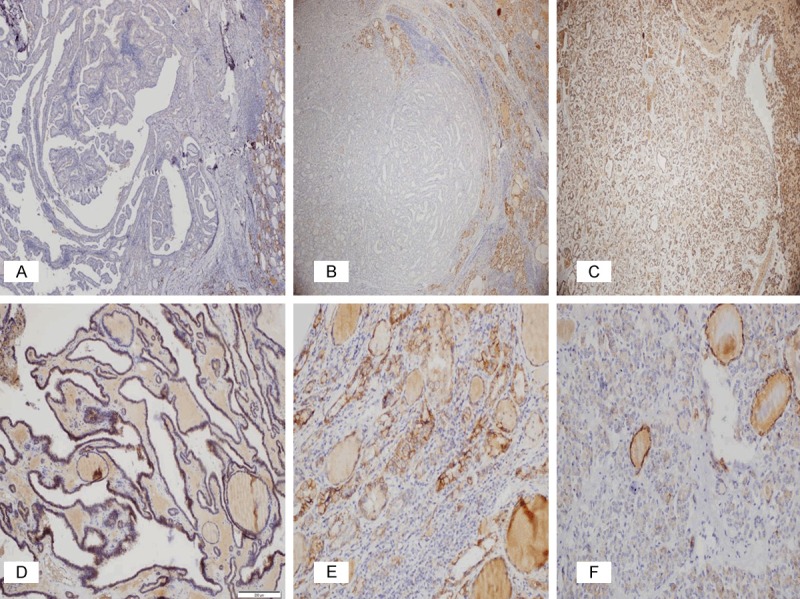
Immunohistochemical expression of CD56 in PTC (A, B), the other benign follicular-patterned lesions with suspicious nuclear features (C-E), and WDT-UMP (F). (A) Negative expression in CVPTC (x40); (B) Negative expression in FVPTC (x20); (C) Diffuse positive membranous expression in FA (x40); (D) Diffuse positive membranous expression in HN (x100); (E) Positive membranous expression in LT (x200); (F) Focal positive membranous expression in WDT-UMP (x200).
The series published by El Demellawy et al. in 2008 reported 100% negativity in PTC cases and 100% positivity in other benign lesions, but also reported that since the WDT-UMP group was not included in that series, studying CD-56 in wider series including this group would be useful for differentiating these suspected lesions from FVPTC [7]. The series included in the present study had 20 cases from this group. When other positive markers were added to the CD56 negativity observed in 65% of these cases, it became possible to re-diagnose 50% of them as PTC.
On the other hand, a statement published by Etem et al. in 2010 concluded that CD56 was not a good marker in the differentiation of PTC and follicular tumors [8]. The selection of all PTC cases from the follicular variant may have led to this different conclusion.
In a series published by Shin et al. in 2011, CD56 negativity in PTC was found at a rate of 95%; however, since FVPTC cases were not included in that series, it was concluded that research was required in wider series including these cases, and that this would be useful for differentiating FVPTC from the other benign follicular proliferative lesions [2]. The series included in the present study had 56 cases and the findings were very significant.
E-cadherin has been known to show lower expression in thyroid neoplasms since Eidelman et al. first reported that it was expressed in normal thyroid tissue [1,16]. In 2012, Ozolins et al. demonstrated that there was a significant reduction in E-cadherin expression in PTC compared to the surrounding thyroid tissue of FA and PTC [1,17]. Nevertheless, there was not any apparent loss of expression in FC compared to FA. The same study showed that CD56 was positive in all thyroid lesions and tumors except PTC, and was significantly reduced in PTC. It was concluded that the triple HBME-1, E-cadherin, and CD56 might be considered helpful along with the morphological findings in both tissue and fine needle aspiration biopsies [1].
The present study investigated E-cadherin loss along with CD56 negativity in PTC, based on the diffusiveness rate and density degree, considering this and other similar studies (Figure 2). Additionally, these two markers were compared with HBME-1, galectin-3, and CK19 positive markers (Figures 3, 4 and 5), which have been reported to be in use in PTC for a long time. It was found that both the sensitivity (91.1%) and the specificity (91.7%) of CD56 negativity were higher than 90% in PTC. It was observed that E-cadherin expression displayed a significant level of loss in diffusiveness rate and density (86.1% and 92.1%, respectively), which was consistent with other studies. Positive staining was observed in 71.3% of PTCs with HBME-1, in 65.3% with galectin-3, and in 84.2% with CK19.
Figure 2.
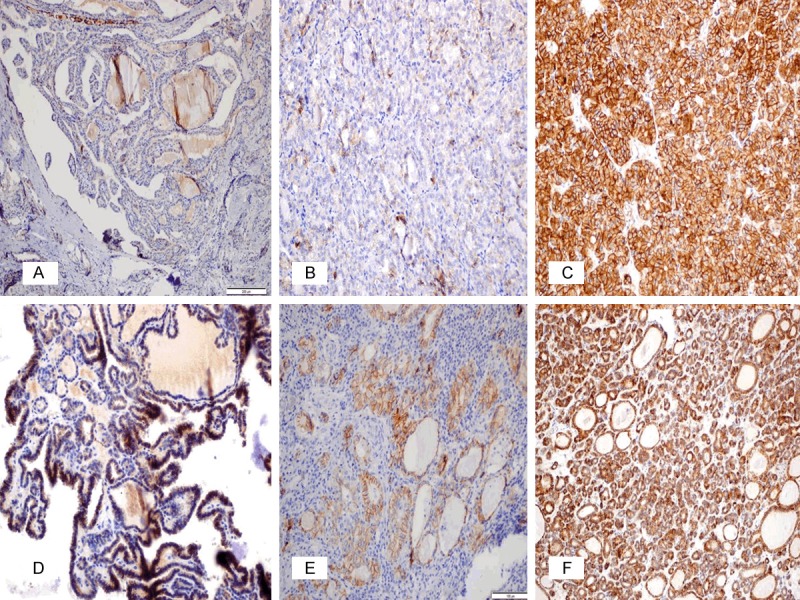
Immunohistochemical expression of E-cadherin in PTC (A, B), the other benign follicular-patterned lesions with suspicious nuclear features (C-E), and WDT-UMP (F). (A) Negative expression in CVPTC (x100); (B) 1 positive (density and ratio) membranous expression in FVPTC (x200); (C) Diffuse intense membranous expression in FA (x200); (D) 3 positive density and 2 positive ratio in HN (x200); (E) 2 positive density and 3 positive ratio in LT (x200); (F) 2 positive density and 3 positive ratio in WDT-UMP (x200).
Figure 3.
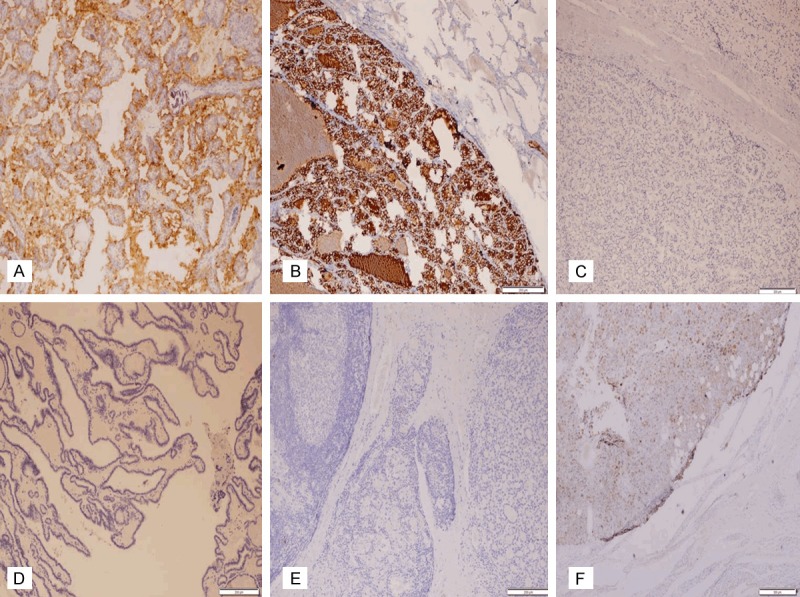
Immunohistochemical expression of HBME-1 in PTC (A, B), the other benign follicular-patterned lesions with suspicious nuclear features (C-E), and WDT-UMP (3F). (A, B) Positive expression in CVPTC and FVPTC (x100); (C-E) Negative expression in FA, HN, LT (x100); (F) Focal weakly positive expression in WDT-UMP (x40).
Figure 4.
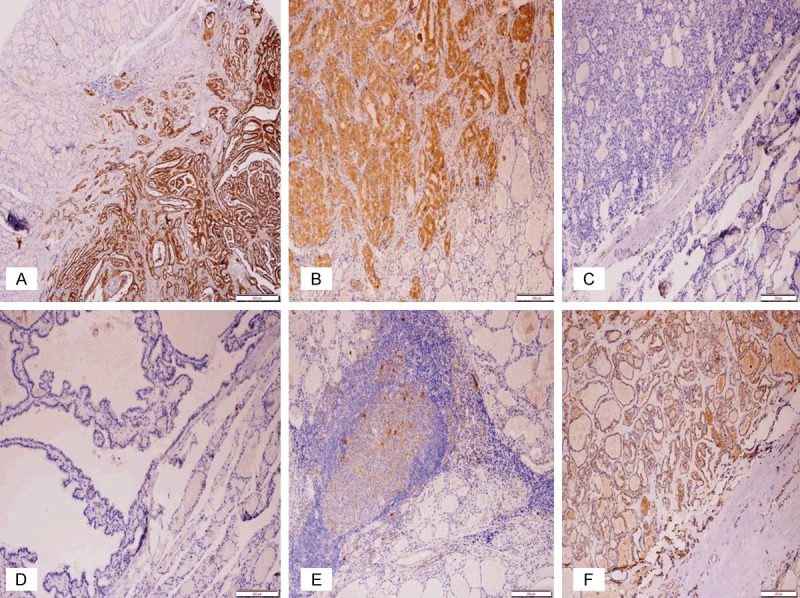
Immunohistochemical expression of galectin-3 in PTC (A, B), the other benign follicular-patterned lesions with suspicious nuclear features (C-E), and WDT-UMP (4F). (A, B) Diffuse strong positive expression in CVPTC (x40) and FVPTC (x100); (C-E) Negative expression in FA, HN, and LT (x100); (F) Diffuse weakly positive in WDT-UMP (x100).
Figure 5.
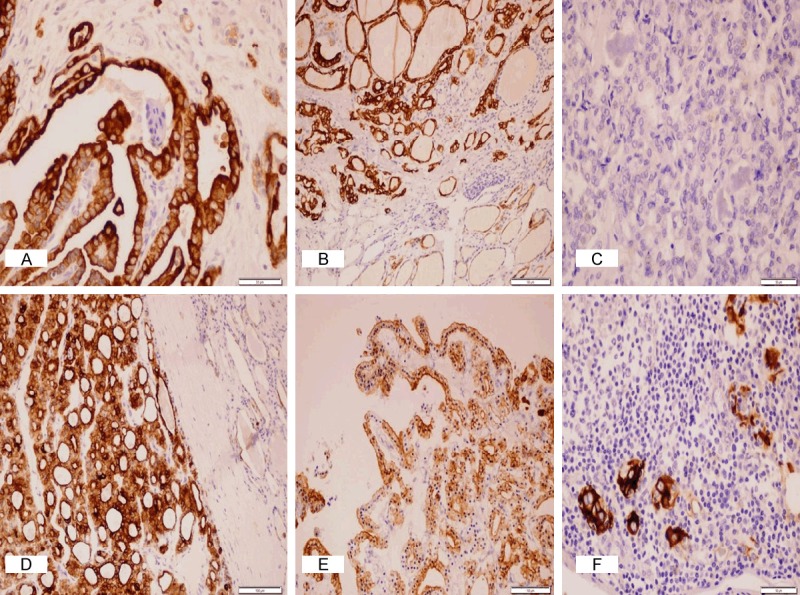
Immunohistochemical expression of CK19 in PTC (A-C) and the other benign follicular-patterned lesions with suspicious nuclear features (D-F). (A, B) Diffuse intense cytoplasmic and membranous expression in CVPTC (x400) and FVPTC (x200); (C) Negative expression in FVPTC (x400); (D) Diffuse intense cytoplasmic and membranous expression in FA (x200); (E) Diffuse cytoplasmic and membranous expression in HN (x200); (F) Focal intense cytoplasmic and membranous expression in LT (x400).
Furthermore, the present study demonstrated that when a positive marker was added to the CD56 negative marker, the sensitivity and specificity of the double markers of CD56 and galectin-3, or of CD56 and HBME-1, were very high (96% and 85%, and 96% and 75%, respectively) and the combined use achieved a more significant result for the differentiation of other benign follicular lesions with suspected nuclear features. The sensitivity of the triple CD56, HBME-1, and galectin-3 markers was 97% and the specificity was 70%. When a group of four markers was used, the sensitivity increased to 99% with CD56, E-cadherin, HBME-1, and galectin-3, whereas the specificity was found to be 50%. The authors think that the highest sensitivity in the PTC diagnosis was shown with the CD56 and galectin-3 pair and the CD56 and HBME-1 pair (96%) or the triple combination of CD56, galectin-3, and HBME-1 (97%) among these combinations. These combinations may be specifically used to differentiate the benign follicular-patterned lesions with suspected nuclear features from FVPTC.
Additionally, the authors found that the reduced E-cadherin expression in PTC was statistically significantly correlated with poor prognostic findings, such as capsule invasion, lymph node metastasis, multiple foci, and a tumor diameter of >10 mm (P<0.05). The correlation of reduced E-cadherin expression with poor prognosis in thyroid cancers was also reported previously by many researchers [18-22]. Furthermore, it has been published that this correlation not only exists in thyroid cancers, but there is a similar association in many other organ tumors [20,23]. However, the study by Klencka et al. in 2012 reported that the loss of E-cadherin expression was associated with the histopathological tumor type rather than the metastatic potential of thyroid cancers [24].
It is believed that the reduced expression of E-cadherin, which is a cell-adhesion molecule, causes loss in cell adhesion, leading to excessive proliferation, cancer progression, and increased metastatic potential. This has been demonstrated in in vitro studies [20,25].
The authors observed CD56 negativity in 13 of 20 cases in the WDT-UMP group. They concluded that 10 (50%) of these cases might be reclassified as FVPTC by adding galectin-3 or HBME-1 positivity, or if the positivity of both markers and a double or triple panel was used in addition to the CD56 negativity. For the remaining 7 cases, FVPTC was excluded in 5 (25%) with the CD56 and E-cadherin positivity together and galectin-3 or HBME-1 negativity. In this way, the authors could proceed to the differential diagnosis by reclassifying 75% of the controversial WDT-UMP cases.
Other than these, when CD56 negativity in 5 follicular-patterned nodules with suspected nuclear features and HBME-1 positivity in 3 cases (5%) were evaluated together as a double panel, the authors thought that these lesions may be reclassified as FVPTCs.
The specificity of CK19 (36.7%) was identified as the lowest, despite the high sensitivity rate (84.2%), due to the positive staining in 63.3% of benign lesions and in 75% of WDT-UMPs. Therefore, CK19 was considered appropriate not to be included in the panel.
In conclusion, histological and cytomorphological criteria are the basis for the diagnosis of PTC and its differentiation from the other follicular-patterned lesions, but the double and triple panels of the CD56 negative marker with HBME-1 and/or galectin-3 positive markers are also very useful. For the diagnosis of PTC, the only marker with both a sensitivity and a specificity of >90% is CD56; the most sensitive panels are the CD56 and HBME-1 or the CD56 and galectin-3 double panels, or the CD56, HBME-1, and galectin-3 triple panel. Furthermore, definitive differential diagnosis of FVPTC can be achieved in the majority of the lesions within the WDT-UMP group and in HNs with suspected nuclear features, FAs, and nodular foci of thyroiditis by using this triple panel due to their high specificities. Due to its low specificity, CK19 is not suitable for use in the differential diagnosis of suspected follicular-patterned lesions. Additionally, the reduced expression of E-cadherin in PTC is statistically significantly (P<0.05) correlated with poor prognostic findings.
The authors continue to design further genetic research for molecular modifications such as BRAF V600E mutation (44.4%-52.8%) and RET/PTC rearrangement (36.1%), found at a high rate in the majority of PTCs, in order to make this study more significant. We believe that our findings will be supported with these studies [26,27].
Disclosure of conflict of interest
None.
References
- 1.Ozolins A, Narbuts Z, Strumfa I, Volanska G, Stepanovs K, Gardovskis J. Immunohistochemical expression of HBME-1, E-cadherin, and CD56 in the differential diagnosis of thyroid nodules. Medicina (Kaunas) 2012;48:507–14. [PubMed] [Google Scholar]
- 2.Shin MK, Kim JW, JU YS. CD56 and High Molecular Weight Cytokeratin as Diagnostic Markers of Papillary Thyroid Carcinoma. Kore J Pathol. 2011;45:477–484. [Google Scholar]
- 3.Rosai J, Ackerman LV. Rosai and Ackerman’s Surgical Pathology. 10th edition. volume 1. Elsevier Mosby; 2011. pp. 504–514. [Google Scholar]
- 4.Cristopher DM. Diagnostic Histopathology of Tumors. 3th edition. volume 2. Churchill Livingstone, Elsevier Limited; 2007. Fletcher; pp. 1000–1015. [Google Scholar]
- 5.DeLellis RA, Lloyd RV, Heitz PU, Eng C. Pathology and genetics of tumours of endocrin organs. Lyon: IARC Press; 2004. pp. 57–66. [Google Scholar]
- 6.Park WY, Jeong SM, Lee JH, Kang HJ, Sin DH, Choi KU. Diagnostic value of decreased expression of CD56 protein in papillary carcinoma of the thyroid gland. Basic Applied Pathol. 2009;2:63–68. [Google Scholar]
- 7.El Demellawy D, Nasr AL, Babay S, Alowami S. Diagnostic utility of CD56 immunohistochemistry in papillary carcinoma of the thyroid. Pathol Res Pract. 2009;205:303–9. doi: 10.1016/j.prp.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Etem H, Özekinci S, Mızrak B, Şentürk S. The Role of CD56, HBME-1, and p63 in Follicular Neoplasms of the Thyroid. Turk J Pathol. 2010;26:238–242. [Google Scholar]
- 9.Atti RM, Shash LS. Potential diagnostic utility of CD56 and claudin-1 in papillary thyroid carcinoma and solitary follicular thyroid nodules. J Egypt Natl Canc Inst. 2012;24:175–184. doi: 10.1016/j.jnci.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Scarpino S, Di Napoli A, Melotti F, Talerico C, Cancrini A, Ruco L. Papillary carcinoma of the thyroid: low expression of NCAM (CD56) is associated with downregulation of VEGF-D production by tumour cells. J Pathol. 2007;212:411–9. doi: 10.1002/path.2183. [DOI] [PubMed] [Google Scholar]
- 11.El Demellawy D, Nasr A, Alowami S. Application of CD56, P63 and CK19 immunohistochemistry in the diagnosis of papillary carcinoma of the thyroid. Diagn Pathol. 2008;3:5. doi: 10.1186/1746-1596-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeromski J, Biczysko M, Stajgis P, Lawniczak M, Biczysko W. CD56 (NCAM) antigen in glandular epithelium of human thyroid: light microscopic and ultrastructural study. Folia Histochem Cytobiol. 1999;37:11–17. [PubMed] [Google Scholar]
- 13.Satoh F, Umemura S, Yasuda M, Osamura RY. Neuroendocrine marker expression in thyroid epithelial tumors. Endocr Pathol. 2001;12:291–9. doi: 10.1385/ep:12:3:291. [DOI] [PubMed] [Google Scholar]
- 14.Yang AH, Chen JY, Lee CH, Chen JY. Expression of NCAM and OCIAD1 in well-differentiated thyroid carcinoma: correlation with the risk of distant metastasis. J Clin Pathol. 2012;65:206–12. doi: 10.1136/jclinpath-2011-200416. [DOI] [PubMed] [Google Scholar]
- 15.Nechifor-Boila A, Borda A, Sassolas G, Hafdi-Nejjari Z, Borson-Chazot F, Lifante JC, Sturm N, Lavérriere MH, Berger N, Decaussin-Petrucci M. Immunohistochemical markers in the diagnosis of papillary thyroid carcinomas: The promising role of combined immunostaining using HBME-1 and CD56. Pathol Res Pract. 2013;209:585–92. doi: 10.1016/j.prp.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Eidelman S, Damsky CH, Wheelock MJ, Damjanov I. Expression of the cell-cell adhesion glycoprotein cell-CAM 120/80 in normal human tissues and tumors. Am J Pathol. 1989;135:101–10. [PMC free article] [PubMed] [Google Scholar]
- 17.Von Wasielewski R, Rhein A, Werner M, Scheumann GF, Dralle H, Potter E. Immunohistochemical detection of E-cadherin in differentiated thyroid carcinomas correlates with clinical outcome. Cancer Res. 1997;57:2501–7. [PubMed] [Google Scholar]
- 18.Scheumman GF, Hoang-Vu C, Cetin Y, Gimm O, Behrends J, von Wasielewski R, Georgii A, Birchmeier W, von Zur Mühlen A, Dralle H, et al. Clinical significance of E-cadherin as a prognostic marker in thyroid carcinomas. J Clin Endocrinol Metab. 1995;80:2168–72. doi: 10.1210/jcem.80.7.7608273. [DOI] [PubMed] [Google Scholar]
- 19.Graff JR, Greenberg VE, Herman JG, Westra WH, Boghaert ER, Ain KB, Saji M, Zeiger MA, Zimmer SG, Baylin SB. Distinct patterns of E-cadherin CpG island methylation in papillary, follicular, Hurthle’s cell, and poorly differentiated human thyroid carcinoma. Cancer Res. 1998;58:2063–6. [PubMed] [Google Scholar]
- 20.Erdem H, Gündogdu C, Sipal S. Correlation of E-cadherin, VEGF, COX-2 expression to prognostic parameters in papillary thyroid carcinoma. Exp Mol Pathol. 2011;90:312–7. doi: 10.1016/j.yexmp.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Sethi K, Sarkar S, Das S, Rajput S, Mazumder A, Roy B, Patra S, Mohanty B, El-Naggar AK, Mandal M. Expressions of CK-19, NF-kappaB, E-cadherin, beta-catenin and EGFR as diagnostic and prognostic markers by immunohistochemical analysis in thyroid carcinoma. J Exp Ther Oncol. 2011;9:187–99. [PubMed] [Google Scholar]
- 22.Tao XF, Liu C, Bai Y, Chen X. [Study of the correlation of papillary thyroid carcinoma’s invasion with Ezrin, Moesin and E-cadherin] . Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2011;46:761–3. [PubMed] [Google Scholar]
- 23.Kefeli M, Karagöz F, Barış S, Yıldız L, Aydın O, Kandemir B. E-cadherin and Ki-67 Expression in colorectal carcinomas and their relationship with stage, histological subtype and grade. Turk. J Pathol Patoloji Derg. 2005;21:8–10. [Google Scholar]
- 24.Slowinska-Klencka D, Sporny S, Stasikowska-Kanicka O, Popowicz B, Klencki M. E-cadherin expression is more associated with histopathological type of thyroid cancer than with the metastatic potential of tumors. Folia Histochem Cytobiol. 2012;50:519–26. doi: 10.5603/20322. [DOI] [PubMed] [Google Scholar]
- 25.Brachman DG. Molecular biology of head and neck cancer. Semin Oncol. 1994;21:320–9. [PubMed] [Google Scholar]
- 26.Dağlar-Aday A, Toptaş B, Oztürk T, Seyhan F, Saygili N, Eronat AP, Akadam-Teker B, Yilmaz-Aydoğan H, Aksoy F, Oztürk O. Investigation of BRAF V600E mutation in papillary thyroid carcinoma and tumor-surrounding nontumoral tissues. DNA Cell Biol. 2013;32:13–8. doi: 10.1089/dna.2012.1776. [DOI] [PubMed] [Google Scholar]
- 27.Guerra A, Zeppa P, Bifulco M, Vitale M. Concomitant BRAF (V600E) Mutation andRET/PTC rearrangement is a frequent occurrence in papillary thyroid carcinoma. Thyroid. 2014;24:254–9. doi: 10.1089/thy.2013.0235. [DOI] [PubMed] [Google Scholar]


