Abstract
Recent studies suggest that Golgi phosphoprotein 3 (GOLPH3) protein is a candidate metastasis gene in human cancer. The goal of this study was to determine the function of GOLPH3 in prostate cancer metastasis and to identify GOLPH3-regulated pathways and genes involved in prostate cancer metastasis. GOLPH3 expression was detected in prostate cancer. To investigate its function, PC-3 cells were stably transfected with shRNA targeting GOLPH3. Cell abilities of invasion and migration were measured in vitro. Downstream regulatory pathways of GOLPH3 were characterized using quantitative RT-PCR and Western blotting analysis. Immunohistochemical studies in prostate cancer specimens revealed a positive correlation of GOLPH3 expression with prostate cancer. GOLPH3 was expressed in prostate cancer cell lines. GOLPH3 repression resulted in the reduction of mRNA level and protein level of MMP9, accompanied with reduced phosphorylation of mTOR, EGFR and Src. Our findings suggest GOLPH3 regulate MMP9 expression which impact cell migration and invasion. This regulation is probably mediated by EGFR and Src signaling pathways.
Keywords: GOLPH3, MMP9, mTOR, EGFR, prostate cancer, metastasis
Introduction
Prostate cancer remains one of the most malignant and lethal cancers in male genitourinary system. The mortality of prostate cancer is not primarily attributed to the tumor growth but, rather, is caused by metastases in other organs. There are some curative treatments for localized prostate cancer, however, there is still no effective cure for advanced metastatic prostate cancer [1]. Presently, we are facing a largely unsolved challenge in combating this ad-vanced metastatic disease. Clarification of molecular mechanisms of metastasis is a crucial step towards establishing a new therapeutic strategy. Hence, the elucidation of the mechanisms underlying metastasis has been the research focus of prostate cancer over the past decades. Cancer metastasis initiates from cancer cells that detach from the primary tumor and are able to breach, pass through extracellular matrix (ECM), and survive in remote organs [2]. Metastatic phenotype of prostate cancer was achieved with an increase of EGFR/PI3K/AKT/mTOR phosphorylation, resistance to apoptosis, and reduction of ERK phosphorylation [3]. Activation of EGFR/AKT/mTOR stimulates Src signaling that promotes cell migration through turnover of cytoskeletal organization and en-hance invasion via promotion of matrix metalloproteinases (MMPs) secretion [4-6].
The Golgi apparatus is a subcellular organelle where post-translational modification and processing and trafficking of glycosylated proteins and lipid molecules proceeds [7]. It also participates extensively in cell differentiation, signaling pathways and the endocytosis pathway [8]. In recent years, with the rapid development in the fields of genomics and proteomics, novel Golgi and its associated proteins have been identified. Golgi phosphoprotein (GOLPH3) is a highly conserved Golgi membrane protein that was originally identified in the Golgi apparatus [9] and considered to be associated with tumorigenesis and cancer progression [10,11]. Also in prostate cancer it was revealed that GOLPH3 was associated with cancer aggressiveness [12]. It indicates GOLPH3 possibly associates with metastases development in prostate cancer.
To examine the possibility of GOLPH3 to regulate metastasis and investigate the underlying mechanism, we constructed a GOLPH3 knockdown stable cell line, detect its motility and invasion and characterized the downstream regulatory pathways.
Material and methods
Immunohistochemical analysis
Prostate adenocarcinoma TMAs were obtained from U.S. Biomax (Rockville, MD, USA) and prepared as recommendations. To examine the GOLPH3 protein expression in the prostate, we used TMAs comprising samples of prostate adenocarcinoma, benign and normal prostate tissue. Immunohistochemistry was performed as previously described [13]. The GOLPH3 primary antibody (Epitomics Inc.) was diluted at a 1:25 and applied to incubation with slides overnight at 4°C. The secondary goat anti-mouse/rabbit IgG polymer was diluted at 1:200 for incubation with slides at room temperature for 30 min. The normal rabbit IgG isotype served as the negative control.
Cell lines and stable transfections
PC-3, DU145 and LNCaP purchased from the cell bank of Chinese Academy of Sciences (Shanghai, China) were routinely maintained in RPMI-1640 medium (Hyclone) supplemented with 10% FBS. Cultures were maintained in a humidified atmosphere of 5% CO2.
To establish GOLPH3-knockdown (GOLPH3-KD) stable cell line, the cell culture was selected by 200 µg/ml hygromycin. Successfully transfected cells were selected in culture medium with the addition of 200 µg/ml hygromycin for one week before the individual clones were isolated, then established and maintained in the medium containing 50 µg/ml of hygromycin. Knockdown of GOLPH3 was determined by detection of the mRNA and protein level using quantitative RT-PCR and Western blotting analysis in triplicate, respectively.
Cell migration and invasion assay
PC-3 cells were seeded in 6-well plates with the appreciate density and cultured overnight. One day after vaccination, PC-3 cells were infected by GOLPH3-RNAi lentiviruses and blank control with virus culture solution was set. Cells (1 × 105 cells) were seeded on INSERT 24WELL 8UM PORE TRANSPARENT (BD Falcon) coated overnight at 4°C, cells were immobilized with 4% PFA and their migration capabilities were measured by 0.1% crystal violet staining assay. Using 10-fold magnification, take pictures for five field of view under arthroscope, counted and then calculate an average.
Invasion assay was conducted using Matrigel invasion chambers coated with the matrix Matrigel (BD Falcon). Briefly, Matrigel was diluted 1:9 in DMEM medium (Invitrogen), added onto the insert filter of transwell and incubated for 1 h at 37°C. 1 × 105 cells were seeded in the upper chamber. The lower chambers were filled with DMEM plus 10% FBS. After 72 h at 37°C, the cells passed through the insert were stained with 0.1% crystal violet, and counted by microscopic visualization.
Quantitative RT-PCR
The total RNA was extracted using Trizol reagent (Invitrogen), and reverse transcription was carried out using a RevertAid Reverse Transcriptase (Fermentas) at 42°C for 1 hour. cDNA was used as the template to amplify the tumor metastasis-related gene and cytokines with the following primers (Table 1). The quantitative PCR reactions were performed in triplicate in a 15 μl reaction volume containing 7.5 μl of 2 × SYBR mix (Takara), 0.8 μl of each primer (10 μM), and 1 μl cDNA. The program was as follows: 94°C for 120 s, followed by 40 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 30 s. The expression values of the tumor metastasis-related gene were normalized to β-actin.
Table 1.
List of the primer sequences for polymerase chain reaction studies
| Target | Sequences (5’-3’) |
|---|---|
| MMP1 | Forward: ATCCCTTCTACCCGGAAGTTG |
| Reverse: TCATCTCTGTCGGCAAATTCG | |
| MMP2 | Forward: CGGCGGTCACAGCTACTTC |
| Reverse: CGCTTCTGGCTGGGTCTGT | |
| MMP3 | Forward: GCGCAAATCCCTCAGGAA |
| Reverse: CATCCACGCCTGAAGGAAGA | |
| MMP9 | Forward: GAGGCGCTCATGTACCCTATGT |
| Reverse: GTGCCGGATGCCATTCAC | |
| MMP10 | Forward: CCCTGGTGCCCACAAAATC |
| Reverse: GAAGGACAAAGCAGGATCACACT | |
| MMP14 | Forward: GCCTGCGTCCATCAACACT |
| Reverse: TCATCAAACACCCAATGCTTGT | |
| Moesin | Forward: ACAAGACCCTGCGCCAGAT |
| Reverse: GCCCATTACATAGACTCAAATTCGT | |
| TIMP2 | Forward: GAAGGAGCCCCATCAATCCT |
| Reverse: CTCCCATTTCTACAAGGCTCAGA | |
| Vimentin | Forward: CGCCAGATGCGTGAAATG |
| Reverse: AGGCGGCCAATAGTGTCTTG | |
| VEGF-A | Forward: CGCAGCTACTGCCATCCAAT |
| Reverse: GTGAGGTTTGATCCGCATAATCT | |
| E-Cadherin | Forward: GGCCAGGAAATCACATCCTA |
| Reverse: GGCAGTGTCTCTCCAAATCC | |
| ACTR2 | Forward: CAAGCTACTTCTGTTGCGAGGATAC |
| Reverse: TTTAATCATGCGAACCGTTTCA | |
| Furin | Forward: CCCCTCAAACCTCCTCTTCTG |
| Reverse: AATGGCTAGGGTAGAAAAGAAAAGG | |
| CD44 | Forward: GACACCATGGACAAGTTTTGG |
| Reverse: CGGCAGGTTATATTCAAATCG | |
| P65 | Forward: AAGAGCAGCGTGGGGACTA |
| Reverse: CACATCAGCTTGCGAAAAGG | |
| P50 | Forward: AGGATTTCGTTTCCGTTATGTATG |
| Reverse: TGTGACCAACTGAACAATAACCTTT | |
| GOLPH3 | Forward: CCTGTTTTGGGTTTCTGGTC |
| Reverse: CCAAAAGCTGTGCGTATGAG | |
| β-Actin | Forward: TCCTTCCTGGGCATGGAGT |
| Reverse: CAGGAGGAGCAATGATCTTGAT |
Western blotting analysis
Briefly, GOLPH3-KD stable prostate cancer cell lines were lysed with 150 μl lysis buffer containing inhibitors of protease and phosphatase. The protein concentrations were measured with the BCA protein assay and adjusted to equivalent amounts. 20 μg protein was electrophoresed, transferred onto nitrocellulose membranes (Millipore) and then incubated with specific antibodies (MMP2, MMP9, Moesin, vimentin, EGFR, phospho-EGFR, FAK, phospho-FAK, Src, phospho-Src, AKT, phospho-AKT, mTOR, phospho-mTOR, p70S6K, phospho-p70S6K; Abcam) at room temperature for 2h. After washing and incubation with a horseradish peroxidase-conjugated secondary antibody, signals were developed with ECL reagents (Millipore).
Statistical analysis
Statistical analyses were performed using SPSS software version 19.0 (SPSS Inc.). Data are presented as the mean ± SD. Student’s t-test was performed for statistical analysis. Regarding the immunohistochemical study, the chi-square analyses were performed to evaluate the statistical significance. P<0.05 denotes statistical significance of differences.
Results
Expression of GOLPH3 is up-regulated in prostate cancer
To determine whether GOLPH3 was up-regulated in prostatic carcinoma, we analyzed GOLPH3 protein level on a prostate tissue microarray containing prostate cancer, benign prostatic hyperplasia and normal prostate tissue with immunohistochemistry. Figure 1A showed various patterns of GOLPH3 expression in normal prostate (a, d), benign prostatic hyperplasia (b, e), and adenocarcinoma tissues (c, f). GOLPH3 protein expression was typically cytoplasmic. In 2 out of 10 (20%) normal prostate tissues and in 6 out of 20 (30%) benign prostatic hyperplasia, positive GOLPH3 immunostaining could be observed in the cytoplasm. Of the 50 prostate carcinoma samples, only 18 (36%) were graded as negative, whereas 32 (64%) were positive for GOLPH3 expression (Table 2) (P<0.05). The normal and benign prostate tissues showed low levels of GOLPH3 staining, in contrast to prostate cancer, which displayed strong GOLPH3 staining. These findings suggest that the GOLPH3 protein is significantly elevated in prostate cancer compared with noncancerous tissues. We further confirmed the expression of GOLPH3 in LNCaP, DU145 and PC-3 cell lines. It was found the mRNA level and protein level of GOLPH3 was overexpressed in prostate cancer cell lines (Figure 1B and 1C). The study of the expression signature of GOLPH3 in prostate cancer indicated that GOLPH3 was significantly upregulated in clinical specimens and prostate cancer cells, suggesting that GOLPH3 act as an oncogene in prostate cancer.
Figure 1.
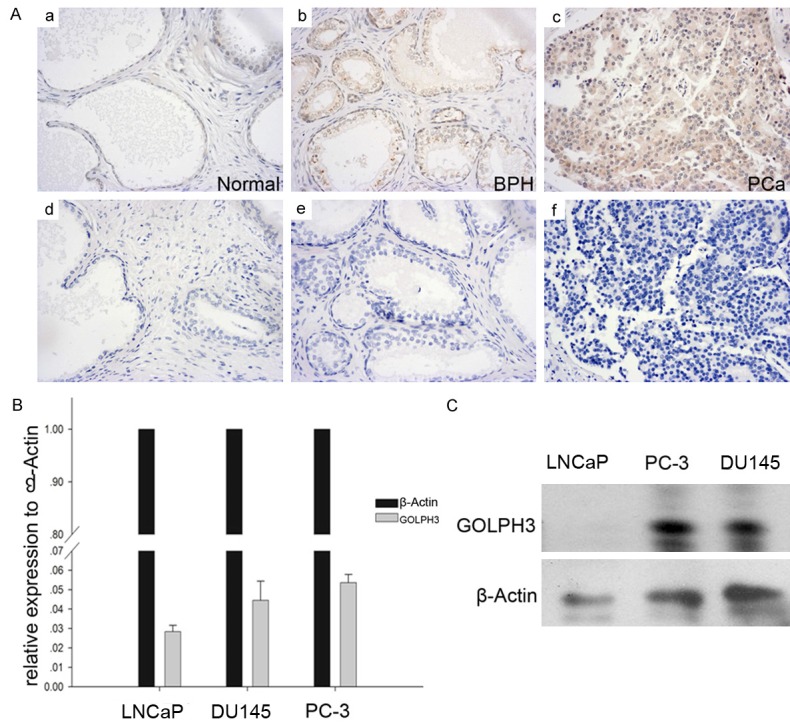
GOLPH3 is overexpressed in clinical specimens and prostate cancer cell lines. A. Immunohistochemical staining indicated that the level of GOLPH3 was obviously upregulated in human prostate cancer specimens versus BPH and normal specimens. B. qRT-PCR validation of GOLPH3 showed that the mean expression of GOLPH3 was higher in prostate cancer cell lines. C. Western blotting analysis showed that the expression of GOLPH3 was increased in human prostate cancer cell lines.
Table 2.
Expression of GOLPH3 in normal prostate, BPH, and PCa
| Pathological type | No. of cases | GOLPH3 | Expression | P-value* |
|---|---|---|---|---|
|
| ||||
| Positive (rate %) | Negative | |||
| Normal | 10 | 2 (20%) | 8 | s. (.001)* |
| BPH | 20 | 6 (30%) | 14 | |
| PCa | 50 | 32 (64%) | 18 | |
P-value derived from χ2 test; s., significance.
Down-regulated GOLPH3 expression inhibits prostate cancer cell migration and invasion
GOLPH3 has been found to be significantly associated with invasion in gastric cancer [14]. It prompted us to investigate the effect of GOLPH3 on prostate cancer cell migration and invasion. We first constructed a GOLPH3-KD stable cell line derived from PC-3 (Figure 2A). Quantitative RT-PCR and Western blotting analysis revealed GOLPH3 mRNA level and protein level were both reduced by roughly 80% in the stable cell line compared to the scramble shRNA control (Figure 2B and 2C).
Figure 2.
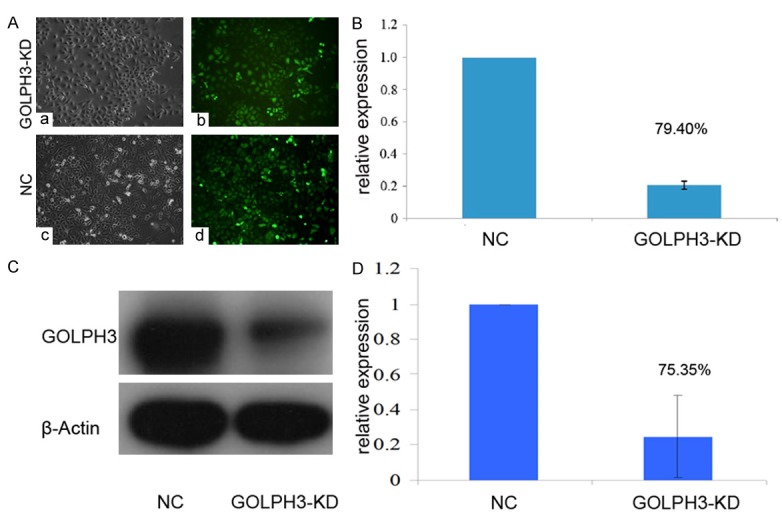
The GOLPH3-KD stable cell line were constructed and authenticated. A. Prostate cancer cells infected with lentiviral vector under a fluorescence microscope. B. Quantitative real-time polymerase chain reaction was performed to validate the efficiency of GOLPH3 interference in prostate cancer cells. C and D. Western blotting was performed to validate the efficiency of GOLPH3 interference in prostate cancer cells. (P<0.05, n = 3).
We then compared the ability of migration and invasion of GOLPH3-KD cells with scramble control through migration and invasion assay. It was shown that the migration of GOLPH3-KD cells was greatly affected compared with control cells after 24 hours (Figure 3A and 3C). Statistical analysis demonstrated that invasion ability of GOLPH3-KD cells was reduced by 60% almost significantly at P<0.05 (Figure 3B). Similarly, the migration of GOLPH3-KD cells markedly decreased to 40% of control cells with highly significance at P<0.05 (Figure 3D).
Figure 3.
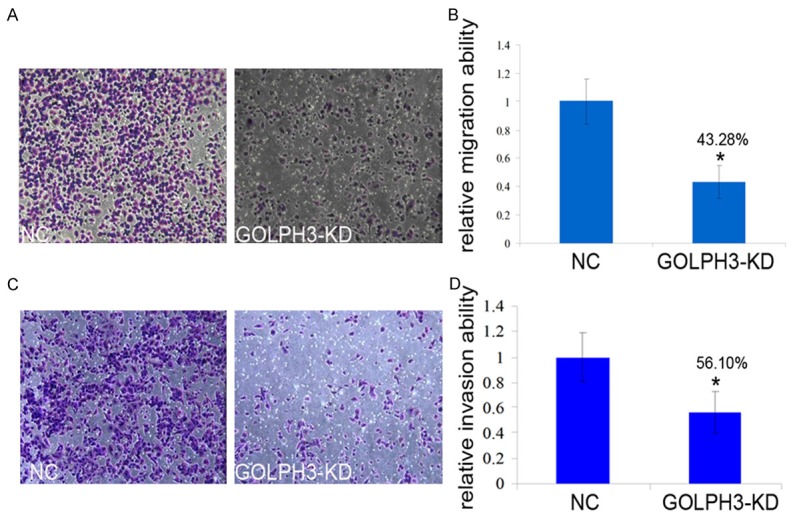
GOLPH3 may promote prostate cancer metastasis. A and B. The migration activity of the cells in the GOLPH3-KD group was reduced by approximately 43.28% in comparison with control group cells (P<0.05). C and D. Cell invasion assays showed that the knockdown of GOLPH3 expression resulted in a decreased capacity for invasion in comparison with the NC group, especially the invasion activity of the cells in the GOLPH3-KD group was reduced by approximately 56.10% in comparison with NC group cells (P<0.05). These data are the means and standard deviations of a representative experiment performed in triplicate (P<0.05, n = 3).
MMP9 was greatly downregulated following GOLPH3 knockdown
To find out the underlying mechanism of GOLPH3 influencing metastasis, we examined the effect of GOLPH3 knockdown on transcription of metastasis associated genes using quantitative PCR. Compared to the scramble control, the mRNA level of expression of MMP2, MMP9, moesin, vimentin, E-cadherin, TIMP2, P65, and GOLPH3 in PC-3 cells transfected with GOLPH3 RNAi at mRNA levels was decreased, of which MMP9 and P65 were greatly suppressed in GOLPH3-KD stable cell line with high significance (P<0.05) (Figure 4A), whereas the mRNA level of furin, MMP1, MMP3, MMP10, MMP14, CD44, P50, VEGF-A, ACTR2 increased (P>0.05) (Figure 4B).
Figure 4.
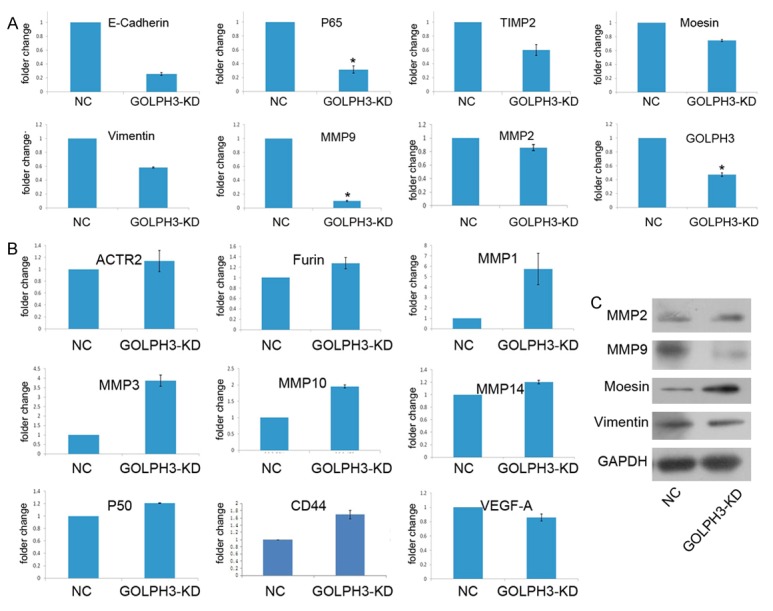
GOLPH3 promotes prostate cancer cell migration and invasion through down-regulation of MMP9. (A and B) qRT-PCR and (C) Western blotting analyses showed that the inhibition of the GOLPH3 reduced MMP9 expression at the mRNA and protein levels in prostate cancer cells.
We further performed protein analysis of four downregualted genes. It was shown only MMP9 significantly reduced, indicating GOLPH3 regulated MMP9 expression in prostate cancer cells (Figure 4C).
GOLPH3 modulates EGFR and Src signaling pathways
GOLPH3 interacts with VPS35 which has been implicated in recycling of receptor [3,15,16], therefore GOLPH3 possibly promotes downstream signaling of receptor tyrosine kinases (RTK). Indeed, GOLPH3 has been found to enhance mTOR signaling [3]. To investigate the effect of GOLPH3 on RTK and its downstream signaling, we examined EGFR phosphorylation and its downstream mTOR, MAPK, Src and FAK signaling.
In the GOLPH3-KD cells, the phosphorylated EGFR was markedly reduced, accompanied by decrease of phosphorylated mTOR and its downstream phosphorylated p70S6K (Figure 5A). Meanwhile, Src signaling was also impaired following GOLPH3 knock down (Figure 5B). However, AKT and FAK signaling were not affected.
Figure 5.
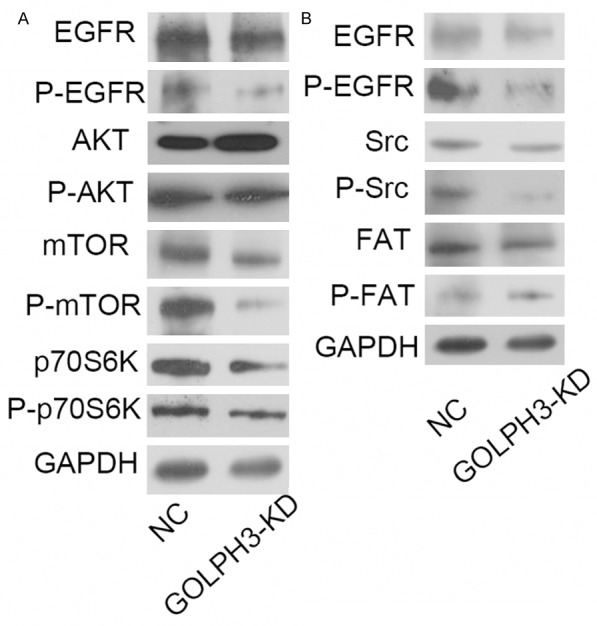
GOLPH3 modulates EGFR and Src signaling pathways. A. Expression levels of total and phosphorylated EGFR, mTOR, AKT, and p70S6K in prostate cancer cells were analyzed by western blotting. B. Expression levels of total and phosphorylated Src, and FAK in prostate cancer cells were analyzed by western blotting. A western blotting analysis showed that the expression of P-EGFR, P-mTOR and P-Src in prostate cancer cells with GOLPH3 inhibition was markedly attenuated in comparison with the NC group.
Discussion
GOLPH3 gene is localized on chromosome 5p13 region and its gain of copy number frequently takes place in various types of solid tumors including the lung, ovary, breast, prostate, and skin (melanoma) [17]. These suggest GOLPH3 is a proto-oncogene. In this study, it was interesting to note that GOLPH3 was positive in 64% cancer samples compared with 20% in normal and 30% in benign samples. This confirmed the previous findings that reported an overexpression of GOLPH3 in 37% of prostate cancer cases. Increased expression of GOLPH3 has been found to be significantly associated with the prognosis of gastric cancer, esophageal squamous cell cancer and ovarian cancer, non-small cell cancer, oral cancer and prostate cancer [8,10,12,14,18,19]. Although in vitro studies in hepatocellular carcinoma cell lines demonstrated that overexpression of GOLPH3 promoted aggressiveness [20,21], its exact functional role in metastasis and underlying mechanism are unclear.
In the study, we employed loss-of-function approaches to examine that GOLPH3 regulate the expression of downstream metastasis associated genes. We observed that knockdown of GOLPH3 in vitro inhibited cell migration and invasion, accompanied with a great loss of MMP9. MMPs function primarily to degrade ECM proteins, and are therefore necessary for cell invasion [22,23]. These suggest GOLPH3 are extensively involved in metastasis of prostate cancer. MMP9 expression secreted by tumor or stroma cells is elevated in both primary and metastases and associated with cancer progression [24-26]. Previous studies showed that interaction between stroma and prostate cancer cells induced pro-MMP9 expression in prostate cancer cells [27]. These suggest MMP9 play a pivotal role in prostate cancer metastasis through which GOLPH3 regulates metastasis. These also would account for association of GOLPH3 with aggressiveness and progression in a variety of cancers. Future work is needed to be determined if MMP9 is critical for GOLPH3-induced prostate cancer cell migration and invasion.
Metastasis is the spread of cancer cells from the primary tumor to distant organs, and thus it is regulated and controlled by intracellular signaling pathways. The molecular mechanism by which GOLPH3 promotes MMP9 expression is an intriguing question. We probed the signaling pathways upstream of MMP9 to clarify the mechanism. Our findings demonstrated that GOLPH3 extensively intersects with cancer regulatory pathways such as EGFR, mTOR and Src that appear crucial in determining the metastatic prostate phenotypes. Phosphorylations of EGFR, mTOR and Src decreased markedly in GOLPH3-KD cells whereas EGFR, mTOR and Src levels did not change. EGFR was ever documented to upregulate MMP9 expression and promotes cancer cell motility and invasion in epithelioid sarcomas [28,29]. Consistently, studies in breast cancer cell line displayed that EGFR knockdown resulted in a decrease of MMP9 expression [30]. One of MMP9 regulation modes by EGFR requires the participation of NFκB [31]. One of NFκB subunits RelA (P65) can bind to the promoter of MMP9 and promotes its transcription [28]. Quantitative PCR revealed that transcription of RelA (P65) was reduced after GOLPH3 knockdown (Figure 4A), indicating NFκB involved in the modulation of MMP9 expression by GOLPH3 in prostate cancer. In addition, MMP9 expression was suppressed in myeloid cells by Src kinase inhibitor Dasatinib, accompanied with inhibition of Src family kinase and cancer cell motility [32]. This indicates Src phosphorylation regulate MMP9 expression and cancer metastasis. In mouse model, knockout of Src negative regulator Carboxy-terminal Src kinase (Csk) resulted in MMP9 and TNF alpha elevation [33]. This indirectly confirmed the role of Src to drive MMP9 expression and activate NFκB. These also imply EGFR and Src could serve as drug targets to treat metastatic prostate cancer. Taken together, GOLPH3 regulates MMP9 expression probably through EGFR and Src signaling pathways and NFκB probably mediates this regulation. The results suggest that GOLPH3 play a pivotal role in the development and progression of prostate cancer. GOLPH3 might become a prognostic marker and a promising therapeutic target which requires a large population to determine.
Overall, our findings confirmed GOLPH3 is associated with prostate cancer development and metastasis, and showed that GOLPH3 controls MMP9 expression through EGFR and Src and consequently regulate metastasis of prostate cancer.
Acknowledgements
This work was supported by the National Natural Science Foundation of China No. 81170697 and Linyi Municipal Science and Technology Development Project NO. 201413015.
Disclosure of conflict of interest
None.
References
- 1.Eisenberger MA, Blumenstein BA, Crawford ED, Miller G, McLeod DG, Loehrer PJ, Wilding G, Sears K, Culkin DJ, Thompson IM Jr, Bueschen AJ, Lowe BA. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med. 1998;339:1036–1042. doi: 10.1056/NEJM199810083391504. [DOI] [PubMed] [Google Scholar]
- 2.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott KL, Kabbarah O, Liang MC, Ivanova E, Anagnostou V, Wu J, Dhakal S, Wu M, Chen S, Feinberg T, Huang J, Saci A, Widlund HR, Fisher DE, Xiao Y, Rimm DL, Protopopov A, Wong KK, Chin L. GOLPH3 modulates mTOR signalling and rapamycin sensitivity in cancer. Nature. 2009;459:1085–1090. doi: 10.1038/nature08109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peiró G, Ortiz-Martínez F, Gallardo A, Pérez-Balaguer A, Sánchez-Payá J, Ponce JJ, Tibau A, López-Vilaro L, Escuin D, Adrover E, Barnadas Figure. GOLPH3 modulates EGFR and Src signaling pathways. A. Expression levels of total and phosphorylated EGFR, mTOR, AKT, and p70S6K in prostate cancer cells were analyzed by western blotting. B. Expression levels of total and phosphorylated Src, and FAK in prostate cancer cells were analyzed by western blotting. A western blotting analysis showed that the expression of P-EGFR, P-mTOR and P-Src in prostate cancer cells with GOLPH3 inhibition was markedly attenuated in comparison with the NC group. A, Lerma E. Src, a potential target for overcoming trastuzumab resistance in HER2-positive breast carcinoma. Br J Cancer. 2014;111:689–695. doi: 10.1038/bjc.2014.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girotti MR, Pedersen M, Sanchez-Laorden B, Viros A, Turajlic S, Niculescu-Duvaz D, Zambon A, Sinclair J, Hayes A, Gore M, Lorigan P, Springer C, Larkin J, Jorgensen C, Marais R. Inhibiting EGF receptor or SRC family kinase signaling overcomes BRAF inhibitor resistance in melanoma. Cancer Discov. 2013;3:158–167. doi: 10.1158/2159-8290.CD-12-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lau SK, Shields DJ, Murphy EA, Desgrosellier JS, Anand S, Huang M, Kato S, Lim ST, Weis SM, Stupack DG, Schlaepfer DD, Cheresh DA. EGFR-mediated carcinoma cell metastasis mediated by integrin alphavbeta5 depends on activation of c-Src and cleavage of MUC1. PLoS One. 2012;7:e36753. doi: 10.1371/journal.pone.0036753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donaldson JG, Lippincott-Schwartz J. Sorting and signaling at the Golgi complex. Cell. 2000;101:693–696. doi: 10.1016/s0092-8674(00)80881-8. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Ma M, Han B. GOLPH3 high expression predicts poor prognosis in patients with resected non-small cell lung cancer: an immunohistochemical analysis. Tumour Biol. 2014;35:10833–10839. doi: 10.1007/s13277-014-2357-3. [DOI] [PubMed] [Google Scholar]
- 9.Wu CC, Taylor RS, Lane DR, Ladinsky MS, Weisz JA, Howell KE. GMx33: a novel family of trans-Golgi proteins identified by proteomics. Traffic. 2000;1:963–975. [PubMed] [Google Scholar]
- 10.Li H, Guo L, Chen SW, Zhao XH, Zhuang SM, Wang LP, Song LB, Song M. GOLPH3 overexpression correlates with tumor progression and poor prognosis in patients with clinically N0 oral tongue cancer. J Transl Med. 2012;10:168. doi: 10.1186/1479-5876-10-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu BS, Hu H, Zhu CY, Gu YL, Li JP. Overexpression of GOLPH3 is associated with poor clinical outcome in gastric cancer. Tumour Biol. 2013;34:515–520. doi: 10.1007/s13277-012-0576-z. [DOI] [PubMed] [Google Scholar]
- 12.Hua X, Yu L, Pan W, Huang X, Liao Z, Xian Q, Fang L, Shen H. Increased expression of Golgi phosphoprotein-3 is associated with tumor aggressiveness and poor prognosis of prostate cancer. Diagn Pathol. 2012;7:127. doi: 10.1186/1746-1596-7-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W, Wang X, Li B, Lu J, Chen G. Diagnostic significance of overexpression of Golgi membrane protein 1 in prostate cancer. Urology. 2012;80:952, e1–7. doi: 10.1016/j.urology.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 14.Peng J, Fang Y, Tao Y, Li K, Su T, Nong Y, Xie F, Lai M. Mechanisms of GOLPH3 associated with the progression of gastric cancer: a preliminary study. PLoS One. 2014;9:e107362. doi: 10.1371/journal.pone.0107362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seaman MN. Recycle your receptors with retromer. Trends Cell Biol. 2005;15:68–75. doi: 10.1016/j.tcb.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Bonifacino JS, Hurley JH. Retromer. Curr Opin Cell Biol. 2008;20:427–436. doi: 10.1016/j.ceb.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott KL, Chin L. Signaling from the Golgi: mechanisms and models for Golgi phosphoprotein 3-mediated oncogenesis. Clin Cancer Res. 2010;16:2229–2234. doi: 10.1158/1078-0432.CCR-09-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang JH, Chen XT, Wen ZS, Zheng M, Deng JM, Wang MZ, Lin HX, Chen K, Li J, Yun JP, Luo RZ, Song LB. High expression of GOLPH3 in esophageal squamous cell carcinoma correlates with poor prognosis. PLoS One. 2012;7:e45622. doi: 10.1371/journal.pone.0045622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma Y, Ren Y, Zhang X, Lin L, Liu Y, Rong F, Wen W, Li F. High GOLPH3 expression is associated with a more aggressive behavior of epithelial ovarian carcinoma. Virchows Arch. 2014;464:443–452. doi: 10.1007/s00428-014-1536-3. [DOI] [PubMed] [Google Scholar]
- 20.Dai T, Zhang D, Cai M, Wang C, Wu Z, Ying Z, Wu J, Li M, Xie D, Li J, Song L. Golgi phosphoprotein 3 (GOLPH3) promotes hepatocellular carcinoma cell aggressiveness by activating NF-κB pathway. J Pathol. 2015;235:490–501. doi: 10.1002/path.4479. [DOI] [PubMed] [Google Scholar]
- 21.Hu GS, Li YQ, Yang YM, Shi W, Liao AJ, Yao YH, Zeng B, Yuan J. High expression of Golgi phosphoprotein-3 is associated with poor survival in patients with hepatocellular carcinoma. Tumour Biol. 2014;35:8625–8632. doi: 10.1007/s13277-014-2105-8. [DOI] [PubMed] [Google Scholar]
- 22.Zucker S, Lysik RM, Zarrabi HM, Moll U, Tickle SP, Stetler-Stevenson W, Baker TS, Docherty AJ. Plasma assay of matrix metalloproteinases (MMPs) and MMP-inhibitor complexes in cancer. Potential use in predicting metastasis and monitoring treatment. Ann N Y Acad Sci. 1994;732:248–262. doi: 10.1111/j.1749-6632.1994.tb24740.x. [DOI] [PubMed] [Google Scholar]
- 23.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest. 2004;114:623–633. doi: 10.1172/JCI22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allott EH, Lysaght J, Cathcart MC, Donohoe CL, Cummins R, McGarrigle SA, Kay E, Reynolds JV, Pidgeon GP. MMP9 expression in oesophageal adenocarcinoma is upregulated with visceral obesity and is associated with poor tumour differentiation. Mol Carcinog. 2013;52:144–154. doi: 10.1002/mc.21840. [DOI] [PubMed] [Google Scholar]
- 26.Kim HC, Kim YS, Oh HW, Kim K, Oh SS, Kim JT, Kim BY, Lee SJ, Choe YK, Kim DH, Kim SH, Chae SW, Kim KD, Lee HG. Collagen triple helix repeat containing 1 (CTHRC1) acts via ERK-dependent induction of MMP9 to promote invasion of colorectal cancer cells. Oncotarget. 2014;5:519–529. doi: 10.18632/oncotarget.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong Z, Nemeth JA, Cher ML, Palmer KC, Bright RC, Fridman R. Differential regulation of matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-1 (TIMP-1) and TIMP-2 expression in co-cultures of prostate cancer and stromal cells. Int J Cancer. 2001;93:507–515. doi: 10.1002/ijc.1358. [DOI] [PubMed] [Google Scholar]
- 28.Bera A, Zhao S, Cao L, Chiao PJ, Freeman JW. Oncogenic K-Ras and loss of Smad4 mediate invasion by activating an EGFR/NF-κB Axis that induces expression of MMP9 and uPA in human pancreas progenitor cells. PLoS One. 2013;8:e82282. doi: 10.1371/journal.pone.0082282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dupouy S, Doan VK, Wu Z, Mourra N, Liu J, De Wever O, Llorca FP, Cayre A, Kouchkar A, Gompel A, Forgez P. Activation of EGFR, HER2 and HER3 by neurotensin/neurotensin receptor 1 renders breast tumors aggressive yet highly responsive to lapatinib and metformin in mice. Oncotarget. 2014;5:8235–8251. doi: 10.18632/oncotarget.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nickerson NK, Mohammad KS, Gilmore JL, Crismore E, Bruzzaniti A, Guise TA, Foley J. Decreased autocrine EGFR signaling in metastatic breast cancer cells inhibits tumor growth in bone and mammary fat pad. PLoS One. 2012;7:e30255. doi: 10.1371/journal.pone.0030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mir SU, Jin L, Craven RJ. Neutrophil gelatinase-associated lipocalin (NGAL) expression is dependent on the tumor-associated sigma-2 receptor S2RPgrmc1. J Biol Chem. 2012;287:14494–14501. doi: 10.1074/jbc.M111.324921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang W, Kujawski M, Wu J, Lu J, Herrmann A, Loera S, Yen Y, Lee F, Yu H, Wen W, Jove R. Antitumor activity of targeting SRC kinases in endothelial and myeloid cell compartments of the tumor microenvironment. Clin Cancer Res. 2010;16:924–935. doi: 10.1158/1078-0432.CCR-09-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yagi R, Waguri S, Sumikawa Y, Nada S, Oneyama C, Itami S, Schmedt C, Uchiyama Y, Okada M. C-terminal Src kinase controls development and maintenance of mouse squamous epithelia. EMBO J. 2007;26:1234–1244. doi: 10.1038/sj.emboj.7601595. [DOI] [PMC free article] [PubMed] [Google Scholar]


