Abstract
Myocardial tissue injury caused by ischemia and hypoxia is a major cause of fatal diseases, including coronary atherosclerosis resulting from myocardial infarction and stroke. Trimetazidine (TMZ), as an anti-ischemic and antioxidant agent, has been demonstrated to preventing ischemia/reperfusion-induced cardiomyocyte apoptosis. However, the anti-apoptosis mechanism of TMZ has not been fully elucidated. The present study demonstrated that miR-21 involved trimetazidine-induced anti-apoptosis during H/R injury in H9C2 cell. In this study, TMZ increased miR-21 expression which further upregulated the Akt signaling activity via suppressing the expression of phosphatase and tensin homolog (PTEN) in H/R H9C2 cell. The increased activity of Akt signaling decreased the ratio of Bax/Bcl-2 and the expression of caspase-3 and inhibited H/R induced apoptosis. In conclusion, this study revealed the mechanism that TMZ up-regulated miR-21 expression, then miR-21 targeted PTEN increasing the PI3K pathway and finally the activation of this pathway counteracted the apoptotic effect of hypoxia/reperfusion.
Keywords: Trimetazidine, H/R injury, miR-21, PTEN/Akt pathway
Introduction
Myocardial ischemia/reperfusion (I/R) injury is an exacerbated tissue injury resulted from inhibition of cardiac function during reperfusion after prolonged myocardial ischemia. It is an important clinical problem associated with procedures such as thrombolysis, angioplasty, and coronary bypass surgery [1]. In the past few years, an increasing number of innovative approaches to cardioprotection against IR injury are under preclinical and clinical investigation.
Trimetazidine, 1-(2,3,4-trimethoxybenzyl) piperazine dihydrochloride (TMZ), is an anti-ischemic agent, and its anti-ischemic effects have been experimentally assessed in various models including cell cultures, isolated and perfused organs, and also in vivo [2-4]. Recent reports revealed TMZ as an antioxidant agent used to protect grafts from myocardial ischemia/reperfusion (I/R) injury. TMZ is a metabolic anti-ischemic drug that exerts its beneficial effects without altering the hemodynamic function of the heart [5]. TMZ decreases ischemic stress and improves cardiac performance during ischemia by reducing fatty acid oxidation through the selective inhibition of mitochondrial 3-ketoacyl CoA thiolase [6]. TMZ also observes cytoprotective effect in several models of myocardial infarction [7,8]. TMZ decreases oxidative damage to mitochondria and protects hearts from I/R-induced damage to mitochondrial respiration [9]. TMZ reduces neutrophil-mediated cardiac reperfusion injury [10]. Recently, it has been shown that pretreatment with trimetazidine for 2 weeks significantly decreases cardiomyocyte apoptosis and improves cardiac performance [11]. Nevertheless, the mechanism of TMZ-mediated anti-apoptosis during H/R injury in H9C2 cell remains unclear.
MiRNAs are a class of endogenous, small non-coding single-stranded RNAs. They contain about 22 nucleotides, with highly conserved sequences among species. MiRNAs interacted with the 3’-untranslated region (UTR) of the target mRNA, resulting in mRNA degradation or the inhibition of protein translation. Accumulative studies suggest that miRNAs have a crucial role in the protection against myocardial H/R injury [12-14]. miR-21 has been found to decrease the myocardial apoptosis in hypoxia/reoxygenation rat [15]. Recent study revealed that miR-21 had a protective role in I/R- and H/R-induced cardiocyte apoptosis [16].
In the present study, we revealed that TMZ exerts cardioprotection against cardiomyocyte H/R injury by targeting miR-21 and activation of PTEN/Akt pathway plays an important role in mediating the anti-apoptotic effect of TMZ in H/R H9C2 cells.
Methods
Cell culture and H/R treatment
The human H9C2 cell line was purchased from The Institute of Cell Biology at the Chinese Academy of Sciences (Shanghai, China). H9C2 cells were cultured in DMEM containing 10% FBS and incubated at 37°C in a humidified incubator with 5% CO2.
In H/R treatment group, H9C2 cells were cultured in DMEM with neither serum nor antibiotics at 37°C with 5% CO2 for 12 h, which were then cultured at 37°C with 1% O2, 94% N2 and 5% CO2 for 4 h. Subsequent to that, the cells were cultured in DMEM containing 10% FBS, incubated at 37°C with 21% O2, 74% N2 and 5% CO2 for 3 h and used in the following experiments.
Western blot analysis
The cells were harvested and lysed with radioimmunoprecipitation assay buffer. The concentration of protein was determined using a bicinchoninic acid assay kit. Following that, proteins of 20 μg/lane were loaded on a 10% SDS-PAGE to be separated, and then electrophoretically transferred to polyvinylidene fluoride membranes. Proteins on the membranes were then probed using primary antibodies, including mouse anti-Bcl-2, Bax, caspase-3, PTEN, p-Akt and β-actin purchased from Sigma-Aldrich, according to the manufacturer’s instructions. Following incubation with secondary antibodies, including rabbit antimouse secondary antibody, the results were visualized with horseradish peroxidase and an enhanced chemiluminescence system, and quantified by the Quantity One software (Bio-Rad, Hercules, CA, USA).
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from H9c2 cells with the TRIzol reagent (Invitrogen, USA) and then reverse-transcribed into cDNA, following the thermal cycle program of 16°C for 30 min, 37°C for 60 min, and 85°C for 5 min, cDNA was stored at -20°C. The real-time quantitative PCR was performed by a fast real-time PCR system (7900HT, ABI, USA) using a TaqMan miRNA assay kit. the protocol was conducted for 35 cycles at 95°C for 3 minutes, 95°C for 12 seconds, and 58°C for 30 seconds. Finally, the relative expression level of miR-21 was normalized to that of internal control U6 by using 2-ΔΔCt cycle threshold method.
Statistical analysis
Data are expressed as mean ± SEM. The significance of the results was analyzed using a student’s t-test. The value of P<0.05 was considered as a significant statistical difference.
Results
miR-21 regulated PTEN and Akt expression in H9C2 cells
PTEN has been demonstrated to be a target of miR-21 and has an inhibitory role in the regulation of the Akt signaling pathway, which acts as a key regulator in cellular survival. Thus, to further investigate the miR-21 involved regulatory of PTEN and Akt pathway in H9C2 cell, the expression of PTEN and the phosphorylation levels of Akt were determined in Figure 1. miR-21 mimics (mimics group) or inhibitor (inhibitor group) was transfected into the H9C2 cells, respectively. In the miR-21 inhibitor group, the PTEN expression levels were significantly upregulated and the p-Akt expression levels were significantly downregulated (Figure 1A). In contrast, the p-Akt expression levels were significantly upregulated and the PTEN expression levels were significantly downregulated in miR-21 mimic group (Figure 1B). These results identified that miR-21 evidently downregulated the PTEN expression while inducing the phosphorylation levels of Akt, which was also reverted by miR-21 inhibitor.
Figure 1.
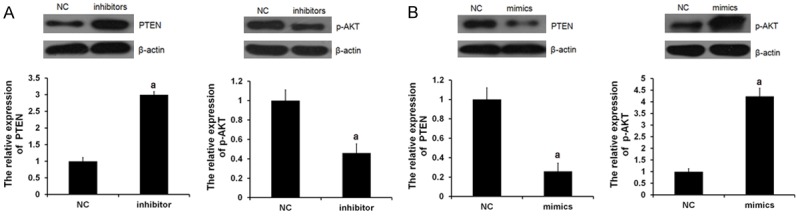
miR-21 regulated PTEN and p-AKT expression. miR-21 mimics or inhibitor was transfected into the H9C2 cells, respectively. NC means negative control group. Protein expression of PTEN (A) and p-AKT (B) were examined by western blot analysis, respectively (aP<0.05 vs. NC group).
TMZ interfered miR-21, PTEN and p-AKT expression in H/R-treated H9C2 cells
As demonstrated in Figure 2A, qRT-PCR analysis revealed that the expression of miR-21 was significantly downregulated by H/R treatment, which was, to a certain degree, reverted by TMZ. Furthermore, as expected, the expression of PTEN was significantly increased (Figure 2B) and the phosphorylation levels of Akt were evidently decreased in the H/R group compared with the control group, which could be restored in H/R+TMZ group (Figure 2C). TMZ is an anti-ischemic agent in cardiomyopathies. So, we speculated that TMZ protected against myocardial H/R by affecting the expression levels of miR-21 and PTEN and phosphorylation levels of the Akt signaling pathway.
Figure 2.
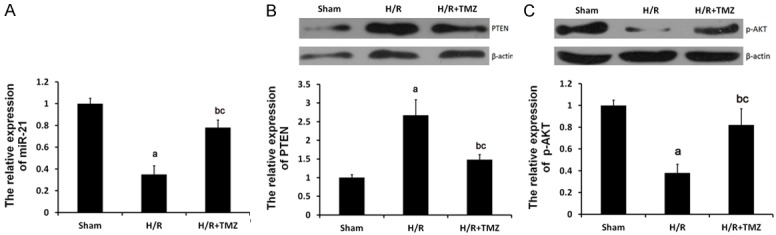
Role of TMZ in H/R-treated H9C2 cells. The H9C2 cells were randomly assigned into three groups: Sham group, in which the cells were treated with 0μM TMZ for 48 h and then cultured under normal oxygenation conditions (5% CO2, and 95% air); H/R group, in which cells were treated with 0 μM TMZ for 48 h and then cultured under H/R conditions; and H/R+TMZ group, in which cells were treated with 10 μM TMZ for 48 h and then subjected to H/R treatment. (A) qRT-PCR assay determined the relative expression of miR-21. Protein expression of PTEN (B) and p-AKT (C) were examined by western blot analysis, respectively (aP<0.01 and bP<0.05 vs. sham group, cP<0.05 vs. H/R group).
MiR-21 inhibitor restored TMZ-interfered PTEN and p-AKT expression in H/R-treated H9C2 cells
To further investigate the role of miR-21 in TMZ protecting against myocardial H/R injury, miR-21 inhibitor was transfected into H/R H9C2 cells. As shown in Figure 3, miR-21 inhibitor could significantly restore the PTEN and p-AKT expression in H/R+TMZ+ inhibitors group.
Figure 3.
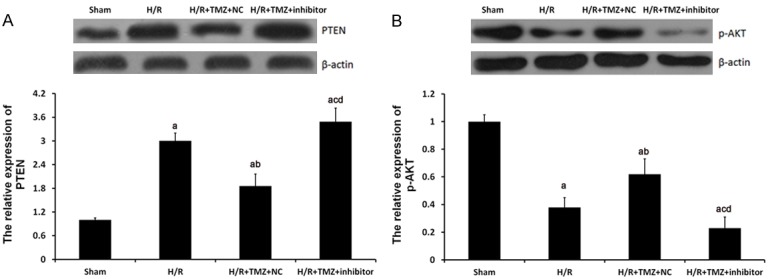
miR-21 involved the regulation of TMZ on PTEN and p-AKT expression in HR-treated H9C2 cells. The H9C2 cells were assigned into four groups: sham group, in which the cell were treated with 0 μM TMZ for 48 h and then cultured under normal oxygenation conditions (5% CO2, and 95% air); H/R group, in which cells were treated with 0 μM TMZ for 48 h and then cultured under H/R conditions; and H/R+TMZ group, in which cells were treated with 10 μM TMZ for 48 h and then subjected to H/R treatment; H/R+TMZ+ inhibitors group, in which cells were transfected with 50 nM miR-21 inhibitors, next, treated with 10 μM TMZ for 48 h and finally subjected to H/R treatment. Protein expression of PTEN (A) and p-AKT (B) were examined by western blot analysis, respectively. aP<0.01 vs. sham group, bP<0.01 and cP<0.05 vs. H/R group, dP<0.01 vs. H/R+TMZ+NC group.
Role of TMZ, miR-21, PTEN and p-AKT in H/R-induced apoptosis of H9C2 cells
In H/R+TMZ+ miR-21 inhibitor + siPTEN group, H9C2 cells were transfected with 50 nM miR-21 inhibitors, next, treated with 10 μM TMZ for 48 h, next, treated with 50 nM siPTEN for 18 h and finally subjected to H/R treatment. As shown in Figure 4A, the expression levels of PTEN were greatly decrease by 50 nM siPTEN and the p-AKT expression was significantly upregulated by 10 nM IGF-1 (Figure 4B).
Figure 4.
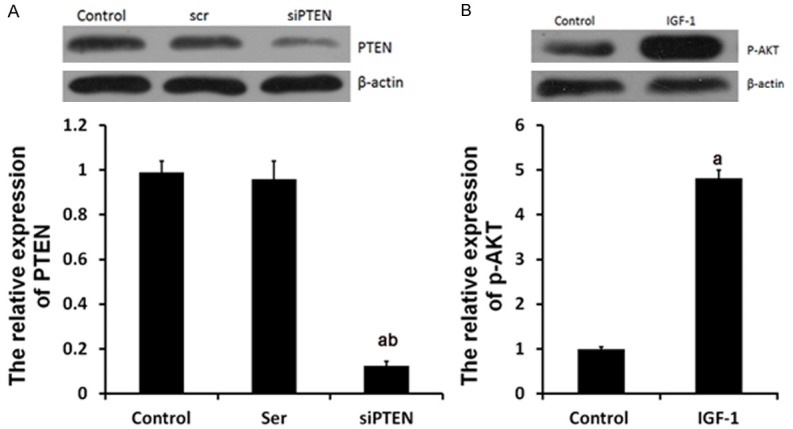
siPTEN and IGF-1 affected the expression of PTEN and p-AKT. Ser group, cells were treated with 50 nM scramble control siPTEN for 18 h. siPTEN group, cells were treated with siPTEN. IGF-1 group, cells were treated with IGF-1. Protein expression of PTEN (A) and p-AKT (B) were examined by western blot analysis, respectively. aP<0.01 vs. control group, bP<0.01 vs. Ser group.
Cardiac H/R injury often results in cardiomyocyte apoptosis. To gain a better understanding of the molecular mechanism of TMZ on myocardial H/R injury, the present study focused on myocardial cell apoptosis. The Bcl-2 family plays a crucial role on the regulation of apoptosis. Bcl-2, the key member in Bcl-2 family, suppresses apoptosis and subsequently enhances cell survival. Bax protein, as a homolog of bcl-2, could bind to bcl-2 forming bax/bcl-2 heterodimers or to itself forming bax/bax homodimers and promote cell death through apoptosis. In this study, western blot analysis was applied to determine the expression levels of Bcl-2 and Bax protein, two key members in the Bcl-2 family. As shown in 5B, the protein expression level of Bcl-2 was decreased and Bax was increased in the H/R group compared with the control group. So, the Bax/Bcl-2 ratio was increased, which were reverted by TMZ in the H/R+TMZ group. This result indicated that Bcl-2 family members involved TMZ-induced anti-apoptosis in H/R-treated cells. Additionally, miR-21 inhibitor significantly upregulated Bax/Bcl-2 ratio in H9C2 cells with the treatment of H/R and, indicating that miR-21 silencing reverted the activity of apoptotic signaling. siPTEN and IGF-1 treatment evidently decreased the expression levels of Bax protein and increased the expression levels of Bcl-2 protein, so the Bax/Bcl-2 ratio was decreased, compared with H/R+ TMZ+ miR-21inhibitor group (Figure 5).
Figure 5.
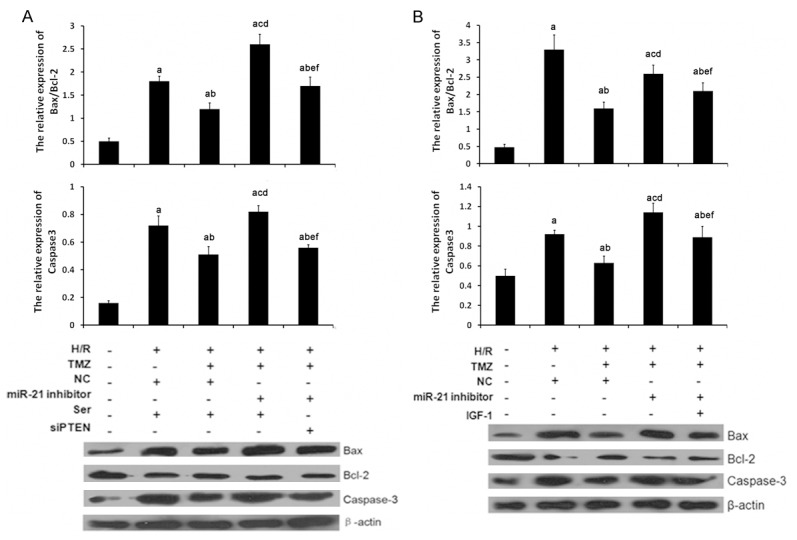
TMZ, miR-21, PTEN and p-AKT involved the regulation of H/R-induced apoptosis in H9C2 cells. H/R, cells were treated with H/R. TMZ, cells were treated with 10 μM TMZ for 48 h. NC, cells were treated with 0 μM TMZ. miR-21 inhibitor, cells were transfected with 50 nM miR-21 inhibitor. Ser, cells were treated with 50 nM scramble control siPTEN for 18 h. siPTEN, cells were treated with 50 nM siPTEN for 18 h. IGF-1, cells were treated with 10 nM for 12 h. A and B. caspase-3, Bax and Bcl-2 expression were examined by western blot analysis, respectively. (aP<0.01 vs. sham group, bP<0.01 and cP<0.05 vs. H/R group, dP<0.01 and eP<0.05 vs. H/R+TMZ group, cP<0.05 vs. H/R+TMZ+miR-21 inhibitor group).
Additionally, the expression levels of caspase-3, as a downstream effector of Bcl-2, were also determined in each group. As shown in Figure 5, the expression levels of caspase-3 were also upregulated in the H/R group as compared with those in the sham group, which were reverted by TMZ in the H/R+TMZ group. miR-21 silencing significantly upregulated caspase-3 expression in H/R+TMZ+ miR-21inhibitor group as compared with those in the H/R+TMZ group, which were reverted with the treatment of siPTEN and IGF-1 in H/R+TMZ+ miR-21inhibitor +siPTEN group and H/R+TMZ+ miR-21inhibitor + IGF-1 group.
These findings supported the idea that miR-21 involved TMZ induced anti-apoptotic effect via regulating the expression of PTEN and p-AKT, which further suppresses pro-apoptotic factors caspase-3 and Bax/Bcl-2 ratio.
Discussion
In the present study, we investigated the signaling pathways by which TMZ triggers anti-apoptosis effect in H/R-induced myocardial injury. Specifically, we focused on the potential role of miR-21 induced PTEN/p-AKT pathway in TMZ-induced protection.
Several drugs have been studied in the protection of the myocardium from H/R injury. The anti-ischemic metabolic drug trimetazidine (TMZ) has been observed to protect the myocardium cells and exert its beneficial effects without altering the hemodynamic function of the heart [5]. TMZ decreases ischemic stress and improves cardiac performance during ischemia by reducing fatty acid oxidation which further selectively inhibits mitochondrial 3-ketoacyl CoA thiolase [6]. It was reported that TMZ could affect mitochondrial function and protect the heart from prolonged ischemia-reperfusion injury [9,17]. TMZ also showed cytoprotective effect in several models of myocardial infarction [8]. Recently, it has been shown that TMZ protected heart against myocardial ischemia/reperfusion injury by suppressing myocardial apoptosis [11,18]. In this study, we further revealed the anti-apoptosis of TMZ in H/R-induced myocardial injury by detecting the protein expression levels of several key apoptotic effectors. Consistent with these results, our result showed that including Bcl-2, Bax and caspase-3, in cardiomyocyte H/R injury. Furthermore, to reveal anti-apoptosis mechanism of TMZ, miR-21 and PTEN/p-AKT pathway was studied in this work.
miR-21 is highly expressed in the adult heart and has a crucial role in the regulation of normal biological functions of the myocardial tissue [19]. Previously, accumulating evidence has shown that miR-21 protected cardiomyocyte from ischemia-induced apoptosis. miR-21 inhibited left ventricular remodeling in the early phase of I/R injury by suppressing cell apoptosis in rats [15]. PTEN has previously been shown to be a direct target of miR-21 [20,21], and downregulate the regulation of Akt signaling, which acts as a key regulator in cellular survival. AKT was reported to inhibit hypoxia-induced apoptosis through miR-21-dependent suppression of FasL [22]. Consistent with these reports, we observed that MiR-21 regulated PTEN and Akt expression in H9C2 cells. Recently, it was revealed that miR-21 had a protective role in I/R- and H/R-induced cardiomyocyte apoptosis via the PTEN/Akt-dependent mechanism [16]. In this study, we observed that TMZ interfered miR-21, PTEN and p-AKT expression and miR-21 inhibitor restored TMZ-interfered PTEN and p-AKT expression in H/R-treated H9C2 cells. These results indicated that miR-21-dependent regulation of PTEN and p-Akt involved protective effects of trimetazidine on H/R injury.
In conclusion, to the best of our knowledge, the present study was the first to reveal that TMZ protects against H/R-induced myocardial cell apoptosis, most likely by inducing miR-21 expression and therefore inhibiting PTEN and upregulating the activity of the Akt signaling pathway, which further suppresses pro-apoptotic factors such as caspase-3, while increase anti-apoptotic factors, including Bcl-2/Bax.
Disclosure of conflict of interest
None.
References
- 1.Zhao L, Peng DQ, Zhang J, Song JQ, Teng X, Yu YR, Tang CS, Qi YF. Extracellular signal-regulated kinase 1/2 activation is involved in intermedin1-53 attenuating myocardial oxidative stress injury induced by ischemia/reperfusion. Peptides. 2012;33:329–335. doi: 10.1016/j.peptides.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 2.Demir T, Turgut B, Ozercan I, Gul FC, Ilhan N, Celiker U. Trimetazidine for prevention of induced ischemia and reperfusion of guinea pig retina. Clin Ophthalmol. 2010;4:21–26. doi: 10.2147/opth.s8409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unal D, Karatas OF, Savas M, Yeni E, Keser BS, Verit A, Erel O, Bitiren M. Protective effects of trimetazidine on testicular ischemia-reperfusion injury in rats. Urol Int. 2007;78:356–362. doi: 10.1159/000100842. [DOI] [PubMed] [Google Scholar]
- 4.Sulikowski T, Domanski L, Ciechanowski K, Adler G, Pawlik A, Safranow K, Dziedziejko V, Chlubek D, Ciechanowicz A. Effect of trimetazidine on xanthine oxidoreductase expression in rat kidney with ischemia--reperfusion injury. Arch Med Res. 2008;39:459–462. doi: 10.1016/j.arcmed.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Lopaschuk GD, Barr R, Thomas PD, Dyck JR. Beneficial effects of trimetazidine in ex vivo working ischemic hearts are due to a stimulation of glucose oxidation secondary to inhibition of long-chain 3-ketoacyl coenzyme a thiolase. Circ Res. 2003;93:e33–37. doi: 10.1161/01.RES.0000086964.07404.A5. [DOI] [PubMed] [Google Scholar]
- 6.Kantor PF, Lucien A, Kozak R, Lopaschuk GD. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res. 2000;86:580–588. doi: 10.1161/01.res.86.5.580. [DOI] [PubMed] [Google Scholar]
- 7.Aubert A, Bernard C, Clauser P, Harpey C, Vaudry H. [A cellular anti-ischemic agent, trimetazidine prevents the deleterious effects of oxygen free-radicals on the internal ear] . Ann Otolaryngol Chir Cervicofac. 1990;107(Suppl 1):28–35. [PubMed] [Google Scholar]
- 8.Pantos C, Bescond-Jacquet A, Tzeis S, Paizis I, Mourouzis I, Moraitis P, Malliopoulou V, Politi ED, Karageorgiou H, Varonos D, Cokkinos DV. Trimetazidine protects isolated rat hearts against ischemia-reperfusion injury in an experimental timing-dependent manner. Basic Res Cardiol. 2005;100:154–160. doi: 10.1007/s00395-004-0505-4. [DOI] [PubMed] [Google Scholar]
- 9.Guarnieri C, Muscari C. Effect of trimetazidine on mitochondrial function and oxidative damage during reperfusion of ischemic hypertrophied rat myocardium. Pharmacology. 1993;46:324–331. doi: 10.1159/000139070. [DOI] [PubMed] [Google Scholar]
- 10.Tritto I, Wang P, Kuppusamy P, Giraldez R, Zweier JL, Ambrosio G. The anti-anginal drug trimetazidine reduces neutrophil-mediated cardiac reperfusion injury. J Cardiovasc Pharmacol. 2005;46:89–98. doi: 10.1097/01.fjc.0000164091.81198.a3. [DOI] [PubMed] [Google Scholar]
- 11.Ruixing Y, Wenwu L, Al-Ghazali R. Trimetazidine inhibits cardiomyocyte apoptosis in a rabbit model of ischemia-reperfusion. Transl Res. 2007;149:152–160. doi: 10.1016/j.trsl.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Hullinger TG, Montgomery RL, Seto AG, Dickinson BA, Semus HM, Lynch JM, Dalby CM, Robinson K, Stack C, Latimer PA, Hare JM, Olson EN, van Rooij E. Inhibition of miR-15 protects against cardiac ischemic injury. Circ Res. 2012;110:71–81. doi: 10.1161/CIRCRESAHA.111.244442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu LF, Liang Z, Lv ZR, Liu XH, Bai J, Chen J, Chen C, Wang Y. MicroRNA-15a/b are up-regulated in response to myocardial ischemia/reperfusion injury. J Geriatr Cardiol. 2012;9:28–32. doi: 10.3724/SP.J.1263.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang B, Hong J, Xiao J, Zhu X, Ni X, Zhang Y, He B, Wang Z. Involvement of miR-1 in the protective effect of hydrogen sulfide against cardiomyocyte apoptosis induced by ischemia/reperfusion. Mol Biol Rep. 2014;41:6845–53. doi: 10.1007/s11033-014-3570-2. [DOI] [PubMed] [Google Scholar]
- 15.Qin Y, Yu Y, Dong H, Bian X, Guo X, Dong S. MicroRNA 21 inhibits left ventricular remodeling in the early phase of rat model with ischemia-reperfusion injury by suppressing cell apoptosis. Int J Med Sci. 2012;9:413–423. doi: 10.7150/ijms.4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Q, Yang K, Li A. microRNA-21 protects against ischemia-reperfusion and hypoxia-reperfusion-induced cardiocyte apoptosis via the phosphatase and tensin homolog/Akt-dependent mechanism. Mol Med Rep. 2014;9:2213–2220. doi: 10.3892/mmr.2014.2068. [DOI] [PubMed] [Google Scholar]
- 17.Argaud L, Gomez L, Gateau-Roesch O, Couture-Lepetit E, Loufouat J, Robert D, Ovize M. Trimetazidine inhibits mitochondrial permeability transition pore opening and prevents lethal ischemia-reperfusion injury. J Mol Cell Cardiol. 2005;39:893–899. doi: 10.1016/j.yjmcc.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Yin RX, Liang WW, Liu TW, Tao XZ, Zhu LG, Al-Ghazali R. Inhibitory effect of trimetazidine on cardiac myocyte apoptosis in rabbit model of ischemia-reperfusion. Chin Med Sci J. 2004;19:242. [PubMed] [Google Scholar]
- 19.Cheng Y, Ji R, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. MicroRNAs are aberrantly expressed in hypertrophic heart: do they play a role in cardiac hypertrophy? Am J Pathol. 2007;170:1831–1840. doi: 10.2353/ajpath.2007.061170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–658. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu CZ, Liu W, Zheng Y, Su JM, Li JJ, Yu L, He XD, Chen SS. PTEN and PDCD4 are bona fide targets of microRNA-21 in human cholangiocarcinoma. Chin Med Sci J. 2012;27:65–72. [PubMed] [Google Scholar]
- 22.Sayed D, He M, Hong C, Gao S, Rane S, Yang Z, Abdellatif M. MicroRNA-21 is a downstream effector of AKT that mediates its antiapoptotic effects via suppression of Fas ligand. J Biol Chem. 2010;285:20281–20290. doi: 10.1074/jbc.M110.109207. [DOI] [PMC free article] [PubMed] [Google Scholar]


